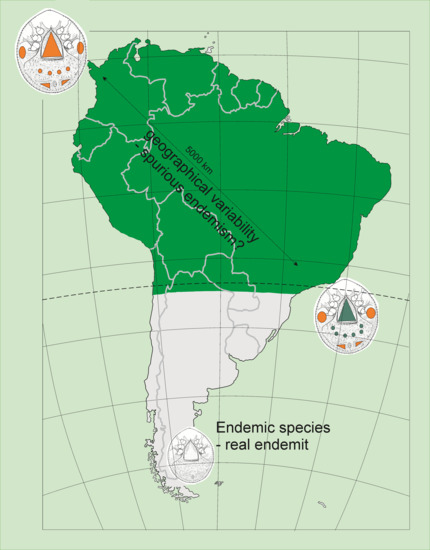
Endemism of Uropodina Mites: Spurious or Real?
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Geographical Ranges of Uropodina
3.2. Spurious or Real Endemism
3.2.1. Example I: Mites from the Genus Rotundabalogia from South America
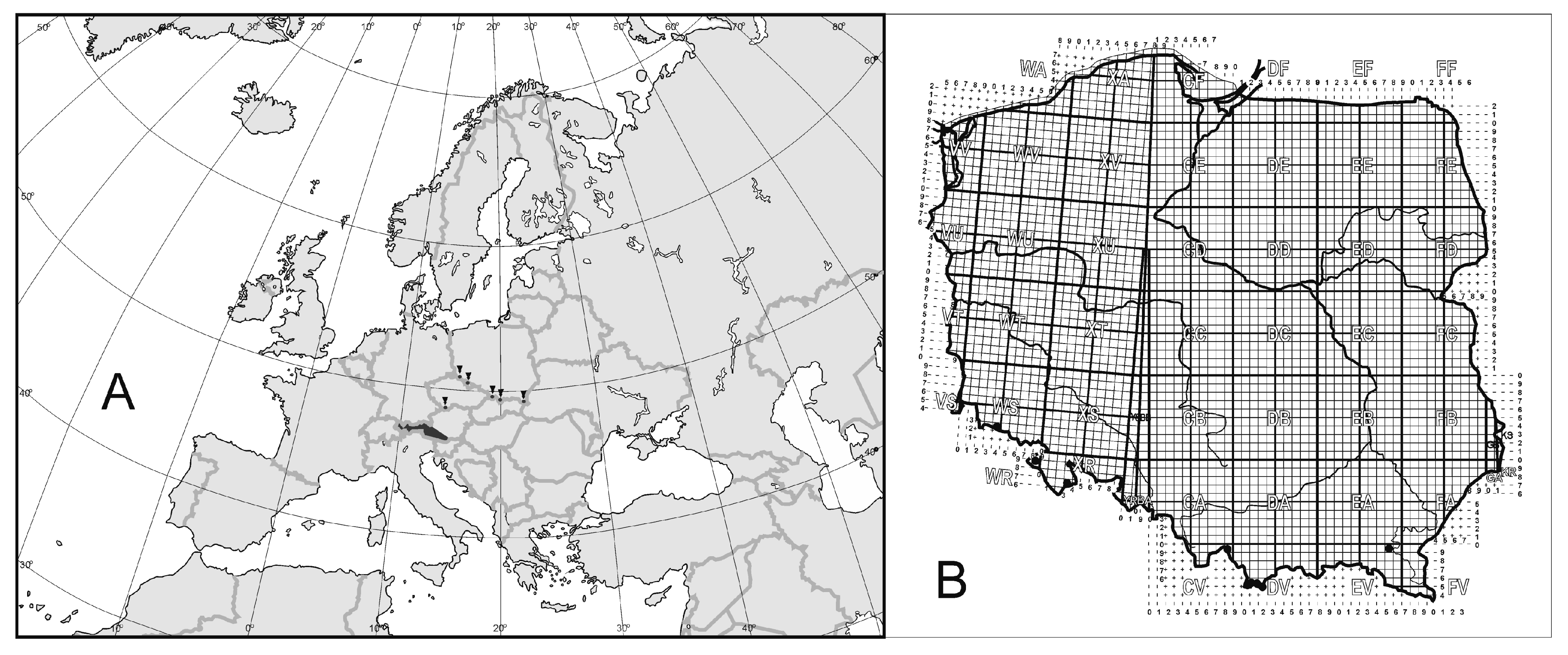
3.2.2. Example II: Uropoda (Phaulodinychus) penicillata Hirschmann et Zirgiebl-Nicol, 1969 from Ecuador
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Müller, O.F. Zoologiae Danicae Prodromus, seu Animalism Daniae et Norvegien Indigenarum. Characteres, Nomina et Synonyma Imprimis Populariarum; Havniae, Typis Hallageriis: Copenhagen, Denmark, 1776; pp. 186–187. [Google Scholar]
- Wiśniewski, J.; Hirschmann, W. Katalog der Ganggattungen, Untergattungen, Gruppen und Arten der Uropodiden der Erde (Taxonomie, Literatur, Grősse, Verbreitung, Vorkommen). Gangsystematic der Parasitiformes 548. Acarologie 1993, 40, 1–220. [Google Scholar]
- Błoszyk, J. Geograficzne i Ekologiczne Zróżnicowanie Zgrupowań Roztoczy z Kohorty Uropodina (Acari: Mesostigmata) w Polsce. 1. Uropodina Lasów Grądowych (Carpinion betuli); Kontekst: Poznań, Poland, 1999; p. 245. [Google Scholar]
- Mašán, P. Mites of the cohort Uropodina (Acarina, Mesostigmata) in Slovakia. Annot. Zool. Bot. Bratislava. 2001, 223, 1–320. [Google Scholar]
- Błoszyk, J.; Bajaczyk, R.; Markowicz, M.; Gulvik, M. Geographical and ecological variability of mites of the suborder Uropodina (Acari: Mesostigmata) in Europe. Biol. Lett. 2003, 40, 15–35. [Google Scholar]
- Błoszyk, J. East Carpathian geographical elements in the mite fauna of Poland. Biol. Lett. 1998, 35, 137–147. [Google Scholar]
- Błoszyk, J.; Bajaczyk, R.; Błoszyk, G.; Napierała, A. Uropodina (Acari: Mesostigmata) parków narodowych Polski na tle innych obszarów. Kosmos 2002, 51, 463–470. [Google Scholar]
- Napierała, A.; Błoszyk, J.; Kozak, J.; Bruin, J. Spatial distribution of mites of the suborder Uropodina (Acari: Mesostigmata) in small isolated forest area. Exp. Appl. Acarol. 2006, 39, 289–295. [Google Scholar] [CrossRef]
- Athias-Binche, F.; Błoszyk, J. Australian Uropodina (Acari: Anactonotrichida). 1. Australocilliba gen.n. (Cillibidae). J. Aust. Ent. Soc. 1988, 27, 1–8. [Google Scholar] [CrossRef]
- Błoszyk, J.; Halliday, R.B.; Dylewska, M. Acroseius womersleyi gen. nov., sp. nov., a new genus and species of Uropodina from Australia (Acari: Trachytidae). Syst. Appl. Acarol. 2005, 10, 41–60. [Google Scholar] [CrossRef]
- Błoszyk, J.; Halliday, R.B.; Napierała, A. Acroseius weiri sp. nov. (Acari: Trachytidae), a new species of Uropodina from eastern Australia, with notes on the biogeography of the genus. Syst. Appl. Acarol. 2013, 18, 273–290. [Google Scholar]
- Błoszyk, J.; Halliday, R.B.; Adamski, Z.; Książkiewicz-Parulska, Z. Capricornella bicornuta, a new genus and species of mite from eastern Australia (Acari: Uropodina). Zootaxa 2017, 4244, 321–338. [Google Scholar] [CrossRef]
- Błoszyk, J.; Szymkowiak, P. Trachytes kaliszewskii n. sp. (Acari: Uropodina) from Great Basin (Utah, USA), with remarks on the habitats and distribution of the members of the genus Trachytes. Great Basin Nat. 1996, 56, 59–72. [Google Scholar]
- Hirschmann, W.; Wiśniewski, J. Weltweite Revision der ganggattung Trichouropoda Berlese1916. II. Die interstructura-Gruppe (Trichouropodini, Uropodinae). GDP T. 492. Acarologie 1986, 33, 81–109. [Google Scholar]
- Hirschmann, W.; Wiśniewski, J. Weltweite Revision der ganggattung Trichouropoda Berlese1916. IV. Die longiseta-Gruppe (Trichouropodini, Uropodinae). GDP T.496. Acarologie 1987, 34, 1–50. [Google Scholar]
- Hirschmann, W.; Wiśniewski, J. Weltweite Revision der ganggattung Trichouropoda Berlese1916 Nachträge zu den von 1986 bis 1988 revidieren Gruppen (Trichouropodini, Uropodinae). GDP T. 503. Acarologie 1988, 35, 85–115. [Google Scholar]
- Pečina, P. Czechoslovak Uropodid mites of the Genus Trachytes Michael, 1894 (Acari: Mesostigmata). Acta Univ. Carol.-Biol. Praha 1970, 1, 39–59. [Google Scholar]
- Trägårdh, I. Acaridienaus dem Sarekgebirge. Naturwiss. Untersuch. Sarek-Geb. Schwed. Lappland 1910, 4, 375–586. [Google Scholar]
- Willmann, C. Milben aus Mineralquellen (2. Mitteilund). Zool. Anz. 1950, 145, 186–195. [Google Scholar]
- Chapple, D.G.; Hoskin, C.J.; Chapple, S.N.J.; Thompson, M.B. Phylogeographic divergence in the widespread delicate skink (Lampropholis delicata) corresponds to dry habitat barriers in eastern Australia. BMC Evol. Biol. 2011, 11, 191. [Google Scholar] [CrossRef]
- Baker, C.H.; Graham, G.C.; Scott, K.D.; Cameron, S.L.; Yeates, D.K.; Merritt, D.J. Distribution and phylogenetic relationships of Australian glow-worms Arachnocampa (Diptera, Keroplatidae). Mol. Phylogenet. Evol. 2008, 48, 506–514. [Google Scholar] [CrossRef]
- Stansic, J. The distribution and patterns of species diversity of land snails in eastern Australia. Mem. Queensl. Mus. 1994, 36, 207–214. [Google Scholar]
- Floyd, A.G. Rainforest Trees of Mainland South-eastern Australia. Forestry Commission of New South Wales; Inkata Press: Melbourne, Australia; Sydney, Australia, 1989; pp. 1–420. [Google Scholar]
- Błoszyk, J.; Halliday, R.B.; Dylewska, M.; Napierała, A. An interesting case of vicariance in the endemic mite genus Acroseius in eastern Australia (Acari: Uropodina: Trachytidae). In Integrative Acarology. Proceedings of the 6th European Congress, European Association of Acarologists; Bertrand, M., Kreiter, S., McCoy, A., Migeon, M., Navajas, M., Tixier, M.S., Vial, L., Eds.; European Association of Acarologists: Montpellier, France, 2008; pp. 44–46. [Google Scholar]
- Hirschmann, W. Gangsystematik der Parasitiformes. Teil 536. 41 Rotundabaloghia-Arten aus Sudamerika (Venezuela, Ekuador, Peru, Bolivien, Brasilien) und Mittelamerika (Guatemala) (Dinychini, Uropodinae). Schriftenreihe für Vergleichende Milbenkunde. Acarologie 1992, 39, 69–95. [Google Scholar]
- Hirschmann, W.; Zirngiebl-Nicol, I. Uropodiden Das Gangsystem der Fanilie Uropodide (Berlese, 1882) Hirschmann u. Zirngiebl-Nicol nov. comb. Brstimmungstabellen Kurzdiagnosen Operculumbestimmungstabellen. GDP T.7. Acarologie 1964, 6, 2–22. [Google Scholar]
- Hirschmann, W.; Zirngiebl-Nicol, I. Uropodiden bestimmungstabellen von 300 Uropodiden-Arten (Larven, Protonymphen, Deutonymphen, Weibchen, Männchen). GDP T.9. Acarologie 1965, 8, 2–33. [Google Scholar]
- Hirschmann, W.; Zirngiebl-Nicol, I. Neunzehn neue Uropoda-Arte. DGP T. 39. Acarologie 1969, 12, 20. [Google Scholar]
- Hirschmann, W. Adulten-Gruppen und Bestimmungstabelle von 63 Uropoda-Arten (Uropodini, Uropodinae). GDP T. 120. Acarologie 1972, 18, 67–74. [Google Scholar]
- Hirschmann, W. Teilgange, Stadien von 21 nneuen Uropoda (Phalodinychus)-Arten (Uropodini, Uropodinae). GDP T. 123. Acarologie 1972, 18, 79–90. [Google Scholar]
- Hirschmann, W. Stadienfamilien und Stadiengattungender Atrichopygidiina, erstellt im Vergleich zum Gangsystem Hirschmann 1979. SDP T.1. Acarologie 1979, 26, 57–70. [Google Scholar]
- Hirschmann, W.; Hiramatsu, N. Weibchen einer neuen Uropoda (Phaulodinychus) -Art der penicillata-Gruppe aus den Philippinen (Uropodini, Uropodinae). GDP T.517. Acarologie 1990, 37, 86–88. [Google Scholar]
- Niedbała, W. Brachychthoniidae Polski (Acari, Oribatei): Studium Ekologiczno-Faunistyczne. Monografie Fauny Polski; Państ. Wydaw Naukowe: Warszawa, Poland; Kraków, Poland, 1976; pp. 1–144. [Google Scholar]
- Olszanowski, Z. A Monograph of the Nothridae and Camisiidae of Poland (Acari: Oribatida: Crotonioidea); Genus (Supplement); BS: Wrocław, Poland, 1996; pp. 1–201. [Google Scholar]
- Niedbała, W. Ptyctimous mites (Acari, Oribatida) of Poland. Fauna Poloniae. Nat. Opt. Dux. Found. 2008, 3, 1–242. [Google Scholar]
- Błaszak, C. Zerconidae (Acari, Mesostigmata) Polski; Państwowe Wydawn. Naukowe: Kraków, Poland, 1974; pp. 1–315. [Google Scholar]
- Błoszyk, J.; Książkiewicz-Parulska, Z.; Adamski, Z.; Napierała, A. Influence of Pleistocene glaciation on the distribution of three species of Labidostomma in Europe (Acari: Labidostommatidae). Syst. Appl. Acarol. 2017, 22, 841–857. [Google Scholar] [CrossRef]










| Proper Species | Similar Species |
|---|---|
| Baloghibrasiluropoda foveolata Hirschmann, 1973—Brazil | Baloghibrasiluropoda foveolatasimilis Hirschmann, 1973—Brazil; Baloghibrasiluropoda foveatoides Hirschmann, 1973—Brazil |
| Baloghkaszabia baloghi Hirschmann, 1973—Brazil | Baloghkaszabia baloghisimilis Hirschmann, 1973—Brazil |
| Castriidinychus castri Hirschmann, 1973—Chile | Castriidinychus castrisimilis Hirschmann, 1973—Chile |
| Castriidinychus dentatus (Hirschmann, 1972)—Chile | Castriidinychus similidentatus Hirschmann, 1973—Chile Castriidinychus dentatoides Hirschmann, 1973—Chile |
| Castriidinychus eupunctatus Hirschmann, 1972—Chile | Castriidinychus eupunctatosimilis Hirschmann, 1972—Chile |
| Clausiadinychus cristatus Sellnick, 1930—Martinique | Clausiadinychus similicristatus Hirschmann, 1973—Brazil |
| Cyllibula (Baloghcyllibula) alta (Sellnick, 1973)—Trinidad | Cyllibula (Baloghcyllibula) altasimilis Hirschmann, 1977)—Bolivia |
| Deraiophorus hirschmanni Hiramatsu, 1977—Japan | Deraiophorus hirschmannisimilis Hiramatsu, 1977—Japan |
| Deraiophorus piriformis Hirschmann, 1973—? | Deraiophorus piriformoides Hirschmann and Hiramatsu, 1990—Philippines |
| Deraiophorus dicornutus Hirschmann, 1973—Bolivia | Deraiophorus dicornutosimilis Hirschmann, 1973—Bolivia |
| Deraiophorus hexacornutus Hirschmann, 1973—New Guinea | Deraiophorus hexacornutosimilis Hirschmann, 1973—New Guinea |
| Deraiophorus penicillatus Hirschmann, 1973—Cejlon | Deraiophorus penicillatasimilis Hirschmann, 1973—Ceylon |
| Deraiophorus loksai Hirschmann, 1973—Brazil, Paragwaj | Deraiophorus loksaisimilis Hirschmann, 1973—Paraguay |
| Deraiophorus kaszabi Hirschmann, 1973—Brazil, Chile | Deraiophorus kaszabisimilis Hirschmann, 1973—Brazil |
| Deraiophorus stammeri Hirschmann and Zirngiebl-Nicol, 1969—Brazil | Deraiophorus stammerisimilis Hirschmann, 1973—Brazil |
| Dinychus carinatus Berlese, 1903—Italy, France, Poland | Dinychus bincheaecarinatus Hirschmann, Wągrowska-Adamczyk and Zirngiebl-Nicol, 1984—Germany, France, Slovakia Dinychus carinatus var. magnus (Athias-Binche, 1980)—France |
| Dicourella baloghi Hirschmann and Zirngiebl-Nicol, 1969—Hungary, Romania, Poland | Dicourella baloghisimilis Wiśniewski, 1984—Poland |
| ?? | Discourella crucisimilis Hirschmann, 1972—Paraguay, Brazil |
| Discourella caputmedusae (Berlese and Leonardi, 1901)—Chile | Discourella caputmedusaesimilis Hirschmann, 1972—Chile |
| Discourella modesta (Leonardi, 1899)—Europe | Discourella modestasimilis (Hiramatsu and Hirschmann, 1979)—Canada |
| Discourella rotunda Hirschmann, 1972—Brazil | Discourella rotunda Hirschmann, 1973—Brazil Discourella rotundiformis Hirschmann, 1973—Brazil |
| Hutufeideria hirschmanni Hiramatsu, 1978—New Guinea | Hutufeideria hirschmannisimilis Hiramatsu, 1980—New Guinea |
| Hutufeideria feideri Hirschmann and Hiramatsu, 1977—New Guinea | Hutufeideria feiderisimilis Hiramatsu, 1981—New Guinea |
| Jerzywisniewska depilata (Trouessart, 1902)—Brazil | Jerzywisniewska depilatasimilis (Wiśniewski, 1902)—Brazil |
| Kaszabjbaloghia kaszabi Hirschmann, 1873—Ecuador | Kaszabjbaloghia kaszabisimilis Hirschmann, 1873—Peru |
| Nenteria oudemansi Hirschmann and Ziringiebl—Nicol, 1969—Netherlands | Nenteria oudemansiformis Hirschmann, 1985—? |
| Nenteria pilosella (Berlese, 1903)—Italy | Nenteria pilosellapides Hirschmann and Hiramatsu, 1978—Italy |
| Nenteria ritzemai (Oudemans, 1903)—Netherlands, Belgia, Niemcy | Nenteria ritzemaisimilis Hirschmann and Hiramatsu, 1978—Japan |
| Nenteria tropica (Oudemans, 1905)—Togo | Nenteria tropicasimilis Wiśniewski and Hirschamnn, 1985—Guinea |
| Oplitis baloghi Zirngiebl–Nicol and Hirschmann, 1973—Paraguay | Oplitis baloghisimilis Zirngiebl–Nicol and Hirschmann, 1973—Chile |
| Oplitis bispirata (Sellnick, 1954)—Brazil | Oplitis similibispirata Zirngiebl–Nicol, 1973—Bolivia |
| Oplitis castrii Zirngiebl—Nicol and Hirschmann, 1973—Chile | Oplitis castriisimilis Zirngiebl–Nicol and Hirschmann, 1973—Brazil |
| Oplitis mahunkai Zirngiebl—Nicol and Hirschmann, 1973—Brazil | Oplitis mahunkaisimilis Zirngiebl–Nicol and Hirschmann, 1973—Brazil |
| Oplitis minutissima (Berlese, 1903)—Netherlands, Italy, United Kingdom, Ukraine | Oplitis japanominutissima Hiramatsu, 1979—Japan Oplitis similiminutissima Hiramatsu, 1979—Japan |
| Oplitis dimidiata Hirschmann, 1991—Belize | Oplitis dimidiatasimilis Hirschmann and Wiśniewski, 1991—Cuba |
| Oplitis kaszabii Zirngiebl—Nicol and Hirschmann, 1973—Peru | Oplitis kaszabiisimilis Zirngiebl—Nicol and Hirschmann, 1973—Brazil |
| Oplitis pecki Hirschmann, 1991—Galapagos Islands | Oplitis peckisimilis Hirschmann, 1991—Galapagos Islands |
| Rotundabaloghia baloghi Hirschmann, 1975—New Guinea | Rotundabaloghia baloghisimilis Hirschmann, 1975—New Guinea Rotundabaloghia baloghioides Hirschmann, 1975—New Guinea |
| Rotundabaloghia incisa Hirschmann, 1992—Peru | Rotundabaloghia incisasimilis Hirschmann, 1992—Peru |
| Rotundabaloghia leteciae Hirschmann, 1992—Colombia | Rotundabaloghia leteciaesimilis Hirschmann, 1992—Colombia |
| Rotundabaloghia soliformis Hirschmann, 1992—Ecuador | Rotundabaloghia soliformoides Hirschmann, 1992—Ecuador |
| Rotundabaloghia haradai Hiramatsu, 1983—Borneo | Rotundabaloghia haradaisimilis Hiramatsu and Hirschmann, 1992—Philippines |
| Rotundabaloghia campanella Hirschmann, 1992—Cameroon | Rotundabaloghia campanellasimilis Hirschmann, 1992—Cameroon |
| Rotundabaloghia kaszabi Hirschmann, 1975—New Guinea | Rotundabaloghia kaszabi Hirschmann, 1975—New Guinea |
| Tetrasejaspis baloghi Hirschmann, 1973—Brazil | Tetrasejaspis baloghisimilis Hirschmann, 1973—Brazil |
| Trachyuropoda arculata Hirschmann, 1975—Brazil | Trachyuropoda similiarculata Hirschmann, 1975—Brazil |
| Trachyuropoda baloghi Hirschmann, 1975—Chile | Trachyuropoda baloghisimilis Hirschmann, 1975—Chile |
| Trachyuropoda coccinea (Michael, 1891)—Europe | Trachyuropoda similicoccinea Hiramatsu, 1979—Japan |
| Trachyuropoda longicornuta Hirschmann, 1976—Spain | Trachyuropoda longicornutasimilis Hirschmann, 1976—?? |
| Trachyuropoda formicaria (Lubbock, 1881)—Europe | Trachyuropoda formicariasimilis Hirschmann, 1975—Russia |
| Trachyuropoda mesofovea Hirschmann, 1976—Paragway | Trachyuropoda mesofoveasimilis Hirschmann, 1976—?? |
| Trachyuropoda schusteri Hirschmann, 1976—Brazil | Trachyuropoda schusterisimilis Hirschmann, 1976—?? |
| Trachyuropoda multituberosa (Willmann, 1951)—Austria | Trachyuropoda multituberculata Hirschmann, 1976—Spain, Austria Trachyuropoda tuberosa Hirschmann, 1976—Spain |
| Trachyuropoda dicarinata Hirschmann, 1976—Peru | Trachyuropoda dicarinatasimilis Hirschmann, 1976—Peru |
| Trichouropoda polytricha (Vitzthum, 1923)—Central Europe, Turkiestan | Trichouropoda polytrichasimilis Hirschmann, 1972—Portugal |
| Trichouropoda lindquisti Hirschmann, 1978—Canada | Trichouropoda lindquistisimilis Hirschmann, 1978—Canada |
| Trichouropoda afossalis Hirschmann, 1978—Ghana | Trichouropoda afossalisimilis Hirschmann and Wiśniewski, 1987—Indie |
| Trichouropoda denticulata Hirschmann, 1978—USA | Trichouropoda denticulatasimilis Wiśniewski and Hirschmann, 1987—Honduras |
| Trichouropoda ditricha Hirschmann and Wiśniewski, 1987—Ghana | Trichouropoda ditrichasimilis Hirschmann and Wiśniewski, 1987—Cameroon |
| Trichouropoda bipilis (Vitzthum, 1921)—Austria | Trichouropoda similibipilis Hirschmann, 1972—USA |
| Trichouropoda obscura (C. L. Koch, 1836)—Austria | Trichouropoda obscurasimilis Hirschmann and Zirngiebl–Nicol, 1961—Central Europe |
| Trichouropoda punctata Hirschmann and Zirngiebl–Nicol, 1961—Spain, Norway | Trichouropoda punctatasimilis Wiśniewski and Hirschmann, 1986—Poland |
| Trichouropoda javensis (Oudemans, 1901)—Java | Trichouropoda similijavensis Hiramatsu and Hirschmann, 1979—Philippines Trichouropoda philippino javensis Hirschmann and Wiśniewski, 1988—Philippines |
| Trichouropoda adifaxa (Vitzthum, 1921)—Spanish Guinea | Trichouropoda adifaxasimilis Hirschmann and Wiśniewski, 1986—Spanish Guinea |
| Trichouropoda endroedyi Hirschmann and Wiśniewski, 1986—Ghana | Trichouropoda endroedyioides Hirschmann and Wiśniewski, 1986—Ghana |
| Trichouropoda ruehmi Hirschmann, 1972—Brazil | Trichouropoda ruehmisimilis Wiśniewski and Hirschmann, 1987—Brazil |
| Trichouropoda serrata Hirschmann and Zirngiebl–Nicol, 1961—Germany | Trichouropoda serratasimilis Hirschmann and Zirngiebl–Nicol, 1961—Canada |
| Trichouropoda stammeri Hirschmann and Zirngiebl–Nicol, 1969—Hungary | Trichouropoda stammerisimilis Hirschmann, 1978—Poland |
| Trichouropoda sturmii Hirschmann and Wiśniewski, 1987—Colombia | Trichouropoda sturmiisimilis Hirschmann and Wiśniewski, 1987—Colombia |
| Trichouropoda sturmii Hirschmann and Zirngiebl–Nicol, 1961 Central and Western Europe, Mongolia, Siberia | Trichouropoda sturmiisimilis Hirschmann and Wiśniewski, 1987—Germany |
| Trichouropoda sellnicki Hirschmann and Zirngiebl–Nicol, 1969—Bahamas | Trichouropoda sellnickioides Wiśniewski and Hirschmann, 1988—Cuba |
| Trichouropoda solaris Hirschmann, 1972—Brazil | Trichouropoda solarissima Hirschmann, 1978—Bolivia |
| Trichouropoda vanna (Lombardini, 1928)—Brazil | Trichouropoda vannaoides Hirschmann, 1978—Bolivia |
| Trichouropoda quadritricha Hirschmann and Wiśniewski, 1988—Ghana | Trichouropoda quadritrichasimilis Hirschmann and Wiśniewski, 1988—Ghana |
| Trigonuropoda crucistructura Hirschmann, 1975—Sri Lanka | Trigonuropoda crucistructuraoides Hirschmann, 1975—Sri Lanka |
| Trigonuropoda schizostructura Hirschmann, 1975—Sri Lanka | Trigonuropoda schizostructurasimilis Hirschmann, 1975—Sri Lanka |
| Trigonuropoda tuberosa Hirschmann, 1975—New Guinea | Trigonuropoda tuberosasimilis Hirschmann, 1975—New Guinea |
| Trigonuropoda monofoveolata Hirschmann, 1975—New Guinea | Trigonuropoda monofoveolatasimilis Hirschmann, 1975—New Guinea |
| Trigonuropoda sanguinea Hirschmann and Hiramatsu, 1977—Japan | Trigonuropoda sanguineasimilis Hirschmann and Hiramatsu, 1977—Taiwan |
| Trigonuropoda tuberculata Hirschmann, 1975—New Guinea | Trigonuropoda tuberculatasimilis Hiramatsu, 1979—Japan |
| Trigonuropoda terra–reginae Domrow, 1957—Australia | Trigonuropoda terra–reginaesimilis Hirschmann, 1975—Australia |
| Trigonuropoda trioculata Hirschmann, 1975—Australia | Trigonuropoda trioculatasimilis Hirschmann, 1975—Australia |
| Trigonuropoda trichobaloghia Hirschmann, 1975—Peru | Trigonuropoda trichobaloghiasimilis Hirschmann, 1975—New Guinea |
| Trigonuropoda pontina Hirschmann, 1975—Ceylon | Trigonuropoda trichopontina Hirschmann, 1975—Sri Lanka |
| Uroactinia hippocrepea (Berlese, 1918)—Thaiti | Uroactinia hippocrepoides (Vitzthum, 1935)—Marquesas Islands |
| Urodiaspis religiosa Hiramatsu, 1979—Japan | Urodiaspis similireligiosa Hiramatsu, 1979—Japan |
| Uroobovella costai Hirschmann and Zirngiebl–Nicol, 1972—Israel, Romania, Greenland | Uroobovella costaisimilis Wiśniewski, 1980—New Guinea |
| Uroobovella faceta Hiramatsu and Hirschmann, 1978—Ecuador | Uroobovella facetaoides Hiramatsu and Hirschmann, 1978—New Guinea |
| Uroobovella pectinata (Hirschmann, 1973)—New Guinea | Uroobovella pectinatasimilis Hiramatsu, 1980—Indonezja |
| Uroobovella fimicola (Berlese, 1903)—Europe | Uroobovella fimicolasimilis Hirschmann and Zirngiebl–Nicol, 1972—Chile |
| Uroobovella flagelliger (Berlese, 1910)—Italy, Sweden, Germany, Switzerland, Europe | Uroobovella flagelligerformis Hirschmann, 1979—Canada |
| Uroobovella foveolata Hirschmann and Zirngiebl–Nicol, 1972—France | Uroobovella foveolatasimilis Hiramatsu, 1980—Japan |
| Uroobovella ipidis (Vitzthum, 1923)—Europe, Lebanon | Uroobovella ipidisimilis Hirschmann and Zirngiebl–Nicol, 1962—Germany, Poland |
| Uroobovella orri Hirschmann, 1972—USA | Uroobovella orrisimilis Hirschmann and Zirngiebl–Nicol, 1975—Australia |
| Uroobovella obovata (Canestrini and Berlese, 1884)—Europe | Uroobovella similiobovata Hirschmann and Zirngiebl–Nicol, 1962—Germany, Rumunia |
| Uroobovella ovalis Hirschmann and Zirngiebl–Nicol, 1962—Germany | Uroobovella similiovalis Hirschmann and Zirngiebl–Nicol, 1979—Germany |
| Uroobovella pauxila Hiramatsu, 1981—New Guinea | Uroobovella pauxiloides Hirschmann, 1981—Vietnam |
| Uroobovella takakii Hiramatsu, 1980—Japan | Uroobovella similitakakensis Hirschmann, 1981—Vietnam |
| Uroobovella portalis Hirschmann, 1973—Brazil | Uroobovella portalisimilis Hirschmann, 1981—Brazil |
| Uroobovella nova (Oudemans, 1902—Europe | Uroobovella novasimilis Hiramatsu, 1979—Japan |
| Uroobovella Zaireensis Hirschmann, 1981—Zaire | Uroobovella similiZaireensis Hirschmann, 1981—USA |
| Uroobovella vitzthumi Hirschmann and Zirngiebl–Nicol, 1962—?? | Uroobovella vitzthumisimilis Hirschmann, 1973—Paraguay |
| Uropoda (Metadinychus) serrata Hirschmann, 1972—Paraguay | Uropoda (Metadinychus) serratasimilis Hiramatsu and Hirschmann, 1972—Bolivia |
| Uropoda (Metadinychus) argasiformis (Berlese, 1916)—Brazil, Bolivia | Uropoda (Metadinychus) similiargasiformis Hirschmann, 1981—Zaire |
| Cilliba cassidea (Hermann, 1804)—Europe | Cilliba cassideasimilis Błoszyk, Stachowiak and Halliday, 2006—Central Europe |
| Uropoda (Phaulodinychus) amani Hirschmann, 1973—East Africa | Uropoda (Phaulodinychus) amanisimilis Wiśniewski, 1980—Bismarck Archipelago |
| Uropoda (Phaulodinychus) difoveolata Hirschmann and Zirngiebl–Nicol, 1969—Brazil | Uropoda (Phaulodinychus) difoveolatasimilis Hirschmann, 1972—Brazil |
| Uropoda (Phaulodinychus) quadridentata Hirschmann, 1973—Ecuador | Uropoda (Phaulodinychus) quadridentatasimilis Hiramatsu, 1980—Brazil |
| Uropoda (Phaulodinychus) hiramatsui Hirschmann, 1976—New Guinea | Uropoda (Phaulodinychus) hiramatsuiformis Hirschmann, 1976—New Guinea Uropoda (Phaulodinychus) hiramatsuioides Hirschmann, 1976—New Guinea Uropoda (Phaulodinychus) hiramatsuisimilis Hirschmann, 1976—New Guinea |
| Uropoda (Phaulodinychus) hamulifera Michael, 1894—Europe | Uropoda (Phaulodinychus) similihamulifera Hiramatsu, 1894—Japan |
| Uropoda (Phaulodinychus) stolida Hiramatsu and Hirschmann, 1978—Peru | Uropoda (Phaulodinychus) stolidasimilis Hiramatsu and Hirschmann, 1979—Peru |
| Uropoda (Uropoda) orbicularis (Muller, 1776)—Europe | Uropoda (Uropoda) japanoorbicularis Hiramatsu, 1979—Japan Uropoda (Uropoda) onishiiorbicularis Hiramatsu, 1980—Japan Uropoda (Uropoda) similiorbicularis Hiramatsu, 1980—Japan |
| Uropoda (Phaulodinychus) penicillata Hirschmann and Zirngiebl–Nicol, 1969—Panama | Uropoda (Phaulodinychus) penicillatasimilis Hirschmann, 1972—Brazil |
| Uropoda (Phaulodinychus) procera Hiramatsu and Hirschmann, 1979—Meksyk | Uropoda (Phaulodinychus) procerasimilis Hiramatsu and Hirschmann, 1979—Mexico |
| Uropoda (Phaulodinychus) regia (Vitzthum, 1921)—Bolivia | Uropoda (Phaulodinychus) regiasimilis Hirschmann, 1972—Brazil |
| Uropoda (Phaulodinychus) Braziliensis (Sellnick, 1962)—Brazil | Uropoda (Phaulodinychus) similiBraziliensis Hirschmann, 1972– Paragway |
| Uropoda (Phaulodinychus) insulana Hiramatsu, 1979—Japan | Uropoda (Phaulodinychus) insulanasimilis Hiramatsu, 1981—Japan |
| Uropoda (Phaulodinychus) porticensis (Berlese, 1903)—Italy | Uropoda (Phaulodinychus) porticensoides Hirschmann, 1993—Switzerland |
| Uropoda (Phaulodinychus) morikawai Hiramatsu, 1978—Japan | Uropoda (Phaulodinychus) similimorikawai Hiramatsu, 1979—Japan |
| Uropoda (Phaulodinychus) tropicana Hiramatsu, 1978—New Guinea | Uropoda (Phaulodinychus) tropicanasimilis Hiramatsu, 1981—New Guinea |
| Uropoda (Phaulodinychus) ungulata Hirschmann and Hiramatsu, 1977—Ecuador | Uropoda (Phaulodinychus) ungulatasimilis Hirschmann and Hiramatsu, 1979—Ecuador |
| No | Country | Location | Species |
|---|---|---|---|
| 1 | Colombia | Monserrate | Rotundabalogia lamellosa Hirschmann, 1992; Rotundabaloghia tetraclavata Hirschmann, 1992; Rotundabaloghia chisacaensis Hirschmann, 1992; Rotundabaloghia monserratensisHirschmann, 1992; Rotundabaloghia monterredondoensis Hirschmann, 1992; Rotundabaloghia silvacola Hirschmann, 1992; Rotundabaloghia humicola Hirschmann, 1992; Rotundabaloghia bosquensis Hirschmann, 1992; Rotundabaloghia fincae Hirschmann, 1992; |
| 2 | Colombia | Alto Belem | Rotundabaloghia forcipata Hirschmann, 1992; Rotundabaloghia hexaspinosa Hirschmann, 1992; Rotundabaloghia belemensis Hirschmann, 1992; Rotundabaloghia altoensis Hirschmann, 1992; |
| 3 | Colombia | El Guerrero | Rotundabaloghia pituitosa Hirschmann, 1992; Rotundabaloghia sexspinosa Hirschmann, 1992; Rotundabaloghia diclavata Hirschmann, 1992; Rotundabaloghia flava Hirschmann, 1992; Rotundabaloghia guerreroensis Hirschmann, 1992; |
| 4 | Colombia | Paramo | Rotundabaloghia hexaspinosa Hirschmann, 1992; |
| 5 | Colombia | La Rusia | Rotundabaloghia hexaspinosa Hirschmann, 1992; |
| 6 | Colombia | Letecia | Rotundabaloghia hexaspinosa Hirschmann, 1992; Rotundabaloghia amazonasae Hirschmann, 1992; Rotundabaloghia leteciae Hirschmann, 1992; Rotundabaloghia leteciasimilis Hirschmann, 1992; |
| 7 | Colombia | Chingaza | Rotundabaloghia sexspinosa Hirschmann, 1992; Rotundabaloghia octospinosa Hirschmann, 1992; Rotundabaloghia tetraclavata Hirschmann, 1992; Rotundabaloghia chingazensis Hirschmann, 1992; |
| 8 | Colombia | La Calera; Bogota; Chisaca | Rotundabaloghia tetraclavata Hirschmann, 1992; Rotundabaloghia diclavata Hirschmann, 1992; Rotundabaloghia chisacaensis Hirschmann, 1992; Rotundabaloghia pajonalis Hirschmann, 1992; |
| 9 | Colombia | La Tagua | Rotundabaloghia tague Hirschmann, 1992; |
| 10 | Colombia | Huila Resina | Rotundabaloghia hullae Hirschmann, 1992; Rotundabaloghia resinae Hirschmann, 1992; |
| 11 | Brazil | Manaus | Rotundabaloghia tetraunguiseta Hirschmann, 1992; Rotundabaloghia manausensis Hirschmann, 1992; |
| 12 | Brazil | Santos | Rotundabaloghia hexaunguiseta Hirschmann, 1992; |
| 13 | Peru | Lima-Pucallpa | Rotundabaloghia limae Hirschmann, 1992; Rotundabaloghia duodecimsetae Hirschmann, 1992; Rotundabaloghia limae Hirschmann, 1992; Rotundabaloghia magnioperculi Hirschmann, 1992; Rotundabaloghia ucayali Hirschmann, 1992; Rotundabaloghia vonalis Hirschmann, 1992; Rotundabaloghia peruensis Hirschmann, 1992; |
| 14 | Ecuador | Quito-Bacza | Rotundabaloghia ovaligynella Hirschmann, 1992; Rotundabaloghia soliformoides Hirschmann, 1992; Rotundabaloghia quitoaensis Hirschmann, 1992; Rotundabaloghia magna Hirschmann, 1992; Rotundabaloghia soliformis Hirschmann, 1992; Rotundabaloghia linguaeformis Hirschmann, 1992; Rotundabaloghia baczaensis Hirschmann, 1992; Rotundabaloghia maculosa Hirschmann, 1992; Rotundabaloghia equadorensis Hirschmann, 1992; |
| 15 | Peru | Iquitos | Rotundabaloghia incisasimilis Hirschmann, 1992; Rotundabaloghia incisa Hirschmann, 1992; Rotundabaloghia iquitosensis Hirschmann, 1992; Rotundabaloghia iquitosensoides Hirschmann, 1992; Rotundabaloghia pucallpae Hirschmann, 1992; Rotundabaloghia maranonensis Hirschmann, 1992; Rotundabaloghia moyobambe Hirschmann, 1992; Rotundabaloghia cajamarcae Hirschmann, 1992; |
| 16 | Peru | Machu Picchu | Rotundabaloghia picchuensis Hirschmann, 1992; |
| 17 | Venezuela | ? | Rotundabaloghia venezuelae Hirschmann, 1992; |
| 18 | Bolivia | ? | Rotundabaloghia boliviensis Hirschmann, 1992; |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Błoszyk, J.; Napierała, A. Endemism of Uropodina Mites: Spurious or Real? Diversity 2020, 12, 283. https://doi.org/10.3390/d12070283
Błoszyk J, Napierała A. Endemism of Uropodina Mites: Spurious or Real? Diversity. 2020; 12(7):283. https://doi.org/10.3390/d12070283
Chicago/Turabian StyleBłoszyk, Jerzy, and Agnieszka Napierała. 2020. "Endemism of Uropodina Mites: Spurious or Real?" Diversity 12, no. 7: 283. https://doi.org/10.3390/d12070283
APA StyleBłoszyk, J., & Napierała, A. (2020). Endemism of Uropodina Mites: Spurious or Real? Diversity, 12(7), 283. https://doi.org/10.3390/d12070283