Do Salamanders Limit the Abundance of Groundwater Invertebrates in Subterranean Habitats?
Abstract
1. Introduction
2. Materials and Methods
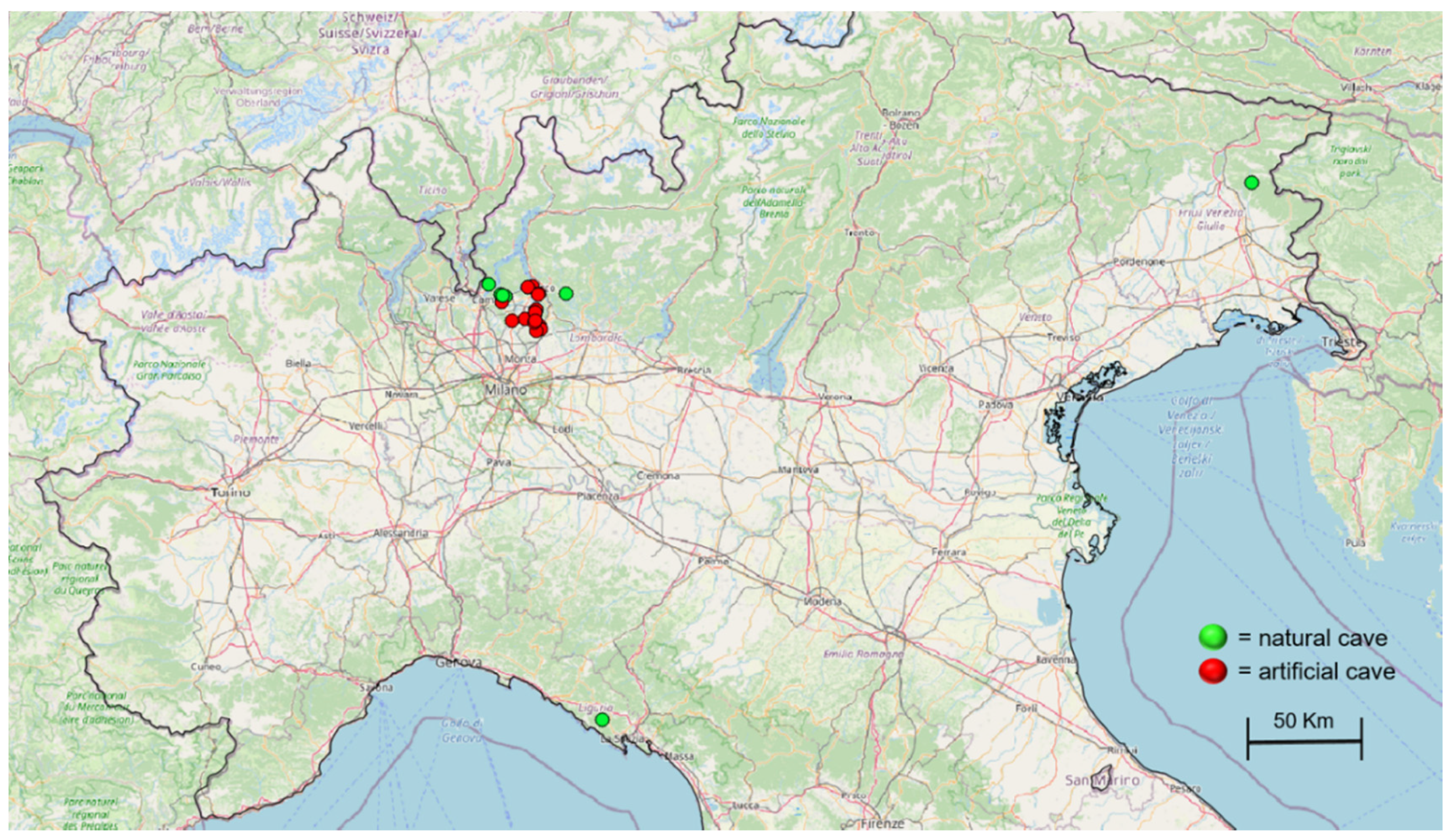
2.1. Sampling Design
2.2. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wells, K.D. The Ecology and Behaviour of Amphibians; The University of Chicago Press: Chicago, IL, USA, 2007. [Google Scholar]
- Davic, R.D.; Welsh, H.H. On the ecological roles of salamanders. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 405–434. [Google Scholar] [CrossRef]
- Wilbur, H.M. Experimental ecology of food webs: Complex systems in temporary ponds-The Robert H. MacArthur Award Lecture-Presented 31 July 1995 Snowbird. Utah. Ecol. 1997, 78, 2279–2302. [Google Scholar]
- Anthony, C.; Hickerson, C.M.; Walton, B.M. Eastern Red-backed salamanders regulate top-down effects in a temperate forest-floor community. Herpetologica 2017, 73, 180–189. [Google Scholar]
- Mancinelli, G.; Costantini, M.L.; Rossi, L. Top-down control of reed detritus processing in a lake littoral zone: Experimental evidence of a seasonal compensation between fish and invertebrate predation. Int. Rev. Hydrobiol. 2007, 92, 117–134. [Google Scholar] [CrossRef]
- Petranka, J.W. Salamanders of the United States and Canada. Washington; Smithsonian Instute Press: Washington, DC, USA, 1998. [Google Scholar]
- Crowther, T.W.; Stanton, D.W.; Thomas, S.M.; A’Bear, A.D.; Hiscox, J.; Jones, T.H.; Voriskova, J.; Baldrian, P.; Boddy, L. Top-down control of soil fungal community composition by a globally distributed keystone consumer. Ecology 2013, 94, 2518–2528. [Google Scholar] [CrossRef]
- Walker, D.M.; Murray, C.M.; Talbert, D.; Tinker, P.; Graham, S.P.; Crowther, T.W. A salamander’s top down effect on fungal communities in a detritivore ecosystem. FEMS Microbiol. Ecol. 2018, 94, fiy168. [Google Scholar] [CrossRef]
- Fuge, R.; Perkins, W. Aluminium and heavy metals in potable waters of the north Ceredigion area, mid-Wales. Environ. Geochem. Health 1991, 13, 56–65. [Google Scholar] [CrossRef]
- Geldreich, E.E. Drinking water microbiology--new directions toward water quality enhancement. Int. J. Food Microbiol. 1989, 9, 295–312. [Google Scholar] [CrossRef]
- Culver, D.C.; Pipan, T. The Biology of Caves and Other Subterranean Habitats, 2nd ed.; Oxford University Press: New York, NY, USA, 2019. [Google Scholar]
- Romero, A. Cave Biology; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Botello, A.; Iliffe, T.M.; Alvarez, F.; Juan, C.; Pons, J.; Jaume, D. Historical biogeography and phylogeny of Typhlatya cave shrimps (Decapoda: Atyidae) based on mitochondrial and nuclear data. J. Biogeogr. 2013, 40, 594–607. [Google Scholar] [CrossRef]
- Trontelj, P.; Blejec, A.; Fiser, C. Ecomorphological Convergence of Cave Communities. Evolution 2012, 66, 3852–3865. [Google Scholar] [CrossRef]
- Howarth, F.G.; Moldovan, O.T. The ecological classification of cave animals and their adaptations. In Cave Ecology; Moldovan, O.T., Kováč, L., Halse, S., Eds.; Springer: Berlin, Germany, 2018; pp. 41–67. [Google Scholar]
- Gorički, S.; Niemiller, M.L.; Fenolio, D.B.; Gluesenkamp, A.G. Salamanders. In Encyclopedia of Caves; White, W.B., Culver, D.C., Pipan, T., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 871–884. [Google Scholar]
- Fenolio, D.B.; Graening, G.O.; Collier, B.A.; Stout, J.F. Coprophagy in a cave-adapted salamander; the importance of bat guano examined through nutritional and stable isotope analyses. Proc. R. Soc. B Biol. Sci. 2006, 273, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Ficetola, G.F.; Lunghi, E.; Canedoli, C.; Padoa-Schioppa, E.; Pennati, R.; Manenti, R. Differences between microhabitat and broad-scale patterns of niche evolution in terrestrial salamanders. Sci. Rep. 2018, 8, 10575. [Google Scholar] [CrossRef] [PubMed]
- Lunghi, E.; Cianferoni, F.; Ceccolini, F.; Mulargia, M.; Cogoni, R.; Barzaghi, B.; Cornago, L.; Avitabile, D.; Veith, M.; Manenti, R.; et al. Field-recorded data on the diet of six species of European Hydromantes cave salamanders. Sci. Data 2018, 5, 180083. [Google Scholar] [CrossRef]
- Niemiller, M.L.; Osbourn, M.S.; Fenolio, D.B.; Pauley, T.K.; Miller, B.T.; Holsinger, J.R. Conservation Status and Habitat Use of the West Virginia Spring Salamander (Gyrinophilus Subterraneus) and Spring Salamander (G. Porphyriticus) in General Davis Cave, Greenbrier Co., West Virginia. Herpetol. Conserv. Biol. 2010, 5, 32–43. [Google Scholar]
- Manenti, R.; Ficetola, G.F.; Marieni, A.; de Bernardi, F. Caves as breeding sites for Salamandra salamandra: Habitat selection, larval development and conservation issues. N. West. J. Zool. 2011, 7, 304–309. [Google Scholar]
- Manenti, R.; Ficetola, G.F. Salamanders breeding in subterranean habitats: Local adaptations or behavioural plasticity? J. Zool. 2013, 289, 182–188. [Google Scholar] [CrossRef]
- Lunghi, E.; Manenti, R.; Ficetola, G.F. Seasonal variation in microhabitat of salamanders: Environmental variation or shift of habitat selection? PeerJ 2015, 3, e1122. [Google Scholar] [CrossRef]
- Salvidio, S.; Costa, A.; Oneto, F.; Pastorino, M.V. Variability of a subterranean prey-redator community in space and time. Diversity 2020, 12, 17. [Google Scholar] [CrossRef]
- Babik, W.; Rafinski, J. Amphibian breeding site characteristics in the Western Carpathians, Poland. Herpetol. J. 2001, 11, 41–51. [Google Scholar]
- Manenti, R.; Melotto, A.; Denoël, M.; Ficetola, G.F. Amphibians breeding in refuge habitats have larvae with stronger antipredator responses. Anim. Behav. 2016, 118, 115–121. [Google Scholar] [CrossRef]
- Steinfartz, S.; Weitere, M.; Tautz, D. Tracing the first step to speciation: Ecological and genetic differentiation of a salamander population in a small forest. Mol. Ecol. 2007, 16, 4550–4561. [Google Scholar] [CrossRef] [PubMed]
- Limongi, L.; Ficetola, G.F.; Romeo, G.; Manenti, R. Environmental factors determining growth of salamander larvae: A field study. Curr. Zool. 2015, 61, 421–427. [Google Scholar] [CrossRef]
- Manenti, R.; Lunghi, E.; Ficetola, G.F. Cave exploitation by an usual epigean species: A review on the current knowledge on fire salamander breeding in cave. Biogeographia 2017, 32, 31–46. [Google Scholar] [CrossRef]
- Manenti, R.; Pennati, R.; Ficetola, G.F. Role of density and resource competition in determining aggressive behaviour in salamanders. J. Zool. 2015, 296, 270–277. [Google Scholar] [CrossRef]
- Manenti, R.; Siesa, M.E.; Ficetola, G.F. Odonata occurence in caves: Active or accidentals? A new case study. J. Cave Karst Stud. 2013, 75, 205–209. [Google Scholar] [CrossRef]
- Barzaghi, B.; Ficetola, G.F.; Pennati, R.; Manenti, R. Biphasic predators provide biomass subsidies in small freshwater habitats: A case study of spring and cave pools. Freshw. Biol. 2017, 62, 1637–1644. [Google Scholar] [CrossRef]
- Culver, D.C.; Pipan, T. Shallow Subterranean Habitats Ecology, Evolution, and Conservation; Oxford University Press: New York, NY, USA, 2014. [Google Scholar]
- Manenti, R.; Pezzoli, E. Think of what lies below, not only of what is visible above, or: A comprehensive zoological study of invertebrate communities of spring habitats. Eur. Zool. J. 2019, 86, 272–279. [Google Scholar] [CrossRef]
- Pezzoli, E. I Molluschi crenobionti e stigobionti presenti in Italia. Censimento delle stazioni: VII aggiornamento. Quad. Della Civ. Stn. Idrobiol. Milano 1996, 21, 111–118. [Google Scholar]
- Lunghi, E.; Corti, C.; Mulargia, M.; Zhao, Y.; Manenti, R.; Ficetola, G.F.; Veith, M. Cave morphology, microclimate and abundance of five cave predators from the Monte Albo (Sardinia, Italy). Biodivers. Data J. 2020, 8, e48623. [Google Scholar] [CrossRef]
- Manenti, R.; de Bernardi, F.; Ficetola, G.F. Pastures vs forests: Do traditional pastoral activities negatively affect biodiversity? The case of amphibians communities. N. West. J. Zool. 2013, 9, 284–292. [Google Scholar]
- Arcangeli, A. Note su alcuni sferomidi cavernicoli italiani. Bollettino dei Musei di zoologia e anatomia comparata della R. Univ. Di Torino 1942, 49, 117–125. [Google Scholar]
- Stoch, F. Isopodi ed anfipodi (Crustacea, Malacostraca) della Provincia di Bergamo: Note sulle specie rinvenute nelle grotte e nelle sorgenti. In I Molluschi Delle Sorgenti e Delle ’Acque Sotterranee’, IX Aggiornamento al Censimento; Pezzoli, E., Spelta, F., Eds.; Monografie di Natura Bresciana: Brescia, Italy, 2000; pp. 231–241. [Google Scholar]
- Luštrik, R.; Turjakl, M.; Kralj-Fišer, S.; Fišer, C. Coexistence of surface and cave amphipods in an ecotone environment. Contrib. Zool. 2011, 80, 133–141. [Google Scholar] [CrossRef]
- Fišer, C.; Kovačec, Ž.; Pustovrh, M.; Trontelj, P. The role of predation in the diet of Niphargus (Amphipoda: Niphargidae). Speleobiol. Notes 2010, 2, 4–6. [Google Scholar]
- Manenti, R.; Barzaghi, B.; Lana, E.; Stocchino, G.A.; Manconi, R.; Lunghi, E. The stenoendemic cave-dwelling planarians (Platyhelminthes, Tricladida) of the Italian Alps and Apennines: Conservation issues. J. Nat. Conserv. 2018, 45, 90–97. [Google Scholar] [CrossRef]
- Barker, R.J.; Schofield, M.R.; Link, W.A.; Sauer, J.R. On the reliability of N-Mixture models for count data. Biometrics 2017, 74, 369–377. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsenn, A.; Skaug, H.J.; Maechler, M.; Bolker, B. glmmTMB Balances Speed and Flexibility Among Packages for Zero-inflatedn Generalized Linear Mixed Modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An {R} Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Manenti, R.; Denoël, M.; Ficetola, G.F. Foraging plasticity favours adaptation to new habitats in fire salamanders. Anim. Behav. 2013, 86, 375–382. [Google Scholar] [CrossRef]
- Manenti, R.; Ficetola, G.F.; Bianchi, B.; de Bernardi, F. Habitat features and distribution of Salamandra salamandra in underground springs. Acta Herpetol. 2009, 4, 143–151. [Google Scholar]
- Melotto, A.; Ficetola, G.F.; Manenti, R. Safe as a cave? Intraspecific aggressiveness rises in predator-devoid and resource-depleted environments. Behav. Ecol. Sociobiol. 2019, 73, 68. [Google Scholar] [CrossRef]
- Costa, A.; Baroni, D.; Romeno, A.; Salvidio, S. Individual diet variation in Salamandra salamandra (Linnaeus, 1758) larvae in a Mediterranean stream. Salamandra 2017, 53, 148–152. [Google Scholar]
- Reinhardt, T.; Steinfartz, S.; Paetzold, A.; Weitere, M. Linking the evolution of habitat choice to ecosystem functioning: Direct and indirect effects of pond-reproducing fire salamanders on aquatic-terrestrial subsidies. Oecologia 2013, 173, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Salvidio, S.; Romano, A.; Baroni, D. Larval diet of Salamandra salamandra (L., 1758): Preliminary results on prey selection and feeding strategy. In Proceedings of the Atti X congresso Nazionale della Societas Herpetologica Italica, Genova, Italy, 15–18 October 2014; Doria, G., Poggi, R., Salvidio, S., Tavano, M., Eds.; Ianieri Edizioni: Pescara, Italy, 2014; pp. 33–38. [Google Scholar]
- Mosslacher, F. Subsurface dwelling crustaceans as indicators of hydrological conditions, oxygen concentrations, and sediment structure in an alluvial aquifer. Int. Rev. Hydrobiol. 1998, 83, 349–364. [Google Scholar] [CrossRef]
- Durkota, J.M.; Wood, P.J.; Johns, T.; Thompson, J.R.; Flower, R.J. Distribution of macroinvertebrate communities across surface and groundwater habitats in response to hydrological variability. Fundam. Appl. Limnol. 2019, 193, 79–92. [Google Scholar] [CrossRef]
- Galassi, D.M.P.; Stoch, F.; Fiasca, B.; di Lorenzo, T.; Gattone, E. Groundwater biodiversity patterns in the Lessinian Massif of northern Italy. Freshw. Biol. 2009, 54, 830–847. [Google Scholar] [CrossRef]
- Manenti, R.; Lunghi, E.; Ficetola, G.F. Distribution of spiders in cave twilight zone depends on microclimatic features and trophic supply. Invertebr. Biol. 2015, 134, 242–251. [Google Scholar] [CrossRef]
- Lunghi, E.; Manenti, R.; Ficetola, G.F. Cave features, seasonality and subterranean distribution of non-obligate cave dwellers. PeerJ 2017, 5, e3169. [Google Scholar] [CrossRef]
- Salvidio, S.; Palumbi, G.; Romano, A.; Costa, A. Safe caves and dangerous forests? Predation risk may contribute to salamander colonization of subterranean habitats. Sci. Nat. 2017, 104, 20. [Google Scholar] [CrossRef]
- Fišer, C. Niphargus—A model system for evolution and ecology. In Encyclopedia of Caves; White, W.B., Culver, D.C., Pipan, T., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 746–755. [Google Scholar]
- Väinölä, R.; Witt, J.D.S.; Grabowski, M.; Bradbury, J.H.; Jażdżewski, K.; Sket, B. Global diversity of amphipods (Amphipoda; Crustacea) in freshwater. Hydrobiologia 2008, 595, 241–255. [Google Scholar] [CrossRef]
- Marković, V.; Novaković, B.; Ilić, M.; Nikolić, V. Epigean Niphargids in Serbia: New Records of Niphargus valachicus Dobreanu & Manolache, 1933 (Amphipoda: Niphargidae), with Notes on its Ecological Preferences. Acta Zool. Bulg. 2018, 70, 45–50. [Google Scholar]
- Fišer, C.; Keber, R.; Kerezi, V.; Moskric, A.; Palandancic, A.; Petkovska, V.; Potocnik, H.; Sket, B. Coexistence of species of two amphipod genera: Niphargus timavi (Niphargidae) and Gammarus fossarum (Gammaridae). J. Nat. Hist. 2007, 41, 2641–2651. [Google Scholar] [CrossRef]
- Fišer, Z.; Novak, L.; Lustrik, R.; Fiser, C. Light triggers habitat choice of eyeless subterranean but not of eyed surface amphipods. Sci. Nat. 2016, 103, 7. [Google Scholar] [CrossRef]
- Romeo, G.; Giovine, G.; Ficetola, G.F.; Manenti, R. Development of the fire salamander larvae at the altitudinal limit in Lombardy (north-western Italy): Effect of two cohorts occurrence on intraspecific aggression. N. West. J. Zool. 2015, 11, 234–240. [Google Scholar]
- Steinfartz, S.; Stemshorn, K.; Kuesters, D.; Tautz, D. Patterns of multiple paternity within and between annual reproduction cycles of the fire salamander (Salamandra salamandra) under natural conditions. J. Zool. 2006, 268, 1–8. [Google Scholar] [CrossRef]
- Manenti, R.; Barzaghi, B.; Tonni, G.; Ficetola, G.F.; Melotto, A. Even worms matter: Cave habitat restoration for a planarian species has increased prey availability but not population density. Oryx 2019, 53, 216–221. [Google Scholar] [CrossRef]
- Reynoldson, J.D.; Young, J.O. A key to the Freshwater Triclads of Britain and Ireland with Notes on Their Ecology; Freshwater Biological Association: Ambleside, UK, 2000. [Google Scholar]
- Gillespie, J.H. Application of stable isotope analysis to study temporal changes in foraging ecology in a highly endangered amphibian. PLoS ONE 2013, 8, 10. [Google Scholar] [CrossRef]
- Prevorčnik, S.; Verovnik, R.; Zagmajster, M.; Sket, B. Biogeography and phylogenetic relations within the Dinaric subgenus Monolistra (Microlistra) (Crustacea: Isopoda: Sphaeromatidae), with a description of two new species. Zool. J. Linn. Soc. 2010, 159, 1–21. [Google Scholar] [CrossRef]
- Mammola, S.; Piano, E.; Cardoso, P.; Vernon, P.; Dominguez, D.; Isaia, M. Climate change going deep: The effects of global climatic alterations on cave ecosystems. Anthr. Rev. 2019, 6, 98–116. [Google Scholar] [CrossRef]


| Variables | Estimate | SE | χ2 | P | |
|---|---|---|---|---|---|
| Niphargus | |||||
| Fire salamander larvae | −3.34 | 0.44 | 78.31 | <0.001 | |
| Distance from surface | <0.01 | <0.01 | 0.01 | 0.94 | |
| Maximum water depth | 0.01 | <0.01 | 4.29 | 0.03 | |
| Surveyed area | −0.05 | 0.05 | 1.043 | 0.30 | |
| Dendrocoelum | |||||
| Fire salamander larvae | −2.39 | 0.84 | 11.53 | <0.01 | |
| Distance from surface | <0.01 | <0.01 | 1.35 | 0.24 | |
| Maximum water depth | <−0.01 | 0.01 | 0.57 | 0.44 | |
| Surveyed area | 0.06 | 0.07 | 0.74 | 0.38 | |
| Monolistra | |||||
| Fire salamander larvae | <−0.01 | <0.01 | 6.24 | 0.01 | |
| Distance from surface | <0.01 | <0.01 | 4.71 | 0.02 | |
| Maximum water depth | <0.01 | <0.01 | 0.67 | 0.41 | |
| Surveyed area | <0.01 | <0.01 | 6.98 | <0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manenti, R.; Lunghi, E.; Barzaghi, B.; Melotto, A.; Falaschi, M.; Ficetola, G.F. Do Salamanders Limit the Abundance of Groundwater Invertebrates in Subterranean Habitats? Diversity 2020, 12, 161. https://doi.org/10.3390/d12040161
Manenti R, Lunghi E, Barzaghi B, Melotto A, Falaschi M, Ficetola GF. Do Salamanders Limit the Abundance of Groundwater Invertebrates in Subterranean Habitats? Diversity. 2020; 12(4):161. https://doi.org/10.3390/d12040161
Chicago/Turabian StyleManenti, Raoul, Enrico Lunghi, Benedetta Barzaghi, Andrea Melotto, Mattia Falaschi, and Gentile Francesco Ficetola. 2020. "Do Salamanders Limit the Abundance of Groundwater Invertebrates in Subterranean Habitats?" Diversity 12, no. 4: 161. https://doi.org/10.3390/d12040161
APA StyleManenti, R., Lunghi, E., Barzaghi, B., Melotto, A., Falaschi, M., & Ficetola, G. F. (2020). Do Salamanders Limit the Abundance of Groundwater Invertebrates in Subterranean Habitats? Diversity, 12(4), 161. https://doi.org/10.3390/d12040161








