Floral Scent and Pollinators of Cypripedium calceolus L. at Different Latitudes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Species
2.2. Study Sites
2.3. Scent Collection and Analysis
2.4. Flower Visitor and Pollinator Observation, Collection and Identification
2.5. Statistical Analysis
3. Results
3.1. Composition of Flower Scent
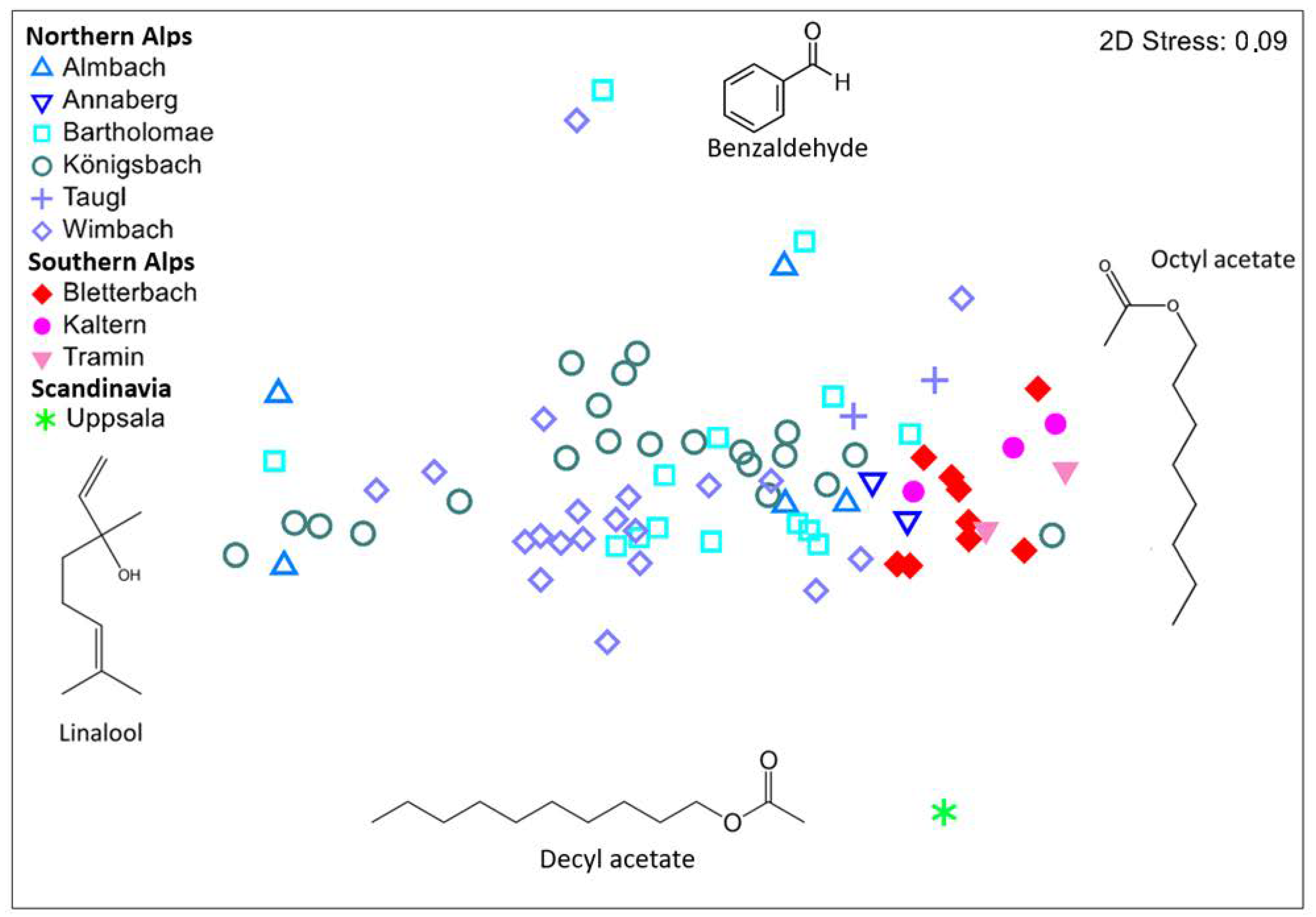
3.2. Variations in Scent among Regions and Populations
3.3. Pollinators
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grant, V.; Grant, K.A. Flower Pollination in the Phlox Family; Columbia University Press: New York, NY, USA, 1965. [Google Scholar]
- Stebbins, G.L. Adaptive radiation of reproductive characteristics in angiosperms, I: Pollination mechanisms. Annu. Rev. Ecol. Syst. 1970, 1, 307–326. [Google Scholar] [CrossRef]
- Johnson, S.D. Pollinator-driven speciation in plants. In Ecology and Evolution of Flowers; Harder, L.D., Barrett, S.C.H., Eds.; Oxford University Press: Oxford, UK, 2006; pp. 295–310. [Google Scholar]
- Zu, P.; Blanckenhorn, W.U.; Schiestl, F.P. Heritability of floral volatiles and pleiotropic responses to artificial selection in Brassica rapa. New Phytol. 2016, 209, 1208–1219. [Google Scholar] [CrossRef]
- Raguso, R.A. Wake up and smell the roses: The ecology and evolution of floral scent. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 549–569. [Google Scholar] [CrossRef]
- Dötterl, S.; Wolfe, L.M.; Jürgens, A. Qualitative and quantitative analyses of flower scent in Silene latifolia. Phytochemistry 2005, 66, 203–213. [Google Scholar] [CrossRef]
- Mant, J.; Peakall, R.; Schiestl, F.P. Does selection on floral odor promote differentiation among populations and species of the sexually deceptive orchid genus Ophrys? Evolution 2005, 59, 1449–1463. [Google Scholar] [CrossRef] [PubMed]
- Gross, K.; Sun, M.; Schiestl, F.P. Why do floral perfumes become different? Region-specific selection on floral scent in a terrestrial orchid. PLoS ONE 2016, 11, e0147975. [Google Scholar] [CrossRef] [PubMed]
- Delle-Vedove, R.; Schatz, B.; Dufay, M. Understanding intraspecific variation of floral scent in light of evolutionary ecology. Ann. Bot. 2017, 120, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majetic, C.J.; Raguso, R.A.; Ashman, T.-L. Sources of floral scent variation. Plant Signal. Behav. 2009, 4, 129–131. [Google Scholar] [CrossRef]
- Gigord, L.D.B.; Macnair, M.R.; Smithson, A. Negative frequency-dependent selection maintains a dramatic flower color polymorphism in the rewardless orchid Dactylorhiza sambucina (L.) Soo. Proc. Natl. Acad. Sci. USA 2001, 98, 6253–6255. [Google Scholar] [CrossRef] [Green Version]
- Braunschmid, H.; Mükisch, B.; Rupp, T.; Schäffler, I.; Zito, P.; Birtele, D.; Dötterl, S. Interpopulation variation in pollinators and floral scent of the lady’s-slipper orchid Cypripedium calceolus L. Arthropod. Plant. Interact. 2017, 11, 363–379. [Google Scholar] [CrossRef] [Green Version]
- Jersáková, J.; Kindlmann, P.; Renner, S.S. Is the colour dimorphism in Dactylorhiza sambucina maintained by differential seed viability instead of frequency-dependent selection? Folia Geobot. 2006, 41, 61–76. [Google Scholar] [CrossRef]
- Pellegrino, G.; Caimi, D.; Noce, M.E.; Musacchio, A. Effects of local density and flower colour polymorphism on pollination and reproduction in the rewardless orchid Dactylorhiza sambucina (L.) Soo. Plant Syst. Evol. 2005, 251, 119–129. [Google Scholar] [CrossRef]
- Espíndola, A.; Pellissier, L.; Alvarez, N. Variation in the proportion of flower visitors of Arum maculatum along its distributional range in relation with community-based climatic niche analyses. Oikos 2011, 120, 728–734. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Gross, K.; Schiestl, F.P. Floral adaptation to local pollinator guilds in a terrestrial orchid. Ann. Bot. 2014, 113, 289–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cribb, P. The Genus Cypripedium; A Botanical Magazine Monograph; Timber Press: Portland, OR, USA, 1997; ISBN 0-88192-403-2. [Google Scholar]
- Bergström, G.; Birgersson, G.; Groth, I.; Anders Nilsson, L. Floral fragrance disparity between three taxa of lady’s slipper Cypripedium calceolus (Orchidaceae). Phytochemistry 1992, 31, 2315–2319. [Google Scholar] [CrossRef]
- Kull, T. Fruit-set and recruitment in populations of Cypripedium calceolus L. in Estonia. Bot. J. Linn. Soc. 1998, 126, 27–38. [Google Scholar] [CrossRef]
- Kull, T. Cypripedium calceolus L. J. Ecol. 1999, 87, 913–924. [Google Scholar] [CrossRef]
- Nilsson, L.A. Anthecological studies on the Lady’s slipper, Cypripedium calceolus (Orchidaceae). Bot. Not. 1979, 132, 329–347. [Google Scholar]
- Antonelli, A.; Dahlberg, C.J.; Carlgren, K.H.I.; Appelqvist, T. Pollination of the lady’s slipper orchid (Cypripedium calceolus) in Scandinavia-taxonomic and conservational aspects. Nord. J. Bot. 2009, 27, 266–273. [Google Scholar] [CrossRef]
- Erneberg, M.; Holm, B. Bee size and pollen transfer in Cypripedium calceolus (Orchidaceae). Nord. J. Bot. 1999, 19, 363–367. [Google Scholar] [CrossRef]
- Heiduk, A.; Kong, H.; Brake, I.; Von Tschirnhaus, M.; Tolasch, T.; Tröger, A.G.; Wittenberg, E.; Francke, W.; Meve, U.; Dötterl, S. Deceptive Ceropegia dolichophylla fools its kleptoparasitic fly pollinators with exceptional floral scent. Front. Ecol. Evol. 2015, 3. [Google Scholar] [CrossRef] [Green Version]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. Permanova + for Primer: Guide to Software and Statisticl Methods; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. Primer v6: User Manual/Tutorial; Primer-E: Plymouth, UK, 2006. [Google Scholar]
- Ackerman, J.D.; Cuevas, A.A.; Hof, D. Are deception-pollinated species more variable than those offering a reward? Plant Syst. Evol. 2011, 293, 91–99. [Google Scholar] [CrossRef]
- Knudsen, J.T. Variation in floral scent composition within and between populations of Geonoma macrostachys (Arecaceae) in the western Amazon. Am. J. Bot. 2002, 89, 1772–1778. [Google Scholar] [CrossRef] [PubMed]
- Salzmann, C.C.; Nardella, A.M.; Cozzolino, S.; Schiestl, F.P. Variability in floral scent in rewarding and deceptive orchids: The signature of pollinator-imposed selection? Ann. Bot. 2007, 100, 757–765. [Google Scholar] [CrossRef] [Green Version]
- Salzmann, C.C.; Cozzolino, S.; Schiestl, F.P. Floral scent in food-deceptive orchids: Species specificity and sources of variability. Plant Biol. 2007, 9, 720–729. [Google Scholar] [CrossRef]
- Dötterl, S.; Vereecken, N.J. The chemical ecology and evolution of bee–flower interactions: A review and perspectives. Can. J. Zool. 2010, 88, 668–697. [Google Scholar] [CrossRef]
- Francke, W.; Reith, W.; Bergström, G.; Tengö, J. Pheromone Bouquet of the Mandibular Glands in Andrena haemorrhoa F. (Hym., Apoidea). Zeitschrift fur Naturforsch. 1981, 36C, 928–932. [Google Scholar] [CrossRef]
- Tengö, J.; Bergström, G. Comparative analyses of complex secretions from heads of Andrena bees (Hym., Apoidea). Comp. Biochem. Physiol. 1977, 57B, 197–202. [Google Scholar] [CrossRef]
- El-Sayed The Pherobase: Database of Pheromones and Semiochemicals. Available online: http://www.pherobase.com (accessed on 1 December 2020).
- Braunschmid, H.; Dötterl, S. Does the rarity of a flower’s scent phenotype in a deceptive orchid explain its pollination success? Front. Plant Sci. 2020. [Google Scholar] [CrossRef]
- Majetic, C.J.; Fetters, A.M.; Beck, O.M.; Stachnik, E.F.; Beam, K.M. Petunia floral trait plasticity in response to soil nitrogen content and subsequent impacts on insect visitation. Flora Morphol. Distrib. Funct. Ecol. Plants 2017, 232, 183–193. [Google Scholar] [CrossRef]
- Sletvold, N.; Grindeland, J.M.; Agren, J. Vegetation context influences the strength and targets of pollinator-mediated selection in a deceptive orchid. Ecology 2013, 94, 1236–1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamieson, M.A.; Burkle, L.A.; Manson, J.S.; Runyon, J.B.; Trowbridge, A.M.; Zientek, J. Global change effects on plant–insect interactions: The role of phytochemistry. Curr. Opin. Insect Sci. 2017, 23, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Knauer, A.C.; Schiestl, F.P. The effect of pollinators and herbivores on selection for floral signals: A case study in Brassica rapa. Evol. Ecol. 2017, 31, 285–304. [Google Scholar] [CrossRef]


| No. | RI a | Compound b | Occurrence % of Samples | Relative Amount (%) Median (Minimum–Maximum) e | CV f | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Southern Alps | Northern Alps | Uppsala | Southern Alps | Northern Alps | Uppsala | Southern Alps | Northern Alps | |||
| Aliphatic Compounds | ||||||||||
| 1 | 855 | (Z)-3-Hexen-1-ol d | 14 | 69 | 0 | 0 (0–*) | * (0–5) | 0 | 3.09 | 1.31 |
| 2 | 866 | 1-Hexanol d | 79 | 86 | pr c | * (0–*) | * (0–1) | 1 | 0.97 | 0.90 |
| 3 | 902 | Heptanal d | 86 | 98 | 0 | * (0–5) | 1 (0–10) | 0 | 1.26 | 0.89 |
| 4 | 913 | Pentyl acetate | 50 | 69 | pr | * (0–*) | * (0–1) | * | 1.19 | 1.48 |
| 5 | 1006 | (Z)-3-Hexenyl acetate d | 100 | 100 | pr | * (*–4) | 1 (*–17) | 1 | 1.19 | 1.15 |
| 6 | 1011 | Hexyl acetate d | 100 | 100 | pr | 3 (2–4) | 3 (1–9) | 3 | 0.36 | 0.55 |
| 7 | 1070 | 1-Octanol d | 100 | 100 | pr | 1 (*–4) | 1 (*–8) | 1 | 0.92 | 0.88 |
| 8 | 1111 | Heptyl acetate d | 100 | 100 | pr | 1 (*–2) | 1 (1–6) | 1 | 0.38 | 0.55 |
| 9 | 1122 | 3-Octanyl acetate | 7 | 5 | 0 | 0 (0–*) | 0 (0–*) | 0 | 3.74 | 5.51 |
| 10 | 1129 | Octyl formate | 0 | 34 | 0 | 0 (0–0) | 0 (0–*) | 0 | NA | 2.00 |
| 11 | 1162 | Octanoic acid d | 14 | 70 | 0 | 0 (0–1) | * (0–2) | 0 | 2.91 | 1.25 |
| 12 | 1200 | (Z/E)-2-Octenyl acetate d | 71 | 52 | 0 | * (0–*) | * (0–*) | 0 | 0.70 | 1.29 |
| 13 | 1210 | Octyl acetate d | 100 | 100 | pr | 53 (40–68) | 29 (1–62) | 30 | 0.15 | 0.48 |
| 14 | 1272 | 1-Decanol d | 93 | 86 | pr | * (0–1) | * (0–1) | * | 0.95 | 0.86 |
| 15 | 1295 | (Z)-3-Nonenyl acetate | 93 | 92 | 0 | * (0–1) | * (0–1) | 0 | 0.56 | 0.81 |
| 16 | 1309 | Nonyl acetate | 86 | 98 | pr | * (0–*) | * (0–2) | 1 | 0.53 | 0.80 |
| 17 | 1409 | Decyl acetate d | 93 | 97 | pr | 11 (0–18) | 6 (0–19) | 28 | 0.51 | 0.77 |
| 18 | 1475 | 1-Dodecanol d | 57 | 78 | pr | * (0–*) | * (0–2) | * | 1.52 | 1.16 |
| 19 | Undecyl acetate | 0 | 0 | pr | 0 (0–0) | 0 (0–0) | * | NA | NA | |
| 20 | 1608 | Dodecanyl acetate | 21 | 42 | pr | 0 (0–*) | 0 (0–1) | 6 | 2.07 | 1.59 |
| 21 | 1808 | Tetradecyl acetate d | 64 | 33 | 0 | * (0–*) | 0 (0–1) | 0 | 0.90 | 2.14 |
| 22 | Hexadecyl acetate | 0 | 0 | pr | 0 (0–0) | 0 (0–0) | * | NA | NA | |
| Aromatic Compounds | ||||||||||
| 23 | 966 | Benzaldehyde d | 100 | 100 | pr | 1 (*–3) | 1 (*–33) | 1 | 0.57 | 2.07 |
| 24 | 1025 | 4-Methylanisole d | 14 | 50 | pr | 0 (0–*) | * (0–1) | 1 | 2.56 | 1.82 |
| 25 | 1037 | Benzyl alcohol d | 93 | 67 | pr | 1 (0–3) | * (0–9) | 1 | 0.72 | 2.02 |
| 26 | 1048 | Phenylacetaldehyde d | 21 | 11 | 0 | 0 (0–*) | 0 (0–1) | 0 | 2.24 | 3.37 |
| 27 | 1074 | p-Cresol d | 0 | 17 | 0 | 0 (0–0) | 0 (0–2) | 0 | NA | 2.67 |
| 28 | 1082 | Benzyl formate | 14 | 14 | 0 | 0 (0–*) | 0 (0–*) | 0 | 2.54 | 3.56 |
| 29 | 1120 | 2-Phenylethanol d | 64 | 53 | pr | * (0–*) | * (0–1) | * | 1.05 | 1.68 |
| 30 | 1167 | 1,4-Dimethoxybenzene d | 14 | 0 | pr | 0 (0–6) | 0 (0–0) | 4 | 2.54 | NA |
| 31 | 1168 | Benzyl acetate d | 93 | 33 | pr | 1 (0–6) | 0 (0–4) | * | 1.34 | 2.31 |
| 32 | 1182 | 2-Phenylethyl formate | 0 | 2 | 0 | 0 (0–0) | 0 (0–*) | 0 | NA | 8.00 |
| 33 | 1205 | Methyl salicylate d | 0 | 5 | 0 | 0 (0–0) | 0 (0–1) | 0 | NA | 7.35 |
| 34 | 1213 | 1-Phenylbutane-2,3-dione d | 29 | 0 | 0 | 0 (0–1) | 0 (0–0) | 0 | 1.69 | NA |
| 35 | 1262 | 2-Phenylethyl acetate d | 86 | 75 | pr | 1 (0–5) | * (0–4) | * | 1.17 | 1.54 |
| 36 | 1329 | 3-Hydroxy-4-phenyl-3-buten-2-one | 14 | 0 | 0 | 0 (0–*) | 0 (0–0) | 0 | 2.55 | NA |
| 37 | 1355 | 3-hydroxy-4-phenylbutan-2-one | 14 | 0 | 0 | 0 (0–1) | 0 (0–0) | 0 | 2.59 | NA |
| 38 | 1366 | Eugenol d | 7 | 27 | 0 | 0 (0–*) | 0 (0–1) | 0 | 3.74 | 2.31 |
| Terpenoids | ||||||||||
| 41 | 987 | 6-Methyl-5-hepten-2-one d | 100 | 95 | 0 | 1 (*–1) | 2 (0–9) | 0 | 0.36 | 0.72 |
| 42 | 993 | β-Myrcene d | 21 | 30 | pr | 0 (0–1) | 0 (0–3) | * | 2.12 | 1.87 |
| 43 | 1016 | δ-3-Carene | 0 | 2 | 0 | 0 (0–0) | 0 (0–*) | 0 | NA | 8.00 |
| 44 | 1018 | Pinocarvone d | 93 | 34 | 0 | * (0–*) | 0 (0–1) | 0 | 0.66 | 3.02 |
| 45 | 1039 | (Z)-β-Ocimene d | 0 | 14 | pr | 0 (0–0) | 0 (0–1) | * | NA | 2.90 |
| 46 | 1045 | Lavender lactone | 36 | 41 | 0 | 0 (0–1) | 0 (0–4) | 0 | 1.71 | 1.77 |
| 47 | 1050 | (E)-β-Ocimene d | 0 | 8 | pr | 0 (0–0) | 0 (0–*) | 8 | NA | 3.92 |
| 48 | 1056 | (Z)-Arbusculone | 7 | 3 | 0 | 0 (0–*) | 0 (0–*) | 0 | 3.74 | 5.61 |
| 49 | 1078 | (Z)-Linalool oxide furanoid d | 0 | 77 | 0 | 0 (0–0) | * (0–1) | 0 | NA | 0.95 |
| 50 | 1094 | (E)-Linalool oxide furanoid d | 93 | 98 | 0 | * (0–1) | 1 (0–4) | 0 | 0.84 | 0.80 |
| 51 | 1103 | Linalool d | 100 | 100 | pr | 12 (6–17) | 33 (1–73) | 9 | 0.28 | 0.45 |
| 52 | 1132 | Allo-Ocimene d | 21 | 33 | 0 | 0 (0–*) | 0 (0–*) | 0 | 2.03 | 1.63 |
| 53 | 1137 | Epoxy-oxoisophorone d | 0 | 5 | 0 | 0 (0–0) | 0 (0–*) | 0 | NA | 5.47 |
| 54 | 1137 | β-Phellandrene d | 0 | 6 | pr | 0 (0–0) | 0 (0–*) | * | NA | 4.28 |
| 55 | 1149–1171 | Lilac aldehyde A–D d | 0 | 0 | 0 (0–*) | 0 (0–0) | 0 | NA | NA | |
| 56 | 1150 | 4-Oxoisophorone d | 36 | 70 | 0 | 0 (0–3) | 1 (0–8) | 0 | 2.59 | 0.99 |
| 57 | 1180 | (Z)-Linalool oxide pyranoid d | 79 | 92 | 0 | * (0–*) | * (0–*) | 0 | 0.79 | 0.75 |
| 58 | 1233 | Nerol d | 0 | 8 | 0 | 0 (0–0) | 0 (0–*) | 0 | NA | 3.86 |
| 59 | 1257 | Geraniol d | 14 | 14 | 0 | 0 (0–*) | 0 (0–*) | 0 | 2.58 | 2.90 |
| 60 | 1292 | (E)-Linalool oxide acetate pyranoid | 29 | 28 | 0 | 0 (0–*) | 0 (0–*) | 0 | 1.91 | 1.96 |
| 61 | 1384 | Geranyl acetate d | 0 | 3 | 0 | 0 (0–0) | 0 (0–*) | 0 | NA | 5.61 |
| 62 | 1449 | Dihydro-β-ionone d | 0 | 0 | pr | 0 (0–0) | 0 (0–0) | * | NA | NA |
| 63 | 1458 | Geranylacetone d | 86 | 95 | 0 | * (0–1) | * (0–1) | 0 | 1.14 | 0.90 |
| 64 | 1462 | (E)-β-Farnesene d | 7 | 14 | pr | 0 (0–*) | 0 (0–*) | * | 3.74 | 3.99 |
| 65 | 1497 | (E)-β-Ionone | 7 | 0 | 0 | 0 (0–1) | 0 (0–0) | 0 | 3.74 | NA |
| 66 | 1498 | α-Farnesene isomer | 0 | 9 | 0 | 0 (0–0) | 0 (0–*) | 0 | NA | 3.34 |
| 67 | 1513 | (E,E)-α-Farnesene d | 0 | 48 | pr | 0 (0–0) | 0 (0–3) | 5 | NA | 1.75 |
| 68 | 1571 | (E)-Nerolidol d | 57 | 2 | 0 | * (0–*) | 0 (0–*) | 0 | 1.24 | 8.00 |
| 69 | 1653 | 4-Oxo-α-damascone | 50 | 0 | 0 | * (0–*) | 0 (0–0) | 0 | 1.08 | NA |
| 70 | 1211–1231 | Lilac alcohol A–D d | 57 | 55 | 0 | * (0–1) | * (0–1) | 0 | 1.97 | 1.66 |
| 71 | 1348–1363 | Lilac alcohol formate A–D | 79 | 81 | 0 | * (0–2) | * (0–1) | 0 | 1.87 | 1.08 |
| 72 | Sesquiterpene hydocarbone | 0 | 0 | pr | 0 (0–0) | 0 (0–0) | 2 | NA | NA | |
| 73 | α-Copaene d | 0 | 0 | pr | 0 (0–0) | 0 (0–0) | * | NA | NA | |
| C5-branched Chain Compounds | ||||||||||
| 74 | 876 | Isoamyl acetate d | 7 | 5 | 0 | 0 (0–6) | 0 (0–*) | 0 | 3.74 | 4.93 |
| Nitrogen-Containing Compounds | ||||||||||
| 75 | 1305 | Indole d | 0 | 13 | 0 | 0 (0–0) | 0 (0–*) | 0 | NA | 2.86 |
| Unknowns | ||||||||||
| Unknowns g (10 substances) | 196 | 2010 | pr1 | 0 (0–*) | 0 (0–*) | 0 | ||||
| Total absolute amount (ng/min) | 95 (31–282) | 101 (21–652) | ||||||||
| Species | Southern Alps | Northern Alps | Scandinavia | |
|---|---|---|---|---|
| Andrena bicolor Fabricius | Hymenoptera | 1 | ||
| Andrena cineraria (Linnaeus) | 2 *** | |||
| Andrena fucata Smith | # | 3 NA | ||
| Andrena haemorrhoa (Fabricius) | # | 61 NA | ||
| Andrena helvola (Linnaeus) | # | 5 *** | ||
| Andrena jacobi Perkins = A. carantonica Perkins | 4 * | 6 * | 12 * | |
| Andrena nigroaenea (Kirby) | 8 ** | |||
| Andrena praecox (Scopoli) | 1 *** | |||
| Andrena sp. | 1 *** | |||
| Andrena tibialis (Kirby) | 1 *** | |||
| Colletes cunicularius Linnaeus | 1 *** | |||
| Halictus rubicundus (Christ) | 1 *** | |||
| Halictus tumulorum (Linnaeus) | # | 11 ** | ||
| Lasioglossum bavaricum (Blüthgen) | 3 ** | |||
| Lasioglossum calceatum/albipes (Scopoli/Fabricius) | 1 *** | 38 ** | 13 NA | |
| Lasioglossum fratellum (Pérez) | 5 ** | |||
| Lasioglossum fulvicorne (Kirby) | 3 *** | 4 ** | 4 ** | |
| Lasioglossum leucozonium (Schrank) | 3 ** | |||
| Lasioglossum morio (Fabricius) | 2 *** | |||
| Lasioglossum quadrinotatum (Kirby) | 1 *** | |||
| Nomada panzeri Lepeletier | 2 *** | 1 *** | ||
| Hoplocampa plagiata (Klug) | 1 *** | |||
| Eristalis rupium Fabricius | Diptera | 1 *** | ||
| Pipiza austriaca Meigen | 2 *** | |||
| Platycheirus albimanus (Fabricius) | 12 * | # |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braunschmid, H.; Guilhot, R.; Dötterl, S. Floral Scent and Pollinators of Cypripedium calceolus L. at Different Latitudes. Diversity 2021, 13, 5. https://doi.org/10.3390/d13010005
Braunschmid H, Guilhot R, Dötterl S. Floral Scent and Pollinators of Cypripedium calceolus L. at Different Latitudes. Diversity. 2021; 13(1):5. https://doi.org/10.3390/d13010005
Chicago/Turabian StyleBraunschmid, Herbert, Robin Guilhot, and Stefan Dötterl. 2021. "Floral Scent and Pollinators of Cypripedium calceolus L. at Different Latitudes" Diversity 13, no. 1: 5. https://doi.org/10.3390/d13010005
APA StyleBraunschmid, H., Guilhot, R., & Dötterl, S. (2021). Floral Scent and Pollinators of Cypripedium calceolus L. at Different Latitudes. Diversity, 13(1), 5. https://doi.org/10.3390/d13010005






