Abstract
Free-living species often receive greater conservation attention than the parasites they support, with parasite conservation often being hindered by a lack of parasite biodiversity knowledge. This study aimed to determine the current state of knowledge regarding parasites of the Southern Hemisphere freshwater fish family Galaxiidae, in order to identify knowledge gaps to focus future research attention. Specifically, we assessed how galaxiid–parasite knowledge differs among geographic regions in relation to research effort (i.e., number of studies or fish individuals examined, extent of tissue examination, taxonomic resolution), in addition to ecological traits known to influence parasite richness. To date, ~50% of galaxiid species have been examined for parasites, though the majority of studies have focused on single parasite taxa rather than assessing the full diversity of macro- and microparasites. The highest number of parasites were observed from Argentinean galaxiids, and studies in all geographic regions were biased towards the highly abundant and most widely distributed galaxiid species, Galaxias maculatus. Parasite diversity generally increased with the number of studies and individual fish examined, however studies which examined parasites from all body tissues could overcome the effects of low study effort. In order to promote further understanding of galaxiid–parasite biodiversity, we provide a series of recommendations, including the use of molecular techniques to verify parasite identity, and highlight the future roles both fish biologists and parasitologists can play.
Keywords:
Galaxiidae; Aplochiton; Brachygalaxias; Galaxias; Galaxiella; Lovettia; Neochanna; Paragalaxias; infection 1. Introduction
Parasites represent an often neglected, yet numerically and functionally important, component of global biodiversity [1]. Given the predominantly negative attention parasites receive, it is unsurprising that free-living species (hosts) have received greater biodiversity conservation attention as opposed to the affiliated parasites they support (e.g., [2]). However, as parasites are dependent on their hosts for survival, host population declines and extinctions likely result in parasite co-extinctions, especially for specialist parasites adapted to single host species [3,4,5]. Parasite conservation efforts may be further hindered by considerable disparities in our knowledge of parasite diversity amongst geographic regions, ecosystem types, host, and parasite groups [6,7,8].
Understanding which factors drive observed differences in parasite diversity has long been of interest in parasitology. At the geographic region scale, parasite diversity is strongly linked to the number of potential host species, which in turn is associated to a region’s size (e.g., [9,10]), with larger geographical regions supporting a greater number of both host and parasite species. At the host species scale, parasite diversity is often linked to a series of host-specific ecological traits (e.g., body size, geographical range, diet), phylogenetic history (e.g., evolutionary age or distinctness), and environmental factors (e.g., latitude [11,12]). In particular, host body size, geographical range size, and population density have been shown to be consistent drivers of parasite species richness across a wide range of host groups [13], with a greater variety of parasites likely to be encountered for those host species which occupy large geographical ranges and/or are large-bodied [14,15].
Research effort also plays a major role in the number of parasites documented in any host species [16], with fish parasites, for instance, often displaying aggregated distributions, both across the geographic range of their hosts and amongst individuals from a single locality [17]. There may also be mismatches between regions of high parasitology research effort and those with high freshwater fish diversity [18]. Furthermore, the taxonomic expertise of parasitology researchers may not encompass all macro- and microparasite groups encountered [19], with such problems even greater for non-parasitologists who may mistake parasites as ingested food items (Paterson pers. obs.) or morphological features of the host [20,21]. The use of molecular sequencing, which has become the standard practice for recently described parasite taxa [22,23], may partially assist in addressing limitations in morphological taxonomic expertise. Molecular sequencing has also revealed the presence of multiple genetically distinct cryptic parasite species from previously morphologically described single species [24].
The current study forms part of a special issue on Galaxiidae fish, a family of diadromous and landlocked freshwater fishes of Gondwanan origin that occur only in the cool temperate waters of the Southern Hemisphere (Oceania, South America, Africa [25,26]), and focuses on the diversity of parasites from the seven known galaxiid genera (Aplochiton, Brachygalaxias, Galaxias [Ga], Galaxiella [Gx], Lovettia, Neochanna, Paragalaxias). With the notable exception of Galaxias maculatus, and to a lesser extent Aplochiton zebra, Galaxias platei (Argentina, Chile and Islas Malvinas/Falkland Islands) and Galaxias brevipinnis (Australia and New Zealand), the majority of galaxiid species have restricted geographic distributions (e.g., Galaxias globiceps—two Chilean localities [27]). Whilst natural speciation and geomorphological processes have shaped galaxiid distribution patterns [28,29], the impacts of anthropogenic stressors (e.g., habitat fragmentation, interactions with exotic fish [30,31,32]) have reduced the geographic extent of many galaxiid species, which are now considered vulnerable or critically endangered [2]. However, previous studies (e.g., [33,34]) suggest that there are many galaxiid species and geographic regions for which a basic understanding of host–parasite interactions is lacking.
The primary objective of this study was to determine the current state of knowledge of parasite diversity in fishes of the family Galaxiidae and, as a consequence, identify knowledge gaps to focus future research attention. Specifically, we aimed to identify which galaxiid species were most understudied at the geographical region level. To do this, we assessed the study effort in terms of number of studies, locations, and fish examined, the extent of tissue examination, and taxonomic resolution of the described parasite assemblages. Furthermore, we evaluated whether ecological traits known to influence parasite richness were important drivers of observed differences in parasite assemblages among galaxiid species.
2. Materials and Methods
2.1. Data Collection
Studies investigating parasite taxa in fishes of the family Galaxiidae were obtained from literature databases (Web of Science, Google Scholar), the Google search engine, and fish-parasite species checklists (e.g., [34]) for 53 galaxiid species recognized in FishBase (pre-March 2020, [35]). The following search terms in English and Spanish were used to obtain studies: (“Aplochiton” OR “Brachygalaxias” OR “Galaxias” OR “Galaxiella” OR “Lovettia” OR “Neochanna” OR “Paragalaxias” OR “galaxiid”) AND (“parasit*” OR “infect*” OR “disease*”), in addition to individual searches for each galaxiid species in each geographic region. To evaluate the full extent of study efforts related to galaxiid parasite research, our database also included records obtained from technical reports (e.g., [36]) and unpublished theses (e.g., [37,38,39]). All possible efforts were made to obtain original publications through direct contact with authors and inter-library requests, however parasite records were included from secondary data sources in instances where original publications were not available (e.g., [40,41]).
Our database includes both macro- (i.e., acanthocephalans, cestodes, copepods, molluscs, monogeneans, nematodes, trematodes, leeches) and microparasite species (e.g., myxozoans, ciliates). However, we acknowledge that there is likely a research attention bias towards macroparasite species [8,42], in addition to standard preservation techniques (e.g., freezing; formalin or ethanol fixing) being more suited for macroparasites.
We considered Argentina (AR), Australia (AU; including Lord Howe Island), Chile (CL), Islas Malvinas/Falkland Islands (M/F), New Caledonia (NC), New Zealand (NZ; including Auckland, Campbell, Chatham, and Stewart Island/s), and South Africa (SA) as separate geographic regions. From each study, we obtained the study location (waterbody name) and the number of individual fish sampled to quantify the study effort for each galaxiid species. Examination effort (full body, focal tissues, single species, or incidental) for each study was classified based on whether parasite assemblages were assessed from all body tissues, examination of focal tissues (e.g., alimentary tract [43], brain [44]), a single target parasite species (e.g., Acanthocephalus galaxii [45], Philureter trigoniopsis [46]), or were incidentally observed from a non-parasitology study (e.g., diet analysis [47]). We also determined the level of taxonomic resolution for each reported parasite taxa (species to phylum), in addition to whether the taxa were verified by molecular sequencing in addition to morphological identification.
To reflect changes in both valid taxonomy and taxonomic description level for each recorded parasite, the following parasite taxa were standardized between studies: Coitocaecum anaspidis (e.g., [48]) to Coitocaecum parvum [49]; Stephanostomum sp. [50] to Acanthostomoides apophalliformis [51,52]; Diphyllobothium (e.g., [53,54]) to Dibothriocephalus [55,56], Nippotaenia sp. [57,58] to Ailinella mirabilis (ex Ga. maculatus [59]) and Galaxitaenia toloi (ex Ga. platei [60]), Echinocasmus sp. [57] to Stephanoprora uruguayense [61], and Diplostomum minutum [62] to Diplostomum sp. [57]. We also standardized “nematode sp.” and “nematodes” to unidentified nematode (e.g., [63,64]); “adult cestodes” and “cestodes” to unidentified cestode (e.g., [65,66]); “encysted larval trematodes” to unidentified trematode (e.g., [67]). Furthermore, “cyst parasites”, “unidentified cysts”, or “species not specified” were standardized to unidentified parasite (e.g., [68,69]) to account for multiple parasite families known to encyst in intermediate or paratenic fish hosts. A full checklist of all parasite taxa described from each galaxiid species is provided in Table S1. In terms of galaxiid hosts, Galaxias scriba [70] and Galaxias o’connori [71] were standardized to Ga. maculatus and Ga. olidus, respectively, to reflect current taxonomy. We also recognize that Argentinean Aplochiton taeniatus populations studied by Ortubay et al. [57] are now considered to be A. zebra [72]. Records from unknown Galaxias sp. (e.g., [73]) and hybrids (e.g., Galaxias depressiceps x Galaxias sp. [68]) were excluded from the database.
2.2. Predictors of Parasite Diversity
We obtained information on the dominant traits recognized to influence parasite diversity in a host species (see [13]). For each fish species, host length (maximum standard length, mm) was obtained from FishBase [35], whereas the geographical range size was estimated for each geographic region as the distance (km) between the minimum and maximum reported latitude for each fish species (where 1° of latitude equals 111.19 km [26,35,74,75,76,77,78]). Population density was not consistently available across galaxiid species and was, therefore, not included in this study.
2.3. Statistical Analysis
All statistical analyses were conducted in R version 3.6.1 [79]. Islas Malvinas/Falkland Islands were excluded from all analyses due to small sample sizes but are included in figures for illustrative purposes. Continuous variables were centered on the mean and scaled by two standard deviations prior to analysis [80]. In all instances, parasite taxa refer to the lowest taxonomic level reported for each documented parasite. The combined effects of geographic region, study effort, host length, and geographic range size on the number of parasite taxa reported in each galaxiid species were tested using a generalized linear model (glm) fitted with a quasipoisson distribution to account for over-dispersed count data. Study effort was modelled in two separate analyses as (i) the number of studies per galaxiid species where parasites were reported or (ii) the number of individual fish examined for parasites per galaxiid species, with the latter representing a reduced dataset, since the number of fish examined was not reported in all data sources (e.g., [34,81,82]). Post hoc pairwise comparisons for interactions between categorical (i.e., geographic region) and continuous (i.e., study effort, host length, geographic range size) variables were made by calculating estimated marginal means using emmeans::lsmeans, whereas estimated marginal means of linear trends were calculated for interactions between two continuous variables (emmeans::emtrends [83]). Differences in the number of study locations per galaxiid species between geographic regions were tested using a generalized linear model fitted with a quasipoisson distribution to account for overdispersion, with significant differences between geographic regions tested with estimated marginal means. Separate glms fitted with a binomial distribution were used to test whether (i) the proportion of parasite taxa described to species level or (ii) the portion of macroparasites out of the total number of parasite taxa described for each galaxiid species differed amongst geographic regions.
3. Results
Parasite taxa were reported from half of all 53 galaxiid species (Argentina (3/3), Australia (12/24), Chile (5/8), Islas Malvinas/Falkland Islands (2/3), and New Zealand (13/22 species; Table 1)). No parasite records were found for the single galaxiid species of South Africa (Galaxias zebratus) or New Caledonia (Galaxias neocaledonicus), the four Australian Paragalaxias species, nor for galaxiid species occurring on Australian or New Zealand offshore islands. The most widely distributed galaxiid species, Ga. maculatus, was also the most commonly studied species in terms of each geographic region, the total number of studies (92/143 studies) and the number of individuals examined (28,931/37,730 individuals; Table 1).

Table 1.
Summary of parasitology studies from Galaxiidae fishes.
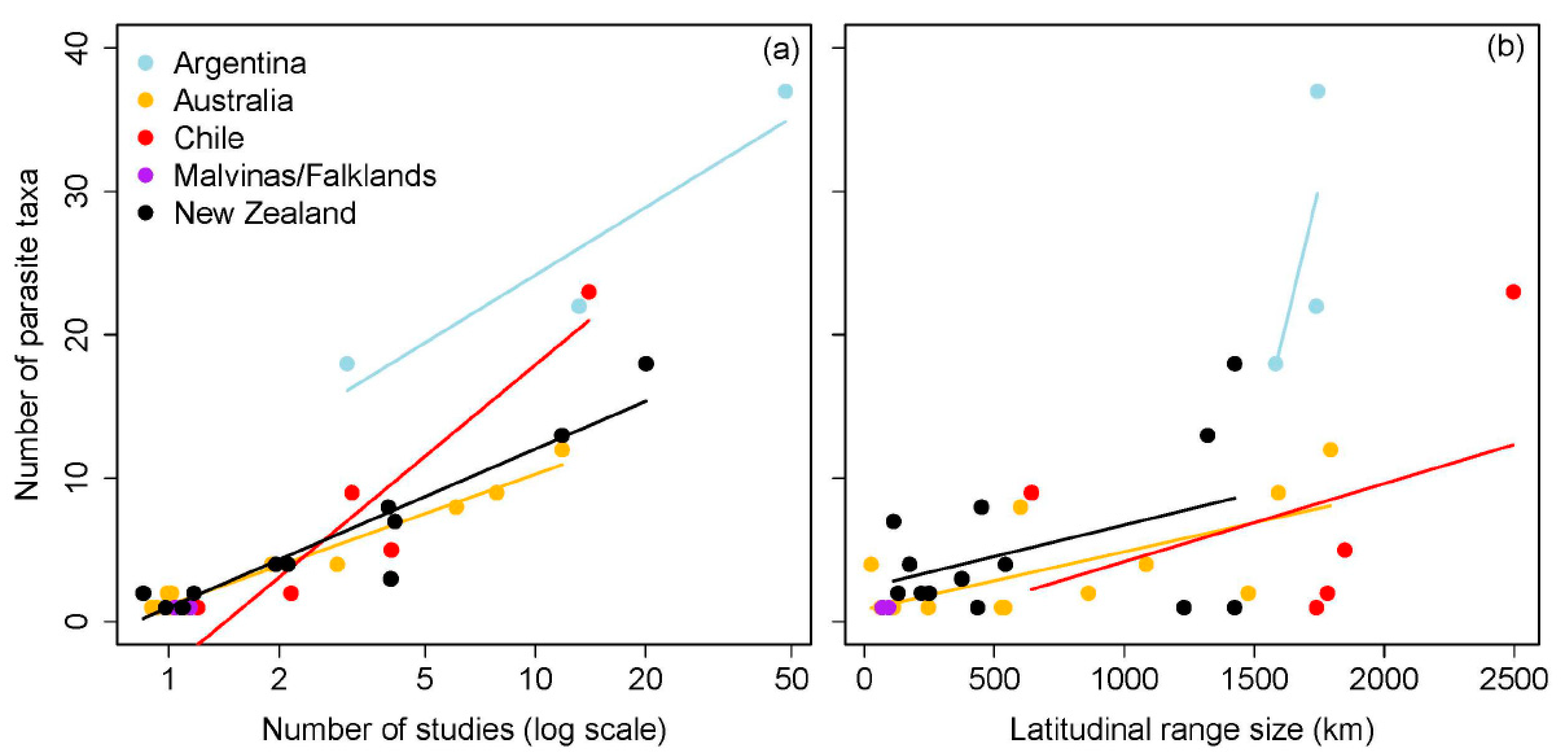
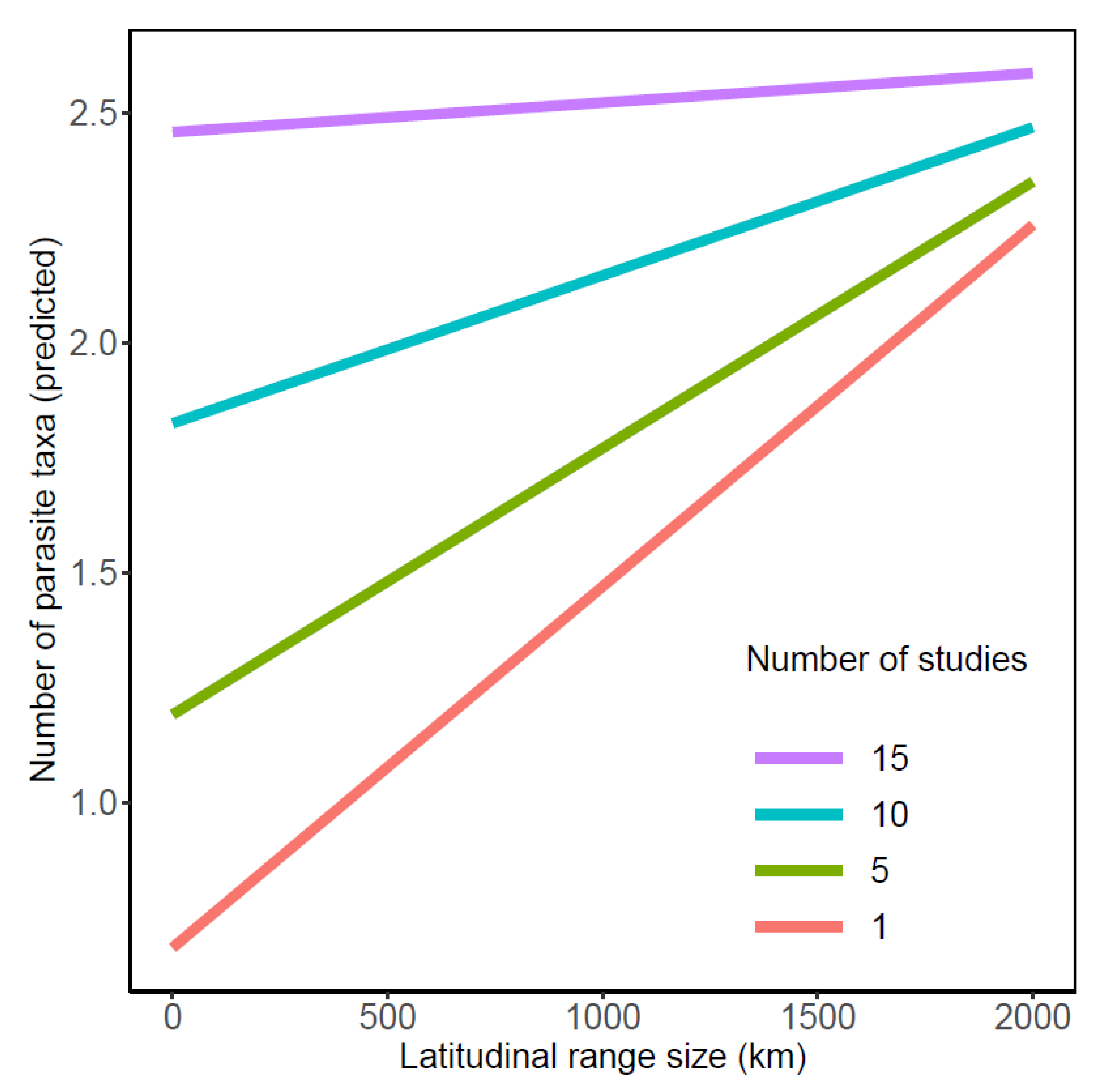
The number of parasite taxa recognized from galaxiid species was influenced by two-way interactions between the number of studies, geographic region, and latitudinal range size (all p < 0.02; Table 2). Whilst a greater number of parasite taxa were documented from Argentinean galaxiids overall (Figure 1a), pairwise comparisons of slope estimates demonstrated that the number of parasites reported from Argentinean galaxiids showed the least variation from the least to the most studied galaxiid species (n studies/n parasite taxa: A. zebra 3/18; Ga. maculatus 48/37) compared to all other geographic regions (Table A1 and Table A2). In contrast, an increasing number of studies had the strongest influence on the number of parasite taxa reported from Chilean galaxiids, ranging from a single A. taeniatus study reporting one parasite to 23 parasite taxa recognized from 14 Ga. maculatus studies. An increasing number of studies had intermediate effects on the number of parasite taxa reported from Australian and New Zealand galaxiids, which did not differ from one another. Whilst an interaction between latitudinal range size and geographic region on the number of parasite taxa reported was also detected (Table 2, Figure 1b), post hoc comparisons suggest this relationship is weak (Table A1 and Table A2). The positive effect of latitudinal range size on the number of parasite taxa reported was shown to decrease with increasing study effort (n studies), with latitudinal range size of galaxiids having little influence on the number of parasite taxa reported when the study effort approached 15 studies per fish host species (Figure 2). Standard length did not influence the number of parasites reported across all studies.

Table 2.
Influence of study effort (number of studies or fish), geographical region, fish length (standard), and latitudinal range size (km) on the number of parasite taxa reported from galaxiid fish. Statistically significant differences in parameters (α = 0.05) are in bold.
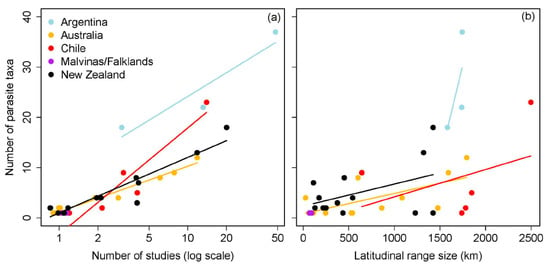
Figure 1.
Influence of the (a) number of studies (log scale) and (b) latitudinal range size (km) on the number of parasite taxa reported for each galaxiid species per geographic region.
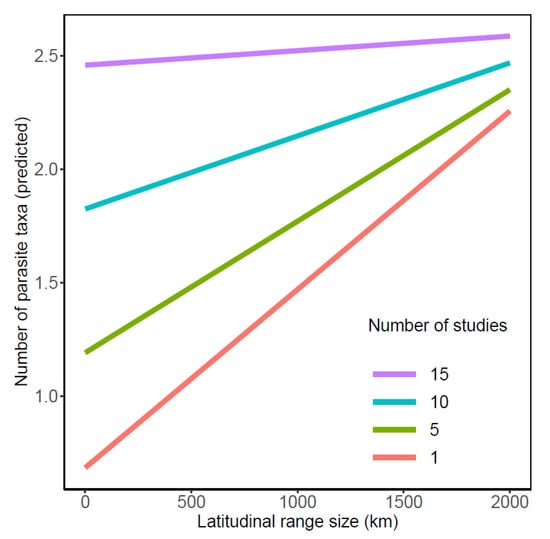
Figure 2.
Influence of latitudinal range size (km) and the number of studies on the predicted number of parasite taxa reported in galaxiid fishes.
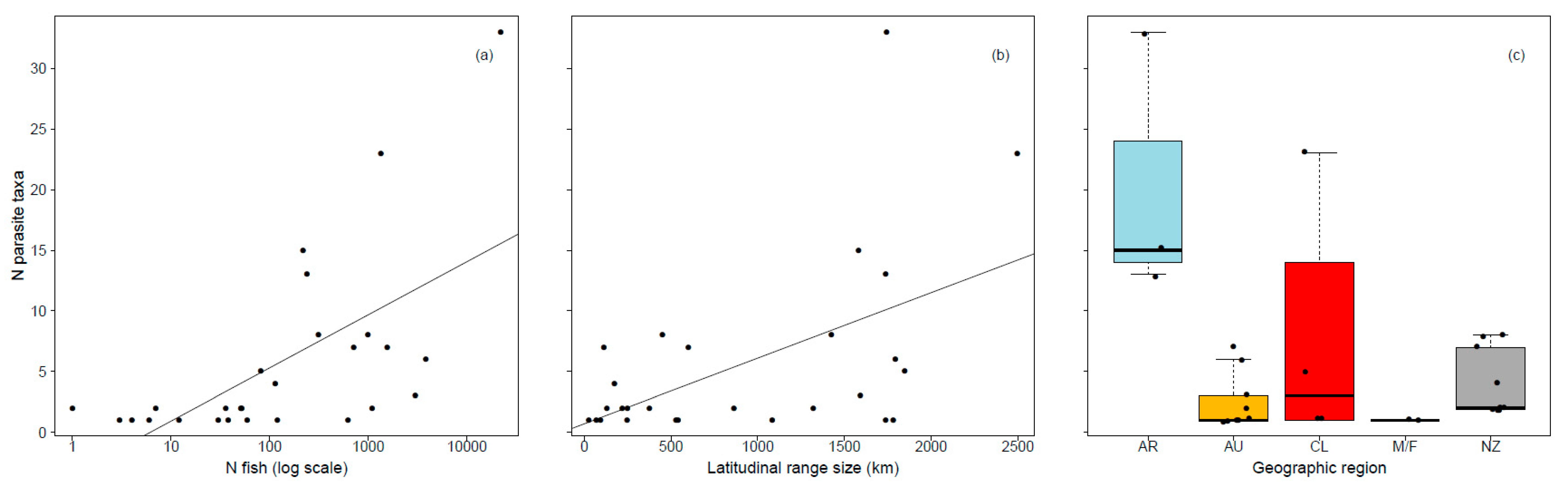
The number of fish examined was reported from 66.4% of studies (n = 40 Argentina, 18 Australia, 16 Chile, 2 Malvinas/Falklands, 19 New Zealand). The number of parasite taxa was positively correlated with both the number of fish examined and latitudinal range size (Table 2, Figure 3) and also differed between geographic regions, with significantly more parasite taxa reported in Argentina than other geographic regions (Tukey HSD post hoc test: all p < 0.05). Standard length did not influence the number of parasite taxa per host species among studies where the number of fish examined was reported.
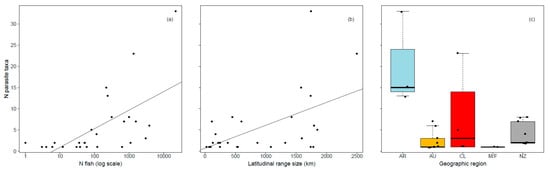
Figure 3.
Influence of the (a) number of fish (log scale), (b) latitudinal range size (km), and (c) geographic region on the number of parasite taxa reported in galaxiid fishes (AR Argentina, AU Australia, CL Chile, M/F Islas Malvinas/Falkland Islands, NZ New Zealand).
The number of locations from which parasites were studied differed among geographic regions (GLMCOUNTRY: F3,29 = 6.49, p = 0.002; Table 1), with a greater number of localities per galaxiid species studied in Argentina compared to New Zealand and Australia (pairwise comparisons, p < 0.001; Table A3), where parasites were frequently assessed from one or two locations only. Thirty or more individual fish were examined from all study locations for three galaxiid species, Galaxiella toourtkoourt (Australia, n locations = 4), A. zebra (Malvinas/Falklands, n = 1), Neochanna burrowsius (New Zealand, n = 4), with parasite assemblages observed from fewer than 30 individual fish per location for most other galaxiid species (Table 1).
Parasites from Argentinean and Chilean galaxiids were most commonly recorded from single species studies, whereas parasites from Australian and New Zealand galaxiids were observed from both studies focusing on single parasite species and examinations of focal tissues (Table 1). Aplochiton zebra from Argentina was the only galaxiid species for which all studies (n = 3) in a single geographic region involved full examinations of all body tissues. Incidental observations of parasites observed during non-parasitology studies contributed a total of seven studies across all galaxiid species. We also note that no parasite taxon was detected from the single parasitological study assessing Australian Galaxiella nigrostriata (n = 779 fish [37]), however this study focused on the detection of a single parasite species.
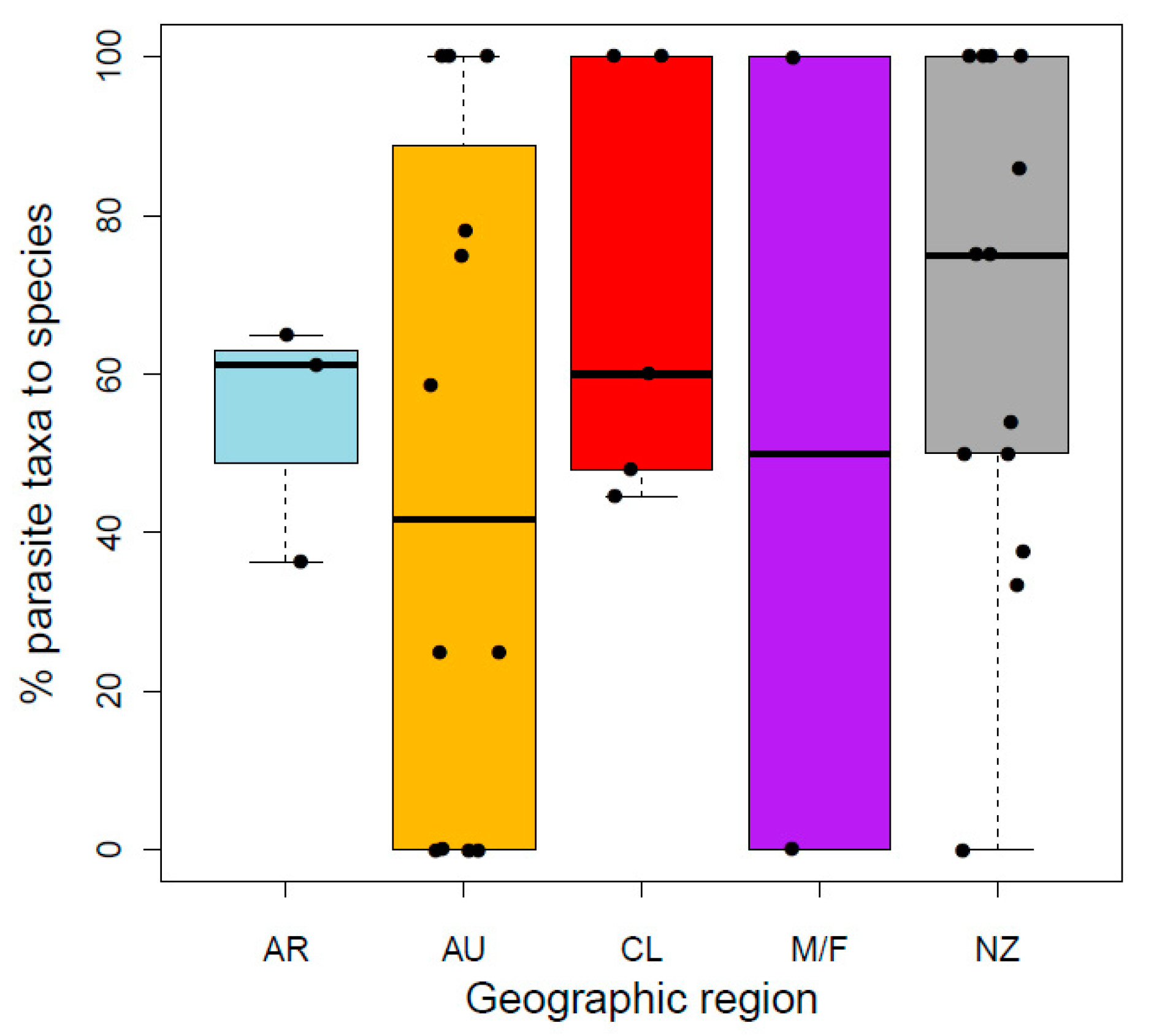
Approximately 60% of parasite taxa described from Galaxiidae were described to species level using morphological taxonomy, with no observed difference in the proportion of parasites described to species-level between regions (GLMREGION: χ2 = 0.99, df =3, p = 0.805; Figure 4). Of these parasites, identification was verified with molecular sequencing for one new (host–parasite (country): Ga. maculatus—Ortholinea lauquen (AR) [84]) and three previously described species (Galaxiella pusilla, Gx. toourtkoourt—Apatemon gracilis (AU) [85], Galaxias occidentalis—Lernaea cyprinacea (AU) [86], Ga. maculatus—H. spinigera (NZ) [87]). Molecular tools were also used to describe a further two new parasite taxa to family level (Ga. maculatus, Ga. occidentalis—Diplostomoidea (AU) [37]) and subfamily levels (Neochanna apoda—Capillariinae (NZ) [88]). Furthermore, all geographic regions consistently reported more macroparasite taxa (mean 86.5%) than microparasites from studied galaxiids with no differences among regions (GLMREGION: χ2 = 1.09, df = 3, p = 0.779; Table 1).
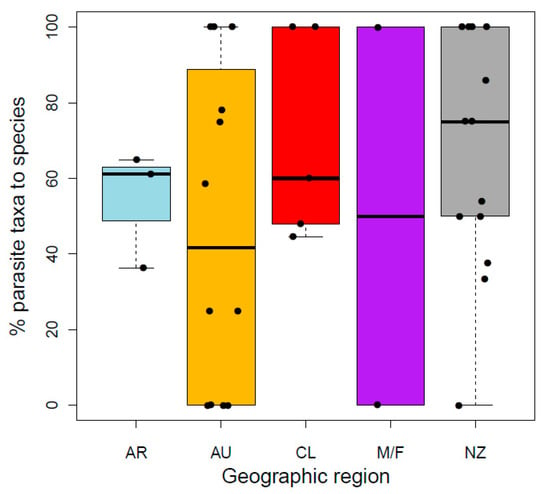
Figure 4.
Percentage of parasite taxa described to species level in each galaxiid species per geographic region (AR Argentina, AU Australia, CL Chile, M/F Islas Malvinas/Falkland Islands, NZ New Zealand).
4. Discussion and Conclusions
Our study demonstrates a number of knowledge gaps in relation to the current understanding of galaxiid–parasite diversity, including the absence of parasitological studies from 26 out of 53 recognized galaxiid species. This suggests that the diversity of parasites supported by this fish family is only partially understood. However, in the global context of the >34,000 recognized fish species, previous studies (e.g., [89,90]) suggest that most of the other 579 fish families are likely to have parasites described from less than half of their recognized species. It is, however, important to note that whilst galaxiids may in fact represent one of the better-known fish families in terms of parasitological investigations, it does not necessary imply that research efforts have been distributed evenly across member species. Our study demonstrates that Ga. maculatus has been extensively studied in most regions, though relatively limited parasitological knowledge is available for other galaxiid species. We also note that almost 20 years after McDowall [33] highlighted the absence of parasitological studies from Australian and New Zealand offshore islands, South Africa, New Caledonia, and Islas Malvinas/Falkland Islands, progress towards characterizing the galaxiid–parasite assemblages has only been made in the latter island group [47,91].
In general, study effort, in terms of the number of studies or the number of fish examined per galaxiid species, was a strong predictor of the number of parasite taxa reported; however, examination effort has the potential to overcome the effects of low study effort in some instances. Whilst the majority of Argentinean studies were focused on single parasite taxa, all three studies (n = 254 fish) investigating the parasites of A. zebra consisted of full examinations of all host tissues. This resulted in a greater number of parasite taxa documented from this fish species relative to study effort in terms of either the number of studies or fish examined and the least discrepancy in the number of parasite taxa reported with regard to study effort for Argentinean galaxiids. Differences in study effort among geographic regions may result from a combination of local researcher interests and the diversity of freshwater fish assemblages in each geographic region. The eight known galaxiid species of South America, distributed across the icthyogeographic Patagonian province and the south of the Chilean Province [92,93], occupy a region of relatively low freshwater fish diversity (29 fish species [26]). In contrast, galaxiids comprise 22 out of New Zealand’s 62 freshwater fish species, with even greater freshwater fish diversity occurring in South Africa (1 galaxiid/180 freshwater fish species), and the majority of Australian states where galaxiids are distributed (e.g., New South Wales: 4/81, Queensland: 2/191, Western Australia: 5/134 [35]). Bearing in mind the recent expansion in galaxiid taxonomic resolution (e.g., [94,95,96]), the number of recognized galaxiid species with undescribed parasite assemblages is set to grow.
Surprisingly, fish length was not found to influence the number of parasite taxa reported in galaxiids, despite larger, long-lived hosts, with associated ontogenetic diet changes often resulting in encounters with a wide range of parasites [97,98,99]. The absence of such patterns may be attributed to the majority of studies focusing on Ga. maculatus, a relatively small (<110 mm), short-lived species (maximum age class 3+ years [100]). The close proximity of research laboratories to this highly abundant, widely distributed galaxiid, combined with an ever-increasing list of described parasite taxa, has ensured that Ga. maculatus has remained highly attractive to fish parasitologists (e.g., [101,102]). If body size and age do in fact influence parasite diversity within the Galaxiidae family, then a considerable number of parasite species may currently be undiscovered, especially from the large, long-lived species such as Ga. platei (max. age = 18 years [103]) and Galaxias argenteus (20 years [104]). Positive correlations between latitudinal range size and the number of parasites also suggest that widely distributed species such as the Chilean A. zebra and A. taeniatus or Australian Ga. brevipinnis (range size >1500 km) have the potential to support a greater diversity of parasite taxa than the New Caledonian Ga. neocaledonicus or New Zealand’s Neochanna rekohua (range size <5 km), however our results display that the effects of latitudinal range size may diminish with increasing study effort.
Our study demonstrated that with the exception of recent taxonomic studies, there has been limited use of molecular techniques to confirm the identity of galaxiid parasites. For example, the trematode responsible for black spot disease in the muscles of Australian galaxiids has now been reclassified (from Diplostomum galaxiae to “Dip01” aligned with Posthodiplostomum spp., and family Strigeidae [37]). However, the majority of parasite taxa reported from galaxiid hosts rely on morphological descriptions only, of which many parasite taxa were first described in other freshwater fish in the same geographic region. Whilst galaxiids may share generalist parasite species capable of infecting fish species from other families (e.g., Acanthocephalus tumescens in Atherinopsidae, Galaxiidae, Diplomystidae, Percichthyidae, and Salmonidae [58]), the increasing recognition of cryptic parasite species suggests that galaxiids may be host to their own unique parasites. For instance, molecular approaches demonstrate that the trematode Stegodexamene anguillae and the acanthocephalan A. galaxii, which are common parasites of New Zealand galaxiid fishes, may consist of multiple cryptic species, each very host-specific (Hernandez-Orts unpublished, [105]).
Our study also suggests that although the current understanding of macroparasite diversity in galaxiids may be patchy, our knowledge of microparasite diversity is even more limited, with a greater proportion of species reported being macroparasite taxa rather than microparasites, from both single parasite studies and full assessments of parasite assemblages (i.e., parasite component population and community [106]). Although the majority of microparasite species have been reported from galaxiids originating from Argentina (e.g., [84,107,108,109]), this may be evidence of study bias towards the research interests of local parasitologists [18], rather than the absence of microparasites from other regions. Reporting bias is also likely to occur with macroparasite taxa, though to a lesser degree, where single target parasite studies are unlikely to report the presence of other encountered parasite taxa [110].
Many galaxiid species for which parasites have not been described represent species that have either been recently described, are of conservation concern, and/or are from remote localities, thus obtaining specimens from which to evaluate parasite diversity may be logistically difficult. Ethical constraints must also be taken into consideration given that, with the exception of some ectoparasites, current practices for assessing parasite diversity in fishes usually involve lethal sampling, though the development of non-lethal methods for detecting fish endoparasites offers a promising solution [111,112]. Whilst our study suggests that incidentally reported parasites in non-parasitology studies have made only minor contributions to knowledge of parasite diversity in galaxiids, greater communication between fish biologists and parasitologists could ensure that full biological information is obtained (i.e., host taxonomy, age structure, diet, and their parasites [113]), provided that collected material is preserved in a way that is useful to parasitologists [114]. Parasitologists must also play their part by sampling all parasites from collected fishes, especially when conservation or ethical considerations are involved [18]. However, there may be some fish species for which parasite diversity must remain unknown or only partially evaluated, since gaining knowledge about parasite diversity should not come at the expense of sampling either a parasite or its host to extinction.
Access to parasite specimens will only partially address galaxiid–parasite knowledge gaps, since the number of taxonomically skilled parasitology researchers and availability of funding to support taxonomic research in a geographical region may be limited. Here, collaborations with research institutes with the necessary morphological and molecular parasite taxonomy expertise is vital and may also help to address the lack of attention directed towards microparasite species. Existing collaborations between Argentinean and Chilean researchers have aided the description of new parasites from Ga. maculatus [115] and B. bullocki [116], with such collaborations largely stemming from a strong history of joint parasitology meetings between these regions. However, additional effort is required to address the gaps in parasite taxonomy expertise, and thus we encourage measures that enable taxonomic experts based outside the geographic range of galaxiids to access samples. We support Poulin et al.’s [18] suggestion of an online database of parasite specimens, which not only documents the host species and geographic localities surveyed for parasites, but also facilitates greater international collaboration among parasitologists. Furthermore, we strongly encourage the deposition of voucher specimens into museum collections, especially from host species that have received little parasitological attention.
Improving our understanding of galaxiid–parasite associations has the potential to shift general attitudes concerning parasites in the conservation and management of galaxiid populations. However, there remains much progress to be made until parasites are no longer regarded as simply threats to galaxiid survival that need to be minimized or eliminated in management efforts [67,117,118]. The recognition that the unique and potentially co-endangered parasite assemblages supported by galaxiid hosts are themselves important in biodiversity conservation [119] will contribute to efforts to co-manage hosts and parasites and ensure the retention of parasites and the ecosystem functions they provide in conservation programs.
Supplementary Materials
The following are available online at https://www.mdpi.com/1424-2818/13/1/27/s1, Table S1. Checklist of parasite taxa detected in fishes of the Galaxiidae family.
Author Contributions
Conceptualization, R.A.P., G.P.V., C.A.R., and V.R.F.; methodology, R.A.P., G.P.V., C.A.R., V.R.F., and R.P.; formal Analysis, R.A.P.; data curation, R.A.P., G.P.V., C.A.R., and V.R.F.; writing—original draft preparation, R.A.P.; writing—review and editing, R.A.P., G.P.V., C.A.R., V.R.F., and R.P.; visualization, R.A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Norwegian Institute for Nature Research, CONICET (PIP 112.201501.00477) and the Universidad Nacional del Comahue (Proyect B–225 UNCo).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in Table 1 and Supplementary Materials Table S1.
Acknowledgments
We thank C. Lagrue, A. Lymbery, F. T. Singsaas, and C. Winkworth for assistance in obtaining references and unpublished data. We also thank our four reviewers for their constructive comments that improved our manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Slope average estimates describing the effect of study effort (number of studies) and latitudinal range size on the number of parasite taxa reported from galaxiid fishes from each geographical region.
Table A1.
Slope average estimates describing the effect of study effort (number of studies) and latitudinal range size on the number of parasite taxa reported from galaxiid fishes from each geographical region.
| Base Variable | Country | Slope Estimate | SE | 95% Confidence Interval (Lower, Upper) |
| N studies | Argentina | 18.94 | 1.10 | 16.68, 21.19 |
| Australia | 6.18 | 0.51 | 5.13, 7.22 | |
| Chile | 9.08 | 0.66 | 7.71, 10.44 | |
| New Zealand | 6.23 | 0.42 | 5.37, 7.09 | |
| Latitudinal range size | Argentina | −31.44 | 31.80 | −96.93, 34.06 |
| Australia | 4.57 | 1.63 | 1.22, 7.93 | |
| Chile | 3.78 | 3.95 | −4.35, 11.92 | |
| New Zealand | 6.42 | 1.75 | 2.82, 10.02 | |
| Latitudinal Range Size | N Studies | Trend Estimate | SE | Asymptotic 95% Confidence Interval (Lower, Upper) |
| 1 | 0.0008 | 0.0002 | 0.0003, 0.001 | |
| 5 | 0.0006 | 0.0002 | 0.0002, 0.001 | |
| 10 | 0.0003 | 0.0003 | −0.0003, 0.0001 | |
| 15 | <0.0001 | 0.0005 | −0.001, 0.001 |

Table A2.
Tukey pairwise comparisons investigating the effect of geographic region on the number of reported parasite taxa per galaxiid species in relation to number of studies. Statistically significant estimates (α = 0.05) are in bold.
Table A2.
Tukey pairwise comparisons investigating the effect of geographic region on the number of reported parasite taxa per galaxiid species in relation to number of studies. Statistically significant estimates (α = 0.05) are in bold.
| Base Variable | Contrast | Estimate | SE | t Ratio | p |
|---|---|---|---|---|---|
| N studies | Argentina–Australia | 12.76 | 1.21 | 10.56 | <0.001 |
| Argentina–Chile | 9.86 | 1.28 | 7.70 | <0.001 | |
| Argentina–New Zealand | 12.70 | 1.17 | 10.83 | <0.001 | |
| Australia–Chile | −2.90 | 0.83 | −3.47 | <0.001 | |
| Australia–New Zealand | −0.05 | 0.66 | −0.08 | 1.000 | |
| Chile–New Zealand | 2.84 | 0.78 | 3.63 | 0.007 | |
| Latitudinal range size | Argentina–Australia | −36.01 | 31.84 | −1.13 | 0.674 |
| Argentina–Chile | −35.22 | 32.04 | −1.10 | 0.693 | |
| Argentina–New Zealand | −37.85 | 31.85 | −1.19 | 0.640 | |
| Australia–Chile | 0.79 | 4.27 | 0.19 | 0.998 | |
| Australia–New Zealand | −1.84 | 2.39 | −0.77 | 0.866 | |
| Chile–New Zealand | −2.63 | 4.32 | −0.61 | 0.928 |

Table A3.
Post host comparisons between geographic regions on the total number of locations from which galaxiid–parasite associations have been studied. Statistically significant estimates (α = 0.05) are in bold.
Table A3.
Post host comparisons between geographic regions on the total number of locations from which galaxiid–parasite associations have been studied. Statistically significant estimates (α = 0.05) are in bold.
| Contrast | Estimate | SE | z Ratio | p |
|---|---|---|---|---|
| Argentina–Australia | 1.88 | 0.48 | 3.93 | <0.001 |
| Argentina–Chile | 1.37 | 0.54 | 2.54 | 0.054 |
| Argentina–New Zealand | 1.96 | 0.48 | 4.10 | <0.001 |
| Australia–Chile | −0.51 | 0.59 | −0.86 | 0.826 |
| Australia–New-Zealand | 0.08 | 0.53 | 0.15 | 0.999 |
| Chile–New-Zealand | 0.59 | 0.59 | 0.99 | 0.753 |
References
- Gómez, A.; Nichols, E. Neglected wild life: Parasitic biodiversity as a conservation target. Int. J. Parasitol. Parasites Wildl. 2013, 2, 222–227. [Google Scholar] [CrossRef] [PubMed]
- IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org/ (accessed on 20 September 2020).
- Colwell, R.K.; Dunn, R.R.; Harris, N.C. Coextinction and persistence of dependent species in a changing world. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 183–203. [Google Scholar] [CrossRef]
- Koh, L.P.; Dunn, R.R.; Sodhi, N.S.; Colwell, R.K.; Proctor, H.C.; Smith, V.S. Species coextinctions and the biodiversity crisis. Science 2004, 305, 1632–1634. [Google Scholar] [CrossRef] [PubMed]
- Dunn, R.R.; Harris, N.C.; Colwell, R.K.; Koh, L.P.; Sodhi, N.S. The sixth mass coextinction: Are most endangered species parasites and mutualists? Proc. R. Soc. Lond. B Biol. Sci. 2009, 276, 3037–3045. [Google Scholar] [CrossRef] [PubMed]
- Leung, T.L.; Mora, C.; Rohde, K. Patterns of diversity and distribution of aquatic invertebrates and their parasites. In Parasite Diversity and Diversification: Evolutionary Ecology Meets Phylogenetics; Morand, S., Krasnov, B., Littlewood, D., Eds.; Cambridge University Press: Cambridge, UK, 2015; pp. 39–57. [Google Scholar]
- Carlson, C.J.; Dallas, T.A.; Alexander, L.W.; Phelan, A.; Phillips, A.J. What would it take to describe the global diversity of parasites? Proc. R. Soc. B 2020, 287, 815902. [Google Scholar] [CrossRef]
- Okamura, B.; Hartigan, A.; Naldoni, J. Extensive uncharted biodiversity: The parasite dimension. Integr. Comp. Biol. 2018, 58, 1132–1145. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, T.; O’Dwyer, K.; Nakagawa, S.; Poulin, R. Host diversity drives parasite diversity: Meta-analytical insights into patterns and causal mechanisms. Ecography 2014, 37, 689–697. [Google Scholar] [CrossRef]
- Poulin, R. Parasite biodiversity revisited: Frontiers and constraints. Int. J. Parasitol. 2014, 44, 581–589. [Google Scholar] [CrossRef]
- Randhawa, H.S.; Poulin, R. Determinants of tapeworm species richness in elasmobranch fishes: Untangling environmental and phylogenetic influences. Ecography 2010, 33, 866–877. [Google Scholar] [CrossRef]
- Morand, S. (macro-) Evolutionary ecology of parasite diversity: From determinants of parasite species richness to host diversification. Int. J. Parasitol. Parasites Wildl. 2015, 4, 80–87. [Google Scholar] [CrossRef]
- Kamiya, T.; O’Dwyer, K.; Nakagawa, S.; Poulin, R. What determines species richness of parasitic organisms? A meta-analysis across animal, plant and fungal hosts. Biol. Rev. 2014, 89, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Guégan, J.-F.; Lambert, A.; Lévêque, C.; Combes, C.; Euzet, L. Can host body size explain the parasite species richness in tropical freshwater fishes? Oecologia 1992, 90, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Price, P.W.; Clancy, K.M. Patterns in number of helminth parasite species in freshwater fishes. J. Parasitol. 1983, 449–454. [Google Scholar] [CrossRef]
- Walther, B.A.; Cotgreave, P.; Price, R.D.; Gregory, R.D.; Clayton, D.H. Sampling effort and parasite species richness. Parasitol. Today 1995, 11, 306–310. [Google Scholar] [CrossRef]
- Sarabeev, V.; Balbuena, J.A.; Morand, S. Testing the enemy release hypothesis: Abundance and distribution patterns of helminth communities in grey mullets (Teleostei: Mugilidae) reveal the success of invasive species. Int. J. Parasitol. 2017, 47, 687–696. [Google Scholar] [CrossRef]
- Poulin, R.; Presswell, B.; Jorge, F. The state of fish parasite discovery and taxonomy: A critical assessment and a look forward. Int. J. Parasitol. 2020, 50, 733–742. [Google Scholar] [CrossRef]
- Poulin, R.; Leung, T. Taxonomic resolution in parasite community studies: Are things getting worse? Parasitology 2010, 137, 1967–1973. [Google Scholar] [CrossRef]
- Allibone, R. Dealing with diversity dwarf galaxias style. Water Atmos. 2002, 10, 18–19. [Google Scholar]
- Eldon, A.E. White spots red faces. Freshw. Catch 1989, 40, 10. [Google Scholar]
- Blasco-Costa, I.; Cutmore, S.C.; Miller, T.L.; Nolan, M.J. Molecular approaches to trematode systematics: ‘best practice’ and implications for future study. Syst. Parasitol. 2016, 93, 295–306. [Google Scholar] [CrossRef]
- Perkins, S.; Martinsen, E.; Falk, B. Do molecules matter more than morphology? Promises and pitfalls in parasites. Parasitology 2011, 138, 1664–1674. [Google Scholar] [CrossRef] [PubMed]
- Nadler, S.A.; de León, G.P. Integrating molecular and morphological approaches for characterizing parasite cryptic species: Implications for parasitology. Parasitology 2011, 138, 1688–1709. [Google Scholar] [CrossRef] [PubMed]
- McDowall, R. Crying wolf, crying foul, or crying shame: Alien salmonids and a biodiversity crisis in the southern cool-temperate galaxioid fishes? Rev. Fish Biol. Fish. 2006, 16, 233–422. [Google Scholar] [CrossRef]
- Cussac, V.E.; Barrantes, M.E.; Boy, C.C.; Górski, K.; Habit, E.; Lattuca, M.E.; Rojo, J.H. New insights into the distribution, physiology and life histories of South American galaxiid fishes, and potential threats to this unique fauna. Diversity 2020, 12, 178. [Google Scholar] [CrossRef]
- Murillo, V.; Ruiz, V. El puye Galaxias globiceps Eigenmann 1927 (Osteichthyes: Galaxiidae): ¿Una especie en peligro de extinción? Gayana 2001, 66, 191–197. [Google Scholar] [CrossRef]
- Waters, J.M.; Wallis, G.P.; Burridge, C.P.; Craw, D. Geology shapes biogeography: Quaternary river-capture explains New Zealand’s biologically ‘composite’ Taieri River. Quat. Sci. Rev. 2015, 120, 47–56. [Google Scholar] [CrossRef]
- Craw, D.; Upton, P.; Burridge, C.P.; Wallis, G.P.; Waters, J.M. Rapid biological speciation driven by tectonic evolution in New Zealand. Nat. Geosci. 2016, 9, 140–144. [Google Scholar] [CrossRef]
- Meijer, C.G.; Warburton, H.J.; Harding, J.S.; McIntosh, A.R. Shifts in population size structure for a drying-tolerant fish in response to extreme drought. Austral Ecol. 2019, 44, 658–667. [Google Scholar] [CrossRef]
- Habit, E.; Piedra, P.; Ruzzante, D.E.; Walde, S.J.; Belk, M.C.; Cussac, V.E.; Gonzalez, J.; Colin, N. Changes in the distribution of native fishes in response to introduced species and other anthropogenic effects. Glob. Ecol. Biogeogr. 2010, 19, 697–710. [Google Scholar] [CrossRef]
- Díaz, G. Revealing the Effects of Loss of Longitudinal Connectivity on Freshwater Fish in Andean River Networks. Ph.D. Thesis, University of Concepción, Concepción, Chile, 2019. [Google Scholar]
- McDowall, R.M. Accumulating evidence for a dispersal biogeography of southern cool temperate freshwater fishes. J. Biogeogr. 2002, 29, 207–219. [Google Scholar] [CrossRef]
- Hine, M.; Jones, J.B.; Diggles, B.K. A Checklist of the Parasites of New Zealand Fishes Including Previously Unpublished Records; The National Institute of Water and Atmospheric Research: Wellington, New Zealand, 2000; p. 95. [Google Scholar]
- Froese, R.; Pauly, D. FishBase. Available online: www.fishbase.org (accessed on 4 March 2020).
- Johnson, W.; Mace, J.; Turner, A. Fisheries Survey of Lake Christabel, West Coast Acclimatisation District, South Island; Fisheries Technical Report 144; Ministry of Agriculture and Fisheries: Wellington, New Zealand, 1976; p. 28.
- Tritt, E. Black Spot Disease in Freshwater Fishes of South-Western Australia: Identification of the Parasite, Host Range and Potential as a Bioindicator for Water Quality. Ph.D. Thesis, Murdoch University, Perth, Australia, 2018. [Google Scholar]
- Semenas, L. Estructura Comunitaria de Parásitos en Galaxias maculatus (Pisces, Galaxiidae) y Percichthys trucha (Pisces, Percichthydae) del lago Escondido (Río Negro, Argentina). Ph.D. Thesis, Universidad Nacional de Buenos Aires, Buenos Aires, Argentina, 1999. [Google Scholar]
- Trochine, C. Infestación por Acanthostomoides apophalliformis (Trematoda, Acanthostomidae) de la Fauna íctica del Sistema del lago Moreno. Licenciate Thesis, Universidad Nacional del Comahue, Bariloche, Argentina, 2000. [Google Scholar]
- Brunsdon, R.V. Studies on Nematode Parasites of New Zealand Fishes. Ph.D. Thesis, Victoria University of Wellington, Wellington, New Zealand, 1956. [Google Scholar]
- Hewitt, G.C.; Hine, P.M. Checklist of parasites of New Zealand fishes and of their hosts. N. Z. J. Mar. Freshw. Res. 1972, 6, 69–114. [Google Scholar] [CrossRef]
- Okamura, B. Hidden infections and changing environments. Integr. Comp. Biol. 2016, 56, 620–629. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Torres, P.; Franjola, R.; Cabezas, X.; Neira, A.; Covarrubias, C. Distribución de la infección por Camallanus corderoi: Nemata; spiruroidea; en distintos hospedadores autóctonos y sectores de la cuenca del río Valdivia, Chile. Bol. Chil. Parasitol. 1990, 45, 55–59. [Google Scholar] [PubMed]
- Flores, V.; Semenas, L. Infection patterns of Tylodelphys barilochensis and T. crubensis (Trematoda: Diplostomatidae) metacercariae in Galaxias maculatus (Osmeriformes: Galaxiidae) from two Patagonian lakes and observations on their geographical distribution in the southern Andean region, Argentina. J. Parasitol. 2002, 88, 1135–1139. [Google Scholar] [CrossRef]
- Paterson, R.A.; Townsend, C.R.; Poulin, R.; Tompkins, D.M. Introduced brown trout alter native acanthocephalan infections in native fish. J. Anim. Ecol. 2011, 80, 990–998. [Google Scholar] [CrossRef]
- Viozzi, G.; Semenas, L. Do environmental differences between lakes in Northwestern Argentinean Patagonia affect the infection of Philureter trigoniopsis (Monogenea) in Galaxias maculatus (Osmeriformes)? J. Parasitol. 2009, 95, 25–31. [Google Scholar] [CrossRef]
- McDowall, R.M. Making a living in Red Pond: A snapshot of the diet of a population of Aplochiton zebra (Teleostei: Galaxiidae) at the Falkland Islands. N. Z. J. Zool. 2005, 32, 23–27. [Google Scholar] [CrossRef]
- Macfarlane, W.V. Life cycle of Coitocaecum anaspidis Hickman, a New Zealand digenetic trematode. Parasitology 1939, 31, 172–184. [Google Scholar] [CrossRef]
- Holton, A.L. A redescription of Coitocaecum parvum Crowcroft, 1945 (Digenea: Allocreadiidae) from crustacean and fish hosts in Canterbury. N. Z. J. Zool. 1984, 11, 1–8. [Google Scholar] [CrossRef]
- Torres, P.; Franjola, R.; Cubillos, V.; Miranda, J.C.; Vera, R. Parasitismo en ecosistemas de agua dulce en Chile. 1. Presencia de metacercarias del género Stephanostomum (Digenea: Acanthocolpidae) en peces. J. Vet. Med. B 1988, 35, 169–177. [Google Scholar] [CrossRef]
- Torres, P.; Franjola, T.R.; Montefusco, A. Infección estacional por metacercarias de Diplostomum (Austrodiplostomum) mordax (Szidat y Nani, 1951) y Tylodelphys destructor Szidat y Nani, 1951 en el pejerrey chileno, Basilichthys australis Eigenmann, 1927 (Pisces: Atherinidae) en el lago Riñigue, Chile. Bol. Chil. Parasitol. 1996, 51, 15–19. [Google Scholar] [PubMed]
- Ostrowski de Núñez, M.; Semenas, L.; Brugni, N.; Viozzi, G.; Flores, V. Redescription of Acanthostomoides apophalliformis (Trematoda, Acanthostomidae) from Percichthys trucha. Acta Parasitol. 1999, 44, 222–228. [Google Scholar]
- Torres, P.; Franjola, R.; Pérez, J.; Auad, S.; Uherek, F.; Miranda, J.C.; Flores, L.; Riquelme, J.; Salazar, S.; Hermosilla, C. Epidemiología de la difilobotriasis en la cuenca del río Valdivia, Chile. Rev. Saude Publica 1989, 23, 45–57. [Google Scholar] [CrossRef]
- Viozzi, G.; Semenas, L.; Brugni, N.; Flores, V.R. Metazoan parasites of Galaxias maculatus (Osmeriformes: Galaxiidae) from Argentinean Patagonia. Comp. Parasitol. 2009, 76, 229–239. [Google Scholar] [CrossRef]
- Waeschenbach, A.; Brabec, J.; Scholz, T.; Littlewood, D.T.J.; Kuchta, R. The catholic taste of broad tapeworms–multiple routes to human infection. Int. J. Parasitol. 2017, 47, 831–843. [Google Scholar] [CrossRef]
- Kuchta, R.; Radačovská, A.; Bazsalovicsová, E.; Viozzi, G.; Semenas, L.; Arbetman, M.; Scholz, T. Host switching of zoonotic broad fish tapeworm (Dibothriocephalus latus) to salmonids, Patagonia. Emerg. Infect. Dis. 2019, 25, 2156–2158. [Google Scholar] [CrossRef]
- Ortubay, S.; Semenas, L.; Ubeda, C.; Quaggiotto, A.; Viozzi, G. Catálogo de Peces Dulceacuícolas de la Patagonia Argentina y sus Parásitos Metazoos; Dirección de Pesca de la provincia de Río Negro: Viedma, Argentina, 1994; Volume 1, p. 110. [Google Scholar]
- Rauque, C.A.; Viozzi, G.P.; Semenas, L.G. Component population study of Acanthocephalus tumescens (Acanthocephala) in fishes from Lake Moreno, Argentina. Folia Parasitol. 2003, 50, 72–78. [Google Scholar] [CrossRef]
- Gil de Pertierra, A.A.; Semenas, L.G. Ailinella mirabilis gen. n., sp. n. (Eucestoda: Pseudophyllidea) from Galaxias maculatus (Pisces: Galaxiidae) in the Andean-Patagonian region of Argentina. Folia Parasitol. 2006, 53, 276–286. [Google Scholar] [CrossRef]
- Gil de Pertierra, A.A.; Semenas, L. Galaxitaenia toloi n. gen. n. sp. (Eucestoda: Pseudophyllidea) from Galaxias platei (Pisces: Osmeriformes, Galaxiidae), in the Patagonian region of Argentina. J. Parasitol. 2005, 91, 900–908. [Google Scholar] [CrossRef]
- Ostrowski de Núñez, M.; Flores, V.; Viozzi, G.; Kreiter, A. Stephanoprora uruguayense Holcman-Spector et Olague, 1989 (Digenea, Echinostomatidae) from Argentina, and comment on species of Stephanoprora from birds of the neotropical region. Acta Parasitol. 2004, 49, 292–299. [Google Scholar]
- Semenas, L.; Úbeda, C.; Ortubay, S.; Noguera, P.; Revenga, J.; Viozzi, G. Estado sanitario de las poblaciones de peces de cuerpos de agua andino patagónicos. In Actas Primeras Jornadas Nacionales de Fauna Silvestre; Uni. Nac. La Pampa: Santa Rosa, Argentina, 1987; pp. 329–347. [Google Scholar]
- Bonnett, M.L.; Lambert, P.W. Diet of giant kokopu, Galaxias argenteus. N. Z. J. Mar. Freshw. Res. 2002, 36, 361–369. [Google Scholar] [CrossRef]
- Rashnavadi, M. The Ecological Impacts of Secondary Salinisation on Halo-Tolerant Fishes in South-Western Australia. Ph.D. Thesis, Murdoch University, Perth, Australia, 2010. [Google Scholar]
- McDowall, R.M. Galaxias maculatus (Jenyns), the New Zealand Whitebait; Fisheries Research Bulletin: Wellington, New Zealand, 1968; Volume 2, p. 84. [Google Scholar]
- Llewellyn, L. Breeding biology, and egg and larval development of Galaxias rostratus Klunzinger, the Murray Jollytail from inland New South Wales. Aust. Zool. 2005, 33, 141–165. [Google Scholar] [CrossRef]
- Raadik, T.A. A Research Recovery Plan for the Barred Galaxias in South-Eastern Australia; Flora and Fauna Technical Report No. 141; Department of Conservation and Natural Resources: Melbourne, Australia, 1995; p. 25.
- Allibone, R.M. Water Abstraction Impacts on Non-Migratory Galaxiids of Otago Streams; Science for Conservation 147; Department of Conservation: Wellington, New Zealand, 2000; p. 43.
- O’Brien, L. The Conservation Ecology of Canterbury Mudfish (Neochanna burrowsius). Ph.D. Thesis, University of Canterbury, Christchurch, New Zealand, 2005. [Google Scholar]
- Johnston, T.H.; Mawson, P.M. Some nematodes parasitic in Australian freshwater fish. Trans. R. Soc. S. Aust. 1940, 64, 340–352. [Google Scholar]
- Duhig, J.V. On two fish of the species Galaxias o’connori (Ogilby) suffering from melanosis. Proc. R. Soc. Qld. 1930, xvi, 42. [Google Scholar]
- Cussac, V.; Ortubay, S.; Iglesias, G.; Milano, D.; Lattuca, M.E.; Barriga, J.P.; Battini, M.; Gross, M. The distribution of South American galaxiid fishes: The role of biological traits and post-glacial history. J. Biogeogr. 2004, 31, 103–121. [Google Scholar] [CrossRef]
- Fryer, G. A new freshwater species of the genus Dolops (Crustacea: Branchiura) parasitic on a galaxiid fish of Tasmania-with comments on disjunct distribution patterns in the southern hemisphere. Aust. J. Zool. 1969, 17, 49–64. [Google Scholar] [CrossRef]
- Atlas of Living Australia. Available online: https://www.ala.org.au/ (accessed on 13 September 2020).
- Coleman, R.; Raadik, T.; Freeman, R. Galaxiella pusilla; The IUCN Red List of Threatened Species. 2019. Available online: https://www.researchgate.net/profile/Tarmo_Raadik/publication/340662947_Galaxiella_pusilla_Dwarf_Galaxias_The_IUCN_Red_List_of_Threatened_Species_2019/links/5e97cb6d92851c2f52a63586/Galaxiella-pusilla-Dwarf-Galaxias-The-IUCN-Red-List-of-Threatened-Species-2019.pdf (accessed on 13 February 2019). [CrossRef]
- McDowall, R.; Allibone, R.; Chadderton, W. Issues for the conservation and management of Falkland Islands freshwater fishes. Aquat. Conserv. 2001, 11, 473–486. [Google Scholar] [CrossRef]
- Keith, P. Threatened fishes of the world: Galaxias neocaledonicus Weber & De Beaufort, 1913 (Galaxiidae). Environ. Biol. Fishes 2002, 63, 26. [Google Scholar] [CrossRef]
- Crow, S. New Zealand Freshwater Fish Database; Version 1.6. Occurrence Dataset; The National Institute of Water and Atmospheric Research (NIWA): Auckland, The Netherlands, 2018; Available online: https://doi.org/10.15468/ms5iqu (accessed on 6 August 2020).
- R Computing Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Gelman, A. Scaling regression inputs by dividing by two standard deviations. Stat. Med. 2008, 27, 2865–2873. [Google Scholar] [CrossRef] [PubMed]
- Scott, E. Observations on fishes of the family Galaxiidae: Part 1. In Papers and Proceedings of the Royal Society of Tasmania; The Royal Society of Tasmania: Tasmania, Australia, 1935; pp. 85–112. [Google Scholar]
- Percival, E. A note on the life history of Diplodon lutulentus Gould. Trans. Proc. R. Soc. N. Z. Inst. 1931, 62, 86–91. [Google Scholar]
- Lenth, R.; Singmann, H.; Love, J.; Buerkner, P.; Herve, M. Emmeans: Estimated Marginal Means, aka Least-Squares Means (Version 1.4.7). 2020. Available online: https://github.com/rvlenth/emmeans (accessed on 18 September 2020).
- Alama-Bermejo, G.; Viozzi, G.; Waicheim, M.; Flores, V.; Atkinson, S. Host-parasite relationship of Ortholinea lauquen sp. nov. (Cnidaria: Myxozoa) and the fish Galaxias maculatus in northwestern Patagonia, Argentina. Dis. Aquat. Organ. 2019, 136, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.; Hoffmann, A. Digenean trematode cysts within the heads of threatened Galaxiella species (Teleostei: Galaxiidae) from south-eastern Australia. Aust. J. Zool. 2016, 64, 285–291. [Google Scholar] [CrossRef]
- McCredden, M. Anchors Away: The Susceptibility and Response to Infection between Native and Co-Introduced Fishes to the Alien Anchor Worm Lernaea cyprinacea. Ph.D. Thesis, Murdoch University, Perth, Australia, 2016. [Google Scholar]
- Luque, J.L.; Vieira, F.M.; Herrmann, K.; King, T.M.; Poulin, R.; Lagrue, C. New evidence on a cold case: Trophic transmission, distribution and host-specificity in Hedruris spinigera (Nematoda: Hedruridae). Folia Parasitol. 2010, 57, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Jorge, F.; White, R.S.A.; Paterson, R.A. Hiding in the swamp: New capillariid nematode parasitizing New Zealand brown mudfish. J. Helminthol. 2018, 92, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Cribb, T.; Bray, R.; Wright, T.; Pichelin, S. The trematodes of groupers (Serranidae: Epinephelinae): Knowledge, nature and evolution. Parasitology 2002, 124, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Eiras, J.; Takemoto, R.; Pavanelli, G.; Adriano, E. About the biodiversity of parasites of freshwater fish from Brazil. Bull. Eur. Assoc. Fish Pathol. 2011, 31, 161–168. [Google Scholar]
- Chaganti, K.; Brickle, P.; Mackenzie, K. Two new species of myxozoan parasites (Myxosporea, Multivalvulida, Bivalvulida) from fishes of the Falkland Islands. Acta Parasitol. 2000, 45, 285–288. [Google Scholar]
- Cussac, V.E.; Habit, E.; Ciancio, J.; Battini, M.A.; Riva Rossi, C.; Barriga, J.P.; Baigún, C.; Crichigno, S. Freshwater fishes of Patagonia: Conservation and fisheries. J. Fish Biol. 2016, 89, 1068–1097. [Google Scholar] [CrossRef]
- Dyer, B.S. Systematic review and biogeography of the freshwater fishes of Chile revision sistematica y biogeografica de los peces dulceacuicolas de Chile. Estud. Oceanol. 2000, 19, 77–98. [Google Scholar]
- Raadik, T.A. Fifteen from one: A revision of the Galaxias olidus Günther, 1866 complex (Teleostei, Galaxiidae) in south-eastern Australia recognises three previously described taxa and describes 12 new species. Zootaxa 2014, 3898, 1–198. [Google Scholar] [CrossRef]
- Waters, J.M.; Rowe, D.L.; Burridge, C.P.; Wallis, G.P. Gene trees versus species trees: Reassessing life-history evolution in a freshwater fish radiation. Syst. Biol. 2010, 59, 504–517. [Google Scholar] [CrossRef] [PubMed]
- Chakona, G.; Swartz, E.R.; Chakona, A. The status and distribution of a newly identified endemic galaxiid in the eastern Cape Fold Ecoregion, of South Africa. Aquat. Conserv. 2018, 28, 55–67. [Google Scholar] [CrossRef]
- Morand, S. Wormy world: Comparative tests of theoretical hypotheses on parasite species richness. In Evolutionary Biology of Host–Parasite Relationships: Theory Meets Reality; Poulin, R., Morand, S., Skorping, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; pp. 63–79. [Google Scholar]
- Lo, C.M.; Morand, S.; Galzin, R. Parasite diversity\host age and size relationship in three coral-reef fishes from French Polynesia. Int. J. Parasitol. 1998, 28, 1695–1708. [Google Scholar] [CrossRef]
- Chen, H.-W.; Liu, W.-C.; Davis, A.J.; Jordán, F.; Hwang, M.-J.; Shao, K.-T. Network position of hosts in food webs and their parasite diversity. Oikos 2008, 117, 1847–1855. [Google Scholar] [CrossRef]
- Rojo, J.H.; Figueroa, D.E.; Boy, C.C. Age and growth of diadromous Galaxias maculatus (Jenyns, 1842) in southernmost South America (54° S) including contribution of age classes to reproduction. Environ. Biol. Fish. 2018, 101, 1149–1160. [Google Scholar] [CrossRef]
- Fernández, M.; Semenas, L.; Viozzi, G. La estructura de las comunidades de helmintos de Galaxias maculatus (Osmeriformes: Galaxiidae) en diferentes sitios de un lago de la Patagonia argentina. Ecol. Austral 2015, 25, 212–220. [Google Scholar] [CrossRef]
- Cirtwill, A.R.; Stouffer, D.B.; Poulin, R.; Lagrue, C. Are parasite richness and abundance linked to prey species richness and individual feeding preferences in fish hosts? Parasitology 2016, 143, 75–86. [Google Scholar] [CrossRef]
- Belk, M.C.; Habit, E.; Ortiz-Sandoval, J.J.; Sobenes, C.; Combs, E.A. Ecology of Galaxias platei in a depauperate lake. Ecol. Freshw. Fish 2014, 23, 615–621. [Google Scholar] [CrossRef]
- McDowall, R.M. New Zealand Freshwater Fishes: A Natural History and Guide; Heinemann Reed: Auckland, New Zealand, 1990. [Google Scholar]
- Herrmann, K.K.; Poulin, R.; Keeney, D.B.; Blasco-Costa, I. Genetic structure in a progenetic trematode: Signs of cryptic species with contrasting reproductive strategies. Int. J. Parasitol. 2014, 44, 811–818. [Google Scholar] [CrossRef]
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef]
- Flores, V.; Viozzi, G. Redescription, seasonality and distribution of Myxobolus magellanicus (Myxosporea) in Galaxias maculatus (Osmeriformes, Galaxiidae) from Patagonian Andean lakes (Argentina). Acta Parasitol. 2001, 46, 159–163. [Google Scholar]
- Flores, V.; Viozzi, G. Infection of Myxobolus galaxii (Myxozoa) in Galaxias maculatus (Osmeriformes: Galaxiidae) from Northwestern Patagonian Andean Lakes (Argentina). J. Parasitol. 2007, 93, 418–421. [Google Scholar] [CrossRef] [PubMed]
- Viozzi, G.P.; Flores, V.R. Myxidium biliare sp. n. (Myxozoa) from gall bladder of Galaxias maculatus (Osmeriformes: Galaxiidae) in Patagonia (Argentina). Folia Parasitol. 2003, 50, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Poulin, R.; Jorge, F. The geography of parasite discovery across taxa and over time. Parasitology 2019, 146, 168–175. [Google Scholar] [CrossRef] [PubMed]
- De Noia, M.; Poole, R.; Kaufmann, J.; Waters, C.; Adams, C.; McGinnity, P.; Llewellyn, M. In-situ non-lethal rapid test to accurately detect the presence of the nematode parasite, Anguillicoloides crassus, in European eel, Anguilla anguilla. bioRxiv 2020. [Google Scholar] [CrossRef]
- Berger, C.S.; Aubin-Horth, N. An eDNA-qPCR assay to detect the presence of the parasite Schistocephalus solidus inside its three spine stickleback host. J. Exp. Biol. 2018, 221, jeb178137. [Google Scholar] [CrossRef]
- Timi, J.T.; Poulin, R. Why ignoring parasites in fish ecology is a mistake. Int. J. Parasitol. 2020, 50, 755–761. [Google Scholar] [CrossRef]
- Kvach, Y.; Ondračková, M.; Janáč, M.; Jurajda, P. Methodological issues affecting the study of fish parasites. III. Effect of fish preservation method. Dis. Aquat. Organ. 2018, 127, 213–224. [Google Scholar] [CrossRef]
- Viozzi, G.P.; Marín, S.L.; Carvajal, J.; Brugni, N.; Mancilla, M. A new genus of dactylogyrid from the gills of Galaxias maculatus (Osmeriformes: Galaxiidae) in Maullín basin, Patagonia, Chile. J. Parasitol. 2007, 93, 542–544. [Google Scholar] [CrossRef]
- Viozzi, G.; Flores, V.; Marín, S.L.; Mancilla, M.; Carvajal, J. Parasites of the Red Jollytail, Brachygalaxias bullocki (Osmeriformes: Galaxiidae), from the Maullín River, Patagonia, Chile. Comp. Parasitol. 2008, 75, 326–328. [Google Scholar] [CrossRef]
- O’Brien, L.K.; Dunn, N.R. Mudfish (Neochanna Galaxiidae) Literature Review; Sci. Conserv. 277; Science & Technical Publication, Department of Conservation: Wellington, New Zealand, 2007.
- Rauque, C.; Viozzi, G.; Flores, V.; Vega, R.; Waicheim, A.; Salgado-Maldonado, G. Helminth parasites of alien freshwater fishes in Patagonia (Argentina). Int. J. Parasitol. Parasites Wildl. 2018, 7, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.L.; Heath, A.C.; Cardoso, P. Methods for the assessment and conservation of threatened animal parasites. Biol. Conserv. 2020, 248, 108696. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).