Trait-Specific Responses of Carabid Beetle Diversity and Composition in Pinus densiflora Forests Compared to Broad-Leaved Deciduous Forests in a Temperate Region
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling
2.3. Data Analysis
3. Results
3.1. Diversity and Abundance of Carabid Beetles
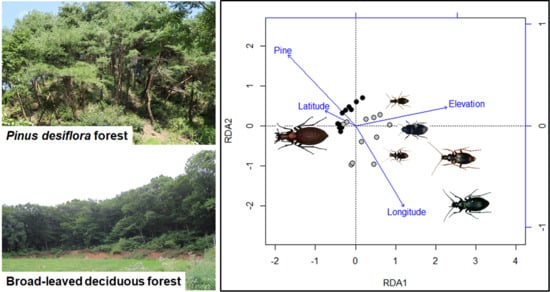
3.2. Species Composition of Carabid Beetles
4. Discussion
4.1. Carabid Beetle Diversity in Different Forest Types
4.2. Ecological and Biological Traits of Carabid Beetles and Forest Types
4.3. Habitat Specialists for Forest Types
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Brockerhoff, E.G.; Jactel, H.; Parrotta, J.A.; Quine, C.P.; Sayer, J. Plantation forests and biodiversity: Oxymoron or opportunity? Biodivers. Conserv. 2008, 17, 925–951. [Google Scholar] [CrossRef]
- Hartmann, H.; Daoust, G.; Bigué, B. Negative or positive effects of plantation and intensive forestry on biodiversity: A matter of scale and perspective. For. Chron. 2010, 86, 354–364. [Google Scholar] [CrossRef] [Green Version]
- Lindenmayer, D.B.; Hobbs, R.J.; Salt, D. Plantation forests and biodiversity conservation. Aust. For. 2003, 66, 62–66. [Google Scholar] [CrossRef] [Green Version]
- Carnus, J.-M.; Parrotta, J.; Brockerhoff, E.G.; Arbez, M.; Jactel, H.; Kremer, A.; Lamb, D.; O’Hara, K.; Walters, B. Planted forests and biodiversity. J. For. Ecol. 2006, 104, 65–77. [Google Scholar]
- MacDicken, K.; Jonsson, Ö.; Piňa, L.; Maulo, S.; Adikari, Y.; Garzuglia, M.; Lindquist, E.; Reams, G.; D’Annunzio, R. The Global Forest Resources Assessment 2015: How Are the World’s Forests Changing? Food and Agriculture Organization of the United Nations: Rome, Italy, 2015. [Google Scholar]
- Korea Forest Service. Statistical Yearbook of Forestry No. 46. Korea Forest Service, 2016. Available online: https://www.forest.go.kr (accessed on 6 April 2017).
- Lee, D.K. Ecological Management of Forests; Seoul National University Press: Seoul, Korea, 2012. [Google Scholar]
- Do, Y. The effect of fragmentation and intensive management on carabid beetles in coniferous forest. Appl. Ecol. Environ. Res. 2013, 11, 451–461. [Google Scholar] [CrossRef]
- Jung, J.-K.; Kim, S.-T.; Lee, S.-Y.; Yoo, J.-S.; Lee, J.-H. Comparison of Ground Beetle Communities (Coleoptera: Carabidae) between Coniferous and Deciduous Forests in Agricultural Landscapes. J. For. Environ. Sci. 2013, 29, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.-K.; Kim, S.-T.; Lee, S.Y.; Park, C.-G.; Park, J.-K.; Lee, J.-H. A comparison of diversity and species composition of ground beetles (Coleoptera: Carabidae) between conifer plantations and regenerating forests in Korea. Ecol. Res. 2014, 29, 877–887. [Google Scholar] [CrossRef]
- Jung, J.-K.; Lee, S.K. Trait-specific response of ground beetles (Coleoptera: Carabidae) to forest fragmentation in the temperate region in Korea. Biodivers. Conserv. 2018, 27, 53–68. [Google Scholar] [CrossRef]
- Choi, S.W.; Park, M.; Kim, H. Differences in moth diversity in two types of forest patches in an agricultural landscape in Southern Korea—Effects of habitat heterogeneity. J. Ecol. Field. Biol. 2009, 32, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.-W. Diversity and composition of larger moths in three different forest types of Southern Korea. Ecol. Res. 2008, 23, 503–509. [Google Scholar] [CrossRef]
- Yi, H.-B.; Kim, H.-J. Species Composition and Species Diversity of Moths (Lepidoptera) on Quercus mongolica forests sand Pinus densiflora forests, in Korean National Long-term Ecological Research Sites (Mt. Nam, Mt. Jiri, Mt. Wolak). Korean J. Appl. Ѐntomol. 2010, 49, 105–113. [Google Scholar] [CrossRef]
- Chang, S.J.; Kim, J.K. Distribution of carabid beetles (Coleoptera: Carabidae) in different forests of central Kangwon-do. J. For. Sci. Kangwon Nat. Univ. 2000, 16, 42–49. [Google Scholar]
- Warren-Thomas, E.; Zou, Y.; Dong, L.; Yao, X.; Yang, M.; Zhang, X.; Qin, Y.; Liu, Y.; Sang, W.; Axmacher, J.C. Ground beetle assemblages in Beijing’s new mountain forests. For. Ecol. Manag. 2014, 334, 369–376. [Google Scholar] [CrossRef] [Green Version]
- Ogai, T.; Kenta, T. The effects of vegetation types and microhabitats on carabid beetle community composition in cool temperate Japan. Ecol. Res. 2016, 31, 177–188. [Google Scholar] [CrossRef]
- Lövei, G.L.; Sunderland, K.D. Ecology and Behavior of Ground Beetles (Coleoptera: Carabidae). Annu. Rev. Ѐntomol. 1996, 41, 231–256. [Google Scholar] [CrossRef] [PubMed]
- Rainio, J.; Niemelä, J. Ground beetles (Coleoptera: Carabidae) as bioindicators. Biodivers. Conserv. 2003, 12, 487–506. [Google Scholar] [CrossRef]
- Schuldt, A.; Assmann, T. Environmental and historical effects on richness and endemism patterns of carabid beetles in the western Palaearctic. Ecography 2009, 32, 705–714. [Google Scholar] [CrossRef]
- Harvey, J.A.; Van Der Putten, W.H.; Turin, H.; Wagenaar, R.; Bezemer, T.M. Effects of changes in plant species richness and community traits on carabid assemblages and feeding guilds. Agric. Ecosyst. Environ. 2008, 127, 100–106. [Google Scholar] [CrossRef]
- Koivula, M.; Punttila, P.; Haila, Y.; Niemelä, J. Leaf litter and the small-scale distribution of carabid beetles (Coleoptera, Carabidae) in the boreal forest. Ecography 1999, 22, 424–435. [Google Scholar] [CrossRef] [Green Version]
- Elek, Z.; Magura, T.; Tóthmérész, B. Impacts of non-native Norway spruce plantation on abundance and species richness of ground beetles (Coleoptera: Carabidae). Web Ecol. 2001, 2, 32–37. [Google Scholar] [CrossRef] [Green Version]
- KOSIS. Korean Statistical Information Service. 2015. Available online: http://kosis.kr/ (accessed on 8 March 2015).
- KMA. Korea Meteorological Administration. 2016. Available online: http://www.kma.go.kr/ (accessed on 28 August 2016).
- Jung, J.-K.; Suk, S.-W.; Kim, B.-Y.; Hong, E.J.; Kim, Y.; Jeong, J.-C. Differences in temporal variation of ground beetle assemblages (Coleoptera: Carabidae) between two well-preserved areas in Mt. Sobaeksan National Park. J. For. Environ. Sci. 2017, 33, 122–129. [Google Scholar]
- Habu, A. Fauna Japonica, Carabidae Truncatipennes Group (Insecta: Coleoptera); Biogeographical Society of Japan: Japan, Tokyo, 1967. [Google Scholar]
- Habu, A. Fauna Japonica, Carabidae: Harpalini (Insecta: Coleoptera); Keigaku Publishing Co. Ltd.: Tokyo, Japan, 1973. [Google Scholar]
- Habu, A. Fauna Japonica, Carabidae: Platynini (Insecta: Coleoptera); Keigaku Publishing Co. Ltd.: Tokyo, Japan, 1978. [Google Scholar]
- Habu, A. Classification of the Callistini of Japan (Coleoptera, Carabidae). Entomol. Rev. Jpn. 1987, 42, 1–36. [Google Scholar]
- Kwon, Y.J.; Lee, S.M. Classification of the subfamily Carabinae from Korea (Coleoptera: Carabidae). Insecta Koreana 1984, 4, 1–363. [Google Scholar]
- Park, J.K. Subfamily Carabinae in Korea (Coleoptera: Carabidae); Economic Insects of Korea 23; Junghaeng-Sa: Seoul, Korea, 2004. [Google Scholar]
- Park, J.K.; Choi, I.J.; Park, J.; Choi, E.Y. Insect Fauna of Korea, vol. 12, no. 16, Arthropoda: Insecta: Coleoptera: Carabidae: Chlaeniini, Truncatipennes Group: Odacanthinae, Lebiinae; Junghaengsa, Inc.: Incheon, Korea, 2014. [Google Scholar]
- Park, J.K.; Paik, J.C. Family Carabidae. Economic Insects of Korea 12; Junghaeng-Sa: Seoul,, Korea, 2001. [Google Scholar]
- Park, J.K.; Park, J. Insect Fauna of Korea, vol. 12, no. 13, Arthropoda: Insecta: Coleoptera: Carabidae: Pterostichinae; Junghaengsa, Inc.: Incheon, Korea, 2014. [Google Scholar]
- Löbl, I.; Smetana, A. Catalogue of Palaearctic Coleoptera. Archostemata-Myxophaga-Adephaga; Apollo Books: Stenstrup, Denmark, 2003; Volume 1. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria. Available online: http://www.R-project.org/ (accessed on 6 April 2017).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 1 May 2020).
- Gotelli, N.J.; Colwell, R.K. Quantifying biodiversity: Procedures and pitfalls in the measurement and comparison of species richness. Ecol. Lett. 2001, 4, 379–391. [Google Scholar] [CrossRef] [Green Version]
- Legendre, P.; Gallagher, E. Ecologically meaningful transformation for ordination of species data. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef]
- Oksanen, J. Multivariate Analysis of Ecological Communities in R: Vegan Tutorial. 2007. Available online: https://www.researchgate.net/publication/260136364_Multivariate_Analysis_of_Ecological_Communities_in_R_Vegan_Tutorial (accessed on 14 April 2017).
- Legendre, P.; Oksanen, J.; Ter Braak, C.J.F. Testing the significance of canonical axes in redundancy analysis. Methods Ecol. Evol. 2010, 2, 269–277. [Google Scholar] [CrossRef]
- Dufrêne, M.; Legendre, P. Species Assemblages and Indicator Species: The Need for a Flexible Asymmetrical Approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Legendre, P.; Legendre, L. Numerical Ecology; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- McGeoch, M.A.; Chown, S.L. Scaling up the value of bioindicators. Trends Ecol. Evol. 1998, 13, 46–47. [Google Scholar] [CrossRef]
- Fuller, R.J.; Oliver, T.H.; Leather, S.R. Forest management effects on carabid beetle communities in coniferous and broadleaved forests: Implications for conservation. Insect Conserv. Divers. 2008, 1, 242–252. [Google Scholar] [CrossRef]
- Kotze, D.J.; O’Hara, R.B. Species decline—But why? Explanation of carabid beetle (Coleoptera, Carabidae) declines in Europe. Oecologia 2003, 135, 138–148. [Google Scholar] [CrossRef]
- Antvogel, H.; Bonn, A. Environmental parameters and micospatial distribution of insects: A case study of carabids in an alluvial forest. Ecography 2001, 24, 470–482. [Google Scholar] [CrossRef]
- Eyre, M.D.; Rushton, S.P.; Luff, M.L.; Telfer, M.G. Investigating the relationships between the distribution of British ground beetle species (Coleoptera, Carabidae) and temperature, precipitation and altitude. J. Biogeogr. 2005, 32, 973–983. [Google Scholar] [CrossRef]
- Usher, M.B.; Keiller, S.W.J. The macrolepidoptera of farm woodlands: Determinants of diversity and community structure. Biodivers. Conserv. 1998, 7, 725–748. [Google Scholar] [CrossRef]
- Lepš, J.; Novotny, V.; Basset, Y. Habitat and successional status of plants in relation to the communities of their leaf-chewing herbivores in Papua New Guinea. J. Ecol. 2001, 89, 186–199. [Google Scholar] [CrossRef]
- Purtauf, T.; Roschewitz, I.; Dauber, J.; Thies, C.; Tscharntke, T.; Wolters, V. Landscape context of organic and conventional farms: Influences on carabid beetle diversity. Agric. Ecosyst. Environ. 2005, 108, 165–174. [Google Scholar] [CrossRef]
- Liu, Y.; Axmacher, J.C.; Wang, C.; Li, L.; Yu, Z. Ground beetles (Coleoptera: Carabidae) in the intensively cultivated agricultural landscape of northern China—Implications for biodiversity conservation. Insect Conserv. Diver. 2010, 3, 34–43. [Google Scholar] [CrossRef]
- Jung, J.-K.; Lee, J.-H. Forest–farm edge effects on communities of ground beetles (Coleoptera: Carabidae) under different landscape structures. Ecol. Res. 2016, 31, 799–810. [Google Scholar] [CrossRef]
- Fujita, A.; Maeto, K.; Kagawa, Y.; Itô, N. Effects of forest fragmentation on species richness and composition of ground beetles (Coleoptera: Carabidae and Brachinidae) in urban landscapes. Ѐntomol. Sci. 2008, 11, 39–48. [Google Scholar] [CrossRef]
- Korea Forest Research Institute. Economic Tree Species 1: Pine Tree. Korea Forest Service, 2012. Available online: http://know.nifos.go.kr (accessed on 1 June 2020).



| Location | Code for Site | Forest Type a | Forest Age Class b | Latitude | Longitude | Elevation (m) | Trap × Days | Sampling Period | |
|---|---|---|---|---|---|---|---|---|---|
| Jeongseon-eup | Gwangha-ri | DJGw | BLF | III | 37°21′39″ N | 128°37′43″ E | 296 | 330 | Jul–Oct 2013 |
| Gasu-ri | DJGa | BLF | III | 37°18′49″ N | 128°37′41″ E | 285 | 330 | Jul–Oct 2013 | |
| Unchi-ri | DJU | BLF | III | 37°16′36″ N | 128°36′48″ E | 278 | 330 | Jul–Oct 2013 | |
| Deokcheon-ri | DJD | BLF | III | 37°15′43″ N | 128°35′23″ E | 273 | 330 | Jul–Oct 2013 | |
| Yeongwol-eup | Geoun-ri | DYG | PF | V | 37°14′09″ N | 128°31′11″ E | 258 | 330 | Jul–Oct 2013 |
| Suju-meyon | Beopheung-ri | YSB1 | PF | IV | 37°22′18″ N | 128°15′45″ E | 473 | 330 | Jul–Oct 2013 |
| Beopheung-ri | YSB2 | BLF | IV | 37°22′23″ N | 128°15′24″ E | 488 | 588 | Apr–Oct 2014 | |
| Unhak-ri | YSU | PF | V | 37°19′53″ N | 128°12′24″ E | 461 | 579 | Jul 2013–Jun 2014 | |
| Mureung-ri | YSM | PF | V | 37°17′28″ N | 128°15′50″ E | 280 | 579 | Jul 2013–Jun 2014 | |
| Jucheon-myeon | Docheon-ri | YJD | PF | V | 37°17′16″ N | 128°13′48″ E | 323 | 579 | Jul 2013–Jun 2014 |
| Yongseok-ri | YJYo | PF | IV | 37°13′12″ N | 128°16′14″ E | 355 | 579 | Jul 2013–Jun 2014 | |
| Hanbando-myeon | Hutan-ri | YHH | PF | IV | 37°11′56″ N | 128°22′03″ E | 245 | 579 | Jul 2013–Jun 2014 |
| Ongjeong-ri | YHO | PF | IV | 37°13′07″ N | 128°21′08″ E | 303 | 579 | Jul 2013–Jun 2014 | |
| Jungdong-myeon | Jikdong-ri | YJJ | BLF | IV | 37°10′13″ N | 128°43′27″ E | 434 | 339 | Jun–Oct 2014 |
| Yeonsang-ri | YJYe | BLF | IV | 37°12′01″ N | 128°35′07″ E | 332 | 339 | Jun–Oct 2014 | |
| Sangdong-eup | Naedeok-ri | YSN | BLF | IV | 37°08′37″ N | 128°48′32″ E | 664 | 339 | Jun–Oct 2014 |
| Deokgu-ri | YSD | BLF | IV | 37°05′29″ N | 128°48′35″ E | 744 | 339 | Jun–Oct 2014 | |
| Gimsatgat-myeon | Nae-ri | YGN | BLF | IV | 37°05′53″ N | 128°42′03″ E | 525 | 339 | Jun–Oct 2014 |
| Waseok-ri | YGW | BLF | III | 37°05′03″ N | 128°36′14″ E | 339 | 339 | Jun–Oct 2014 | |
| Nam-myeon | Gwangcheon-ri | YNG | PF | V | 37°09′21″ N | 128°25′38″ E | 266 | 234 | Aug–Oct 2014 |
| Changwon-ri | YNC | PF | V | 37°10′31″ N | 128°22′06″ E | 298 | 234 | Aug–Oct 2014 | |
| Buk-myeon | Deoksang-ri | YBD | PF | III | 37°17′19″ N | 128°23′43″ E | 350 | 234 | Aug–Oct 2014 |
| Dependent Variables | Carabid Catches (Mean ± S.E.) | Statistics | ||
|---|---|---|---|---|
| PF (n = 11) | BLF (n = 11) | t-value | p | |
| Abundance | ||||
| All species | 540.6 ± 96.46 | 326.7 ± 77.63 | 1.728 | 0.1002 |
| Endemic species | 7.6 ± 2.47 | 69.0 ± 27.40 | −2.231 | 0.0494 |
| Widespread species | 533.0 ± 95.44 | 257.7 ± 56.44 | 2.483 | 0.0243 |
| Brachypterous species | 53.3 ± 14.87 | 172.0 ± 70.11 | −1.657 | 0.1261 |
| Macropterous species | 487.4 ± 87.95 | 154.7 ± 33.31 | 3.537 | 0.0037 |
| Forest specialists | 519.0 ± 91.69 | 301.5 ± 79.25 | 1.795 | 0.0881 |
| Open-habitat species | 21.6 ± 12.47 | 25.3 ± 8.67 | −0.239 | 0.8135 |
| Carnivorous species | 539.5 ± 96.24 | 318.4 ± 75.34 | 0.942 | 0.3573 |
| Herbivorous species | 1.2 ± 0.38 | 8.4 ± 2.76 | −3.002 | 0.0084 |
| Species richness | ||||
| All species | 10.0 ± 0.88 | 15.4 ± 1.99 | −2.466 | 0.0274 |
| Endemic species | 1.1 ± 0.31 | 4.5 ± 0.90 | −3.534 | 0.0039 |
| Widespread species | 8.9 ± 0.72 | 10.9 ± 1.32 | −1.327 | 0.2038 |
| Brachypterous species | 4.5 ± 0.58 | 8.0 ± 1.41 | −2.330 | 0.0362 |
| Macropterous species | 5.5 ± 0.78 | 7.4 ± 1.13 | −1.325 | 0.2021 |
| Forest specialists | 7.2 ± 0.60 | 11.3 ± 1.71 | −2.262 | 0.0423 |
| Open-habitat species | 2.8 ± 0.72 | 4.1 ± 0.99 | −1.041 | 0.3116 |
| Carnivorous species | 9.2 ± 0.77 | 13.3 ± 1.75 | −2.459 | 0.0265 |
| Herbivorous species | 0.82 ± 0.26 | 2.1 ± 0.55 | −0.9464 | 0.3629 |
| Variables | RDA 1 | RDA 2 | RDA 3 | RDA 4 |
|---|---|---|---|---|
| Eigenvalue | 0.0921 | 0.0471 | 0.0218 | 0.0114 |
| % variation explain † | 24.17 | 12.37 | 5.72 | 3.00 |
| Environmental variables | ||||
| Forest type | ** −0.64 | *** 0.54 | −0.02 | −0.23 |
| Elevation | *** 0.87 | 0.14 | 0.05 | −0.32 |
| Latitude | −0.29 | 0.12 | *** 0.76 | 0.23 |
| Longitude | * 0.45 | ** −0.62 | −0.20 | −0.25 |
| Species richness | ||||
| Total | *** 0.85 | −0.18 | 0.06 | −0.16 |
| Endemic | *** 0.86 | −0.07 | 0.08 | −0.14 |
| Widespread | *** 0.80 | −0.23 | 0.06 | −0.17 |
| Brachypterous | *** 0.79 | −0.06 | 0.09 | −0.20 |
| Macropterous | * 0.43 | −0.32 | −0.02 | −0.05 |
| Forest specialists | *** 0.83 | −0.01 | 0.02 | −0.18 |
| Open-habitat | 0.24 | * −0.43 | 0.08 | −0.04 |
| Carnivorous species | *** 0.76 | −0.11 | 0.04 | −0.20 |
| Herbivorous species | ** 0.55 | * −0.48 | 0.07 | 0.00 |
| Forest Types | Indicator Value | p-Value | Number of Individuals (Proportion in Observation, %) | |
|---|---|---|---|---|
| QF | PF | |||
| Broad-leaved deciduous forest (QF) | ||||
| Eucarabus cartereti cartereti | 0.998 | 0.001 | 416 (100.0) | 2 (9.1) |
| Harpalus discrepans | 0.749 | 0.023 | 30 (63.6) | 4 (27.3) |
| Pristosia vigil | 0.729 | 0.060 | 77 (54.5) | 2 (18.2) |
| Leistus niger niger | 0.674 | 0.033 | 8 (45.5) | |
| Cymindis collaris | 0.603 | 0.093 | 8 (36.4) | |
| Pinus densiflora-dominated forest (PF) | ||||
| Coptolabrus smaragdinus branickii | 0.699 | 0.081 | 6 (27.3) | 51 (54.5) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, J.-K.; Lee, J.-H. Trait-Specific Responses of Carabid Beetle Diversity and Composition in Pinus densiflora Forests Compared to Broad-Leaved Deciduous Forests in a Temperate Region. Diversity 2020, 12, 275. https://doi.org/10.3390/d12070275
Jung J-K, Lee J-H. Trait-Specific Responses of Carabid Beetle Diversity and Composition in Pinus densiflora Forests Compared to Broad-Leaved Deciduous Forests in a Temperate Region. Diversity. 2020; 12(7):275. https://doi.org/10.3390/d12070275
Chicago/Turabian StyleJung, Jong-Kook, and Joon-Ho Lee. 2020. "Trait-Specific Responses of Carabid Beetle Diversity and Composition in Pinus densiflora Forests Compared to Broad-Leaved Deciduous Forests in a Temperate Region" Diversity 12, no. 7: 275. https://doi.org/10.3390/d12070275
APA StyleJung, J. -K., & Lee, J. -H. (2020). Trait-Specific Responses of Carabid Beetle Diversity and Composition in Pinus densiflora Forests Compared to Broad-Leaved Deciduous Forests in a Temperate Region. Diversity, 12(7), 275. https://doi.org/10.3390/d12070275