Acknowledgments
For their invaluable assistance in the field and laboratory the authors thank A. Atencio, C. Bland, A. Corder, K. Davis, A. Duncanson, B. Fegley, J. Fegley, N. Fretz, B. Greene, P. Grindle, A. Haddon, K. Hinson, C. Logan, M. Litvaitis, A. Loken, I. Luther, B. Maser, C. Miller, S. Moran, J. Morton, J. Nguyen, E. Oldach, C. Peterson, R. Razo, A. Rodriguez, D. Rudulph, E. Smith, A. Sullivan, K. Tucker, B. VanDusen, E. Vorst, A. Walsh, A. Weiss, S. Whisonant, and A. Whitson. We also thank Gunde Rieger and the Instutute of Zoology, University of Innsbruck, for making available unpublished material left by Reinhard Rieger.
Figure 1.
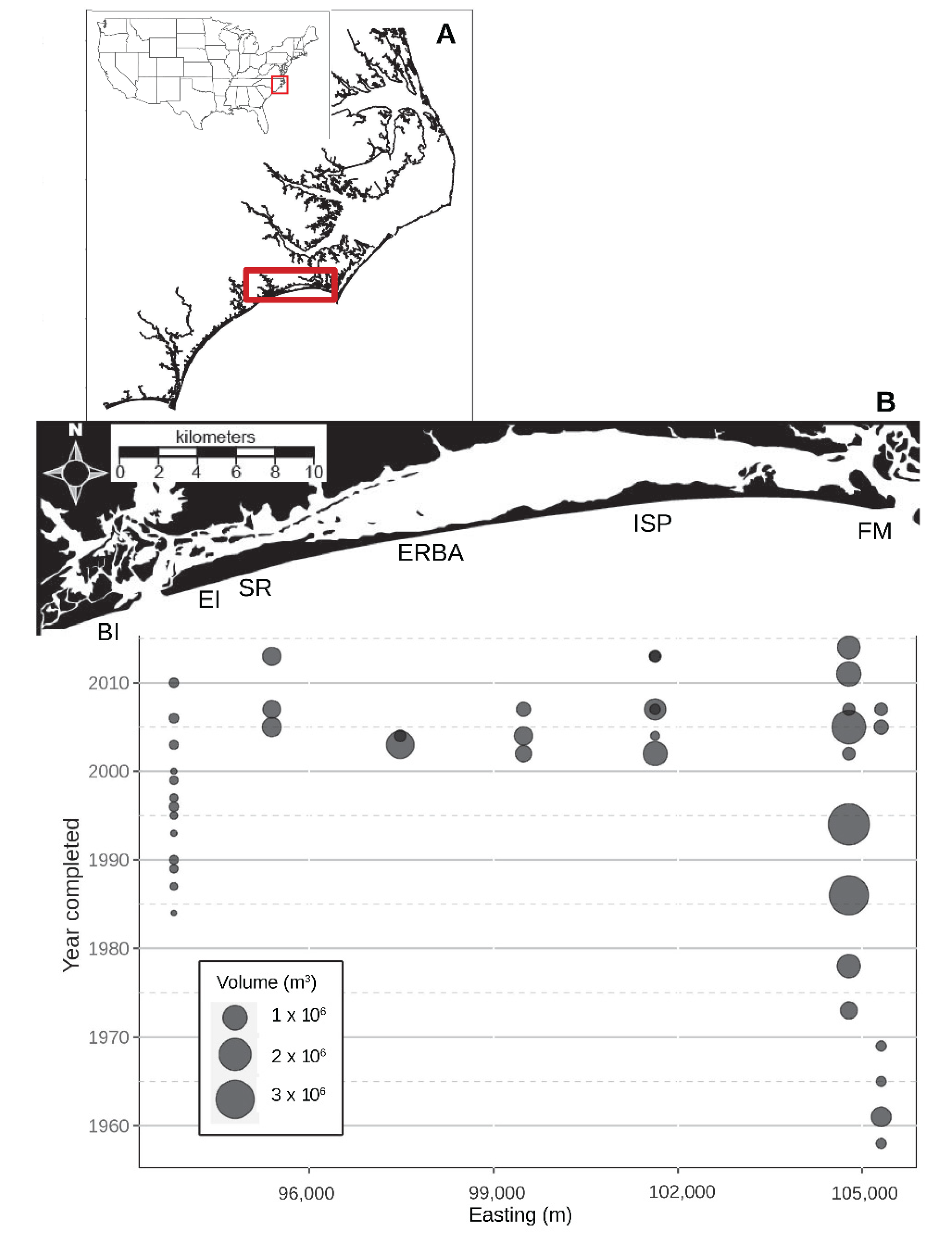
Location of Bogue Banks, North Carolina, USA (A) and the nourishment history of the island (B). The bubbles on the bubble plot below indicate the approximate midpoint of the spoil area for each recorded nourishment event as well as the approximate volume (m3) of material emplaced. Study beaches referred to in the text: BI—Bear Island; EI—Emerald Isle; SR—Spinnaker’s Reach; ERBA—Eastern Regional Beach Access; ISP—Iron Steamer Pier; and FM—Fort Macon.
Figure 1.
Location of Bogue Banks, North Carolina, USA (A) and the nourishment history of the island (B). The bubbles on the bubble plot below indicate the approximate midpoint of the spoil area for each recorded nourishment event as well as the approximate volume (m3) of material emplaced. Study beaches referred to in the text: BI—Bear Island; EI—Emerald Isle; SR—Spinnaker’s Reach; ERBA—Eastern Regional Beach Access; ISP—Iron Steamer Pier; and FM—Fort Macon.
Figure 2.
Comparison of the mean (±1 SE) grain size distributions of sediments used in the experimental cage study (unmanipulated, native sediments are in the central panel). Sediment fraction codes: VFS = very fine sand, FS = fine sand, MS = medium sand, CS = coarse sand, VCS = very coarse sand, VFG = very fine gravel, and FG = fine gravel.
Figure 2.
Comparison of the mean (±1 SE) grain size distributions of sediments used in the experimental cage study (unmanipulated, native sediments are in the central panel). Sediment fraction codes: VFS = very fine sand, FS = fine sand, MS = medium sand, CS = coarse sand, VCS = very coarse sand, VFG = very fine gravel, and FG = fine gravel.
Figure 3.
Comparisons of mean grain size (μm) versus sorting (φ) from the beach surface (0–10 cm) reported in separate studies spanning >60 years (
A). Teal samples were taken from beach areas that had never been nourished. Red samples were taken from beach areas that had been nourished from <1 to >10 years prior. Percent composition of sediment fractions for samples taken at EI and ISP in the 1970s and again, at each beach, in the 2010s (
B). Sediment fraction codes are the same as in
Figure 2.
Figure 3.
Comparisons of mean grain size (μm) versus sorting (φ) from the beach surface (0–10 cm) reported in separate studies spanning >60 years (
A). Teal samples were taken from beach areas that had never been nourished. Red samples were taken from beach areas that had been nourished from <1 to >10 years prior. Percent composition of sediment fractions for samples taken at EI and ISP in the 1970s and again, at each beach, in the 2010s (
B). Sediment fraction codes are the same as in
Figure 2.
Figure 4.
Comparison of median grain size (A) and sorting (B), both measured in phi, from the beach surface to depth at several intertidal heights at Iron Steamer Pier Beach. The historical data (blue) are from Lindgren (1972). The 2013 and 2014 (yellow and green, respectively) data, collected from the same location as the Lindgren data, are means and have standard errors associated with them. Tidal heights: MHW = mean high water; MHWN = mean high water neap; MTL = mean tidal level, MLWN = mean low water neap; and MLW = mean low water.
Figure 4.
Comparison of median grain size (A) and sorting (B), both measured in phi, from the beach surface to depth at several intertidal heights at Iron Steamer Pier Beach. The historical data (blue) are from Lindgren (1972). The 2013 and 2014 (yellow and green, respectively) data, collected from the same location as the Lindgren data, are means and have standard errors associated with them. Tidal heights: MHW = mean high water; MHWN = mean high water neap; MTL = mean tidal level, MLWN = mean low water neap; and MLW = mean low water.
Figure 5.
Relative grain size distributions of sediments collected in the low intertidal of several Bogue Banks beaches in June of 2006: (
A) beach codes same as in
Figure 1, grain size category codes same as in
Figure 2. Panels (
B–
D) illustrate some sediment parameters for the “finer” and “coarser” samples collected from each beach. Green lines indicate that the respective value is lower in “finer” sediment samples, red lines indicate higher values in the “finer” sediment samples.
Figure 5.
Relative grain size distributions of sediments collected in the low intertidal of several Bogue Banks beaches in June of 2006: (
A) beach codes same as in
Figure 1, grain size category codes same as in
Figure 2. Panels (
B–
D) illustrate some sediment parameters for the “finer” and “coarser” samples collected from each beach. Green lines indicate that the respective value is lower in “finer” sediment samples, red lines indicate higher values in the “finer” sediment samples.
Figure 6.
Mean (±1 standard error) vertical sediment penetrability of low intertidal sediments at random intervals alongshore several Bogue Banks beaches in June of 2006 (beach codes same as in
Figure 1).
Figure 6.
Mean (±1 standard error) vertical sediment penetrability of low intertidal sediments at random intervals alongshore several Bogue Banks beaches in June of 2006 (beach codes same as in
Figure 1).
Figure 7.
Historical comparison of the more abundant meiofaunal taxa (
A–
D) and grain size parameters (
E,
F) from samples collected in the same Iron Steamer Pier beach locations (tide and depth) in 1969 and 2013. Colors and tidal height abbreviations as in
Figure 5.
Figure 7.
Historical comparison of the more abundant meiofaunal taxa (
A–
D) and grain size parameters (
E,
F) from samples collected in the same Iron Steamer Pier beach locations (tide and depth) in 1969 and 2013. Colors and tidal height abbreviations as in
Figure 5.
Figure 8.
nMDS ordination of taxon abundances collected in samples from ISP beach intertidal in 1969 and 2013. “Minor” taxa here include tardigrades, archiannelids, juvenile bivalves, and juvenile polychaetes, all of which only occurred infrequently in low numbers. Non-metric scales serve to ease visual estimation of dissimilarity distances but are not associated with any specific units.
Figure 8.
nMDS ordination of taxon abundances collected in samples from ISP beach intertidal in 1969 and 2013. “Minor” taxa here include tardigrades, archiannelids, juvenile bivalves, and juvenile polychaetes, all of which only occurred infrequently in low numbers. Non-metric scales serve to ease visual estimation of dissimilarity distances but are not associated with any specific units.
Figure 9.
Univariate (A) and multivariate (B) comparisons of abundances of major taxa collected from the same tidal height at ISP in March 1976 and March 2012; non-metric scales serve to ease visual estimation of dissimilarity distances but are not associated with any specific units.
Figure 9.
Univariate (A) and multivariate (B) comparisons of abundances of major taxa collected from the same tidal height at ISP in March 1976 and March 2012; non-metric scales serve to ease visual estimation of dissimilarity distances but are not associated with any specific units.
Figure 10.
Univariate (A–D) comparisons of mean taxon abundances collected in low intertidal, surface finer and coarser sediment samples from four different beaches in 2006. Finer (E) and coarser (F) multivariate results are disaggregated to ease visual comparison; non-metric scales serve to ease visual estimation of dissimilarity distances but are not associated with any specific units.
Figure 10.
Univariate (A–D) comparisons of mean taxon abundances collected in low intertidal, surface finer and coarser sediment samples from four different beaches in 2006. Finer (E) and coarser (F) multivariate results are disaggregated to ease visual comparison; non-metric scales serve to ease visual estimation of dissimilarity distances but are not associated with any specific units.
Figure 11.
Univariate (A) and multivariate (B) comparisons of mean taxon abundances collected in low intertidal, surface finer and coarser sediment samples from ISP in 2012; non-metric scales serve to ease visual estimation of dissimilarity distances but are not associated with any specific units.
Figure 11.
Univariate (A) and multivariate (B) comparisons of mean taxon abundances collected in low intertidal, surface finer and coarser sediment samples from ISP in 2012; non-metric scales serve to ease visual estimation of dissimilarity distances but are not associated with any specific units.
Figure 12.
Abundances of major taxa (A–D) associated with finer and coarser sediments at depth into the beach from the sediment surface. Regression lines of ln abundance onto depth are partitioned by sediment type, coarser = solid, finer = dashed.
Figure 12.
Abundances of major taxa (A–D) associated with finer and coarser sediments at depth into the beach from the sediment surface. Regression lines of ln abundance onto depth are partitioned by sediment type, coarser = solid, finer = dashed.
Figure 13.
nMDS ordination of the abundances of turbellarian species found in finer and coarser sediments collected from the lower intertidal of EI beach in 2012; non-metric scales serve to ease visual estimation of dissimilarity distances but are not associated with any specific units. Species codes, “sp__”, reference to the sequence of species listed in
Table 7. Names of undescribed taxa used here are labels and not being made available for formal taxonomic purposes.
Figure 13.
nMDS ordination of the abundances of turbellarian species found in finer and coarser sediments collected from the lower intertidal of EI beach in 2012; non-metric scales serve to ease visual estimation of dissimilarity distances but are not associated with any specific units. Species codes, “sp__”, reference to the sequence of species listed in
Table 7. Names of undescribed taxa used here are labels and not being made available for formal taxonomic purposes.
Figure 14.
Mean (±1 SE) and observed abundances (dots) for major taxa (A–E) observed in the different treatments of the sediment selection experiment. Horizontal lines and associated probabilities (above each line) indicate results of separate Kruskal–Wallis comparisons of respective means. nMDS ordination of species assemblages associated with each experimental sediment type shown in (F); non-metric scales serve to ease visual estimation of dissimilarity distances but are not associated with any specific units. ns—p > 0.05, *—p < 0.05, **—p < 0.01, ***—p < 0.001.
Figure 14.
Mean (±1 SE) and observed abundances (dots) for major taxa (A–E) observed in the different treatments of the sediment selection experiment. Horizontal lines and associated probabilities (above each line) indicate results of separate Kruskal–Wallis comparisons of respective means. nMDS ordination of species assemblages associated with each experimental sediment type shown in (F); non-metric scales serve to ease visual estimation of dissimilarity distances but are not associated with any specific units. ns—p > 0.05, *—p < 0.05, **—p < 0.01, ***—p < 0.001.
Figure 15.
Results of metabarcoding meiofaunal samples collected from unnourished (BI) and previously nourished (ISP) beaches. Rarefaction curves derived from observed OTU’s for each beach (A). OTU reads (C) and number of OTU’s (D) observed at each tidal height (Sta I—MHW, Sta II—intermediate between MHW and MTL, Sta III—MTL, Sta V—MLW, Sta VI—wave swash) for each beach indicated along with probabilities of separate, paired t-test comparisons for each beach. nMDS ordination for the OTU assemblages (B); non-metric scales serve to ease visual estimation of dissimilarity distances but are not associated with any specific units.
Figure 15.
Results of metabarcoding meiofaunal samples collected from unnourished (BI) and previously nourished (ISP) beaches. Rarefaction curves derived from observed OTU’s for each beach (A). OTU reads (C) and number of OTU’s (D) observed at each tidal height (Sta I—MHW, Sta II—intermediate between MHW and MTL, Sta III—MTL, Sta V—MLW, Sta VI—wave swash) for each beach indicated along with probabilities of separate, paired t-test comparisons for each beach. nMDS ordination for the OTU assemblages (B); non-metric scales serve to ease visual estimation of dissimilarity distances but are not associated with any specific units.
Figure 16.
Mean (±1 SE) abundances of major taxa in the lower intertidal of sites directly receiving dredge spoil (impact sites), sites bordering the impact area (near control sites), and sites 3–5 km distant from nourishment activities. Time codes are: T0—before nourishment; T1—during nourishment; T2—the day after nourishment ended; and T3—5 weeks after nourishment ended.
Figure 16.
Mean (±1 SE) abundances of major taxa in the lower intertidal of sites directly receiving dredge spoil (impact sites), sites bordering the impact area (near control sites), and sites 3–5 km distant from nourishment activities. Time codes are: T0—before nourishment; T1—during nourishment; T2—the day after nourishment ended; and T3—5 weeks after nourishment ended.
Figure 17.
Mean (±1 SE) percent grain size composition of sediment samples taken from the distant and impact sites before, during, just after, and 5 weeks after nourishment.
Figure 17.
Mean (±1 SE) percent grain size composition of sediment samples taken from the distant and impact sites before, during, just after, and 5 weeks after nourishment.
Table 1.
Summary of the individual characteristics of the multiple meiofauna sampling projects conducted on Bogue Banks beaches that are reported in this study. Beaches: EI—Emerald Isle; ERBA—Eastern Regional Beach Access; FM—Fort Macon; SR—Spinnaker’s Reach; ISP—Iron Steamer Pier; PKS—Pine Knoll Shores; BI—Bear Island (not located on Bogue Banks). Tidal heights: MHW—mean high water; MWHN—mean high water neap; MTL—mean tide level; MLWN—mean low water neap; MLW—mean low water; STL—shallow subtidal; “Sediment-Selected” indicates whether equal numbers of replicate samples were collected after visually selecting for patently finer or coarser sediments. Numbers in the “Notes” column indicate which questions, defined in the introduction, were addressed by the respective study.
Table 1.
Summary of the individual characteristics of the multiple meiofauna sampling projects conducted on Bogue Banks beaches that are reported in this study. Beaches: EI—Emerald Isle; ERBA—Eastern Regional Beach Access; FM—Fort Macon; SR—Spinnaker’s Reach; ISP—Iron Steamer Pier; PKS—Pine Knoll Shores; BI—Bear Island (not located on Bogue Banks). Tidal heights: MHW—mean high water; MWHN—mean high water neap; MTL—mean tide level; MLWN—mean low water neap; MLW—mean low water; STL—shallow subtidal; “Sediment-Selected” indicates whether equal numbers of replicate samples were collected after visually selecting for patently finer or coarser sediments. Numbers in the “Notes” column indicate which questions, defined in the introduction, were addressed by the respective study.
| Year | Beach | Tidal Heights | Sediment Depths (cm) | Sampled | Sediment-Selected? | Notes | Source |
|---|
| 1969–1970 | ISP | supra- to sub-littoral | surface to water table | sediment, fauna | no | 1 | Lindgren, PhD thesis |
| 1969–1970 | EI, ISP | MHW–MLW | depended on tidal height | sediment, fauna | no | 1 | Rieger, Unpublished |
| 1976 | EI | MTL, MLWN | depended on tidal height | fauna | no | 1—resin slides | Rieger, Unpublished |
| 2006 | SR, ERBA, ISP, FM | MLW | 0–10 | sediment, fauna | yes | 2 | Fegley, this paper |
| 2008 | PKS, AB | MLW | 0–10 | sediment, fauna | no | 2 | Fegley, this paper |
| 2012 | EI | MTL | 0–5 | sediment, fauna | yes | 1, 3, 4—resin slides 1 | Smith III, this paper |
| 2012 | EI | MTL | 0–5 | sediment, fauna | yes | 3—microscope | Smith III, this paper |
| 2012 | EI | MHW–MLW | 0–2, 2–4, 4–10 | sediment | no | 1—for 1970 comparison | Smith III, this paper |
| 2013 | ISP | MHW–MLW | depended on tidal height | sediment | yes | 1, 3 | Smith III, this paper |
| 2013 | ISP | MHW–MLW | surface to water table | sediment, fauna | yes | 1, 3—for 1969–70 comparison | Fegley, this paper |
| 2014 | ISP | MTL–MLWN | depended on tidal height | sediment, fauna | yes | 3 | Fegley, this paper |
| 2017 | ISP, BI | MHW–sublittoral | depended on tidal height | sediment, fauna | no | 2, 4—metabar-coding | Smith III, this paper |
Table 2.
Results of separate, 2-way (year versus tidal heights) Scheirer–Ray–Hare tests comparing faunal or granulometric variables collected in a 1969 study and a repeat of that study in 2013. Probabilities < 0.05 are in bold.
Table 2.
Results of separate, 2-way (year versus tidal heights) Scheirer–Ray–Hare tests comparing faunal or granulometric variables collected in a 1969 study and a repeat of that study in 2013. Probabilities < 0.05 are in bold.
| Variable | Year | Tidal Ht. | Year × Tidal Ht. |
|---|
| Copepoda | 0.029 | 0.139 | 0.009 |
| Nematoda | 0.067 | 0.192 | 0.436 |
| turbellaria | 0.014 | 0.001 | 0.371 |
| Gastrotricha | 0.232 | 0.001 | 0.486 |
| median grain size | 0.004 | 0.628 | 0.829 |
| grain sorting | 0.249 | 0.213 | 0.076 |
Table 3.
Comparison of turbellarian species found within the last decade at ISP to those in collections made in the same location or nearby beaches in the 1970s. “BB” = found by Rieger at other sites on Bogue Banks; “nf” = not found; “OTU” = not observed alive, but present in OTUs from ISP (see
Section 3.5); “spp.” = at least two congeners appear in metabarcoding data. Names of undescribed taxa used here are labels and not being made available for formal taxonomic purposes.
Table 3.
Comparison of turbellarian species found within the last decade at ISP to those in collections made in the same location or nearby beaches in the 1970s. “BB” = found by Rieger at other sites on Bogue Banks; “nf” = not found; “OTU” = not observed alive, but present in OTUs from ISP (see
Section 3.5); “spp.” = at least two congeners appear in metabarcoding data. Names of undescribed taxa used here are labels and not being made available for formal taxonomic purposes.
| Clade | Rieger 1970 Survey | Contemporary Species List |
|---|
| Catenulida | BB | Retronectes atypica |
| Acoelomorpha | Anaperus gelb | Anaperus singularis |
| nf | Haploginaria schillingi |
| nf | Praeconvoluta cf. tigrina |
| BB | Paratomella rubra |
| Macrostomorpha | Myozona gelb | Myozona ISP spp. |
| BB | Paromalostomum sp. |
| nf | Psammomacrostomum spp. (OTU) |
| BB | Haplopharynx cf. rostratus |
| Prolecithophora | Plagiostomum sand | Plagiostomum “corculum” |
| Proseriata | Otoplanid 1 | Parotoplana “stately Oto” |
| Otoplanid II | Kataplana celeretrix |
| BB | Prosogynopora riseri |
| Paramonotus sp. | Monocelididae n.g., n.sp |
| Archimonocelis ISP | Monocelidid 4-testes |
| Monocelis bitestis | Monocelidid 2-testes |
| Nematoplana | Nematoplana spp. |
| Polystylifora sp. | Polystyliphora cf. karlingi spp. |
| nf | Prosogynopora riseri |
| nf | Cirrifera cf. xanthoderma |
Rhabdocoela:
Kalyptorhynchia | Cicerina “orthocirri” | Cicerina debrae |
| Cheliplana sp. | Cheliplana “blind October” |
| Cheliplanilla ISP | Cheliplanilla “schwanzi” |
| Carcharodorhynchus ISP | Carcharodorhynchus “ungleich ISP” |
| nf | Carcharodorhynchus “small” |
| Thylacorhynchus “schwanzi” | Thylacorhynchus “schwanzi” |
| Schizorhynchoides “atoptus” | Schizorhynchoides “lupus” |
| Proschizorhynchus “faeroennsis” | Carolinorhynchus follybeachensis |
| Kalypto macropharynx | Lehardyia alleithoros |
| nf | Lehardyia sp. 2 |
| Kalypto blind ISP | nf |
| nf | Karkinorhynchus “carolinensis” |
| Proschizo juvenile | Proschizorhynchella “shaunae” |
| Uncinorhynchus sp. | Drepanorhynchides cf. hastatus |
| Neognathorhynchus sp. | Gnathorhynchus “caudafiliformis” |
| Eukalypto spitz | Placorhynchus cf. doei? |
| Eukalypto spirale | nf |
| Eukalypto riese | Eukalypto riese |
| nf | Cystiplana cf. rubra |
| BB | Eukalypto schrag |
| Rhabdocoela: Dalytyphloplanida | Promesosto juvenile | Promesostoma ISP |
| Rüssekopf ISP | Coronhelmis sp. |
| BB | Dalytyphloplanida n.gen., n.sp. |
| BB | Dumpy typhloplanid |
Table 4.
Results of separate, 2-way Scheirer–Ray–Hare tests comparing faunal or granulometric variables collected from four Bogue Bank beaches spanning the length of the island with each beach having unique nourishment histories. Probabilities < 0.05 are in bold.
Table 4.
Results of separate, 2-way Scheirer–Ray–Hare tests comparing faunal or granulometric variables collected from four Bogue Bank beaches spanning the length of the island with each beach having unique nourishment histories. Probabilities < 0.05 are in bold.
| Variable | Beach | Sediment Type | Beach × Sediment Type |
|---|
| Copepoda | 0.008 | 0.347 | 0.888 |
| nauplii | 0.010 | 0.807 | 0.635 |
| Nematoda | 0.258 | 0.530 | 0.493 |
| turbellaria | 0.009 | 0.830 | 0.011 |
| Gastrotricha | 0.034 | 0.890 | 0.017 |
| median grain size | 0.002 | 0.001 | 0.919 |
| grain sorting | 0.002 | <0.001 | 0.665 |
| percent gravel | 0.006 | <0.001 | 0.590 |
Table 5.
Mean (±1 SE) density (# individuals per 25 mL) of each major taxon found in visually distinct finer and coarser surface sediments from the lower intertidal of EI. Probabilities derive from separate, Mann–Whitney comparisons testing the null hypothesis of no difference in faunal abundances between finer and coarser sediments. n = 5 for each sediment type. Significant results (p < 0.05) are in bold.
Table 5.
Mean (±1 SE) density (# individuals per 25 mL) of each major taxon found in visually distinct finer and coarser surface sediments from the lower intertidal of EI. Probabilities derive from separate, Mann–Whitney comparisons testing the null hypothesis of no difference in faunal abundances between finer and coarser sediments. n = 5 for each sediment type. Significant results (p < 0.05) are in bold.
| Taxon | Finer Sediment | Coarser Sediment | Probability |
|---|
| Archiannelida | 5.6 (1.4) | 0.6 (0.3) | 0.011 |
| Crustacea | 20.0 (3.9) | 98.8 (41.5) | 0.144 |
| Nematoda | 47.2 (6.9) | 27.8 (4.5) | 0.075 |
| Turbellaria | 43.8 (7.5) | 32.4 (3.2) | 0.347 |
| Gastrotricha | 31.2 (10.4) | 1.6 (1.1) | 0.012 |
Table 6.
Parameters of linear regressions of ln-transformed abundances of major taxa onto depth with sediment type as a cofactor. When the interaction of the overall model is significant the parameters of the separate regressions (finer or coarser) are presented. ns—p > 0.05, *—p < 0.05, **—p < 0.01, ***—p < 0.001.
Table 6.
Parameters of linear regressions of ln-transformed abundances of major taxa onto depth with sediment type as a cofactor. When the interaction of the overall model is significant the parameters of the separate regressions (finer or coarser) are presented. ns—p > 0.05, *—p < 0.05, **—p < 0.01, ***—p < 0.001.
| Taxon | Sediment × Depth | Variable | Intercept | Slope |
|---|
| Copepoda | 0.038 * | Finer | 2.26 *** | 0.003 ns |
| Coarser | 2.59 *** | −0.049 *** |
| Nematoda | 0.0004 *** | Finer | 2.32 *** | 0.004 ns |
| Coarser | 3.09 *** | −0.045 *** |
| turbellaria | 0.0024 ** | Finer | 0.76 *** | 0.046 *** |
| Coarser | 2.45 *** | −0.009 ns |
| Gastrotricha | 0.66 ns | Depth | 0.25 * | 0.017 * |
| Sediment type | 0.25 * | −0.231 ns |
| Sediment type × Depth | 0.25 * | 0.006 ns |
Table 7.
Turbellarian species (undescribed species listed with helping names) found in finer and coarser sediments in the lower intertidal of EI in March 2012. Numbers represent the mean (±1 SE) number of individuals per 25 mL.
Table 7.
Turbellarian species (undescribed species listed with helping names) found in finer and coarser sediments in the lower intertidal of EI in March 2012. Numbers represent the mean (±1 SE) number of individuals per 25 mL.
| “Species” | Finer Sediments | Coarser Sediments |
|---|
| Eukalypto unID’d | 2.8 (2.1) | 0 |
| Oto2/Oto3 undetermined | 2.6 (2.1) | 0 |
| Gnatho Schwanzi | 0.6 (0.4) | 0 |
| Polycystididae | 0.4 (0.2) | 0 |
| Macrostomorpha (not Myozona, Microstomum, or Paromalostomum) | 0.2 (0.2) | 0 |
| Eyed Eukalypt small | 0.2 (0.2) | 0 |
| Otoplanid n. sp. “hannahfloydae” | 8.4 (2.1) | 5.0 (1.8) |
| Immature Oto | 6.6 (2.1) | 4.4 (1.7) |
| “Monocelid” (skinny) | 6.2 (1.3) | 1.0 (0.6) |
| Eukalypto Zange | 4.8 (1.7) | 2.2 (1.3) |
| Stubby | 4.0 (0.9) | 4.0 (0.3) |
| Polystyliphora spp. | 1.8 (0.4) | 1.4 (0.4) |
| Unknown proseriate | 1.2 (0.5) | 0.2 (0.2) |
| Oto straight bundle | 1.2 (0.6) | 2.6 (0.7) |
| Thylacorhynchus “schwanzi” | 1.0 (0.3) | 2.6 (0.8) |
| Eyed Typhloplanoid (immature) | 0.4 (0.2) | 0.2 (0.2) |
| Drepanorhynchus hastatus | 0.4 (0.4) | 0.4 (0.2) |
| Schizo 2-belt | 0.4 (0.2) | 1.2 (0.7) |
| EukalyptoSpitz | 0.2 (0.2) | 0.4 (0.2) |
| Nematoplana spp. | 0.2 (0.2) | 3.4 (0.5) |
| TTK | 0 | 0.2 (0.2) |
| Diopisthoporus gymnopharyngeus | 0 | 0.2 (0.2) |
| Carolinorhynchus follybeachensis | 0 | 0.2 (0.2) |
| Odd little guy (Neodalyellioida) | 0 | 0.2 (0.2) |
| UnID’d typhloplanoid | 0 | 0.2 (0.2) |
| Plagiostomum “corculum” | 0 | 0.4 (0.2) |
| Large flat acoel | 0 | 0.6 (0.4) |
| Cicerina debrae | 0 | 0.8 (0.4) |
Table 8.
Selected turbellarian zero-radius OTUs, from ISP or BI, that matched our in-house database of local 18S sequences, showing top blast hit, match to morphospecies for which we have 18S sequences, and four cases (shaded) of congeneric pairs occurring on these beaches. OTUs clustered with -unoise3, as implemented in Usearch 64 v.10. GenBank Blast assignment as of 4.17.20. * Specimen collected from Bogue Inlet, photo-vouchered, and sequenced.
Table 8.
Selected turbellarian zero-radius OTUs, from ISP or BI, that matched our in-house database of local 18S sequences, showing top blast hit, match to morphospecies for which we have 18S sequences, and four cases (shaded) of congeneric pairs occurring on these beaches. OTUs clustered with -unoise3, as implemented in Usearch 64 v.10. GenBank Blast assignment as of 4.17.20. * Specimen collected from Bogue Inlet, photo-vouchered, and sequenced.
| OTU # | Top Blast Hit (GenBank) | ISP/EI Local 18S Database Hit | OTU to 18S |
|---|
| 387 | Catenula lemnae isolate K04_69 536/564 2 gaps | Retronectes atypica Doe & Rieger | 369/369 bp |
| 36 | Coelogynopora tenuis isolate CTHE1 555/561 1 gap | Cirrifera cf. xanthoderma | 561/561 bp |
| 152 | Monoceolopsis otoplanoides isolate MOHE1 515/563 4 gaps | Monocelididae n.g. “Stubby” | 562/562 bp |
| 158 | Myozona lutheri voucher MTP LS 692 550/563 | Myozona n.sp “Myozona ISP” | 562/563 bp |
| 719 | Myozona lutheri voucher MTP LS 692 550/563 | Myozona n.sp. 2 | 548/563 bp against above |
| 35 | Nematoplana coelogynoporoides isolate NCRO2 545/561 3 gaps | Nematoplana n.sp. “Nematoplana ISP” | 561/561 bp |
| 45 | Nematoplana coelogynoporoides isolate NCRO2 545/561 3 gaps | Nematoplana n.sp. 2 | 557/561 bp against above |
| 57 | Parotoplanella progermaria isolate PLBA1 539/561 2 gaps | Parotoplaninae n.sp “Stately OTO” | 549/549 bp |
| 58 | Polystyliphora karlingi McDaniel 551/560 | Polystyliphora cf karlingi EI | 560/560 bp |
| 126 | Polystyliphora karlingi McDaniel 550/560 | Polystyliphora n.sp. 2 | 550/560 bp against above |
| * | Psammomacrostomum sp. 1 TJ-2015 558/563 | Psammomacrostomum sp EI “MCMPH” | |
| 253 | Psammomacrostomum sp. 1 TJ-2015 558/563 | Psammomacrostomum n.sp. ISP | 548/563 bp against above |
| 795 | Schizorhynchidae sp. 3 JPS-2015 550/564 4 gaps | Karkinorhynchus n.sp. “ESTPG” | 561/562 bp |