Effectiveness of Photoprotective Strategies in Three Mixotrophic Planktonic Ciliate Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ciliate Sampling
2.2. Ciliate Handling Prior to Experiments
2.3. Experimental Design
2.3.1. General Experimental Setup
2.3.2. Experiment 1 (exp 1) to Test the Ciliates’ Overall Resistance to UVR
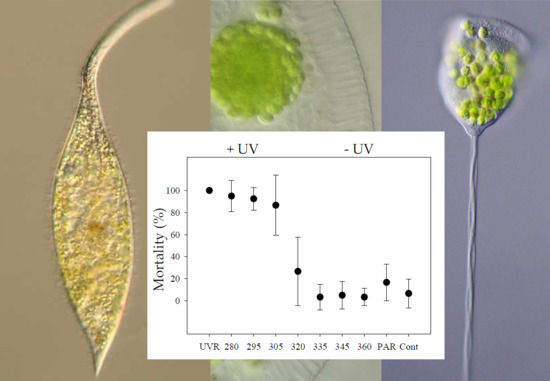
2.3.3. Experiment 2 (exp 2) to Identify the UVR Wavelength-Specific Response of P. trachelioides
2.3.4. Experiment 3 (exp 3) to Test for the Ciliates’ Recovery Potential by “Dark Repair” (All Species) and PER (P. trachelioides)
2.4. Statistical Analyses
3. Results
3.1. Stokesia Vernalis
3.2. Vorticella Chlorellata
3.3. Pelagodileptus trachelioides
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sommaruga, R. The role of solar UV radiation in the ecology of alpine lakes. Photochem. Photobiol. 2001, 62, 35–42. [Google Scholar] [CrossRef]
- Rautio, M.; Tartarotti, B. UV radiation and freshwater zooplankton: Damage, protection and recovery. Freshwater Rev. 2010, 3, 105–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laurion, I.; Ventura, M.; Catalan, J.; Psenner, R.; Sommaruga, R. Attenuation of ultraviolet radiation in mountain lakes: Factors controlling the among- and within-lake variability. Limnol. Oceanogr. 2000, 45, 1274–1288. [Google Scholar] [CrossRef]
- Rose, K.C.; Williamson, C.E.; Saros, J.E.; Sommaruga, R.; Fischer, J.M. Differences in UV transparency and thermal structure between alpine and subalpine lakes: Implications for organisms. Photochem. Photobiol. Sci. 2009, 8, 1244–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tartarotti, B.; Cabrera, S.; Psenner, R.; Sommaruga, R. Survivorship of Cyclops abyssorum tatricus (Cyclopoida, Copepoda) and Boeckella gracilipes (Calanoida, Copepoda) under ambient levels of solar UVB radiation in two high-mountain lakes. J. Plankton Res. 1999, 21, 549–560. [Google Scholar] [CrossRef] [Green Version]
- Sonntag, B.; Posch, T.; Klammer, S.; Teubner, K.; Psenner, R. Phagotrophic ciliates and flagellates in an oligotrophic deep alpine lake: Contrasting variability with seasons and depths. Aquat. Microb. Ecol. 2006, 43, 193–207. [Google Scholar] [CrossRef]
- Müller, H.; Schöne, A.; Pinto-Coelho, R.M.; Schweizer, A.; Weisse, T. Seasonal succession of ciliates in Lake Constance. Microb. Ecol. 1991, 21, 119–138. [Google Scholar] [CrossRef]
- Müller, H.; Geller, W.; Schöne, A. Pelagic ciliates in Lake Constance: Comparison of epilimnion and hypolimnion. Verh. Int. Verein. Limnol. 1991, 24, 846–849. [Google Scholar] [CrossRef]
- Carrias, J.F.; Amblard, C.; Bourdier, G. Seasonal dynamics and vertical distribution of planktonic ciliates and their relationship to microbial food resources in the oligo-mesotrophic Lake Pavin. Arch. Hydrobiol. 1998, 143, 227–255. [Google Scholar] [CrossRef]
- Sonntag, B.; Summerer, M.; Sommaruga, R. Are freshwater mixotrophic ciliates less sensitive to solar UV radiation than heterotrophic ones? J. Euk. Microbiol. 2011, 58, 196–202. [Google Scholar] [CrossRef]
- Reisser, W. Studies on the ecophysiology of endocytobiotic associations of ciliates and algae. II. Potential features of adaptation of symbiotic and free-living Chlorella spp. to the endocytobiotic habitat formed by Paramecium bursaria. Endocyt. Cell Res. 1987, 4, 317–329. [Google Scholar]
- Yamada, T.; Onimatsu, H.; Van Etten, J.L. Chlorella viruses. Adv Virus Res. 2006, 66, 293–336. [Google Scholar] [CrossRef] [PubMed]
- Pröschold, T.; Darienko, T.; Silva, P.C.; Reisser, W.; Krienitz, L. The systematics of “Zoochlorella” revisited employing an integrative approach. Environ. Microbiol. 2011, 13, 350–364. [Google Scholar] [CrossRef] [PubMed]
- Sonntag, B.; Summerer, M.; Sommaruga, R. Sources of mycosporine-like amino acids in planktonic Chlorella-bearing ciliates (Ciliophora). Freshwater Biol. 2007, 52, 1476–1485. [Google Scholar] [CrossRef] [Green Version]
- Summerer, M.; Sonntag, B.; Hörtnagl, P.; Sommaruga, R. Symbiotic ciliates receive protection against UV damage from their algae: A test with Paramecium bursaria and Chlorella. Protist 2009, 160, 233–243. [Google Scholar] [CrossRef]
- Sommaruga, R.; Sonntag, B. Photobiological aspects of the mutualistic association between Paramecium bursaria and Chlorella. In Microbiology Monographs: Endosymbionts of Paramecium; Fujishima, M., Ed.; Springer: Berlin, Germany, 2009; Volume 12, pp. 111–130. [Google Scholar] [CrossRef]
- Mitchell, D.L.; Karentz, D. The induction and repair of DNA photodamage in the environment. In Environmental UV photobiology; Young, A.R., Björn, L.O., Moan, J., Nultsch, W., Eds.; Plenum Press: New York, NY, USA, 1993; pp. 345–377. [Google Scholar]
- Laurion, I.; Lami, A.; Sommaruga, R. Distribution of mycosporine-like amino acids and photoprotective carotenoids among freshwater phytoplankton assemblages. Aquat. Microb. Ecol. 2002, 26, 283–294. [Google Scholar] [CrossRef] [Green Version]
- Slaveykova, V.; Sonntag, B.; Gutiérrez, J.C. Stress and protists: No life without stress. Eur. J. Protistol. 2016, 55, 39–49. [Google Scholar] [CrossRef]
- Hylander, S. Mycosporine-like amino acids (MAAs) in zooplankton. Mar. Drugs 2020, 18, 72. [Google Scholar] [CrossRef] [Green Version]
- Karentz, D. Ultraviolet tolerance mechanisms in Antarctic marine organisms. In Ultraviolet Radiation in Antarctica: Measurements and Biological Effects; Weiler, C.S., Panhale, P.A., Eds.; American Geophysical Union: Washington, DC, USA, 1994; pp. 93–110. [Google Scholar]
- Sanders, R.W.; Macaluso, A.L.; Sardina, T.J.; Mitchell, D.L. Photoreactivation in two freshwater ciliates: Differential responses to variations in UV-B flux and temperature. Aquat. Microb. Ecol. 2005, 40, 283–292. [Google Scholar] [CrossRef] [Green Version]
- Hamsher, S.E.; Cheng, Y.T.S.; Sanders, R.W. Effects of temperature and photorepair radiation on a marine ciliate exposed to UVB radiation. J. Euk. Microbiol. 2018, 65, 458–467. [Google Scholar] [CrossRef]
- Smith-Sonneborn, J. DNA repair and longevity assurance in Paramecium tetraurelia. Science 1979, 203, 1115–1117. [Google Scholar] [CrossRef] [PubMed]
- Giese, A.C. Differential susceptibility of a number of protozoans to ultraviolet radiations. J. Cell Comp. Physiol. 1938, 12, 129–138. [Google Scholar] [CrossRef]
- Giese, A.C. The ultraviolet action spectrum form retardation of division of Paramecium. J. Cell Comp. Physiol. 1945, 26, 47–55. [Google Scholar] [CrossRef]
- Giese, A.C.; McCaw, B.; Cornell, R. Retardation of division of three ciliates by intermittent and continuous ultraviolet radiations at different temperatures. J. Gen. Physiol. 1963, 46, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Häder, D.P.; Häder, M.A. Effects of solar radiation on motility in: Stentor coeruleus. Photochem. Photobiol. 1991, 54, 423–428. [Google Scholar] [CrossRef]
- Sommaruga, R.; Oberleiter, A.; Psenner, R. Effect of UV Radiation on the bacterivory of a heterotrophic nanoflagellate. Appl. Environ. Microbiol. 1996, 62, 4395–4400. [Google Scholar] [CrossRef] [Green Version]
- Di Guiseppe, G.; Cervia, D.; Vallesi, A. Divergences in the response to ultraviolet radiation between polar and non-polar ciliated protozoa. Microb. Ecol. 2012, 63, 334–338. [Google Scholar] [CrossRef]
- Kammerlander, B.; Tartarotti, B.; Sonntag, B. The impact of UV radiation on Paramecium populations from alpine lakes. J. Euk. Microbiol. 2018, 65, 250–254. [Google Scholar] [CrossRef] [Green Version]
- Sommaruga, R.; Buma, A.G.J. UV-induced cell damage is species-specific among aquatic phagotrophic protists. J. Euk. Microbiol. 2000, 47, 450–455. [Google Scholar] [CrossRef]
- Sonntag, B.; Summerer, M.; Sommaruga, R. Factors involved in the distribution pattern of ciliates in the water column of a transparent alpine lake. J. Plankton Res. 2011, 33, 541–546. [Google Scholar] [CrossRef] [Green Version]
- Sonntag, B.; Kammerlander, B.; Summerer, M. Bioaccumulation of ultraviolet sunscreen compounds (mycosporine-like amino acids) by the heterotrophic freshwater ciliate Bursaridium living in alpine lakes. Inland Waters 2017, 7, 55–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wickham, S.; Carstens, M. Effects of ultraviolet-B radiation on two arctic microbial food webs. Aquat. Microb. Ecol. 1998, 16, 163–171. [Google Scholar] [CrossRef]
- Carreto, J.I.; Carignan, M.O. Mycosporine-like amino acids: Relevant secondary metabolites. Chemical and ecological aspects. Mar. Drugs 2010, 9, 387–446. [Google Scholar] [CrossRef] [PubMed]
- Wada, N.; Sakamoto, T.; Matsugo, S. Mycosporine-like amino acids and their derivates as natural antioxidants. Antioxidants 2015, 4, 603–646. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pichel, F. A model for internal self-shading in planktonic organisms and its implications for the usefulness of ultraviolet sunscreens. Limnol. Oceanogr. 1994, 39, 1704–1717. [Google Scholar] [CrossRef]
- Lalegerie, F.; Stiger-Pouvreau, V.; Connan, S. Temporal variation in pigment and mycosporine-like amino acid composition of the red macroalga Palmaria palmata from Brittany (France): Hypothesis on the MAA biosynthesis pathway under high irradiance. J. Appl. Phycol. 2020. [Google Scholar] [CrossRef]
- Pathak, J.; Ahmed, H.; Rajneesh Singh, S.P.; Häder, D.P.; Sinha, R.P. Genetic regulation of scytonemin and micosporine-like amino acids (MAAs) biosynthesis in cyanobacteria. Plant Gene 2019, 17, 100172. [Google Scholar] [CrossRef]
- Foissner, W.; Berger, H.; Schaumburg, J. Identification and Ecology of Limnetic Plankton Ciliates; Bayer, Landesamt für Wasserwirtschaft: Munich, Germany, 1999; Volume 3/99, pp. 1–793. [Google Scholar]
- Vd’ačný, P.; Foissner, W. Monograph of the dileptids (Protista, Ciliophora, Rhynchostomatia). Denisia 2012, 31, 1–529. [Google Scholar]
- Wenrich, H.D. Observations of some freshwater ciliates (Protozoa). I. Teuthophrys trisulca Chatton and de Beauchamp and Stokesia vernalis n.g., n. sp. Trans. Am. Microsc. Soc. 1929, 48, 221–241. [Google Scholar]
- Packroff, G.; Wilbert, N. Taxonomische Studien über die Ciliatenfauna (Protozoa, Ciliophora) der Eifelmaare. Arch. Protistenkd. 1991, 140, 121–139. [Google Scholar] [CrossRef]
- Butkay, M. Beobachtungen an Pelagodileptus trachelioides (Ciliophora). Lauterbornia 2004, 49, 129–139. [Google Scholar]
- Golińska, K. Effect of puromycin on regeneration processes in Dileptus anatinus Golinska 1971. Acta Protozool. 1974, 12, 289–360. [Google Scholar]
- Golińska, K. The course of in situ remodeling of injured mouthparts in Dileptus (Ciliata, Gymnostomata). Acta Protozool. 1978, 17, 47–67. [Google Scholar]
- Golińska, K. Assessment of cell proportions during regeneration of Dileptus anser (Ciliata). Wilhelm Roux’s Archives 1979, 187, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Golińska, K.; Kink, J. The regrowth of oral structures in Dileptus cygnus after partial excision. Acta Protozool. 1975, 15, 143–163. [Google Scholar]
- Tatar, V. The Biology of Stentor; Pergamon Press Ltd.: Oxford, GB, USA, 1961; pp. 1–413. [Google Scholar]
- Wei, W.; Jiang, C.; Yang, W.; Miao, W.; Xiong, J. Proteomic identification and expression of oral apparatus constituents in cell regeneration of giant ciliate Stentor coeruleus (strain WHEL). Gene 2020, 743, 144624. [Google Scholar] [CrossRef]
- Dragesco, J. Ciliés libres de Thonon et ses environs. Protistologica 1966, 2, 59–95. [Google Scholar]
- Krainer, K.-H. Alpha-Taxonomie und Ökologie Neuer Sowie Mehrerer Wenig Bekannter Pelagischer Ciliaten (Protozoa: Ciliphora aus den Klassen Kinetofragminophora, Oligohymenophora, Polyhymenophora) Einiger Grundwasserbaggerteiche des Nördlichen Leibnitzer Feldes (Steiermark, Österreich). Ph.D. Thesis, University of Graz, Graz, Austria, 1988. [Google Scholar]
- Skibbe, O. An improved quantitative protargol stain for ciliates and other planktonic protists. Arch. Hydrobiol. 1994, 130, 339–347. [Google Scholar]



| Ciliate Species | Sample | Total | Shinorine | Palythine | Asterina-330 | Palythene | Usujirene |
|---|---|---|---|---|---|---|---|
| S. vernalis | UVR | 1.86 | 1.86 (99%) 1 | - | - | Traces | Traces |
| PAR | 1.60 | 1.60 (99%) 1 | - | Traces | Traces | Traces | |
| Control | 1.00 | 1.00 (99%) 1 | - | - | Traces | - | |
| P. trachelioides | UVR | 3.16 | 2.89 (91%) 1 | 0.27 (9%) 2 | Traces | Traces | |
| PAR | 2.49 | 2.22 (89%) 1 | 0.27 (11%) 2 | Traces | Traces | ||
| Control | 2.58 | 2.38 (92%) | 0.20 (8%) | - | Traces | Traces | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sonntag, B.; Sommaruga, R. Effectiveness of Photoprotective Strategies in Three Mixotrophic Planktonic Ciliate Species. Diversity 2020, 12, 252. https://doi.org/10.3390/d12060252
Sonntag B, Sommaruga R. Effectiveness of Photoprotective Strategies in Three Mixotrophic Planktonic Ciliate Species. Diversity. 2020; 12(6):252. https://doi.org/10.3390/d12060252
Chicago/Turabian StyleSonntag, Bettina, and Ruben Sommaruga. 2020. "Effectiveness of Photoprotective Strategies in Three Mixotrophic Planktonic Ciliate Species" Diversity 12, no. 6: 252. https://doi.org/10.3390/d12060252
APA StyleSonntag, B., & Sommaruga, R. (2020). Effectiveness of Photoprotective Strategies in Three Mixotrophic Planktonic Ciliate Species. Diversity, 12(6), 252. https://doi.org/10.3390/d12060252