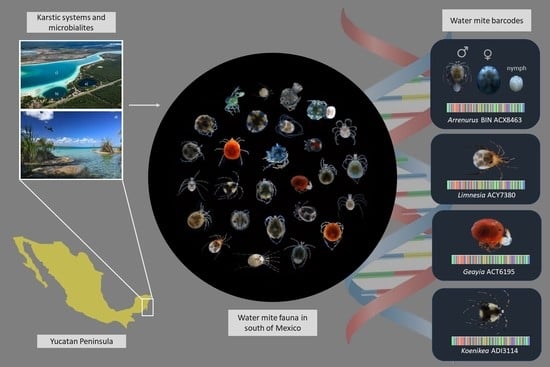
Water Mite Diversity (Acariformes: Prostigmata: Parasitengonina: Hydrachnidiae) from Karst Ecosystems in Southern of Mexico: A Barcoding Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Samples
2.2. Specimen Preparational Analysis
2.3. DNA Extraction and Amplification
2.4. Sequencing and Data Analysis
3. Results
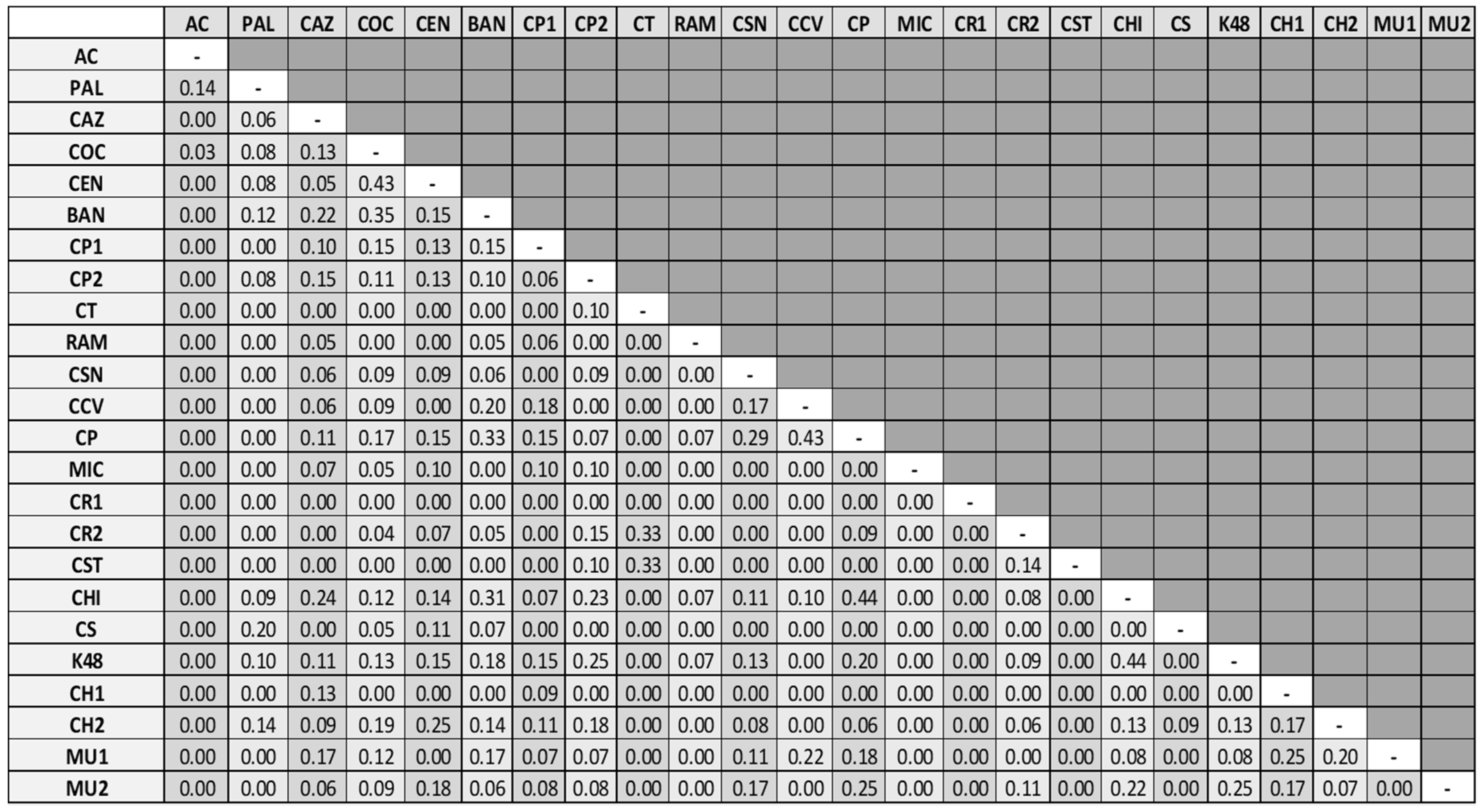
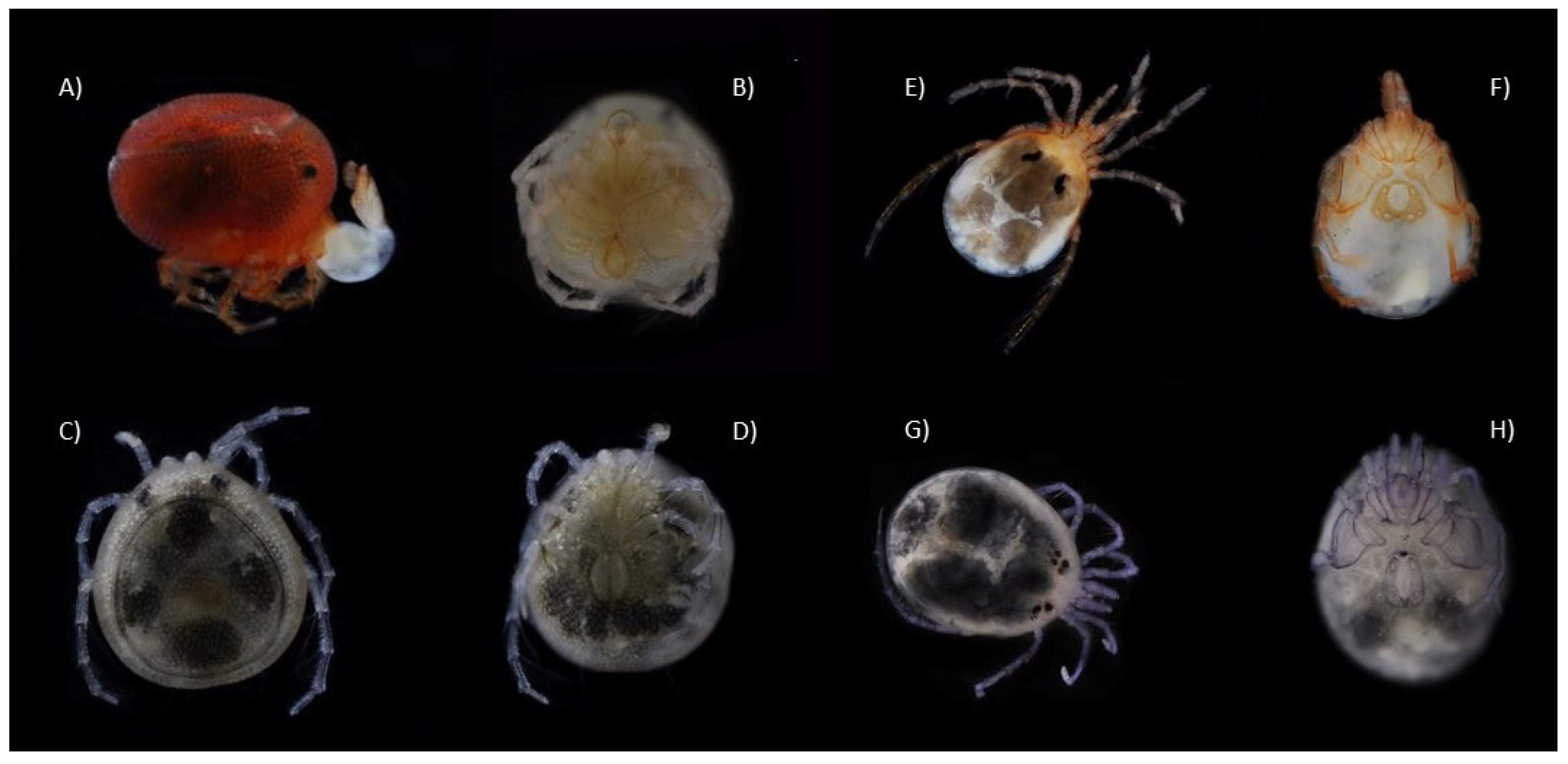
3.1. Water Mite BINs Richness
3.2. BIN Assemblies in the PY
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Di Sabatino, A.; Smit, H.; Gerecke, R.; Goldschmidt, T.; Matsumoto, N.; Cicolani, B. Global diversity of water mites (Acari, Hydrachnidia; Arachnida) in freshwater. Hydrobiologia 2008, 595, 303–315. [Google Scholar] [CrossRef]
- Proctor, H.C.; Smith, I.M.; Cook, D.R.; Smith, B.P. Subphylum Chelicerata, Class Arachnida. In Thorp and Covich´s Freshwater Invertebrades: Ecology and General Biology; Thorp, J., Rogers, D.C., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 599–660. ISBN 9780123850270. [Google Scholar]
- Goldschmidt, T. The biodiversity of Neotropical water mites. In Acarid Phylogeny and Evolution: Adaptations in Mites and Ticks; Bernini, F., Nannelli, R., Nuzzaci, G., de Lillo, E., Eds.; Kluwer Academic Publisher: Dordrecht, The Netherlands, 2002; pp. 91–99. [Google Scholar]
- Goldschmidt, T.; Ramírez-Sánchez, M.; Pedroza-Ramos, A.; Rico-Sánchez, A.E.; Ojeda, P.M.; Pérez-Munguia, R.M.; Pimiento-Ortega, M.; Carlos-Delgado, A.M.; Durán-Suáres, S. Primer registro de ácaros acuáticos (Acari: Hydrachnidia) del estado de Querétaro, México First records of water mites (Acari: Hydrachnidia) from Queretaro, Mexico. Dugesiana 2015, 22, 21–27. [Google Scholar]
- Rivas, G.; Hoffmann, A. Los ácaros acuáticos de México. Estado actual de su conocimiento. Mexicoa 2000, 2, 33–39. [Google Scholar]
- Cook, D.R. Studies on Neotropical Water Mites; The American Entomological Institute: Gainesville, FL, USA, 1980. [Google Scholar]
- Otero-Colina, G. Datos originales sobre los ácaros acuáticos (Prostigmata:Parasitengona) del sureste de México. Folia Entomol. Mex. 1988, 76, 195–223. [Google Scholar]
- Ramírez-Sánchez, M.M.; Rivas, G. New species of subgenus Megaluracarus (Acari: Hydrachnidiae: Arrenuridae: Arrenurus) from Mexico. Zootaxa 2013, 3718, 317–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gondwe, B.R.N.; Lerer, S.; Stisen, S.; Marín, L.; Rebolledo-Vieyra, M.; Merediz-Alonso, G.; Bauer-Gottwein, P. Hydrogeology of the south-eastern Yucatan Peninsula: New insights from water level measurements, geochemistry, geophysics and remote sensing. J. Hydrol. 2010, 389, 1–17. [Google Scholar] [CrossRef]
- Perry, E.; Velazquez-Oliman, G.; Marin, L. The hydrogeochemistry of the karst aquifer system of the northern yucatan peninsula, Mexico. Int. Geol. Rev. 2002, 44, 191–221. [Google Scholar] [CrossRef]
- Castro-Contreras, S.I.; Gingras, M.K.; Pecoits, E.; Aubet, N.R.; Petrash, D.A.; Castro-contreras, S.M.; Dick, G.; Planavsky, N.; Konhauser, K.O. Textural and Geochemical Features of Freshwater Microbialites. Palaios 2014, 29, 192–209. [Google Scholar] [CrossRef] [Green Version]
- Gischler, E.; Gibson, M.A.; Oschmann, W. Giant holocene freshwater microbialites, Laguna Bacalar, Quintana Roo, Mexico. Sedimentology 2008, 55, 1293–1309. [Google Scholar] [CrossRef]
- Elías-Gutiérrez, M.; Valdez-Moreno, M.; Topan, J.; Young, M.R.; Cohuo-Colli, J.A. Improved protocols to accelerate the assembly of DNA barcode reference libraries for freshwater zooplankton. Ecol. Evol. 2018, 8, 3002–3018. [Google Scholar] [CrossRef]
- Pešić, V.; Smit, H. Evidence of cryptic and pseudocryptic speciation in Brachypodopsis baumi species complex (Acari, Hydrachnidia, Aturidae) from Borneo, with description of three new species. Syst. Appl. Acarol. 2016, 21, 1092–1106. [Google Scholar] [CrossRef]
- Pešić, V.; Asadi, M.; Cimpean, M.; Dabert, M.; Esen, Y.; Gerecke, R.; Martin, P.; Savić, A.; Smit, H.; Stur, E. Six species in one: Evidence of cryptic speciation in the Hygrobates fluviatilis complex (Acariformes, Hydrachnidia, Hygrobatidae). Syst. Appl. Acarol. 2017, 22, 1327–1337. [Google Scholar] [CrossRef]
- Pešić, V.; Broda, L.; Dabert, M.; Gerecke, R.; Martin, P.; Smit, H. Re-established after hundred years: Definition of Hygrobates prosiliens Koenike, 1915, based on molecular and morphological evidence, and redescription of H. longipalpis (Hermann, 1804) (Acariformes, Hydrachnidia). Syst. Appl. Acarol. 2019, 24, 1490–1511. [Google Scholar] [CrossRef]
- Stålstedt, J.; Bergsten, J.; Ronquist, F. “Forms” of water mites (Acari: Hydrachnidia): Intraspecific variation or valid species? Ecol. Evol. 2013, 3, 3415–3435. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; DeWaard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. B Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [Green Version]
- DeWaard, J.R.; Ratnasingham, S.; Zakharov, E.V.; Borisenko, A.V.; Steinke, D.; Telfer, A.C.; Perez, K.H.J.; Sones, J.E.; Young, M.R.; Levesque-Beaudin, V.; et al. A reference library for the identification of Canadian invertebrates: 1.5 million DNA barcodes, voucher specimens, and genomic samples. Sci. Data 2019, 6, 308. [Google Scholar] [CrossRef] [Green Version]
- Stryjecki, R.; Bańkowska, A.; Gryzińska, M.; Sarnacka, E.; Rutkowska, M.; Zawal, A. The use of molecular techniques in the taxonomy of water mites (Hydrachnidia, Acari). Acta Biol. 2016, 23, 117–126. [Google Scholar] [CrossRef] [Green Version]
- Pešić, V.; Smit, H. A second Palaearctic species of the genus Wettina Piersig, 1892 based on morphological and molecular data (Acari, Hydrachnidia: Wettinidae). Syst. Appl. Acarol. 2018, 23, 724–732. [Google Scholar] [CrossRef]
- Blattner, L.; Gerecke, R.; von Fumetti, S. Hidden biodiversity revealed by integrated morphology and genetic species delimitation of spring dwelling water mite species (Acari, Parasitengona: Hydrachnidia). Parasites Vectors 2019, 12, 1–13. [Google Scholar] [CrossRef]
- Montes-Ortiz, L.; Elías-Gutiérrez, M. Faunistic survey of the zooplankton community in an oligotrophic sinkhole, cenote azul (Quintana roo, Mexico), using different sampling methods, and documented with DNA barcodes. J. Limnol. 2018, 77, 428–440. [Google Scholar] [CrossRef]
- Martin, P.; Dabert, M.; Dabert, J. Molecular evidence for species separation in the water mite Hygrobates nigromaculatus Lebert, 1879 (Acari, Hydrachnidia): Evolutionary consequences of the loss of larval parasitism. Aquat. Sci. 2010, 72, 347–360. [Google Scholar] [CrossRef]
- Young, M.R.; Proctor, H.C.; DeWaard, J.R.; Hebert, P.D.N. DNA barcodes expose unexpected diversity in canadian mites. Mol. Ecol. 2019, 28, 5347–5359. [Google Scholar] [CrossRef]
- Ratnasingham, S.; Hebert, P.D.N. A DNA-based registry for all animal species: The barcode index number (BIN) system. PLoS ONE 2013, 8, e66213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montes-Ortiz, L.; Goldschmidt, T.; Elías-Gutiérrez, M. First evidence of parasitation of a bosmina (Cladocera) by a water mite larva in a karst sinkhole, in Quintana Roo (Yucatán Peninsula, México). Acarologia 2019, 59, 111–114. [Google Scholar] [CrossRef]
- Gutiérrez-Aguirre, M.A.; Cervantes-Martínez, A.; Elías-Gutiérrez, M.; Lugo-Vázquez, A. Remarks on mastigodiaptomus (Calanoida: Diaptomidae) from Mexico using integrative taxonomy, with a key of identification and three new species. PeerJ 2020, 8, e8416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanova, N.V.; Dewaard, J.R.; Hebert, P.D.N. An inexpensive, automation-friendly protocol for recovering high-quality DNA. Mol. Ecol. Notes 2006, 6, 998–1002. [Google Scholar] [CrossRef]
- Więcek, M.; Szydło, W.; Dabert, J.; Proctor, H. Delimiting species of water mites of the genus Hydrodroma (Acari: Hydrachnidiae: Hydrodromidae) from North America and Europe: Integrative evidence of species status from COI sequences and morphology. Zool. Anz. 2020, 284, 16–29. [Google Scholar] [CrossRef]
- Esen, Y.; Erman, O. A new species of the genus Arrenurus Dugès, 1834 (Acari: Hydrachnidia: Arrenuridae) for the Turkish fauna: Arrenurus (Truncaturus) corsicus (E. Angelier, 1951). Turk. J. Zool. 2013, 37, 372–375. [Google Scholar] [CrossRef]
- Erman, O.; Arslan, B.; Gülle, P. Türkiye ve Bazı Palearktik Bölge Ülkelerinin Arrenurus Duges (Arrenuridae: Hydrachnidia: Acari) Türlerinin Karşılaştırılması. Mehmet Akif Ersoy Üniversitesi Fen Bilim. Enstitüsü Derg. 2020, 11, 22–33. [Google Scholar] [CrossRef] [Green Version]
- Wiles, P.R. The water mites (Acari: Hydrachnidia) of New Guinea. Raffles Bull. Zool. 1997, 45, 375–418. [Google Scholar]
- Walter, D.E.; Proctor, H.C. Mites: Ecology, Evolution & Behaviour: Life at a Microscale, 2nd ed.; Springer: Dordrecht, The Netherlands, 2013; ISBN 9789400771642. [Google Scholar]
- Goldschmidt, T. Water mites (Acari, Hydrachnidia): Powerful but widely neglected bioindicators—a review. Neotrop. Biodivers. 2016, 2, 12–25. [Google Scholar] [CrossRef]
- Pozojević, I.; Juršić, L.; Vučković, N.; Dorić, V.; Gottstein, S.; Ternjej, I.; Mihaljević, Z. Is the spatial distribution of lentic water mite assemblages (Acari: Hydrachnidia) governed by prey availability? Exp. Appl. Acarol. 2019, 77, 487–510. [Google Scholar] [CrossRef] [PubMed]







| Number | Site | Lat N | Long W | BINs |
|---|---|---|---|---|
| 1 | Acapulquito | 18.4321 | 88.5312 | 11 |
| 2 | El palmar | 18.4407 | −88.5273 | 6 |
| 3 | Cenote Azul | 18.651 | −88.4098 | 14 |
| 4 | Cenote Cocalitos | 18.652 | −88.408 | 21 |
| 5 | Cenote Escuela Normal | 18.651 | −88.409 | 9 |
| 6 | North Bacalar Lake | 18.9176 | −88.171 | 14 |
| 7 | Cenote Pucte 1 | 19.079 | −87.994 | 11 |
| 8 | Cenote Pucte 2 | 19.091 | −87.994 | 9 |
| 9 | Cenote el Toro | 19.098 | −88.021 | 2 |
| 10 | Ramonal | 19.3921 | −88.6242 | 10 |
| 11 | Cenote Sijil Noh Ha | 19.475 | −88.052 | 3 |
| 12 | Cenote Chancah Veracruz | 19.486 | −87.988 | 4 |
| 13 | Cenote del Padre | 19.604 | −88.003 | 6 |
| 14 | Minicenote | 19.607 | −87.989 | 2 |
| 15 | Cenote Tres Reyes 1 | 19.668 | −87.881 | 3 |
| 16 | Cenote Tres Reyes 2 | 19.692 | −87.877 | 6 |
| 17 | Santa Teresa | 19.723 | −87.813 | 2 |
| 18 | Chichancanab | 19.924 | −88.7708 | 7 |
| 19 | Cueva de las serpientes | 19.93 | −88.806 | 1 |
| 20 | Cenote km 48 | 19.943 | −87.794 | 6 |
| 21 | Chunyaxche Lagoon 1 | 20.042 | −87.581 | 3 |
| 22 | Chunyaxche Lagoon 2 | 20.06 | −87.576 | 12 |
| 23 | Muyil Lagoon 1 | 20.069 | −87.594 | 8 |
| 24 | Muyil Lagoon 2 | 20.075 | −87.607 | 4 |
| Family | Genera | BIN | Location |
|---|---|---|---|
| Limnocharidae | Limnochares | ADI4862 * | 3 |
| AEA4515 * | 14 | ||
| AEB4511 * | 24 | ||
| ACY6840 | 3, 6, 4 | ||
| Hydrodromidae | Hydrodroma | ADF3732 * | 3, 4, 23, 6, 18, 2, 20, 8. |
| Hydryphantidae | Hydryphantes | AEA5005 * | 3 |
| Torrenticolidae | Torrenticola | AEA7372 * | 1 |
| Unknown genera | AEA4395 * | 1 | |
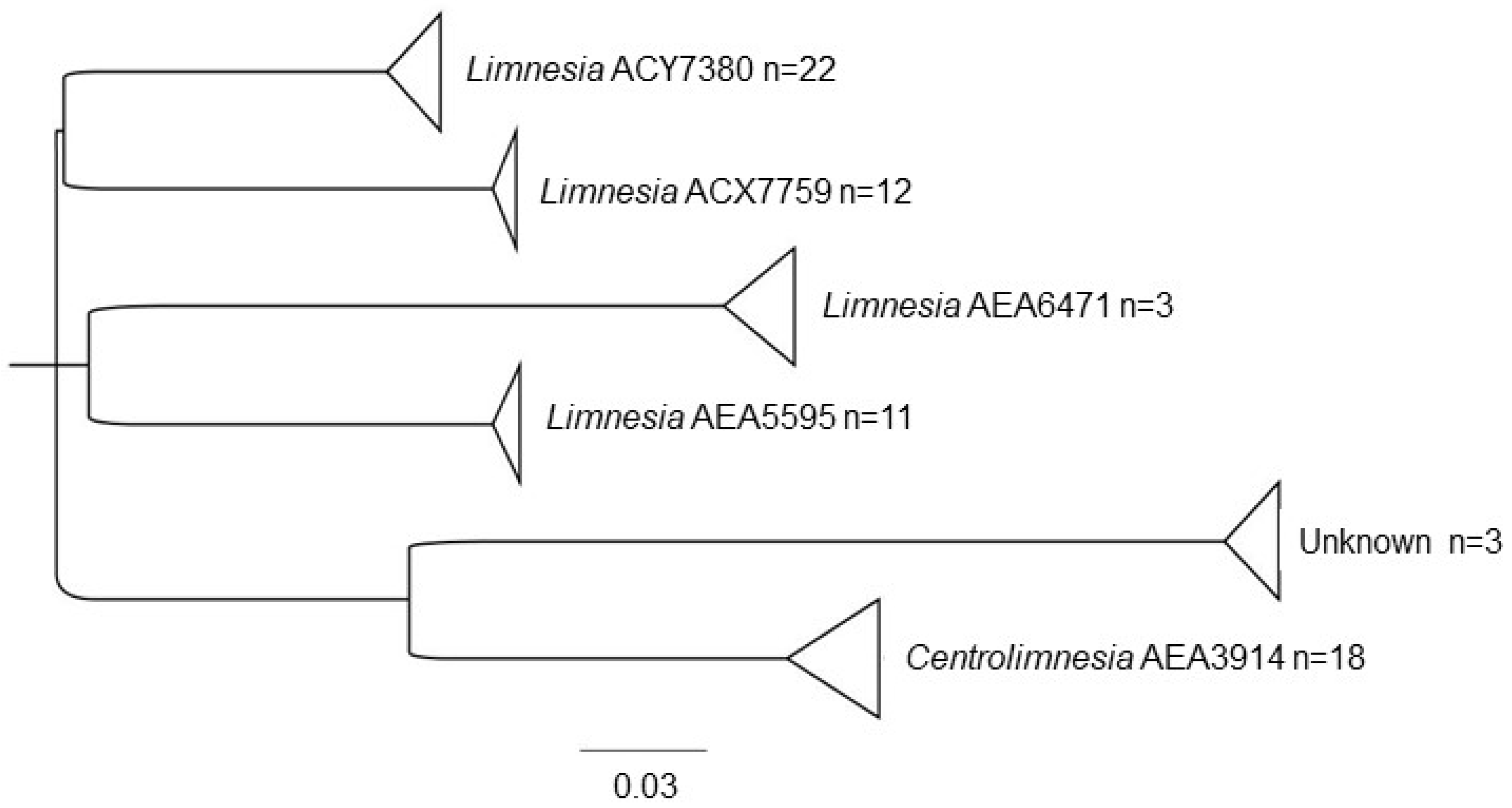
| Limnesiidae | Limnesia | AEA5595 * | 13, 6, 18, 7, 12. |
| AEA6471 * | 10 | ||
| ACX7759 | 5,4 | ||
| ACY7380 | 19, 5, 22, 4, 2, 6 | ||
| Centrolimnesia | AEA3914 * | 9, 8, 16, 17 | |
| Unknown genera | AEA4382 * | 16, 9 | |
| Krendowskiidae | Krendowskia | ACX8435 | 20, 13, 5, 16, 24, 18, 6, 4 |
| Geayia | ACT6195 | 1 | |
| Mideopsidae | Mideopsis | AEA6512 * | 1 |
| ACX8679 | 20, 13, 18, 5, 4, 24, 11, 23, 22, 8. | ||
| ACY7169 | 7, 4, 22, 5. | ||
| Unknown genera | AEB4633 * | 12 | |
| Hygrobatidae | Hygrobates | AEA3689 * | 1 |
| AEA3690 * | 2 | ||
| AEA3924 * | 1, 2 | ||
| ACX7887 | 3, 18 | ||
| ADO7098 | 6 | ||
| Atractides | ACX7786 | 5, 4 | |
| Unknown genera | AEA4089 * | 23 | |
| AEA5236 * | 21, 22, 23 | ||
| Pionidae | Piona | AEB1670 * | 6 |
| ACX8296 | 13, 12, 3, 6, 4, 23, 24, 7. | ||
| Unknown genera | AEA4809 * | 22 | |
| Unionicolidae | Unionicola | ACX8035 * | 4 |
| AEB4634 * | 8 | ||
| ACX8034 | 5, 4, 3, 7, 8, 14 | ||
| ACX9008 | 4, 5, 6 | ||
| ADM7936 | 21, 22, 23, 3 | ||
| ADP1665 | 4, 7, 22, 6 | ||
| Koenikea | ADI2928 * | 3 | |
| ACY7384 | 4, 5, 22 | ||
| ADI3114 | 2, 22, 6, 20, 3, 18, 8, 1. | ||
| Neumania | AEA8101 * | 20, 7, 10 | |
| AEA5358 * | 10 | ||
| ACY6829 | 6, 4 | ||
| Unknown genera | AEA4829 * | 22, 8, 16 | |
| AEA6062 * | 23, 22. | ||
| AEA6668 * | 16 | ||
| AEA7951 * | 16 | ||
| AEB1594 * | 8 | ||
| ACY7381 | 4 | ||
| AEA3726 * | 7 | ||
| AEA4514 * | 1 | ||
| Eylaidae | Eylais | ADD9174 * | 4 |
| Unknown genera | AEA4696 * | 15 | |
| AEA5669 * | 15 | ||
| Arrenuridae | Arrenurus | ACX8462 * | 4 |
| ACX8780 * | 4, 2, 1 | ||
| ADI3752 * | 3 | ||
| AEA3972 * | 10 | ||
| AEA7182 * | 7, 20 | ||
| AEA7842 | 10 | ||
| AEA7843 * | 10 | ||
| AEA7844 * | 1 | ||
| AEA8234 * | 10 | ||
| ACL2418 | 4 | ||
| ACX8463 | 6, 4, 18, 23, 3, 12, 11, 13. | ||
| ACX8464 | 4, 3, 13, 10, 6, 18. | ||
| ACX8788 | 5 | ||
| ACY6809 | 7, 4, 21, 22, 24, 4,3 | ||
| AEB7095 | 1 | ||
| ADI4458 * | 3 | ||
| AEA4828 * | 17 | ||
| Anisitsiellidae | Mamersellides | AEA6955 * | 10 |
| AEA6956 * | 10 | ||
| Unknown | AEA4343 * | 11 | |
| AEA3823 * | 15 | ||
| AEB1898 | 8 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montes-Ortiz, L.; Elías-Gutiérrez, M. Water Mite Diversity (Acariformes: Prostigmata: Parasitengonina: Hydrachnidiae) from Karst Ecosystems in Southern of Mexico: A Barcoding Approach. Diversity 2020, 12, 329. https://doi.org/10.3390/d12090329
Montes-Ortiz L, Elías-Gutiérrez M. Water Mite Diversity (Acariformes: Prostigmata: Parasitengonina: Hydrachnidiae) from Karst Ecosystems in Southern of Mexico: A Barcoding Approach. Diversity. 2020; 12(9):329. https://doi.org/10.3390/d12090329
Chicago/Turabian StyleMontes-Ortiz, Lucia, and Manuel Elías-Gutiérrez. 2020. "Water Mite Diversity (Acariformes: Prostigmata: Parasitengonina: Hydrachnidiae) from Karst Ecosystems in Southern of Mexico: A Barcoding Approach" Diversity 12, no. 9: 329. https://doi.org/10.3390/d12090329
APA StyleMontes-Ortiz, L., & Elías-Gutiérrez, M. (2020). Water Mite Diversity (Acariformes: Prostigmata: Parasitengonina: Hydrachnidiae) from Karst Ecosystems in Southern of Mexico: A Barcoding Approach. Diversity, 12(9), 329. https://doi.org/10.3390/d12090329