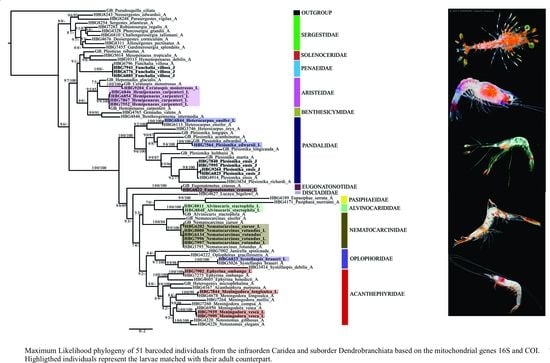
3.2. Larval Morphology
| Acanthephyridae Spence Bate, 1888 |
| Meningodora Smith, 1882 |
| Meningodora longisulca Kikuchi, 1985 |
| (Figure 4) |
Material examined: Gulf of Mexico: HBG 7844, R/V Point Sur, DP05-09May17-MOC10-B175N-095-N3, 28. 95125 and −87.91466, 09 May 2018, 6–1451 m, MOCNESS plankton net, L. Timm, coll.
Zoea. Size: 8 mm (Carapace length); 26 mm (Total length). N = 1.
Carapace (
Figure 4A). Rostrum straight, reaching the end of the cornea, unarmed; epigastric spine present; eyes pedunculate.
Pleon (
Figure 4A) with 6 somites, no spines or setae. Pleopods 1–4 missing in the specimen, pleopod 5 without setae.
Antennule (
Figure 4B). Peduncle 3-segmented, article 1 the longest, slender, with 23 plumose setae; article 2 with 8 plumose setae and article 3 with 9 plumose setae and two flagella distally.
Antenna (
Figure 4C). Protopod 3-segmented with a flagellum; exopod flattened with 73 plumose setae.
Mandible (
Figure 4D) without mandibular palp; incisor with 7 terminal teeth.
Maxillule (
Figure 4E). Coxal endite with 5 simple setae; basial endite with 15 (10 simple setae plus 5 conical setae) and protopod with one simple setae.
Maxilla (
Figure 4F). Coxal endite with 6 simple setae; basial endite bilobed with 3 + 4 simple setae; endopod with 2 (1 + 1) simple setae; scaphognathite (damage in the specimen) margin with 26 plumose setae.
First maxilliped (
Figure 4G). Coxa with 7 simple setae; basis with 28 simple setae; endopod unsegmented with 3 (2 + 1) plumose setae; exopod unsegmented with 35 plumose setae.
Second maxilliped (
Figure 4H). Coxa with one simple setae; basis with 3 simple setae; endopod 5-segmented with 0, 1, 0, 1, 1 simple setae; exopod missing in the specimen.
Third Maxilliped (
Figure 4I). Coxa and basis without setae; endopod 4-segmented with 0, 0, 0, 12, simple setae; exopod missing in the specimen.
First to fifth Pereopods missing in the specimen.
Uropod (
Figure 4J). Endopod well developed with 53 plumose setae; exopod, slightly wider than endopod, with 80 plumose setae.
Telson (
Figure 4K) elongate, subtriangular, armed with two pairs of dorsolateral spines close to the posterior margin. Posterior margin with a pointed projection, armed with two principal spines in each corner.
Figure 4.
Meningodora longisulca: (A) lateral view; (B) antennule; (C) antenna, (D) mandible; (E) maxillule; (F) maxilla; (G) first maxilliped; (H) second maxilliped; (I) third maxilliped; (J) uropods; (K) telson.
Figure 4.
Meningodora longisulca: (A) lateral view; (B) antennule; (C) antenna, (D) mandible; (E) maxillule; (F) maxilla; (G) first maxilliped; (H) second maxilliped; (I) third maxilliped; (J) uropods; (K) telson.
Material examined: Gulf of Mexico, HBG 7939, R/V Point Sur, DP05-08May17-MOC10-B003D-092-N4, 27. 9271 and −87.0178, 8 May 2017, 600–400 m, MOCNESS plankton net, L. Timm, coll. Gulf of Mexico, HBG 7999, R/V Point Sur, DP05-03May17-MOC10-B065N-087-N3, 28.53128 and −88.0236, 3 May 2017, 1000–600 m, MOCNESS plankton net, L. Timm, coll.
Decapodite. Size. 14 mm (Carapace length); 43 mm (Total length). N = 2.
Carapace (
Figure 5A). Rostrum slightly beyond the cornea and armed with 8 dorsal and one ventral spines; strong branchiostegal spine; eyes pedunculate.
Pleon (
Figure 5A) with 6 somites, no spines or setae. Pleopods 1–2 well developed, pleopods 3–5 missing in the specimen.
Antennule (
Figure 5B). Peduncle 3-segmented, article 1 the longest, slender, with 12–16 plumose setae; article 2 with 5–6 plumose setae and article 3, subequal in size with article 2, with 11–15 plumose setae and two flagella distally.
Antenna (
Figure 5C). Protopod 3-segmented (flagellum missing in the specimen); exopod flattened with 59–74 plumose setae.
Mandible (
Figure 5D). Mandibular palp 3-segmented, armed with 2, 4, 3 simple setae; incisor with 7 terminal teeth.
Maxillule (
Figure 5E). Coxal endite with 38 serrulated setae; basial endite with 16 conical setae and a subterminal simple seta; protopod unarmed.
Maxilla (
Figure 5F). Coxal endite with 21 plumose setae; basial endite bilobed with 16 + 19 serrulated setae; exopod with 5 plumose setae; scaphognathite (damage in the specimen) margin with 102 plumose setae.
First maxilliped (
Figure 5G). Coxa with 2 plus 5 plumose setae; basis with 42–46 serrulated setae; endopod with 7 (2 + 3 + 2) plumose setae; exopod with 36–38 plumose setae.
Second maxilliped (
Figure 5H). Coxa without setae; basis with 4–6 simple setae; endopod 5-segmented with 5–11 simple, 0–5, 3–5 simple, 4–12 simple, 9–11 plumose setae; exopod unsegmented and armed with 12–16 plumose setae.
Third maxilliped (
Figure 5I). Coxa without setae; basis with 3 simple setae; endopod 3-segmented with 33 simple, 10 simple, 21 (7 simple + 14 plumose) setae; exopod unsegmented and armed with 15 plumose setae.
Figure 5.
Meningodora vesca: (A) lateral view; (B) antennule; (C) antenna; (D) mandible; (E) maxillule; (F) maxilla; (G) first maxilliped; (H) second maxilliped; (I) third maxilliped.
Figure 5.
Meningodora vesca: (A) lateral view; (B) antennule; (C) antenna; (D) mandible; (E) maxillule; (F) maxilla; (G) first maxilliped; (H) second maxilliped; (I) third maxilliped.
First pereopod (
Figure 6A). Coxa with 7–9 simple setae; basis with 4 simple setae; endopod 5-segmented with 10 (5 plumose plus 5 simple), 14–29 simple, 7–13 plumose, 7–10 simple, 2–4 simple setae; exopod unsegmented and unarmed.
Second pereopod (
Figure 6B). Coxa with 4 simple setae. Basis with 3 simple setae; endopod 5-segmented with 6, 12, 2, 10, 3 simple setae; exopod unsegmented with 5 simple setae.
Third pereopod (
Figure 6C). Coxa with 3 simple setae. Basis with 5 simple setae; endopod 5-segmented with 4 (3 spines plus one simple seta), one spine, 0, 0, 0 setae; exopod unsegmented and unarmed.
Fourth pereopod missing in the specimen.
Fifth pereopod (
Figure 6D). Coxa and basis with one simple seta each one; endopod 4-segmented with 7 (3 spines plus 4 simple setae), 4 spines, 2 simple setae, 19 (8 simple setae plus 11 plumose setae).
Uropod (
Figure 6E). Endopod well developed with 53–65 plumose setae; exopod, slightly wider than endopod, with 80–82 plumose setae.
Telson (
Figure 6F) Damaged in the specimen. Elongate, subtriangular, armed with 3 pairs of dorsolateral spines. Posterior margin with a pointed projection.
Figure 6.
Meningodora vesca: (A) first Pereopod; (B) second Pereopod; (C) third Pereopod; (D) fifth Pereopod; (E) uropods; (F) telson.
Figure 6.
Meningodora vesca: (A) first Pereopod; (B) second Pereopod; (C) third Pereopod; (D) fifth Pereopod; (E) uropods; (F) telson.
| Ephyrina Smith, 1885 |
| Ephyrina ombango Crosnier and Forest, 1973 |
| (Figure 7) |
Material examined: Gulf of Mexico: HBG7902, R/V Point Sur, DP05-01May17-MOC10-B081D-084-N3, 28.5116, −87.0153, 1 May 2017, 1000–600 m, MOCNESS plankton net, L. Timm, coll.
Zoea. Size. 4 mm (Carapace length); 16 mm (Total length). N = 1.
Carapace (
Figure 7A). Rostrum small, not reach the cornea, unarmed; anteroventral margin bearing small pterygostomian spine; eyes pedunculate.
Pleon (
Figure 7A) with 6 somites, no spines or setae. Pleopods 1–3 missing in the specimen, pleopods 4–5 without setae.
Antennule (
Figure 7B). Peduncle 3-segmented, article 1 the longest, slender, with 5 simple setae; article 2 also with 3 simple setae and article 3 with two flagella distally.
Antenna (
Figure 7C). Protopod 2-segmented (flagellum missing in the specimen); exopod flattened with 46 plumose setae.
Mandible (
Figure 7D,E). Mandibular palp 3-segmented, with 4, 1, 8 plumose setae; right incisor with 6 teeth and left incisor with 8 teeth.
Maxillule (
Figure 7F). Coxal endite with 24 (10 plumose plus 14 serrulated) setae; basial endite with 18 conical serrulated setae and a subterminal simple setae; protopod with 4 simple setae.
Maxilla (
Figure 7G). Coxal endite with 33 plumose setae; basial endite bilobed with 12 + 25 plumose setae; endopod with 5 (1 + 1 + 1 + 2) plumose setae; scaphognathite margin with 88 plumose setae.
First maxilliped (
Figure 7H). Coxa with 16 plumose setae; basis with 42 plumose setae; endopod unsegmented with 1, 1, 1, 3, plumose setae; exopod unsegmented with 42 simple setae.
Second maxilliped (
Figure 7I). Coxa with 4 plumose setae; basis with 12 plumose setae; endopod 5-segmented with 8, 1, 7, 11, 0 plumose setae, except in the article 4 where all the setae were serrulated; exopod unsegmented, armed distally with 2 plumose setae.
Third maxilliped (
Figure 7J). (Damaged in the specimen) Coxa without setae; basis with 4 simple setae; endopod 4-segmented with 14, 23, 20, 7, plumose setae.
First to Fifth Pereopod missing in the specimen.
Uropod (
Figure 7K) with rami subequal. Endopod (Damaged in the specimen) with 85 plumose setae; exopod, slightly wider than endopod, with 75 plumose setae.
Telson (
Figure 7L) elongate, subtriangular, armed with 8 pairs of dorsolateral spines. Posterior margin armed with a terminal spine.
Figure 7.
Ephyrina ombango: (A) lateral view; (B) antennule; (C) antenna; (D) left mandible; (E) right mandible (cutting edge); (F) maxillule; (G) maxilla; (H) first maxilliped; (I) second maxilliped; (J) third maxilliped; (K) uropods; (L) telson.
Figure 7.
Ephyrina ombango: (A) lateral view; (B) antennule; (C) antenna; (D) left mandible; (E) right mandible (cutting edge); (F) maxillule; (G) maxilla; (H) first maxilliped; (I) second maxilliped; (J) third maxilliped; (K) uropods; (L) telson.
| Alvinocarididae Christoffersen, 1986 |
| Alvinocaris Williams and Chace, 1982 |
| Alvinocaris stactophila Williams, 1988 |
| (Figure 8 and Figure 9) |
Material examined: Gulf of Mexico: HBG 8811, R/V Point Sur, DP06-20Jul18-MOC10-B001D-101-N0, 28. 95125 and −87.91466, 29.01879 and −88.02719, 20 July 2018, 6–1451 m, MOCNESS plankton net, L. Timm coll; Gulf of Mexico: HBG 8848, R/V Point Sur, DP06-24Jul18-MOC10-B251N-106-N3, 28. 540167, −88.47116 and 28.5122, −88.6337, 24 July 2018, 602–1001 m, MOCNESS plankton net, L. Timm, coll.
Decapodite. Size. 7 mm (Carapace length); 19 mm (Total length). N = 2.
Carapace (
Figure 8A). Rostrum straight, armed dorsally with 11–12 spines, longer than antennular peduncle; antennal spine small; anteroventral margin bearing small pterygostomian spine; eyes pedunculate.
Pleon (
Figure 8A) with 6 somites, no spines or setae. Pleopods 1–3 missing in the specimen, pleopods 4–5 well developed.
Antennule (
Figure 8B). Peduncle 3-segmented, article 1 the longest, slender, article 2 also with plumose setae in both margins and article 3, the smallest, with two flagella distally. Flagella short, almost same size.
Antenna (
Figure 8C). Protopod 3-segmented with a flagellum; exopod flattened with 63–65 plumose setae, endopod unarmed and unsegmented.
Mandible (
Figure 8D). Mandibular palp 2-segmented, article 1 unarmed, article 2 with 4 simple setae; incisor with 5 terminal teeth.
Maxillule (
Figure 8E). Coxal endite with 13 simple setae; basial endite with 11 simple setae and protopod with 6 setae (1 + 1 + 1 +2).
Maxilla (
Figure 8F). Coxal endite with 21–22 simple setae; basial endite bilobed with 13 + 10 simple setae; endopod with 8 (3 + 1 + 2 + 2) plumose setae; scaphognathite margin with 116–120 plumose setae and 18–20 simple terminal long setae.
First maxilliped (
Figure 8G). Coxa with 7–13 simple setae; basis with 28–29 plumose setae; endopod unsegmented with 1, 2, 1, 1, 2 (1 outer plus 1 terminal) plumose setae; exopod unsegmented with 27–31 simple setae.
Second maxilliped (
Figure 8H). Coxa without setae; basis with 2 simple setae; endopod 5-segmented with 6–11, 1–3, 0–2, 0, 1–3 simple setae; exopod unsegmented, armed distally with 2–4 plumose natatory setae.
Third maxilliped (
Figure 8I). Coxa without setae; basis with 4–5 simple setae; endopod 5-segmented with 2, 2, 5, 7, 2 simple setae; exopod unsegmented, armed distally with 6 plumose natatory setae.
First pereopod (
Figure 8J). Coxa and basis without setae; endopod 5-segmented with 2, 2, 1, 6, 0 simple setae; exopod unsegmented, armed distally with 2–6 plumose natatory setae.
Figure 8.
Alvinocaris stactophila: (A) lateral view; (B) antennule; (C) antenna; (D) mandible; (E) maxillule; (F) maxilla; (G) first maxilliped; (H) second maxilliped; (I) third maxilliped; (J) first pereopod.
Figure 8.
Alvinocaris stactophila: (A) lateral view; (B) antennule; (C) antenna; (D) mandible; (E) maxillule; (F) maxilla; (G) first maxilliped; (H) second maxilliped; (I) third maxilliped; (J) first pereopod.
Second pereopod (
Figure 9A). Coxa without setae. Basis with 2 simple setae; endopod 5-segmented with 2, 6, 0, 7, 3 simple setae; exopod unsegmented, armed distally with 2–6 plumose natatory setae.
Third pereopod (
Figure 9B). Coxa without setae. Basis with 2 simple setae; endopod 5-segmented with 3, 5, 1, 7, 0 simple setae; exopod unsegmented, armed distally with 1–8 long, plumose natatory setae.
Fourth pereopod (
Figure 9C). Coxa without setae. Basis with 3 simple setae; endopod 5-segmented with 4, 5, 1, 6, 0 simple setae; exopod unsegmented, armed distally with 2–6 long, plumose natatory setae.
Fifth pereopod (
Figure 9D). Coxa and basis unarmed; endopod 5-segmented with 4, 1, 1, 8, 0 simple setae.
Uropod (
Figure 9E) with rami subequal. Endopod well developed with 54–58 plumose setae; exopod, slightly wider than endopod, with 64–68 plumose setae.
Telson (
Figure 9F) elongate, subrectangular, armed with 4 pairs of dorsolateral spines. Posterior margin convex, armed with 2 principal spines in each corner and 6 small spines on distal margin between.
Figure 9.
Alvinocaris stactophila: (A) second pereopod; (B) third pereopod; (C) fourth pereopod; (D) fifth pereopod; (E) uropods; (F) telson.
Figure 9.
Alvinocaris stactophila: (A) second pereopod; (B) third pereopod; (C) fourth pereopod; (D) fifth pereopod; (E) uropods; (F) telson.
| Eugonatonotidae Chace, 1937 |
| Eugonatonotus Schmitt, 1926 |
| Eugonatonotus crassus (A. Milne Edwards, 1881) |
| (Figure 10 and Figure 11) |
Material examined: Gulf of Mexico: HBG 6822, R/V Point Sur, DP04-08Aug16, MOC10-SE1N-063-N0, from 26.9878 and −87.9494 to 27.0591 and −88.0856, 8 August 2016, 1504.9-N/A m, MOCNESS plankton net, H. Bracken-Grissom, coll.
Zoea. Size. 6 mm (Carapace length); 19 mm (Total length). N = 1.
Carapace (
Figure 10A). Rostrum short and unarmed; eyes pedunculate.
Pleon (
Figure 10A) with 6 somites, no spines or setae. Pleopods 1–3 missing in the specimen, pleopods 4–5 without setae.
Antennule (
Figure 10B). Peduncle 3-segmented, article 1 the longest, slender, with 17 plumose setae; article 2 with 6 plumose setae and article 3, subequal in size with article 2, with 6 plumose setae and two flagella distally, flagella short, almost same size.
Antenna (
Figure 10C). Protopod 3-segmented with a flagellum; exopod flattened with 35 plumose setae.
Mandible (
Figure 10D). Mandibular palp 3-segmented, article 1 and 2 unarmed, article 3 with 4 simple setae; incisor with 7 terminal teeth.
Maxillule (
Figure 10E). Coxal endite with 6 simple setae; basial endite with 6 simple setae and protopod with 12 setae (2 + 2 + 12).
Maxilla (
Figure 10F). (Damaged in the specimen) Coxa l without setae; basial endite with 16 simple setae; scaphognathite margin with 57 plumose setae.
First maxilliped (
Figure 10G). Coxa with 4simple setae plus one plumose set; basis with 14 plumose setae; endopod 4-segmented with 7, 4, 7, 3, 2 simple setae, except the last article that bear 2 plumose and one simple setae; exopod unsegmented with 26 simple setae
Second maxilliped (
Figure 10H). Coxa without setae; basis with 11 simple setae and 2 plumose setae; endopod 4-segmented with 6 simple, 13 simple, 2 simple, 10 (9 simple plus one plumose) setae; exopod unsegmented and unarmed.
Third maxilliped (
Figure 10I). Coxa without setae; basis with 7 simple setae; endopod 5-segmented with 6, 8, 4, 17, 5 simple setae; exopod unsegmented and unarmed.
First pereopod (
Figure 10J). Coxa and basis without setae; endopod 5-segmented with 2, 2, 1, 6, 0 simple setae; exopod unsegmented, armed distally with 6 plumose natatory setae.
Figure 10.
Eugonatonotus crassus: (A) lateral view; (B) antennule; (C) antenna; (D) mandible; (E) maxillule; (F) maxilla; (G) first maxilliped; (H) second maxilliped; (I) third maxilliped; (J) first pereopod.
Figure 10.
Eugonatonotus crassus: (A) lateral view; (B) antennule; (C) antenna; (D) mandible; (E) maxillule; (F) maxilla; (G) first maxilliped; (H) second maxilliped; (I) third maxilliped; (J) first pereopod.
Second pereopod (
Figure 11A). Coxa without setae. Basis with one simple setae; endopod 5-segmented with 5, 8, 5, 16, 1 simple setae; exopod unsegmented and unarmed.
Third pereopod (
Figure 11B). Coxa without setae. Basis with one simple setae; endopod 5-segmented with 6, 5, 1, 10, 1 simple setae; exopod unsegmented, armed distally with 15 simple setae.
Fourth pereopod (
Figure 11C). Coxa and basis without setae; endopod 5-segmented with 1, 1, 3, 10, 4 simple setae; exopod unsegmented and unarmed.
Fifth pereopod (
Figure 11D). Coxa without setae; basis with 3 simple setae and one plumose setae; endopod 5-segmented with 8, 11, 3, 10, 0 simple setae.
Uropod (
Figure 11E) with rami subequal. Endopod well developed with 54 plumose setae; exopod, slightly wider than endopod, with 68 plumose setae.
Telson (
Figure 11D) elongate, subtriangular. Posterior margin, armed with 2 principal spines in each corner and 6 small spines.
Figure 11.
Eugonatonotus crassus: (A) second pereopod; (B) third pereopod; (C) fourth pereopod; (D) first pereopod; (E) uropods; (F) telson.
Figure 11.
Eugonatonotus crassus: (A) second pereopod; (B) third pereopod; (C) fourth pereopod; (D) first pereopod; (E) uropods; (F) telson.
| Nematocarcinidae Smith, 1884 |
| Nematocarcinus A. Milne-Edwards, 1881 |
| Nematocarcinus cursor A. Milne-Edwards, 1881 |
| (Figure 12 and Figure 13) |
Material examined: Florida Straits: HBG 6202, R/V Walton Smith, BLV01-19Jul16-STNB-D005, from 25.421423 and −79.648933 to 25.405617 and −79. 661217, 19 July 2016, 700–500 m, Trawl plankton net, H. Bracken-Grissom, coll.
Zoea. Size. 7 mm (Carapace length); 21 mm (Total length). N = 1.
Carapace (
Figure 12A). Rostrum shorter than the cornea, armed dorsally with 5 spines, epigastric spine present; eyes pedunculate; pterygostomial spine present.
Pleon (
Figure 12A) with 6 somites, no spines or setae. Pleopod 4 missing in the specimen, pleopods 1–2 and 4–5 without setae.
Antennule (
Figure 12B). Peduncle 3-segmented, article 1 the longest, slender, with four pointed projections and with 16 plumose setae; article 2 with one plumose setae and article 3, subequal in size with article 2, with 8 plumose setae and two flagella distally, flagella almost same size.
Antenna (
Figure 12C). Protopod 3-segmented, segment 1 unarmed, segment 2 with two plumose setae, segment 3 with a flagellum; exopod flattened with 66 plumose setae.
Mandible (
Figure 12D,E). Mandibular palp absent; left and right incisor with 3 terminal teeth.
Maxillule (
Figure 12F). Coxal endite with 26 conical serrulated setae; basial endite with 11 simple setae and 13 conical serrulated setae; protopod with two articles, article 1 with two serrulated setae and article 2 with 6 serrulated setae.
Maxilla (
Figure 12G). Coxa with 31 plumose setae; basial endite bilobed with 10 and 16 serrated setae respectively; scaphognathite margin with 127 plumose setae.
First maxilliped (
Figure 12H). Coxa with 18 plumose setae; basis with 13 plumose and 17 serrulated setae; endopod 4-segmented with 6, 2, 2, 3, plumose setae, except the last segment that bear serrulated setae; exopod with 10 plumose setae.
Second maxilliped (
Figure 12I). Coxa with 3 plumose setae; basis with 9 plumose setae; endopod 5-segmented with 5 plumose, 2 plumose, 1 plumose, 8 (5 plumose plus 3 serrulated), 5 serrulated setae; exopod unsegmented and unarmed.
Third maxilliped (
Figure 12J). Coxa with 8 plumose setae; basis with 5 plumose setae; endopod 5-segmented with 4, 3, 3, plumose setae, one serrulated setae; last article subdivided in three small articles with 3, 2 and 2 serrulated setae; exopod armed with 10 plumose setae.
Figure 12.
Nematocarcinus cursor: (A) lateral view; (B) antennule; (C) antenna; (D) left mandible; (E) right mandible (cutting edge); (F) maxillule; (G) maxilla; (H) first maxilliped; (I) second maxilliped; (J) third maxilliped.
Figure 12.
Nematocarcinus cursor: (A) lateral view; (B) antennule; (C) antenna; (D) left mandible; (E) right mandible (cutting edge); (F) maxillule; (G) maxilla; (H) first maxilliped; (I) second maxilliped; (J) third maxilliped.
First pereopod (
Figure 13A). Coxa with 2 plumose setae, basis with 3 plumose setae; endopod 5-segmented with 5, 3, 2, 3, 4 plumose setae, except the last two segments that have serrulated setae; exopod, with 15 plumose setae.
Second pereopod (
Figure 13B). Coxa with 2 plumose setae. Basis with 3 plumose setae; endopod 5-segmented with 3, 3, 3, 3, 3 plumose setae, except the last two segments that have serrulate setae; exopod with 9 plumose setae.
Third pereopod (
Figure 13C). Coxa with 4 plumose setae, basis with one plumose setae; endopod 5-segmented with 6, 5, 5, 2, 3 plumose setae, except the last two segments that have serrulated setae; exopod with 13 plumose setae.
Four pereopod (
Figure 13D). Coxa with 3 plumose setae, basis without setae; endopod 5-segmented with 6, 10, 3, 4, 3 plumose setae, except the last two segment that have serrulated setae; exopod with 7 plumose setae.
Fifth pereopod (
Figure 13E). Coxa without setae; basis with 5 plumose setae; endopod 5-segmented with 2, 3, 7, 4, 3 plumose setae setae, except the last two segments that have serrulated setae.
Uropods (
Figure 13F). Endopod well developed with 72 plumose setae, slightly wider than exopod; exopod, with 76 plumose setae.
Telson (
Figure 13G) elongate, subtriangular. Lateral margin with 8 pairs of spines. Posterior margin, armed with 2 principal spines in each corner and 6 small spines.
Figure 13.
Nematocarcinus cursor: (A) first pereopod; (B) second pereopod; (C) third pereopod; (D) fourth pereopod; (E) fifth pereopod; (F) uropods; (G) telson.
Figure 13.
Nematocarcinus cursor: (A) first pereopod; (B) second pereopod; (C) third pereopod; (D) fourth pereopod; (E) fifth pereopod; (F) uropods; (G) telson.
Material examined: Gulf of Mexico: HBG 7555, R/V Point Sur, DP04-11Aug16, MOC10-SW3D-068-N5, from 27.01226 and −88.4618 to 26.9255 and −88.5970, 11 August 2016, 199.8–5 m, MOCNESS plankton net, H. Bracken-Grissom, coll.
Zoea. Size. 7 mm (Carapace length); 21 mm (Total length). N = 1.
Carapace (
Figure 14A). Rostrum shorter than the cornea, armed dorsally with four spines, epigastric spine present; eyes pedunculate; pterygostomial spine present.
Pleon (
Figure 14A) with 6 somites, no spines or setae. Pleopods 3–4 missing in the specimen, pleopods 1, 2 and 5 without setae.
Antennule (
Figure 14B). Peduncle 3-segmented, article 1 the longest, slender, with four pointed projections and with 16 plumose setae; article 2 with one plumose setae and article 3, subequal in size with article 2, with 8 plumose setae and two flagella distally, flagella almost same size.
Antenna (
Figure 14C). Protopod 3-segmented, segment 1 unarmed, segment 2 with two plumose setae, segment 3 with a flagellum; exopod flattened with 66 plumose setae.
Mandible (
Figure 14D,E). Mandibular palp absent; left and right incisor with 3 terminal teeth.
Maxillule (
Figure 14F). Coxal endite with 28 conical serrulated setae; basial endite with 11 simple setae and 13 conical serrulated setae; protopod with two articles, article 1 with two serrulated setae and article 2 with 6 serrulated setae.
Maxilla (
Figure 14G). Coxal with 31 plumose setae; basial endite bilobed with 8 and 10 serrated setae respectively; scaphognathite margin with 122 plumose setae.
First maxilliped (
Figure 14H). Coxa with 18 plumose setae; basis with 13 plumose and 17 serrulated setae; endopod 4-segmented with 5, 3, 1, 2, plumose setae, except the last article that bear 2 serrulated setae; exopod with 15 plumose setae.
Second maxilliped (
Figure 14I). Coxa with 3 plumose setae; basis with 9 plumose setae; endopod 5-segmented with 3 plumose, 3 plumose, 1 plumose, 5 (2 plumose plus 3 serrulated), 5 serrulated setae; exopod unsegmented and unarmed.
Third maxilliped (
Figure 14J). Coxa with 8 plumose setae; basis with 5 plumose setae; endopod 5-segmented with 3, 2, 2, plumose setae, one serrulated setae; last article subdivided in three small articles with 3, 1 and 2 serrulated setae; exopod armed with 14 plumose setae.
Figure 14.
Nematocarcinus rotundus: (A) lateral view; (B) antennule; (C) antenna; (D) left mandible; (E) right mandible (cutting edge); (F) maxillule; (G) maxilla; (H) first maxilliped; (I) second maxilliped; (J) third maxilliped.
Figure 14.
Nematocarcinus rotundus: (A) lateral view; (B) antennule; (C) antenna; (D) left mandible; (E) right mandible (cutting edge); (F) maxillule; (G) maxilla; (H) first maxilliped; (I) second maxilliped; (J) third maxilliped.
First pereopod (
Figure 15A). Coxa and basis with 3 plumose setae each one; endopod 5-segmented with 5, 3, 4, 3, 4 plumose setae, except the last two segments that have serrulated setae; exopod, with 15 plumose setae.
Second pereopod (
Figure 15B). Coxa with 2 plumose setae. Basis with 3 plumose setae; endopod 5-segmented with 4, 5, 0, 2, 3 plumose setae, except the last two segments that have serrulate setae; exopod damage in the specimen.
Third pereopod (
Figure 15C). Coxa with 2 plumose setae, basis with 3 plumose setae; endopod 5-segmented with 6, 7, 4, 2, 3 plumose setae, except the last two segments that have serrulated setae; exopod with 18 plumose setae.
Four pereopod (
Figure 15D). Coxa and basis with one plumose seta each; endopod 5-segmented with 7, 7, 5, 4 (one plumose and 3 serrulated), 3 plumose setae, except the last segment that have serrulated setae; exopod with 8 plumose setae.
Fifth pereopod (
Figure 15E). Coxa without setae; basis with 5 plumose setae; endopod 5-segmented with 3, 4, 4, plumose setae, 4 (one plumose and 3 serrulated), 3 serrulated setae; exopod with 8 plumose setae.
Uropods (
Figure 15F). Endopod well developed with 72 plumose setae, slightly wider than exopod; exopod, with 76 plumose setae.
Telson (
Figure 15G). (Damaged in the specimen) elongate, subtriangular. Lateral margin with 7 pairs of spines. Posterior margin damage in the specimen.
Figure 15.
Nematocarcinus rotundus: (A) first pereopod; (B) second pereopod; (C) third pereopod; (D) fourth pereopod; (E) fifth pereopod; (F) uropods; (G) telson.
Figure 15.
Nematocarcinus rotundus: (A) first pereopod; (B) second pereopod; (C) third pereopod; (D) fourth pereopod; (E) fifth pereopod; (F) uropods; (G) telson.
Material examined: Gulf of Mexico: HBG 6134, R/V Point Sur, DP03-06May16-MOC10-B079N-045-N3, 27. 4613 and −86.8992, 27.5005 and −86.9771; 6 May 2016, 601.4–996.1 m. MOCNESS plankton net, L. Timm, coll. Gulf of Mexico: HBG 7996, R/V Point Sur, (DP05-06May17-MOC10-B287N-089-N3), 28.1179 and −87.3899; 6 May 2017, 1000–600 m, MOCNESS plankton net, L. Timm, coll. Gulf of Mexico: HBG 7997, R/V Point Sur, (DP05-06May17-MOC10-B287N-089-N3), 28. 1179 and −87.3899, 6 May 2017, 1000–600 m, MOCNESS plankton net, L. Timm, coll. Gulf of Mexico: HBG 8000, R/V Point Sur, DP05-03May17-MOC10-B065N-087-N3, 28. 5312 and −88.0236, 5 May 2017, 1000–600 m, MOCNESS plankton net, L. Timm, coll.
Decapodite. Size: 8 mm (Carapace length); 26 mm (Total length). N = 4.
Carapace (
Figure 16A). Rostrum straight, armed with 7–12 dorsal spines, sligthly longer than antennular peduncle; eyes pedunculate.
Pleon (
Figure 16A) with 6 somites, no spines or setae. Pleopods well developed.
Antennule (
Figure 16B). Peduncle 3-segmented, article 1 the longest, slender, with 15–24 plumose setae; article 2 with 15–17 plumose setae and article 3, subequal in size with article 2, with 8–16 plumose setae and two flagella distally, flagella almost same size.
Antenna (
Figure 16C). Protopod 3-segmented, segment 1 unarmed, segment 2 with two plumose setae, segment 3 with a flagellum; exopod flattened with 66–83 plumose setae.
Mandible (
Figure 16D). Mandibular palp 3-segmented, with 1, 8, 13 simple setae; incisor with 7 terminal teeth.
Maxillule (
Figure 16E). Coxal endite with 8 serrulated setae; basial endite with 15 conical setae; protopod with 3 plumose setae.
Maxilla (
Figure 16F). Coxal endite with 36 plumose setae; basial endite bilobed with 23 (12 plumose plus 11 conical) + 36 plumose setae; endopod with 6 plumose setae; scaphognathite margin with 149 plumose setae.
First maxilliped (
Figure 16G). Coxa without setae; basis with 47 (10 conical plus 10 plumose plus 27 serrulated) setae; endopod unsegmented with 21 plumose setae; exopod unsegmented with 21 simple setae
Second maxilliped (
Figure 16H). Coxa without setae; basis with 8 simple setae; endopod 5-segmented with 11, 6, 2, all plumose, 25 (5 simple plus 20 serrulated setae), 11 serrulated; exopod unsegmented and unarmed.
Third maxilliped (
Figure 16I). Coxa without setae; basis with 3 simple setae; endopod 4-segmented with 16, 20, 9 all simple, 29 serrulated setae; exopod missing in the specimen.
First to fifth Pereopods missing in the specimens.
Uropods (
Figure 16J). Endopod well developed with 81–96 plumose setae, slightly wider than exopod; exopod, with 72–75 plumose setae.
Telson (
Figure 16K) elongate, subtriangular. Lateral margin with 5 pairs of spines. Posterior margin, armed with 2 principal spines in each corner and 2 distal spines.
Figure 16.
Nematocarcinus rotundus: (A) lateral view; (B) antennule; (C) antenna; (D) mandible; (E) maxillule; (F) maxilla; (G) first maxilliped; (H) second maxilliped; (I) third maxilliped; (J) uropods; (K) telson.
Figure 16.
Nematocarcinus rotundus: (A) lateral view; (B) antennule; (C) antenna; (D) mandible; (E) maxillule; (F) maxilla; (G) first maxilliped; (H) second maxilliped; (I) third maxilliped; (J) uropods; (K) telson.
| Oplophoridae Dana, 1852 |
| Systellaspis Spence Bate, 1888 |
| Systellaspis braueri (Balss, 1914) |
| (Figure 17 and Figure 18) |
Material examined: Gulf of Mexico: HBG6823, R/V Point Sur, DP04-08Aug16-MOC10-SE1N-063-N0, from 26.9878, −87.9494 to 27.0591, −88.0856, 8 August 2016, 1504-NA m, MOCNESS plankton net, H. Bracken-Grissom, coll.
Decapodite. Size. 8 mm (Carapace length); 26 mm (Total length). N = 1.
Carapace (
Figure 17A). Rostrum straight, armed dorsally with 9 spines and ventrally with one small spine, same length of the eye; antennal spine small, anteroventral margin bearing one small spine and a pterygostomian spine; eyes pedunculate.
Pleon (
Figure 17A) with 6 somites, no spines or setae. Pleopods 1–2 missing in the specimen, pleopods 3–5 well developed.
Antennule (
Figure 17B). Peduncle 3-segmented, article 1 the longest armed with 5 simple setae, article 2 also with 3 simple setae and article 3 the smallest, with one simple setae and two flagella distally, flagella subequal in size.
Antenna (
Figure 17C). Protopod 3-segmented, flagellum missing in the specimen; exopod flattened with 52 plumose setae and a pointed process distally.
Mandible (
Figure 17D). Mandibular palp 3-segmented, article 1 armed with 3 simple setae, article 2 with 2 lateral simple setae and article 3 with 6 simple setae plus 3 plumose setae, right incisor with 9 teeth.
Maxillule (
Figure 17E). Coxal endite with 19 plumose setae; basial endite with 18 conical serrulate setae plus 2 plumose setae and protopod with one plumose subterminal seta.
Maxilla (
Figure 17F). Coxal endite with 10 plumose setae; basial endite bilobed with 11 + 19 (17 plumose plus 2 simple) setae; endopod with 3 plumose setae; scaphognathite margin with 124 plumose setae.
First maxilliped (
Figure 17G). Coxa with 8 plumose setae; basis with 28 plumose setae; endopod unsegmented with 12 plumose setae; exopod unsegmented, armed with 14 plumose setae.
Second maxilliped (
Figure 17H). Coxa without setae; basis with 6 plumose setae; endopod 5-segmented with 18, 8, 2, plumose setae plus 23, 12 serrulate setae; exopod unsegmented, armed distally with 8 plumose natatory setae.
Figure 17.
Systellaspis braueri: (A) lateral view; (B) antennule; (C) antenna; (D) mandible; (E) maxillule; (F) maxilla; (G) first maxilliped; (H) second maxilliped.
Figure 17.
Systellaspis braueri: (A) lateral view; (B) antennule; (C) antenna; (D) mandible; (E) maxillule; (F) maxilla; (G) first maxilliped; (H) second maxilliped.
Third maxilliped (
Figure 18A). Coxa with 3 plumose setae; basis with 6 plumose setae, endopod 3-segmented with 40 (22 inner setae, 3 of them serrulate setae, all the others plumose + 18 outer plumose setae), 9 serrulate setae and 23 serrulate setae; exopod unsegmented, armed distally with 7 plumose natatory setae.
First pereopod (
Figure 18B). Coxa with 3 and basis with 6 plumose setae; endopod 5-segmented with 10 plumose setae, 18 plumose setae, 5, 11, 1 serrulate setae; exopod unsegmented and unarmed.
Second pereopod (
Figure 18C). Coxa with 8 plumose setae, basis with 4 plumose setae; endopod 5-segmented with 17 plumose setae, 15 plumose setae and 4, 7, 1 serrulate setae.
Third pereopod missing in the specimen.
Fourth pereopod (
Figure 18D). Coxa with 9 simple setae, basis with 4 simple setae; endopod 5-segmented with 10 (5 spines + 5 simple setae), 12 (4 spines + 8 simple setae), 1 simple setae, 5 spines, 0, 0; exopod unsegmented and unarmed.
Fifth pereopod (
Figure 18E). Coxa and basis without setae; endopod 5-segmented with 5 (2 spine + 4 simple setae), 2, 3, 12, 8 simple setae; exopod unsegmented and unarmed.
Uropod (
Figure 18F). Endopod well developed with 54 plumose setae; exopod with 42 plumose setae
Telson (
Figure 18G) elongate, subtriangular, with 11 pairs of lateral spines, 1 pair of large mobile spines and 10 pairs of spines on the distal part near the tip of the telson; one small spine on the distal margin.
Figure 18.
Systellaspis braueri: (A) third maxilliped; (B) first pereopod; (C) second pereopod; (D) fourth pereopod; (E) fifth pereopod; (F) telson; (G) uropods.
Figure 18.
Systellaspis braueri: (A) third maxilliped; (B) first pereopod; (C) second pereopod; (D) fourth pereopod; (E) fifth pereopod; (F) telson; (G) uropods.
| Pandalidae Haworth, 1825 |
| Heterocarpus ensifer A. Milne-Edwards, 1881 |
| (Figure 19 and Figure 20) |
Material examined: Gulf of Mexico: HBG6844, R/V Point Sur, DP04-17Aug16-MOC10-B252N-080-N5, from 28.5272, −87.4972 to 28. 3842, −87.4866, 17 August 2016, 199.5–5 m, MOCNESS plankton net, L. Timm, coll.
Zoea. Size. 22 mm (Carapace length); 36 mm (Total length). N = 1.
Carapace (
Figure 19A). Rostrum large armed dorsally with 21 spines and 9 ventral spines, one spine near the posterior margin of the carapace, suborbital spine strong.
Pleon (
Figure 19A) with a pointed projection on segments 3 and 4. Other segments without spines or setae. Pleopods 1–4 missing in the specimen, pleopod 5 without setae.
Antennule (
Figure 19B). Peduncle 3-segmented, article 1 the longest, slender, with 9 plumose setae in both margins, article 2 with 2 plumose setae and article 3, the smallest, with 3 plumose setae and with two flagella distally.
Antenna (
Figure 19C). Protopod 3-segmented, article 1 and 2 unarmed, article 3 with 5 small spines and a flagellum; exopod flattened, subtriangular, with a slender and pointed projection on its distal region and 13 pointed projections on the superior margin and 64 plumose setae in the inferior margin.
Mandible (
Figure 19D, E) without palp, right mandible with 6 teeth and left mandible with 4 teeth.
Maxillule (
Figure 19F). Coxal endite with 19 conical serrulated setae; basial endite with 12 conical serrulated setae; protopod with 4 plumose setae.
Maxilla (
Figure 19G). Coxal endite bilobed with 17 plumose plus 2 serrated and one plumose setae; basial endite bilobed with 10 plus 12 plumose setae; endopod with 8 (2 + 2 + 1 + 1 + 2) plumose setae, segmentation not well defined; scaphognathite margin with 143 plumose setae.
First maxilliped (
Figure 19H). Coxa with 7 plumose setae; basis with 23 plumose setae; exopod with 50 plumose setae; endopod 4-segmented, armed with 22 setae, five of them plumose all the others simple.
Second maxilliped (
Figure 19I). Coxa with one plumose seta; basis with 10 plumose plus 4 serrulated setae; endopod 5-segmented with 4, 3, 2, 4, 8 plumose setae, except the first and the last articles which have one serrated seta each; exopod armed distally with 17 plumose setae.
Third Maxilliped (
Figure 19J). Coxa with 3 simple setae; basis with 9 simple setae; endopod 4-segmented with 13, 9, 21, 2 simple setae; exopod armed distally with 6 plumose setae.
Figure 19.
Heterocarpus ensifer: (A) lateral view; (B) antennule; (C) antenna; (D) right mandible; (E) left mandible (cutting edge); (F) maxillule; (G) maxilla; (H) first maxilliped; (I) second maxilliped; (J) third maxilliped.
Figure 19.
Heterocarpus ensifer: (A) lateral view; (B) antennule; (C) antenna; (D) right mandible; (E) left mandible (cutting edge); (F) maxillule; (G) maxilla; (H) first maxilliped; (I) second maxilliped; (J) third maxilliped.
First pereopod (
Figure 20A). Coxa without setae; Basis with 5 simple setae; endopod 5-segmented with 5, 8, 14, 27, 4 simple setae; exopod armed distally with 10 plumose setae.
Second pereopod (
Figure 20B). Coxa without setae; basis with 5 simple setae; endopod 5-segmented with 10, 8, 8, 7, 3 simple setae; exopod armed distally with 6 plumose setae.
Third pereopod (
Figure 20C). Coxa without setae; basis with 4 setae; endopod 5-segmented with 5, 21, 7, 23, 5 simple setae; exopod armed distally with 6 plumose setae.
Fourth pereopod (
Figure 20D). Coxa without setae; basis with 2 simple setae; endopod 5-segmented with 9 (6 simple setae plus 3 spines), 17 (10 simple setae plus 7 spines), 7, 27, 5 simple setae; exopod armed distally with 7 plumose setae.
Fifth pereopod (
Figure 20E). Coxa with one simple setae; Basis with 6 simple setae; endopod 5-segmented with 11 (3 spines plus 8 simple setae), 14, 7, 34, 8 simple setae.
Uropod (
Figure 20F). Endopod and exopod well developed, exopod with 84 plumose setae and endopod with 90 plumose setae.
Telson (
Figure 20G) enlarged, subtriangular, with 4 pairs of lateral spines and posterior margin bearing row of 5 diminute spines and one pairs of spines on outer margin.
Figure 20.
Heterocarpus ensifer: (A) first pereopod; (B) second pereopod; (C) third pereopod; (D) fourth pereopod; (E) fifth pereopod; (F) uropods; (G) telson.
Figure 20.
Heterocarpus ensifer: (A) first pereopod; (B) second pereopod; (C) third pereopod; (D) fourth pereopod; (E) fifth pereopod; (F) uropods; (G) telson.
| Plesionika Spence Bate, 1888 |
| Plesionika edwardsii (J.F. Brandt in von Middendorf, 1851) |
| (Figure 21 and Figure 22) |
Material examined: Gulf of Mexico: HBG 7584, R/V Point Sur, DP04-09Aug16-MOC10-SE3N-065-N5, from 26.9997, −86.9912 to 26.9903, −87.1491; 9 August 2016, 199.2–5 m, MOCNESS plankton net, H. Bracken-Grissom, coll.
Decapodite. Size: 15 mm (Carapace length); 58 mm (Total length). N = 1.
Carapace (
Figure 21A). Rostrum long and unarmed, slender, longer than carapace; antennal spine small; anteroventral margin bearing 1 strong pterygostomian spine; eyes pedunculate.
Pleon (
Figure 21A) with 6 somites, no spines or setae. Pleopods 1–4 missing in the specimen, pleopod 4 well developed.
Antennule (
Figure 21B). Peduncle 3-segmented, article 1, the longest, armed with 27 (15 outer plus 12 inner) plumose setae and one spine, article 2 with 9 (6 outer plus 3 inner) plumose setae and article 3 with 5 outer plumose setae and two flagella distally.
Antenna (
Figure 21C). Protopod 3-segmented with a flagellum; exopod flattened with 71 plumose setae and a pointed process distally.
Mandible. Palp absent; right and left slightly asymmetrical, right incisor with 3 terminal teeth (
Figure 21D); left incisor with 4 teeth (
Figure 21E).
Maxillule (
Figure 21F). Coxal endite with 12 conical serrate setae; basial endite with 7 conical serrate setae and 4 simple setae; endopod unsegmented, with 1 + 3 serrated setae.
Maxilla (
Figure 21G). Coxal endite bilobed with 12 plumose plus 3 simple setae; basial endite bilobed with 4 + 7 simple setae; endopod unsegmented with 6 (2 + 2 + 2) simple setae; scaphognathite margin with 120 plumose setae.
First maxilliped (
Figure 21H). Coxa with 3 large plumose plus 3 simple setae; basis with 12 plumose setae; endopod 4-segmented with 6 (5 simple plus one conical serrate) 3 (2 simple plus one conical serrate), 2 (one simple pus one conical serrate), 3 simple setae; endopod armed with 21 plumose setae and exopod armed distally with 12 plumose setae.
Second maxilliped (
Figure 21I). Coxa with one plumose seta; basis with 11 (4 simple plus 4 plumose plus 3 conical serrated) setae; endopod 5-segmented with 3 (one conical serrated plus 2 simple), 2 simple, 1 simple, 7 simple, 8 (5 conical serrated and 3 simple) setae; exopod unarmed.
Third maxilliped (
Figure 21J). Coxa without setae; basis with 5 simple setae; endopod 5-segmented with 2 simple, 19 (13 simple setae plus 6 spines), 11 simple, 12 simple, 0 setae; exopod armed with 9 plumose setae.
Figure 21.
Plesionika edwardsii: (A) lateral view; (B) antennule; (C) antenna; (D) left mandible (cutting edge); (E) rigth mandible (cutting edge); (F) maxillule; (G) maxilla; (H) first maxilliped; (I) second maxilliped; (J) third maxilliped.
Figure 21.
Plesionika edwardsii: (A) lateral view; (B) antennule; (C) antenna; (D) left mandible (cutting edge); (E) rigth mandible (cutting edge); (F) maxillule; (G) maxilla; (H) first maxilliped; (I) second maxilliped; (J) third maxilliped.
First pereopod (
Figure 22A). Coxa and basis unarmed; endopod 5-segmented with 5, 14 (7 spines plus 7 simple setae), 12 (4 spines plus 8 simple setae), 9 simple, 0 setae; exopod unarmed.
Second pereopod (
Figure 22B). Coxa unarmed, basis with 2 simple setae; endopod 5-segmented with 4 spines, 11 (6 spines plus 5 simple setae), 19 (6 spines plus 13 simple setae), 2 simple setae, 0 setae; exopod unarmed.
Third pereopod (
Figure 22C). Basis armed with 2 simple setae; endopod 5-segmented with 6 simple setae, 18 spines, 5 spines, 19 (9 spines plus 10 setae), 0 setae; exopod unarmed.
Fourth pereopod (
Figure 22D). Coxa and basis unarmed; endopod 5-segmented with 3 spines, 19 (9 spines plus 10 simple setae), 5 (4 spines plus one simple seta), 9 (7 spines plus 2 simple setae), 0 simple setae; exopod unarmed.
Fifth pereopod (
Figure 22E). Coxa unarmed, basis with 2 simple setae; endopod 5-segmented with 4 simple setae, 21 (10 spines plus 11 simple setae), 8 (5 spines plus 3 simple setae), 15 (9 spines plus 6 simple setae), 0 simple setae; exopod absent.
Uropods (
Figure 22F). Endopod well developed with 96 plumose setae; exopod, with 84 plumose setae
Telson (
Figure 22G) elongate, subtriangular, with three pairs of lateral spines; distally with one central large spine and 3 pairs of small spines and one spine on each corner.
Figure 22.
Plesionika edwardsii: (A) first pereopod; (B) second pereopod; (C) third pereopod; (D) fourth pereopod; (E) fifth pereopod; (F) telson; (G) Uropods.
Figure 22.
Plesionika edwardsii: (A) first pereopod; (B) second pereopod; (C) third pereopod; (D) fourth pereopod; (E) fifth pereopod; (F) telson; (G) Uropods.
Material examined: Gulf of Mexico: HBG6825, R/V Point Sur, DP04-07Aug16-MOC10-SW4N-061-N0, 26.8887, −89.0389, and 26.9936, −88.9987, 7 August 2016, 1500.8-NA m, MOCNESS plankton net, H. Bracken-Grissom, coll. Gulf of Mexico: HBG7845, R/V Point Sur, DP05-10May17-MOC10-B175D-096-N2, 28.9922 and −87.4786, 29.0336 and −87.6491, 10 May 2017, 1199–995 m, MOCNESS plankton net, L. Timm, coll. Gulf of Mexico: HBG7995, R/V Point Sur, DP05-06May17-MOC10-B287N-089-N3, 28.1179 and −87.3899, 28.0467 and −87.5559, 6 May 2017, 1000–600 m, MOCNESS plankton net, L. Timm, coll. Gulf of Mexico: HBG9264, R/V Point Sur, DP06-20Jul18-MOC10-B175N-102-N0, 29.0045 and −87.4658, 20 July 2018, 600 m, MOCNESS plankton net, H. Bracken-Grissom, coll.
Juvenile. Size. 12 mm (Carapace length); 36 mm (Total length). N = 4.
Carapace (
Figure 23A). Rostrum long, slender, with 3 basal spines, slightly curved upwards and longer than antennular peduncle; antennal spine present; eyes pedunculate.
Pleon (
Figure 23A) with 6 somites, no spines or setae. Pleopods 3–4 missing in the specimen, pleopods 1–2 and 5 well developed.
Antennule (
Figure 23B). Peduncle 3-segmented, article 1 with 16–18 plumose setae, article 2 with 9 plumose setae and article 3 with two flagella.
Antenna (
Figure 23C). Protopod 3-segmented; article 1 with two sharp projections, article 2 with 4 simple setae and article 3 with 5 simple setae. exopod flattened with 63–66 plumose setae and a pointed process distally.
Mandible (
Figure 23D). Palp 3-segmented, article 1 unarmed, article 2 with 3 simple setae and article 3 with 16 simple setae, right incisor with 5 terminal teeth.
Maxillule (
Figure 23E). Coxal endite with 10–12 simple setae plus 10–18 serrulate setae; basial endite with 15–18 simple setae plus 10–12 conical setae; endopod unsegmented, with 6 simple setae plus one plumose seta; exopod absent.
Maxilla (
Figure 23F). Coxal endite with 12–16 plumose setae; basial endite bilobed both armed with 28–30 and 28–32 serrulated setae respectively; endopod unsegmented with 4 (1 + 1 + 2) plumose setae; scaphognathite margin with 89–93 plumose setae.
First maxilliped (
Figure 23H). Coxa with 15–17 serrulate setae; basis endite with 43–52 serrulate setae; endopod with 28–32 plumose setae; exopod unsegmented, armed distally with 10–13 plumose setae.
Second maxilliped (
Figure 23G). Coxa with 4 serrulated setae; basis with 14 serrulated setae; endopod 5-segmented with 1 plumose seta, 6 plumose setae and 4–5, 11–20, 5–10 serrulated setae; exopod armed with 8–10 plumose setae.
Figure 23.
Plesionika ensis: (A) lateral view; (B) antennule; (C) antenna; (D) mandible; (E) maxillule; (F) maxilla; (G) first maxilliped; (H) second maxilliped.
Figure 23.
Plesionika ensis: (A) lateral view; (B) antennule; (C) antenna; (D) mandible; (E) maxillule; (F) maxilla; (G) first maxilliped; (H) second maxilliped.
Third maxilliped (
Figure 24A). Coxa without setae; basis with 7 simple setae; endopod 3-segmented with 24, 13, 12, simple setae; exopod unsegmented, armed distally 16 simple setae.
First pereopod missing in the specimen.
Second pereopod (
Figure 24B). Coxa and basis without setae; endopod 5-segmented with 14, 0, 7, 0 (with 8 divisions), 24, 6 simple setae.
Third and fourth pereopods missing in the specimen.
Fifth pereopod (
Figure 24C). Coxa without setae, basis with 10 simple setae, endopod 5-segmented with 13, 26, 26, 26, 3 simple setae.
Uropod (
Figure 24D). Endopod well developed with 67–76 plumose setae; exopod, with 92–97 plumose setae.
Telson (
Figure 24E) elongate, subtriangular, with 3 pairs of lateral spines and 2 pairs of distal spines.
Figure 24.
Plesionika ensis: (A) third maxilliped; (B) second pereopod; (C) fifth pereopod; (D) Uropods; (E) telson.
Figure 24.
Plesionika ensis: (A) third maxilliped; (B) second pereopod; (C) fifth pereopod; (D) Uropods; (E) telson.
| Aristeidae Wood-Mason in Wood-Mason and Alcock, 1891 |
| Hemipenaeus Spence Bate, 1881 |
| Hemipenaeus carpenteri Wood-Mason in Wood-Mason and Alcock, 1891 |
| (Figure 25 and Figure 26) |
Material examined: Gulf of Mexico: HBG 6846, R/V Point Sur, DP04-09Aug16-MOC10-SE3N-065-N3, 26.9997, −86.9912 and 26.9909, −87.1491, 9 August 2016, 1000.5–3 m, MOCNESS plankton net, H. Bracken-Grissom, coll.
Mysis. Size. 6 mm (Carapace length); 16 mm (Total length). N = 1.
Carapace (
Figure 25A) with two lateral swollen process near the posterior margin, rostrum long, extend until the end of the article 1 of the antennule, slightly curved; anteroventral margin bearing 1 strong pterygostomial spine and 1 postorbital spine; eyes pedunculate.
Pleon (
Figure 25A) with 6 somites, no spines or setae. Pleopods 1–5 without setae.
Antennule (
Figure 25B). Peduncle 3-segmented, article 1 the longest, slender, with 21 plumose setae in both margins, article 2 with 11 plumose setae in both margins and article 3, the smallest with 5 plumose setae and two flagella distally. Flagella short, same size, inner 5-segmented and outer 6-segmented with plumose setae.
Antenna (
Figure 25C). Protopod 3-segmented with a flagellum; exopod with 66 plumose setae.
Mandible (
Figure 25D). Palp 3-segmented, article 1 unarmed, article 2 with 5 simple setae and article 3 with 10 simple setae.
Maxillule (
Figure 25E). Coxal endite with 15 (10 serrated plus 5 plumose) setae; basial endite with 11 conical setae and one plumose subdistal setae.
Maxilla (
Figure 25F). Coxal endite bilobed with 21 (15 plumose plus 6 serrated) setae; basial endite bilobed with 15 (7 plus 8 serrated) setae; endopod with 6 (1 + 1 + 1 + 3) plumose setae, segmentation not well defined; scaphognathite margin with 89 plumose setae.
First maxilliped (
Figure 25G). Coxa with two endites and 12 (5 + 7) plumose setae; basis with 21 serrated setae; endopod 4-segmented with 1, 2, 4, 3 plumose setae; exopod unsegmented, armed with 7 plumose setae.
Second maxilliped (
Figure 25H). Coxa with 4 plumose setae; basis with 6 plumose setae; endopod 5-segmented with 4, 15, 2, 5, 9 plumose setae, except the last two articles which have serrated setae; exopod unsegmented, armed distally with 9 long plumose natatory setae.
Third maxilliped (
Figure 25I). Coxa with 1 plumose seta; basis with 5 serrated setae; endopod 5-segmented with 5, 5, 9, 7, 9 serrated setae; exopod unsegmented, armed distally with 12 long plumose natatory setae.
Figure 25.
Hemipenaeus carpenteri: (A) lateral view; (B) antennule; (C) antenna; (D) maxillule; (E) mandible; (F) maxilla; (G) first maxilliped; (H) second maxilliped; (I) third maxilliped.
Figure 25.
Hemipenaeus carpenteri: (A) lateral view; (B) antennule; (C) antenna; (D) maxillule; (E) mandible; (F) maxilla; (G) first maxilliped; (H) second maxilliped; (I) third maxilliped.
First pereopod (
Figure 26A). Basis with 2 simple setae; endopod 5-segmented with 1, 1, 1, 2, 3 setae; exopod armed distally with 10 plumose setae.
Second pereopod (
Figure 26B). Basis unarmed; endopod 5-segmented with 2, 1, 1, 3, 2 setae; exopod armed distally with 14 plumose setae.
Third pereopod (
Figure 26C). Basis unarmed; endopod 5-segmented with 0, 1, 1, 1, 4 (2 inner + 2 terminal) setae; exopod armed distally with 11 plumose setae.
Fourth pereopod (
Figure 26D). Basis unarmed; endopod 5-segmented with 0, 1, 1, 0, 2 setae; exopod armed distally with 9 plumose setae.
Fifth pereopod (
Figure 26E). Basis unarmed; endopod 5-segmented with 0, 1, 0, 0, 1 setae; exopod armed distally with 12 plumose setae.
Uropod (
Figure 26F). Endopod and exopod well developed, both missing setae.
Telson (
Figure 26G) enlarged, subrectangular, with two pairs of lateral spines and posterior margin bearing row of 4 pairs of minute spinules and 2 pairs of spines on outer margin.
Figure 26.
Hemipenaeus carpenteri: (A) first pereopod; (B) second pereopod; (C) third pereopod; (D) fourth pereopod; (E) fifth pereopod; (F) uropods; (G) telson.
Figure 26.
Hemipenaeus carpenteri: (A) first pereopod; (B) second pereopod; (C) third pereopod; (D) fourth pereopod; (E) fifth pereopod; (F) uropods; (G) telson.
Material examined: Gulf of Mexico: HBG 6854, R/V Point Sur DP04-08Aug16-MOC10-SE1N-063-N5, 26. 9878, −87.9494, and 27.0591, −88.0856, 8 August 2016, 202.7–5 m, MOCNESS plankton net, H. Bracken-Grissom, coll. Gulf of Mexico: HBG 7552, R/V Point Sur DP04-11Aug16-MOC10-SW3D-068-N5, 27. 0122, −88.4618, and 26.9255, −88.5970, 11 August 2016, 199.8–5 m, MOCNESS plankton net, H. Bracken-Grissom, coll. Gulf of Mexico: HBG 7867, R/V Point Sur DP05-11May17-MOC10-B175D-098-N0, 26. 9690, −87.4396, 11 May 2017, 1500–0 m, MOCNESS plankton net, L. Timm, coll.
Mysis. Size: 9 mm (Carapace length); 21 mm (Total length). N = 3.
Carapace (
Figure 27A) with two lateral swollen process near the posterior margin, rostrum long, extend until the end of the article 1 of the antennule; orbital spine as a projected bump; antennal spine is a small bump; anteroventral margin bearing 1 strong and curved pterygostomial spine; eyes pedunculate.
Pleon (
Figure 27A) with 6 somites, no spines or setae. Pleopods without setae.
Antennule (
Figure 27B). Peduncle 3-segmented, article 1 the longest, slender, with 3 simple and 9–12 plumose setae, article 2 also with 6 plumose setae in the outer margins and article 3, the smallest with 3 lateral simple setae and two distal flagella, outer flagella unarmed and inner flagella with 4 lateral simple setae and 2 distal setae.
Antenna (
Figure 27C). Protopod 3-segmented with a flagellum; exopod with 62–69 plumose setae.
Mandible (
Figure 27D). Palp 2-segmented, article 1 with 7–10 plumose setae and article 2 with 13–15 plumose setae (7 lateral plus 6 terminal).
Maxillule (
Figure 27E). Coxal endite with 7 curved conical spines and 1 subterminal simple setae; basial endite with 11 plumose setae.
Maxilla (
Figure 27F). Coxal endite bilobed with 6 + 8 simple setae; basial endite bilobed with 6 + 8 plumose setae; endopod with 5 (2 + 1 + 2) plumose setae, segmentation not well defined; scaphognathite margin with 89–92 plumose setae.
First maxilliped (
Figure 27G). Coxa with 8–10 plumose setae; basis with 14–18 plumose setae in the margin and 10–12 simple setae; endopod unsegmented with 11 (4 + 2 + 1 + 1 + 3) simple setae; exopod unsegmented, armed with 8 plumose setae.
Second maxilliped (
Figure 27H). Coxa without setae; basis with 5–8 simple setae; endopod 5-segmented with 5–6, 5–7, 5, 7–12, 8–9 serrulated setae; exopod unsegmented, armed distally with 7–9 plumose setae.
Third maxilliped (
Figure 27I). Coxa without setae; basis with 4 simple setae; endopod 5-segmented with 5, 3, 4, 6, 8, all simple setae; exopod unsegmented armed distally with 5–7 plumose setae.
Figure 27.
Hemipenaeus carpenteri: (A) lateral view; (B) antennule; (C) antenna; (D) mandible; (E) maxillule; (F) maxilla; (G) first maxilliped; (H) second maxilliped; (I) third maxilliped.
Figure 27.
Hemipenaeus carpenteri: (A) lateral view; (B) antennule; (C) antenna; (D) mandible; (E) maxillule; (F) maxilla; (G) first maxilliped; (H) second maxilliped; (I) third maxilliped.
First pereopod (
Figure 28A). Coxa and basis without setae; endopod 5-segmented with 0, 0, 2, 3, 2 setae; exopod unsegmented, armed with 7–10 plumose natatory setae.
Second pereopod (
Figure 28B). Coxa without setae, basis with 2 simple setae; endopod 5-segmented with 3, 2, 3, 1, 4 simple setae; exopod unsegmented, armed with 7–9 plumose natatory setae.
Third pereopod (
Figure 28C). Coxa and basis without setae; endopod 5-segmented with 0, 1, 1, 3, 3 simple setae; exopod unsegmented, armed with 9–12 long, plumose natatory setae.
Fourth pereopod (
Figure 28B). Coxa and basis without setae; endopod 5-segmented with 0, 1, 1, 0, 1 simple seta; exopod unsegmented, armed with 11–12 long plumose natatory setae.
Fifth pereopod (
Figure 28A). Coxa and basis unarmed; endopod 5-segmented with 0, 0, 0, 0, 1 simple setae; exopod unsegmented armed with 10–12 long plumose natatory setae.
Uropod (
Figure 28F). Endopod well developed with 80–85 plumose setae; exopod with 60–63 plumose setae.
Telson (
Figure 28G) elongate, subtriangular, with 3 pairs of lateral spines and 5 pairs of distal spines.
Figure 28.
Hemipenaeus carpenteri: (A) first pereopod; (B) second pereopod; (C) third pereopod; (D) fourth pereopod; (E) fifth pereopod; (F) uropods; (G) telson.
Figure 28.
Hemipenaeus carpenteri: (A) first pereopod; (B) second pereopod; (C) third pereopod; (D) fourth pereopod; (E) fifth pereopod; (F) uropods; (G) telson.
Material examined: Gulf of Mexico: HBG 9204, R/V Point Sur, DP06-24Jul18-MOC10-B251N-106-N1, 28.5401, −88.4711 and 28.5122, −88.6337, 24 July 2018, 1201–1475 m, MOCNESS plankton net, H. Bracken-Grissom, coll.
Mysis. Size. 6 mm (Carapace length); 20 mm (Total length). N = 1.
Carapace (
Figure 29A) with two small lateral swollen process near the posterior margin, rostrum long, extend until the end of the article 1 of the antennule, slightly curved; anteroventral margin bearing one small pterygostomian spine; eyes pedunculate.
Pleon (
Figure 29A) with 6 somites, small spine on dorsal third somite. Pleopods 4–5 missing in the specimen, pleopods 1–3 without setae.
Antennule (
Figure 29B). Peduncle 3-segmented, article 1 the longest, slender, with 35 plumose setae in both margins, article 2 with 18 plumose setae in both margins and article 3, the smallest with 6 plumose setae and two flagella distally.
Antenna (
Figure 29C). Protopod 2-segmented with a flagellum; exopod with 86 plumose setae and a pointed process distally.
Mandible (
Figure 29D). Palp 4-segmented, articles 1- 3 unarmed, article 4 with 7 simple setae.
Maxillule (
Figure 29E). Coxal endite with 13 conical setae; basial endite with 15 conical setae, protopod with two simple setae.
Maxilla (
Figure 29F). (Damaged in the specimen). Coxal endite and the bilobed basial endite bilobed unarmed; endopod with 5 (1 + 2 + 2) simple setae, segmentation not well defined; scaphognathite margin with 38 plumose setae.
First maxilliped (
Figure 29G). (Damaged in the specimen). Coxa and basis unarmed; endopod unsegmented with 17 plumose setae; exopod 4 segmented with 0, 2,14, 8 plumose setae.
Second maxilliped (
Figure 29H). (Damaged in the specimen). Coxa and basis unarmed; endopod 4-segmented with 3, 1, 1, 2 simple setae; exopod unsegmented and unarmed.
Third maxilliped missing in the specimen.
First pereopod (
Figure 29I). Coxa and basis unarmed; endopod 5-segmented with 2, 1, 0, 0, 0 setae; exopod unsegmented and unarmed.
Second pereopod (
Figure 30A). Coxa unarmed; Basis with 3 setae; endopod 5-segmented with 3, 0, 4, 0, 0 setae; exopod unsegmented and unarmed.
Third pereopod (
Figure 30B). Coxa and basis unarmed; endopod 5-segmented with 2, 0, 0, 0, 0 setae; exopod unsegmented and unarmed.
Fourth pereopod (
Figure 30C). (Damaged in the specimen) Coxa and basis unarmed; endopod 5-segmented with 3, 0, 1, 0, 0 setae; exopod unsegmented and unarmed.
Fifth pereopod (
Figure 30D). (Damaged in the specimen). Coxa and basis unarmed; endopod 5-segmented with 2, 5, 0, 0, 0 setae; exopod unsegmented and unarmed.
Figure 29.
Cerataspis monstrosus: (A) lateral view; (B) antennule; (C) antenna; (D) mandible; (E) maxillule; (F) maxilla; (G) first maxilliped; (H) second maxilliped; (I) first pereopod.
Figure 29.
Cerataspis monstrosus: (A) lateral view; (B) antennule; (C) antenna; (D) mandible; (E) maxillule; (F) maxilla; (G) first maxilliped; (H) second maxilliped; (I) first pereopod.
Uropod (
Figure 30E). Endopod well developed with 96 plumose setae; exopod with 120 plumose setae.
Telson (
Figure 30F). (Damaged in the specimen) Subrectangular, distal margin bearing row of 13 min spines and 3 pairs of spines on lateral margin, small simple setae between the lateral spines.
Figure 30.
Cerataspis monstrosus: (A) second pereopod; (B) third pereopod; (C) fourth pereopod; (D) fifth pereopod; (E) uropods; (F) telson.
Figure 30.
Cerataspis monstrosus: (A) second pereopod; (B) third pereopod; (C) fourth pereopod; (D) fifth pereopod; (E) uropods; (F) telson.
| Family Penaeidae Rafinesque, 1815 |
| Genus Funchalia J. Y. Johnson, 1868 |
| Funchalia villosa (Bouvier, 1905) |
| (Figure 31 and Figure 32) |
Material examined: Gulf of Mexico: HBG 6776, R/V Point Sur, DP04-06Aug16-MOC10-SW6N-059-N4, from 26.9936, −89.9941 to 27.0451, −90.0844, 6 August 2016, 601–4 m, MOCNESS plankton net, H. Bracken-Grissom, coll. Gulf of Mexico: HBG 6885, R/V Point Sur, DP04-06Aug16-MOC10-SW6D-058-N0, from 26.9942, −89, 9938 to 27.0611, −90.0923, 6 August 2017, 1510.6-NA m, MOCNESS plankton net, H. Bracken-Grissom, coll. Gulf of Mexico: HBG 7941, R/V Point Sur, DP05-08May17-MOC10-B081N-083-N0, from 28.5187, −87, 9897, 8 May 2017, 1500–0 m, MOCNESS plankton net, L. Timm, coll.
Juvenile. Size. 11 mm (Carapace length); 32 mm (Total length). N = 3.
Carapace (
Figure 31A) with rostrum short, armed with 5–7 dorsal spines, epigastric tooth present.
Pleon (
Figure 31A) with 6 somites, without spines or setae. Pleopods 2 and 4 missing in the specimen, pleopods 1, 3 and 5 well development.
Antennule (
Figure 31B). Peduncle 3-segmented, article 1 the longest, slender, with 28 simple plus 6 plumose setae, article 2 with 24 simple setae and article 3, the smallest with 10 simple setae and two flagella distally.
Antenna (
Figure 31C). Protopod 3-segmented with a flagellum; exopod with 30–48 plumose setae.
Mandible (
Figure 31D). Palp 2-segmented, articles 1armed with 3–8 simple setae and article 2 with 18–44 plumose setae.
Maxillule (
Figure 31E): Coxal endite with 26–43 (12–22 serrulated plus 14–21 conical serrulated) setae; basial endite with 18 plumose setae setae.
Maxilla (
Figure 31F). Coxal endite with one simple setae, basial endite bilobed with 6–12 + 8–16 simple setae; endopod with one simple setae, segmentation not well defined; scaphognathite margin with 65–126 plumose setae.
First maxilliped (
Figure 31G). Coxa with 6 simple setae, basis with 14–26 simple setae; endopod unsegmented with 5 simple setae; exopod with 11–19 simple setae.
Second maxilliped (
Figure 31H). Coxa without setae, basis with 5–7 simple setae; endopod 4-segmented with 11–18, 0–3, 12–22 serrated, 6–16 serrated setae; exopod unsegmented and unarmed.
Third maxilliped (
Figure 31I). Coxa and basis without setae, endopod 5-segmented with 7–10, 3–5, 11–16, 11–21, 9–21 simple setae; exopod with 8–34 setae.
Figure 31.
Funchalia villosa: (A) lateral view; (B) antennule; (C) antenna; (D) mandible; (E) maxillule; (F) maxilla; (G) first maxilliped; (H) second maxilliped; (I) third maxilliped.
Figure 31.
Funchalia villosa: (A) lateral view; (B) antennule; (C) antenna; (D) mandible; (E) maxillule; (F) maxilla; (G) first maxilliped; (H) second maxilliped; (I) third maxilliped.
First pereopod (
Figure 32A). Coxa and basis with 2 setae; endopod 5-segmented with 4–5, 4–8, 7–15, 6–11, 3–7 setae.
Second pereopod (
Figure 32B). Coxa and basis with 2 simple setae; endopod 5-segmented with 3–6, 9–20 (3–9 spines plus 6–11 simple), 8–21, 6–8, 4–5 simple setae.
Third pereopod (
Figure 32C). Coxa with 2 simple setae, basis without setae; endopod 5-segmented with 4–14, 10–16, 7–14, 7–9, 1–7 simple setae.
Fourth pereopod (
Figure 32D). Coxa with 2 simple setae, basis with one seta; endopod 5-segmented with 6–15, 16–39, 8–10, 12–21, 0 simple setae.
Fifth pereopod (
Figure 32E). Coxa with 3–6 simple setae, basis with 2–4 setae; endopod 5-segmented with 5–14, 10–16, 3–13, 3–9, 0 simple setae.
Uropod (
Figure 32F). Endopod well developed with 30–126 plumose setae; exopod with 54–143 plumose setae.
Telson (
Figure 32G) enlarged, subtriangular, distal margin with a pointed projection, 3 pairs of spines near the distal margin, lateral margins with small simple setae.
Figure 32.
Funchalia villosa: (A) first pereopod; (B) second pereopod; (C) third pereopod; (D) fourth pereopod; (E) fifth pereopod; (F) uropods; (G) telson.
Figure 32.
Funchalia villosa: (A) first pereopod; (B) second pereopod; (C) third pereopod; (D) fourth pereopod; (E) fifth pereopod; (F) uropods; (G) telson.