Using Ecological Niche Models for Population and Range Estimates of a Threatened Snake Species (Crotalus oreganus) in Canada
Abstract
:1. Introduction
2. Materials and Methods
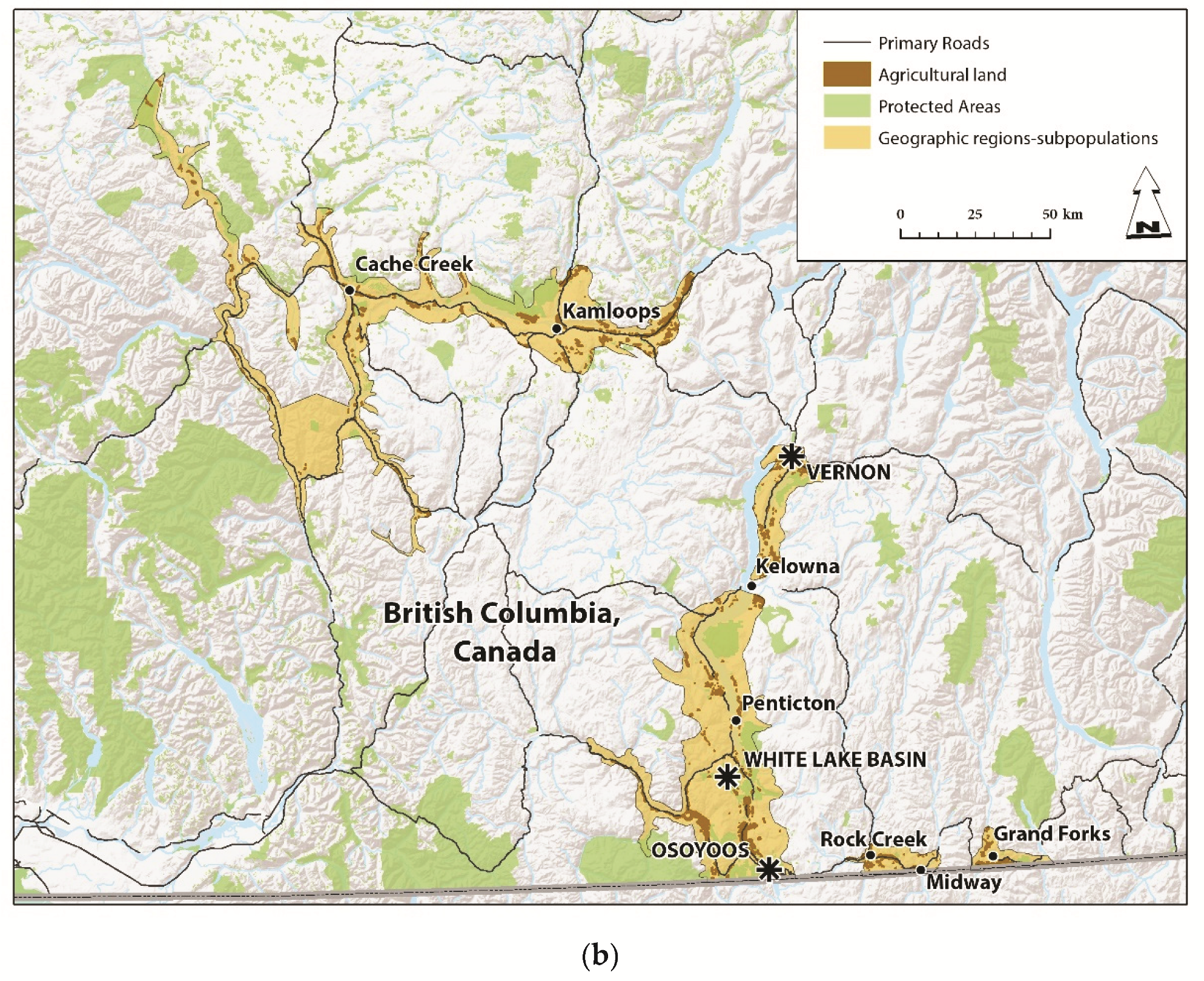
2.1. Western Rattlesnake Distribution in Study Area
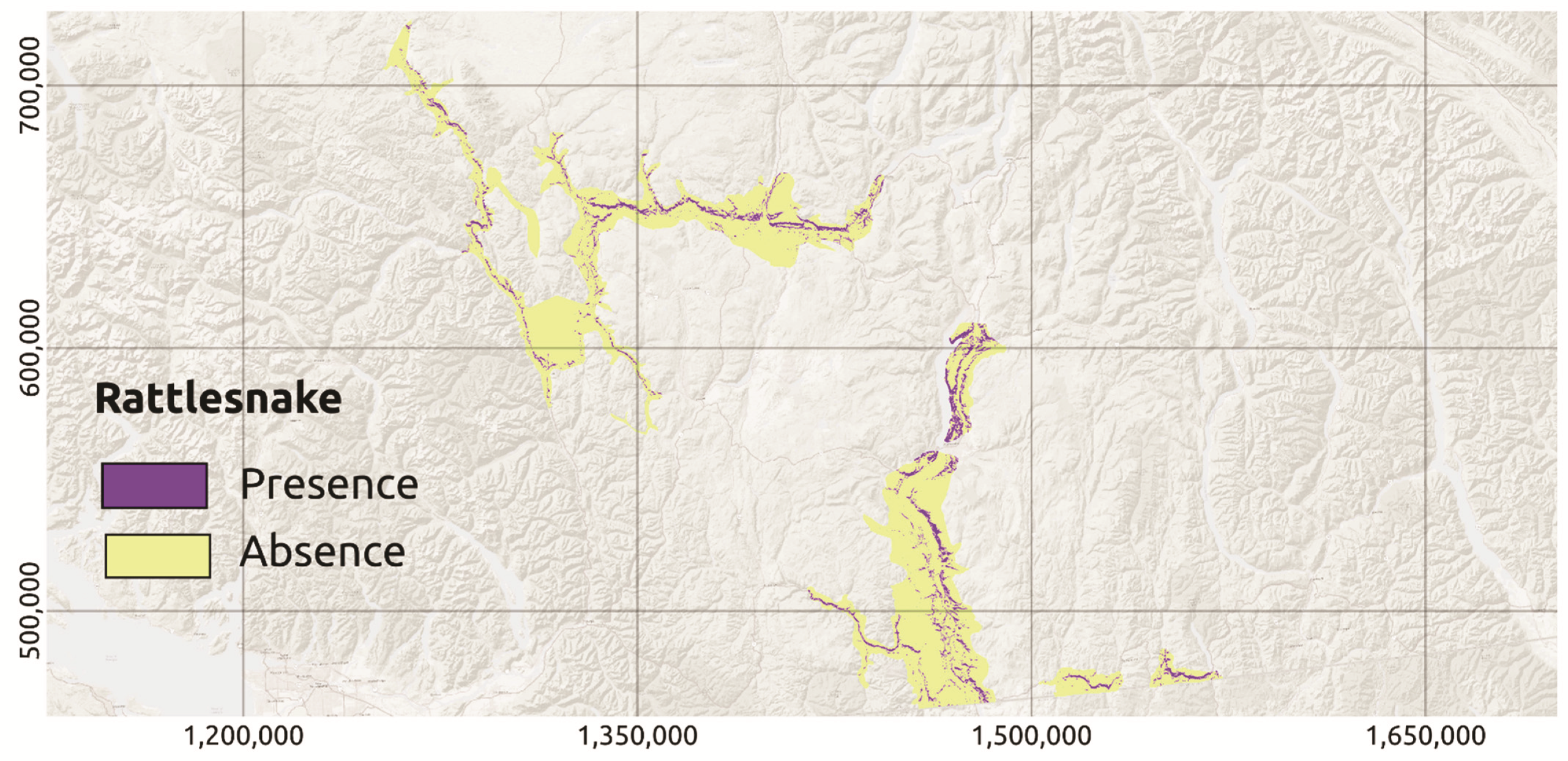
2.2. Species Observations
2.3. Environmental Covariates
2.4. Model Development
2.5. Population Estimate for BC
2.6. Scenarios of Protected Areas, Roads and Agricultural Land
3. Results
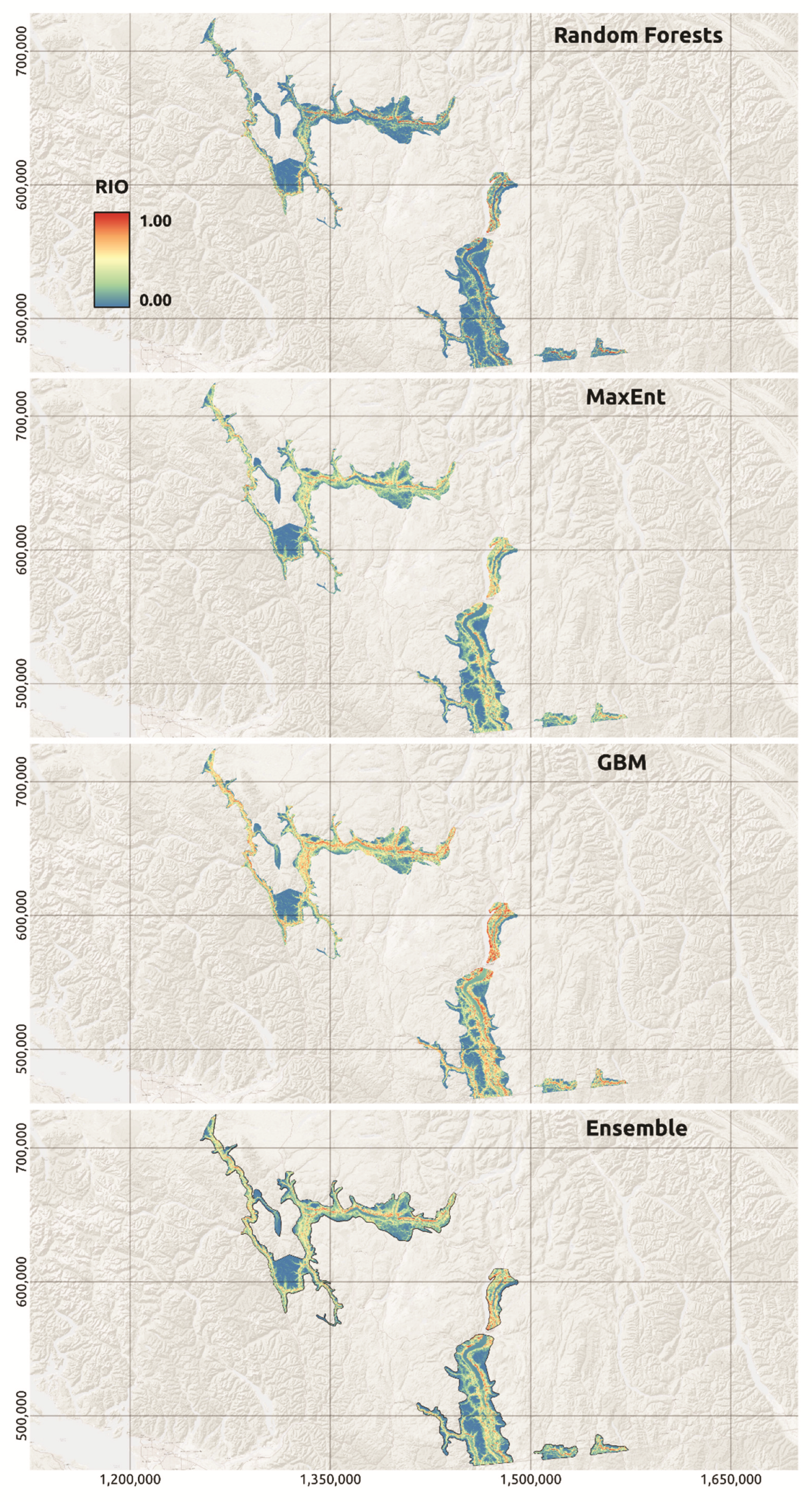
3.1. Relative Index of Occurrence (RIO) Models
3.2. Range-Wide Rattlesnake Population Estimate in Canada and Scenarios
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Butchart, S.H.M.; Walpole, M.; Collen, B.; Van Strien, A.; Scharlemann, J.P.W.; Almond, R.E.A.; Baillie, J.E.M.; Bomhard, B.; Brown, C.; Bruno, J.; et al. Global Biodiversity: Indicators of Recent Declines. Science 2010, 328, 1164–1168. [Google Scholar] [CrossRef] [PubMed]
- Pimm, S.L.; Jenkins, C.N.; Abell, R.; Brooks, T.M.; Gittleman, J.L.; Joppa, L.N.; Raven, P.H.; Roberts, C.M.; Sexton, J.O. The Biodiversity of Species and Their Rates of Extinction, Distribution, and Protection. Science 2014, 344. [Google Scholar] [CrossRef] [PubMed]
- Waldron, A.; Miller, D.C.; Redding, D.; Mooers, A.; Kuhn, T.S.; Nibbelink, N.; Roberts, J.T.; Tobias, J.A.; Gittleman, J.L. Reductions in Global Biodiversity Loss Predicted from Conservation Spending. Nature 2017, 551, 364–367. [Google Scholar] [CrossRef]
- Diaz, S.; Settele, J.; Brondizio, E.; Ngo, H.T.; Gueze, M.; Agard, J.; Arneth, A.; Balvanera, P.; Brauman, K.; Butchart, S.; et al. Summary for Policymakers of the Global Assessment Report on Biodiversity and Ecosystem Services; Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services: Bonn, Germany, 2019. [Google Scholar]
- Gibbons, D.W.; Sandbrook, C.; Sutherland, W.J.; Akter, R.; Bradbury, R.; Broad, S.; Clements, A.; Crick, H.Q.P.; Elliott, J.; Gyeltshen, N.; et al. The Relative Importance of COVID-19 Pandemic Impacts on Biodiversity Conservation Globally. Conserv. Biol. 2021. [Google Scholar] [CrossRef]
- Maxwell, S.L.; Fuller, R.A.; Brooks, T.M.; Watson, J.E.M. Biodiversity: The Ravages of Guns, Nets and Bulldozers. Nature 2016, 536, 143–145. [Google Scholar] [CrossRef]
- Shamoon, H.; Maor, R.; Saltz, D.; Dayan, T. Increased Mammal Nocturnality in Agricultural Landscapes Results in Fragmentation Due to Cascading Effects. Biol. Conserv. 2018, 226, 32–41. [Google Scholar] [CrossRef]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of Climate Change on the Future of Biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef] [Green Version]
- Staudinger, M.D.; Carter, S.L.; Cross, M.S.; Dubois, N.S.; Duffy, J.E.; Enquist, C.; Griffis, R.; Hellmann, J.J.; Lawler, J.J.; O’Leary, J.; et al. Biodiversity in a Changing Climate: A Synthesis of Current and Projected Trends in the US. Front. Ecol. Environ. 2013, 11, 465–473. [Google Scholar] [CrossRef] [Green Version]
- Margules, C.; Pressey, R. Systematic Conservation planning. Nature 2000, 405, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Possingham, H.P.; Wilson, K.A.; Andelman, S.J.; Vynne, C.H. Protected areas: Goals, limitations, and design. In Principles of Conservation Biology; Groom, M.J., Meffe, G.K., Carroll, C.R., Eds.; Sinauer Associates Inc.: Sunderland, MA, USA, 2006; pp. 509–533. [Google Scholar]
- Czech, B.; Krausman, P.R.; Devers, P.K. Economic Associations among Causes of Species Endangerment in the United States. BioScience 2000, 50, 593–601. [Google Scholar] [CrossRef] [Green Version]
- Leverington, F.; Costa, K.L.; Courrau, J.; Pavese, H.; Nolte, C.; Marr, M.; Coad, L.; Burgess, N.; Bomhard, B.; Hockings, M.; et al. Management Effectiveness Evaluation in Protected Areas—A Global Study. Second Edition 2010. Environ. Manag. 2010, 46, 685–698. [Google Scholar] [CrossRef]
- Carwardine, J.; O’Connor, T.; Legge, S.; Mackey, B.; Possingham, H.P.; Martin, T.G. Prioritizing Threat Management for Biodiversity Conservation. Conserv. Lett. 2012, 5, 196–204. [Google Scholar] [CrossRef] [Green Version]
- Gibbons, J.W.; Scott, D.E.; Ryan, T.J.; Buhlmann, K.A.; Tuberville, T.D.; Metts, B.S.; Greene, J.L.; Mills, T.; Leiden, Y.; Poppy, S.; et al. The Global Decline of Reptiles, Déjà Vu Amphibians. BioScience 2000, 50, 653–666. [Google Scholar] [CrossRef] [Green Version]
- Lesbarrères, D.; Ashpole, S.L.; Bishop, C.A.; Blouin-Demers, G.; Brooks, R.J.; Echaubard, P.; Govindarajulu, P.; Green, D.M.; Hecnar, S.J.; Herman, T.; et al. Conservation of Herpetofauna in Northern Landscapes: Threats and Challenges from a Canadian Perspective. Biol. Conserv. 2014, 170, 48–55. [Google Scholar] [CrossRef]
- Gregory, P.T. Biology and conservation of a cold climate snake fauna. In Ecology, Conservation and Status of Reptiles in Canada; Seburn, C.N.L., Bishop, C.A., Eds.; SSAR, Herpetological Conservation 2: Salt Lake City, UT, USA, 2007; pp. 41–56. [Google Scholar]
- Gregory, P.T. Northern Lights and Seasonal Sex: The Reproductive Ecology of Cool-Climate Snakes. Herpetol. 2009, 65, 1–13. [Google Scholar] [CrossRef]
- Bunnell, F.L.; Campbell, R.W.; Squires, K.A. Conservation Priorities for Peripheral Species: The Example of British Columbia. Can. J. For. Res. 2004, 34, 2240–2247. [Google Scholar] [CrossRef]
- Gibson, S.Y.; Van Der Marel, R.C.; Starzomski, B.M. Climate Change and Conservation of Leading-Edge Peripheral Populations. Conserv. Biol. 2009, 23, 1369–1373. [Google Scholar] [CrossRef]
- Thornton, D.H.; Wirsing, A.J.; Lopez-Gonzalez, C.; Squires, J.R.; Fisher, S.; Larsen, K.W.; Peatt, A.; Scrafford, M.A.; Moen, R.A.; Scully, A.E.; et al. Asymmetric Cross-Border Protection of Peripheral Transboundary Species. Conserv. Lett. 2018, 11, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Fraser, D. Species at the Edge: The Case for Listing of “Peripheral” Species. In Proceedings of the Conference on the Biology and Management of Species and Habitats at Risk, Kamloops, BC, Canada, 15–19 February 1999; pp. 49–54. [Google Scholar]
- Schmidt, D.A.; Govindarajulu, P.; Larsen, K.W.; Russello, M.A. Genotyping-in-Thousands by Sequencing Reveals Marked Population Structure in Western Rattlesnakes to Inform Conservation Status. Ecol. Evol. 2020, 10, 7157–7172. [Google Scholar] [CrossRef]
- Parker, W.S.; Plummer, M.V. Population ecology. In Snakes Ecology and Evolutionary Biology; Seigel, R.A., Collins, J.T., Novak, S.S., Eds.; MacMillan Publishing Company: New York, NY, USA, 1987; pp. 253–301. [Google Scholar]
- DeGregorio, B.A.; Putman, B.J.; Kingsbury, B.A. Which Habitat Selection Method Is Most Applicable to Snakes. Herpetol. Conserv. Biol. 2011, 6, 372–382. [Google Scholar]
- IUCN. The IUCN Red List of Threatened Species. Available online: http://www.iucnredlist.org/technical-documents/categories-and-criteria (accessed on 15 April 2021).
- Lind, A.J.; Welsh, H.H.; Tallmon, D.A. Garter Snake Population Dynamics from a 16-Year Study: Considerations for Ecological Monitoring. Ecol. Appl. 2005, 15, 294–303. [Google Scholar] [CrossRef] [Green Version]
- Maida, J.R.; Kirk, D.A.; McKibbin, O.; Row, J.R.; Larsen, K.W.; Stringham, C.; Bishop, C.A. Population Estimate, Survivorship and Generation Time of the Northern Pacific Rattlesnake (Crotalus o. oreganus) at Its Northern-Most Range Limits. Herpetol. Conserv. Biol. 2018, 13, 662–672. [Google Scholar]
- Reading, C.J.; Luiselli, L.M.; Akani, G.C.; Bonnet, X.; Amori, G.; Ballouard, J.M.; Filippi, E.; Naulleau, G.; Pearson, D.; Rugiero, L. Are Snake Populations in Widespread Decline? Biol. Lett. 2010, 6, 777–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durso, A.M.; Willson, J.D.; Winne, C.T. Needles in Haystacks: Estimating Detection Probability and Occupancy of Rare and Cryptic Snakes. Biol. Conserv. 2011, 144, 1508–1515. [Google Scholar] [CrossRef]
- McCluskey, E.; Matthews, S.; Ligocki, I.; Holding, M. The Importance of Historical Land Use in the Maintenance of Early Successional Habitat for an Endangered Rattlesnake. Glob. Ecol. Conserv. 2016, 13, e00370. [Google Scholar] [CrossRef]
- Luiselli, L.; Vignoli, L.; Rugiero, L.; Meek, R. Declining Occupancy Rates in the Hibernacula of Aspic Vipers (Vipera aspis) in Italy and France; Evidence for Climatic Effects? Herpetol. J. 2018, 28, 137–142. [Google Scholar]
- Saha, A.; McRae, L.; Dodd, C.K.; Gadsden, H.; Hare, K.M.; Lukoschek, V.; Böhm, M. Tracking Global Population Trends: Population Time-Series Data and a Living Planet Index for Reptiles. J. Herpetol. 2018, 52, 259–268. [Google Scholar] [CrossRef] [Green Version]
- Shine, R.; Lemaster, M.; Wall, M.; Langkilde, T.; Mason, R. Why Did the Snake Cross the Road? Effects of Roads on Movement and Location of Mates by Garter Snakes (Thamnophis sirtalis parietalis). Ecol. Soc. 2004, 9. [Google Scholar] [CrossRef]
- Harvey, J.A. Thermal Influences on Summer Habitat Use by Western Rattlesnakes (Crotalus oreganus) in British Columbia. Master’s Thesis, Thompson Rivers University, Kamloops, BC, Canada, 2015. [Google Scholar]
- Harvey, J.A.; Larsen, K.W. Rattlesnake Migrations and the Implications of Thermal Landscapes. Mov. Ecol. 2020, 8, 1–13. [Google Scholar] [CrossRef]
- Waldron, J.L.; Welch, S.M.; Bennett, S.H.; Kalinowsky, W.G.; Mousseau, T.A. Life History Constraints Contribute to the Vulnerability of a Declining North American Rattlesnake. Biol. Conserv. 2013, 159, 530–538. [Google Scholar] [CrossRef]
- Gienger, C.M.; Beck, D.D. Northern Pacific Rattlesnakes (Crotalus oreganus) Use Thermal and Structural Cues to Choose Overwintering Hibernacula. Can. J. Zool. 2011, 1090, 1084–1090. [Google Scholar] [CrossRef] [Green Version]
- Lomas, E.; Maida, J.R.; Bishop, C.A.; Larsen, K.W. Movement Ecology of Northern Pacific Rattlesnakes (Crotalus o. oreganus) in Response to Disturbance. Herpetologica 2019, 75, 153–161. [Google Scholar] [CrossRef]
- Gomez, L.; Larsen, K.W.; Gregory, P.T. Contrasting Patterns of Migration and Habitat Use in Neighboring Rattlesnake Populations. J. Herpetol. 2015, 49, 371–376. [Google Scholar] [CrossRef]
- Gardiner, L.E.; Somers, C.M.; Parker, D.L.; Poulin, R.G. Microhabitat Selection by Prairie Rattlesnakes (Crotalus viridis) at the Northern Extreme of Their Geographic Range. J. Herpet. 2015, 49, 131–137. [Google Scholar] [CrossRef]
- Row, J.R.; Blouin-Demers, G.; Weatherhead, P.J. Demographic Effects of Road Mortality in Black Ratsnakes (Elaphe obsoleta). Biol. Conserv. 2007, 137, 117–124. [Google Scholar] [CrossRef]
- Tinkle, D.W. Long-Term Field Studies. BioScience 1979, 29, 717. [Google Scholar] [CrossRef]
- Pechmann, J.H.K.; Wilbur, H.M. Putting Declining Amphibian Populations in Perspective: Natural Fluctuations and Human Impacts. Herpetol. 1994, 50, 65–84. [Google Scholar]
- Hammerson, G.A.; Frost, D.R.; Hollingsworth, B. Crotalus Oreganus. The IUCN Red List of Threatened Species 2007: E.T64326A12769216. Available online: https://www.iucnredlist.org/species/64326/12769216 (accessed on 27 July 2021).
- Committee on the Status of Endangered Wildlife in Canada (COSEWIC). COSEWIC Assessment and Status Report on the Western Rattlesnake Crotalus oreganus in Canada; Committee on the Status of Endangered Wildlife in Canada: Ottawa, ON, Canada, 2015. [Google Scholar]
- Bertram, N.; Larsen, K.W.; Surgenor, J. Identification of Critical Habitats and Conservation Issues for the Western Rattlesnake and Great Basin Gopher Snake within the Thompson-Nicola Region of British Columbia; Final Report for the British Columbia Ministry of Water, Land and Air Protection and the Habitat Trust Conservation Fund of British Columbia: Kamloops, BC, Canada, 2001; 53p. [Google Scholar]
- Gomez, L.M. Habitat Use and Movement Patterns of the Northern Pacific Rattlesnake in British Columbia. Master’s Thesis, Thompson Rivers University, Kamloops, BC, Canada, 2007. [Google Scholar]
- Lomas, E.V. Effects of Disturbance on the Northern Pacific Rattlesnake (Crotalus oreganus oreganus) in British Columbia. Master’s Thesis, Thompson Rivers University, Kamloops, BC, Canada, 2013. [Google Scholar]
- BC Ministry of Forest and Range Biogeoclimatic Ecosystem Classification Program. Available online: https://www.for.gov.bc.ca/hre/becweb/ (accessed on 14 October 2020).
- Atkins, M.C.P. Temporal and Spatial Responses of Western Rattlesnakes (Crotalus oreganus) to Landscape Management Changes. Master’s Thesis, Thompson Rivers University, Kamloops, BC, Canada, 2021. [Google Scholar]
- The Grassland Conservation Council of British Columbia. Mitigating the Fragmentation and Development of BC’s Grasslands Problem Analysis and Strategic Plan; Grasslands Conservation Council of British Columbia: Kamloops, BC, Canada, 2005; 103p. [Google Scholar]
- Tôrres, N.M.; De Marco, P.; Santos, T.; Silveira, L.; de Almeida Jácomo, A.T.; Diniz-Filho, J.A.F. Can Species Distribution Modelling Provide Estimates of Population Densities? A Case Study with Jaguars in the Neotropics. Divers. Distrib. 2012, 18, 615–627. [Google Scholar] [CrossRef]
- Rodríguez, J.P.; Brotons, L.; Bustamante, J.; Seoane, J. The Application of Predictive Modelling of Species Distribution to Biodiversity Conservation. Divers. Distrib. 2007, 13, 243–251. [Google Scholar] [CrossRef]
- Feng, X.; Park, D.S.; Walker, C.; Peterson, A.T.; Merow, C.; Papeş, M. A Checklist for Maximizing Reproducibility of Ecological Niche Models. Nat. Ecol. Evol. 2019, 3, 1382–1395. [Google Scholar] [CrossRef] [Green Version]
- Spear, S.; Cosentino, B.; Hall, B.; Kavanagh, D.; McRae, B.; Shirk, A. Appendix A. 9. Habitat Connectivity for Western Rattlesnake (Crotalus oreganus) in the Columbia Plateau Ecoregion. In Washington Connected Landscapes Project: Analysis of the Columbia Plateau Ecoregion; Washington’s Department of Fish and Wildlife, and Department of Transportation: Olympia, WA, USA, 2010; pp. 1–27. [Google Scholar]
- Spear, S.F.; Parker, J.M.; Peterson, C.R.; Jenkins, C.L.; Waits, L.P. Modeling the Effect of Roads on Habitat Area and Connectivity of Midget Faded Rattlesnakes (Crotalus oreganus concolor) across Southwest Wyoming. In The Biology of Rattlesnakes II; Dreslik, M.J., Hayes, W.K., Beaupre, S.J., Mackessy, S.P., Eds.; ECO Herpetological Publishing and Distribution: Rodeo, NM, USA, 2017; pp. 22–33. ISBN 9781938850547. [Google Scholar]
- Thomasson, V. Habitat Suitability Modeling for the Eastern Hog-Nosed Snake, Heterodon platirinos, in Ontario. Master’s Thesis, University of Ottawa, Ottawa, ON, Canada, 2012. [Google Scholar]
- Bisrat, S.A.; White, M.A.; Beard, K.H.; Richard Cutler, D. Predicting the Distribution Potential of an Invasive Frog Using Remotely Sensed Data in Hawaii. Divers. Distrib. 2012, 18, 648–660. [Google Scholar] [CrossRef]
- Préau, C.; Trochet, A.; Bertrand, R.; Isselin-Nondedeu, F. Modeling Potential Distributions of Three European Amphibian Species Comparing ENFA and MaxEnt. Herpetol. Conserv. Biol. 2018, 13, 91–104. [Google Scholar]
- Sarquis, J.A.; Cristaldi, M.A.; Arzamendia, V.; Bellini, G.; Giraudo, A.R. Species Distribution Models and Empirical Test: Comparing Predictions with Well-Understood Geographical Distribution of Bothrops alternatus in Argentina. Ecol. Evol. 2018, 8, 10497–10509. [Google Scholar] [CrossRef] [Green Version]
- Yen, P.P.W.; Huettmann, F.; Cooke, F. A Large-Scale Model for the at-Sea Distribution and Abundance of Marbled Murrelets (Brachyramphus marmoratus) during the Breeding Season in Coastal British Columbia, Canada. Ecol. Modell. 2004, 171, 395–413. [Google Scholar] [CrossRef]
- Winton, S.A. Impacts of Road Mortality on the Western Rattlesnake (Crotalus oreganus) in British Columbia. Master’s Thesis, Thompson Rivers University, Kamloops, BC, Canada, 2018. [Google Scholar]
- Winton, S.A.; Taylor, R.; Bishop, C.A.; Larsen, K.W. Estimating Actual versus Detected Road Mortality Rates for a Northern Viper. Glob. Ecol. Conserv. 2018, 16, e00476. [Google Scholar] [CrossRef]
- Bishop, C.A.; Williams, K.E.; Kirk, D.A.; Nantel, P.; Reed, E.; Elliott, J.E. A Population Model of the Impact of a Rodenticide Containing Strychnine on Great Basin Gophersnakes (Pituophis catenifer deserticola). Ecotoxicology 2016, 25, 1390–1405. [Google Scholar] [CrossRef]
- British Columbia Government. British Columbia Conservation Data Centre. Available online: https://www2.gov.bc.ca/gov/content/environment/plants-animals-ecosystems/conservation-data-centre (accessed on 24 May 2019).
- Government of British Columbia Wildlife Data and Information. Available online: https://www.canada.ca/en/environment-climate-change/services/species-risk-public-registry.html (accessed on 24 May 2019).
- Diller, L.V.; Wallace, R.L. Comparative Ecology of Two Snake Species (Crotalus viridis and Pituophis melanoleucus) in Southwestern Idaho. Herpetologic 1996, 52, 343–360. [Google Scholar]
- Diller, L.V.; Wallace, R.L. Growth, Reproduction, and Survival in a Population of Crotalus viridis oreganus in North Central Idaho. Herpetol. Monogr. 2002, 16, 26–45. [Google Scholar] [CrossRef]
- Wallace, R.L.; Diller, L.V. Variation in Emergence, Egress, and Ingress among Life-History Stages and Sexes of Crotalus viridis oreganus in Northern Idaho. J. Herpetol. 2001, 35, 583–589. [Google Scholar] [CrossRef]
- Putman, B.J.; Lind, C.; Taylor, E.N. Does Size Matter? Factors Influencing the Spatial Ecology of Northern Pacific Rattlesnakes (Crotalus oreganus oreganus) in Central California. Copeia 2013, 485–492. [Google Scholar] [CrossRef]
- Hecker, L.J.; Bean, W.T.; Marks, S.B. Compensatory Microhabitat Selection by Northern Pacific Rattlesnakes (Crotalus oreganus oreganus) in a Cool and Wet Macroclimate. J. Herpetol. 2020, 54, 39–49. [Google Scholar] [CrossRef]
- Maida, J.R.; Bishop, C.A.; Larsen, K.W.; Maida, J.R.; Bishop, C.A.; Larsen, K.W. Migration and Disturbance: Impact of Fencing and Development on Rattlesnake Spring Movements in British Columbia. Can. J. Zool. 2020, 98, 1–12. [Google Scholar] [CrossRef]
- Brown, J.R.; Bishop, C.A.; Brooks, R.J. Effectiveness of Short-Distance Translocation and Its Effects on Western Rattlesnakes. J. Wildl. Manag. 2009, 73, 419–425. [Google Scholar] [CrossRef]
- Lomas, E.; Larsen, K.W.; Bishop, C.A. Persistence of Northern Pacific Rattlesnakes Masks the Impact of Human Disturbance on Weight and Body Condition. Anim. Conserv. 2015, 18, 548–556. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing: Version 3.0.1; R Development Core Team: Vienna, Austria, 2016. [Google Scholar]
- Aiello-Lammens, A.M.E.; Vilela, B.; Anderson, R.P.; Bjornson, R.; Weston, S.; Aiello-lammens, M.M.E.; America, S. Functions for Spatial Thinning of Species Occurrence Records for Use in Ecological Models. Package ‘SpThin’. Version 0.2.0. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Thomasson, V.; Blouin-Demers, G. Using Habitat Suitability Models Considering Biotic Interactions to Inform Critical Habitat Delineation: An Example with the Eastern Hog-Nosed Snake (Heterodon platirhinos) in Ontario, Canada. Can. Wildl. Biol. Manag. 2015, 4, 1–17. [Google Scholar]
- Wills, C.A.; Beaupre, S.J. An Application of Randomization for Detecting Evidence of Thermoregulation in Timber Rattlesnakes (Crotalus horridus) from Northwest Arkansas. Physiol. Biochem. Zool. 2000, 73, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Putman, B.J.; Clark, R.W. Behavioral Thermal Tolerances of Free-Ranging Rattlesnakes (Crotalus oreganus) during the Summer Foraging Season. J. Therm. Biol. 2017, 65, 8–15. [Google Scholar] [CrossRef]
- Karger, D.N.; Conrad, O.; Böhner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Zimmermann, N.E.; Linder, H.P.; Kessler, M. Data from: Climatologies at High Resolution for the Earth’s Land Surface Areas; Dryad: Berlin-Brandenburg, Germany, 2017. [Google Scholar]
- Karger, D.N.; Conrad, O.; Böhner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Zimmermann, N.E.; Linder, H.P.; Kessler, M. Climatologies at High Resolution for the Earth’s Land Surface Areas. Sci. Data. 2017, 4, 170122. [Google Scholar] [CrossRef] [Green Version]
- Conrad, O.; Bechtel, B.; Bock, M.; Dietrich, H.; Fischer, E.; Gerlitz, L.; Wehberg, J.; Wichmann, V.; Böhner, J. System for Automated Geoscientific Analyses (SAGA) v 2.1.4. Geosci. Model Dev. 2015, 8, 1991–2007. [Google Scholar] [CrossRef] [Green Version]
- Bauder, J.M.; Akenson, H.; Peterson, C.R. Movement Patterns of Prairie Rattlesnakes (Crotalus v. viridis) across a Mountainous Landscape in a Designated Wilderness Area. J. Herpetol. 2015, 49, 377–387. [Google Scholar] [CrossRef]
- Bauder, J.M.; Breininger, D.R.; Bolt, M.R.; Legare, M.L.; Jenkins, C.L.; Rothermel, B.B.; McGarigal, K. Multi-Level, Multi-Scale Habitat Selection by a Wide-Ranging, Federally Threatened Snake. Landsc. Ecol. 2018, 33, 743–763. [Google Scholar] [CrossRef]
- Ministry of Forests, Lands, Natural Resource Operations and Rural Development (Government of British Columbia). Digital Elevation Model. Available online: https://www2.gov.bc.ca/gov/content/data/geographic-data-services/topographic-data/elevation/digital-elevation-model (accessed on 2 August 2021).
- Macartney, J.M. The Ecology of the Northern Pacific Rattlesnake, Crotalus Viridis Oreganus, in British Columbia. Master’s Thesis, University of Victoria, Victoria, BC, Canada, 1985. [Google Scholar]
- Commission for Environmental Cooperation (CEC). North American Environmental Atlas. Available online: http://www.cec.org/files/atlas/?z=3&x=93.1641&y=61.9803&lang=en&layers=polbounds%2Clandcover2015ls&opacities=100%2C100&labels=false (accessed on 20 September 2020).
- Latifovic, R.; Pouliot, D.; Olthof, I. Circa 2010 Land Cover of Canada: Local Optimization Methodology and Product Development. Remote Sens. 2017, 9, 1098. [Google Scholar] [CrossRef] [Green Version]
- Clark, R.W.; Brown, W.S.; Stechert, R.; Zamudio, K.R. Roads, Interrupted Dispersal, and Genetic Diversity in Timber Rattlesnakes. Conserv. Biol. 2010, 24, 1059–1069. [Google Scholar] [CrossRef]
- Paterson, J.E.; Baxter-Gilbert, J.; Beaudry, F.; Carstairs, S.; Chow-Fraser, P.; Edge, C.B.; Lentini, A.M.; Litzgus, J.D.; Markle, C.E.; McKeown, K.; et al. Road Avoidance and Its Energetic Consequences for Reptiles. Ecol. Evol. 2019, 9, 9794–9803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews, K.M.; Gibbons, J.W. How Do Highways Influence Snake Movement? Behavioral Responses to Roads and Vehicles. Copeia 2005, 2005, 772–782. [Google Scholar] [CrossRef] [Green Version]
- GeoBC. GeoBC Atlas Integrated Transportation Network (Dgtl_Road_Atlas.Gdb); GeoBC: Victoria, BC, Canada, 2018.
- Wiersma, Y.F.; Huettmann, F.; Drew, C.A. Introduction. Landscape Modeling of Species and Their Habitats: History, Uncertainty, and Complexity. In Predictive Species and Habitat Modeling in Landscape Ecology: Concepts and Applications; Drew, C.A., Wiersma, Y.F., Huettmann, F., Eds.; Springer: New York, NY, USA, 2011; pp. 1–313. ISBN 9781441973894. [Google Scholar]
- Fielding, A.H.; Bell, J.F. A Review of Methods for the Assessment of Prediction Errors in Conservation Presence/Absence Models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Cutler, A.; Cutler, D.R.; Stevens, J.R. Random Forests. In Ensemble Machine Learning: Methods and Applications; Zhang, C., Ma, Y., Eds.; Springer Science+Business Media, LLC: Berlin, Germany, 2012; pp. 157–175. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of Species Distribution with Maxent: New Extensions and a Comprehensive Evaluation. Ecograpy 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R.; Hastie, T. A Working Guide to Boosted Regression Trees. J. Anim. Ecol. 2008, 77, 802–813. [Google Scholar] [CrossRef]
- Araújo, M.B.; New, M. Ensemble Forecasting of Species Distributions. Trends Ecol. Evol. 2007, 22, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Thuiller, W.; Georges, D.; Engler, R.; Breiner, F. Ensemble Platform for Species Distribution Modeling. Package ‘biomod2’. Version 3.4.6. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Hao, T.; Elith, J.; Guillera-Arroita, G.; Lahoz-Monfort, J.J. A Review of Evidence about Use and Performance of Species Distribution Modelling Ensembles like BIOMOD. Divers. Distrib. 2019, 25, 839–852. [Google Scholar] [CrossRef]
- Zurell, D.; Franklin, J.; König, C.; Bouchet, P.J.; Dormann, C.F.; Elith, J.; Fandos, G.; Feng, X.; Guillera-Arroita, G.; Guisan, A.; et al. A Standard Protocol for Reporting Species Distribution Models. Ecography. 2020, 43, 1261–1277. [Google Scholar] [CrossRef]
- Muscarella, A.R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P.; Muscarella, M.R. Automated Runs and Evaluations of Ecological Niche Models, Package ‘ENMeval’. Version 0.3.1. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Phillips, S.J.; Dudík, M.; Elith, J.; Graham, C.H.; Lehmann, A.; Leathwick, J.; Ferrier, S. Sample Selection Bias and Presence-Only Distribution Models: Implications for Background and Pseudo-Absence Data. Ecol. Appl. 2009, 19, 181–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syfert, M.M.; Smith, M.J.; Coomes, D.A. The Effects of Sampling Bias and Model Complexity on the Predictive Performance of MaxEnt Species Distribution Models. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [Green Version]
- Ernst, C.H.; Ernst, E.M. Venomous Reptiles of the United States, Canada, and Northern Mexico, Vol. 2, Crotalus; Ernst, C.H., Ernst, E.M., Eds.; John Hopkins University Press: Baltimore, NJ, USA, 2011; 424p. [Google Scholar]
- Breiman, L. Statistical Modelling: The Two Cultures. Stat. Sci. 2001, 16, 199–215. [Google Scholar] [CrossRef]
- Kandel, K.; Huettmann, F.; Suwal, M.K.; Ram Regmi, G.; Nijman, V.; Nekaris, K.A.I.; Lama, S.T.; Thapa, A.; Sharma, H.P.; Subedi, T.R. Rapid Multi-Nation Distribution Assessment of a Charismatic Conservation Species Using Open Access Ensemble Model GIS Predictions: Red Panda (Ailurus fulgens) in the Hindu-Kush Himalaya Region. Biol. Conserv. 2015, 181, 150–161. [Google Scholar] [CrossRef]
- Leclere, J.; Oberdorff, T.; Belliard, J.; Leprieur, F. A Comparison of Modeling Techniques to Predict Juvenile 0+ Fish Species Occurrences in a Large River System. Ecol. Inform. 2011, 6, 276–285. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. A Coefficient of Agreement for Nominal Scales. Educ. Psychol. Meas. 1960, 20, 37–46. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the Accuracy of Species Distribution Models: Prevalence, Kappa and the True Skill Statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Lu, N.; Jia, C.X.; Lloyd, H.; Sun, Y.H. Species-Specific Habitat Fragmentation Assessment, Considering the Ecological Niche Requirements and Dispersal Capability. Biol. Conserv. 2012, 152, 102–109. [Google Scholar] [CrossRef]
- Lütolf, M.; Kienast, F.; Guisan, A. The Ghost of Past Species Occurrence: Improving Species Distribution Models for Presence-Only Data. J. Appl. Ecol. 2006, 43, 812–815. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A. A Practical Guide to MaxEnt for Modeling Species’ Distributions: What It Does, and Why Inputs and Settings Matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Hirzel, A.H.; le Lay, G.; Helfer, V.; Randin, C.; Guisan, A. Evaluating the Ability of Habitat Suitability Models to Predict Species Presences. Ecol. Model. 2006, 199, 142–152. [Google Scholar] [CrossRef]
- Huettmann, F.; Gottschalk, T. Predictive Species and Habitat Modeling in Landscape Ecology: Concepts and Applications. In Predictive Modeling in Landscape Ecology; Springer: New York, NY, USA, 2011; pp. 189–208. [Google Scholar]
- Dormann, C.F.; Calabrese, J.M.; Guillera-Arroita, G.; Matechou, E.; Bahn, V.; Bartoń, K.; Beale, C.M.; Ciuti, S.; Elith, J.; Gerstner, K.; et al. Model Averaging in Ecology: A Review of Bayesian, Information-Theoretic, and Tactical Approaches for Predictive Inference. Ecol. Monogr. 2018, 88, 485–504. [Google Scholar] [CrossRef] [Green Version]
- Winton, S.; Skurikhina, A.; Larsen, K. White Lake Snake Road Mortality: 2015–2018 Synthesis Report; Prepared for Environment and Climate Change Canada, Wildlife Research Division, Science and Technology Branch; Minister of the Environment and Climate Change: Gatineau, QC, Canada, 2019. [Google Scholar]
- Liu, C.; Newell, G.; White, M. On the Selection of Thresholds for Predicting Species Occurrence with Presence-Only Data. Ecol. Evol. 2016, 6, 337–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hobbs, J. Den Survey and Population Estimate of the Northern Pacific Rattlesnake in BC; Unpublished Report; Government of British Columbia: Victoria, BC, Canada, 2013. [Google Scholar]
- Environment and Climate Change Canada. Canadian Protected and Conserved Areas Database. Available online: https://www.canada.ca/en/environment-climate-change/services/national-wildlife-areas/protected-conserved-areas-database.html (accessed on 4 October 2020).
- Rudolph, D.C.; Burgdorf, S.J.; Conner, R.N.; Schaefer, R.R. Preliminary Evaluation of the Impact of Roads and Associated Vehicular Traffic on Snake Populations in Eastern Texas. In Proceedings of the Third International Conference on Wildlife Ecology and Transportation; Fl-ER-73-99; Evink, G.L., Garrett, P., Zeigler, D., Eds.; Florida Department of Transportation: Tallahassee, FL, USA, 1999; pp. 129–136. [Google Scholar]
- Fortney, A.N.; Poulin, R.G.; Martino, J.A.; Parker, D.L.; Somers, C.M. Proximity to Hibernacula and Road Type Influence Potential Road Mortality of Snakes in Southwestern Saskatchewan. Can. Field-Nat. 2012, 126, 194–203. [Google Scholar] [CrossRef]
- Meredith, M.; Kruschke, J. Highest (Posterior) Density Intervals. Package ‘HDInterval’. Version 0.2.2 R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Yousefi, M.; Ahmadi, M.; Nourani, E.; Behrooz, R.; Rajabizadeh, M.; Geniez, P.; Kaboli, M. Upward Altitudinal Shifts in Habitat Suitability of Mountain Vipers since the Last Glacial Maximum. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [Green Version]
- Winton, S.A.; Bishop, C.A.; Larsen, K.W. When Protected Areas Are Not Enough: Low-Traffic Roads Projected to Cause a Decline in a Northern Viper Population. Endanger. Species Res. 2020, 41, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Renner, I.W.; Warton, D.I. Equivalence of MAXENT and Poisson Point Process Models for Species Distribution Modeling in Ecology. Biometrics 2013, 69, 274–281. [Google Scholar] [CrossRef]
- Heinrichs, J.A.; Lawler, J.J.; Schumaker, N.H. Intrinsic and Extrinsic Drivers of Source-Sink Dynamics. Ecol. Evol. 2016, 6, 892–904. [Google Scholar] [CrossRef] [Green Version]
- Environment and Climate Change Canada. Recovery Strategy for the Western Rattlesnake (Crotalus oreganus), the Great Basin Gophersnake (Pituophis Catenifer Deserticola) and the Desert Nightsnake (Hypsiglena chlorophaea) in Canada (Proposed); Species at Risk Act Recovery Strategy Series; Environment and Climate Change Canada: Ottawa, ON, Canada, 2017; Part 1, 28p, Part 2, A. 37p. B. 36p. C. 28p. Available online: https://www.registrelep-sararegistry.gc.ca/virtual_sara/files/plans/rs_south_interior_snakes_e_proposed.pdf (accessed on 4 September 2021).
- Reed, E.T. Population Model for Female Great Basin Gophersnake; Unpublished Report to the Committee on the Status of Endangered Wildlife in Canada: Ottawa, ON, Canada, 2013. [Google Scholar]
- Williams, K.E.; Hodges, K.E.; Bishop, C.A. Small Reserves around Hibernation Sites May Not Adequately Protect Mobile Snakes: The Example of Great Basin Gophersnakes (Pituophis catenifer deserticola) in British Columbia. Can. J. Zool. 2012, 90, 304–312. [Google Scholar] [CrossRef]
- Deguise, I.E.; Kerr, J.T. Protected Areas and Prospects for Endangered Species Conservation in Canada. Conserv. Biol. 2006, 20, 48–55. [Google Scholar] [CrossRef]
- Maxwell, S.L.; Cazalis, V.; Dudley, N.; Hoffmann, M.; Ana, S.L.; Stolton, S.; Visconti, P.; Woodley, S.; Maron, M.; Strassburg, B.B.N.; et al. Area-Based Conservation in the Twenty-first Century. Nature 2020, 586, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Austin, M.A.; Buffett, D.A.; Nicolson, D.J.; Scudder, G.G.E.; Stevens, V. Taking Nature’s Pulse: The Status of Biodiversity in British Columbia. 2008, pp. 1–268. Available online: www.biodiversitybc.org (accessed on 4 September 2021).
- Lea, T. Historical (Pre-Settlement) Ecosystems of the Okanagan Valley and Lower Similkameen Valley of British Columbia—Pre-European Contact to the Present. Davidsonia 2008, 19, 3–36. [Google Scholar]
- Zeng, Q.; Zhang, Y.; Sun, G.; Duo, H.; Wen, L.; Lei, G. Using Species Distribution Model to Estimate the Wintering Population Size of the Endangered Scaly-Sided Merganser in China. PLoS ONE 2015, 10, 1–12. [Google Scholar] [CrossRef]
- Mi, C.; Huettmann, F.; Sun, R.; Guo, Y. Combining Occurrence and Abundance Distribution Models for the Conservation of the Great Bustard. PeerJ 2017, 2017, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Cushman, S.A.; Huettmann, F. Spatial Complexity, Informatics, and Wildlife Conservation; Springer: New York, NY, USA, 2010; ISBN 9784431877714. [Google Scholar]
- Drew, C.A.; Wiersma, Y.F.; Huettmann, F. Predictive Species and Habitat Modeling in Landscape Ecology: Concepts and Applications; Springer: New York, NY, USA, 2011. [Google Scholar]
- Schuster, R.; Hanson, J.O.; Strimas-Mackey, M.; Bennett, J.R. Exact integer linear programming solvers outperform simulated annealing for solving conservation planning problems. PeerJ 2020, 8, e9258. [Google Scholar] [CrossRef]
- Gonzales, E.K.; Arcese, P.; Schulz, R.; Bunnell, F.L. Strategic Reserve Design in the Central Coast of British Columbia: Integrating Ecological and Industrial Goals. Can. J. For. Res. 2003, 33, 2129–2140. [Google Scholar] [CrossRef]
- Lyet, A.; Thuiller, W.; Cheylan, M.; Besnard, A. Fine-Scale Regional Distribution Modelling of Rare and Threatened Species: Bridging GIS Tools and Conservation in Practice. Divers. Distrib. 2013, 19, 651–663. [Google Scholar] [CrossRef]
- Baltensperger, A.P.; Huettmann, F. Predictive Spatial Niche and Biodiversity Hotspot Models for Small Mammal Communities in Alaska: Applying Machine-Learning to Conservation Planning. Landsc. Ecol. 2015, 30, 681–697. [Google Scholar] [CrossRef]
- Cattarino, L.; Hermoso, V.; Carwardine, J.; Kennard, M.J.; Linke, S. Multi-Action Planning for Threat Management: A Novel Approach for the Spatial Prioritization of Conservation Actions. PLoS ONE 2015, 10, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Firn, J.; Maggini, R.; Chadès, I.; Nicol, S.; Walters, B.; Reeson, A.; Martin, T.G.; Possingham, H.P.; Pichancourt, J.B.; Ponce-Reyes, R.; et al. Priority Threat Management of Invasive Animals to Protect Biodiversity under Climate Change. Glob. Chang. Biol. 2015, 21, 3917–3930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Firn, J.; Martin, T.G.; Chadès, I.; Walters, B.; Hayes, J.; Nicol, S.; Carwardine, J. Priority Threat Management of Non-Native Plants to Maintain Ecosystem Integrity across Heterogeneous Landscapes. J. Appl. Ecol. 2015, 52, 1135–1144. [Google Scholar] [CrossRef] [Green Version]
- Shackelford, N.; Standish, R.J.; Ripple, W.; Starzomski, B.M. Threats to Biodiversity from Cumulative Human Impacts in One of North America’s Last Wildlife Frontiers. Conserv. Biol. 2018, 32, 672–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Study Area | Year | Area (km2) | Population Size (CMR) 1 | Density (snakes/km2) | SE 2 | |||
|---|---|---|---|---|---|---|---|---|
| A | A + J | A | A + J | A | A + J | |||
| Osoyoos | 2002–2012 | 4.50 | 262 | N/A | 58.2 | N/A | ||
| White Lake Basin | 2015–2018 | 9.07 | 313 | 545 | 34.5 | 60.1 | 4.6 | 8.6 |
| Vernon—provincial park and ranch | 1983 | 11.4 | 964 | 1777 | 84.6 | 155.8 | 17.2 | 44.3 |
| Vernon—provincial park and ranch | 2020 | 11.4 | 751 | 1066 | 65.9 | 93.5 | 43.8 | 66.6 |
| Kalamalka Provincial Park | 1983 | 7.6 | 445 | 938 | 58.6 | 123.4 | 15.2 | 37.2 |
| Kalamalka Provincial Park | 2020 | 7.6 | 320 | 470 | 42.1 | 61.9 | 34.2 | 47.7 |
| Vernon ranch | 1983 | 3.9 | 506 | 856 | 129.9 | 219.6 | 13.2 | 26.2 |
| Vernon ranch | 2020 | 3.9 | 391 | 592 | 100.4 | 151.7 | 19.9 | 31.7 |
| Random Forests | MaxEnt | GBMs 1 | Ensemble | |
|---|---|---|---|---|
| White Lake Basin | 0.24 ± 0.25 | 0.33 ± 0.22 | 0.43 ± 0.25 | 0.34 ± 0.23 |
| Osoyoos | 0.65 ± 0.26 | 0.48 ± 0.14 | 0.64 ± 0.13 | 0.60 ± 0.15 |
| Vernon—provincial park | 0.44 ± 0.32 | 0.52 ± 0.21 | 0.64 ± 0.19 | 0.55 ± 0.22 |
| Vernon—ranch | 0.32 ± 0.29 | 0.46 ± 0.17 | 0.54 ± 0.22 | 0.45 ± 0.22 |
| Entire study area | 0.26 ± 0.23 | 0.25 ± 0.22 | 0.33 ± 0.26 | 0.18 ± 0.24 |
| Variable | Random Forests | MaxEnt | GBMs 1 | Ensemble |
|---|---|---|---|---|
| Precipitation | 0.19 | 0.11 | 0.10 | 0.08 |
| Annual insolation | 0.34 | 0.15 | 0.21 | 0.20 |
| Elevation | 0.52 | 0.51 | 0.48 | 0.50 |
| Land cover classification | 0.24 | 0.14 | 0.21 | 0.19 |
| Ruggedness | 0.23 | 0.12 | 0.19 | 0.15 |
| Road density | 0.17 | 0.09 | 0.09 | 0.07 |
| Threshold (TSS) 1 | # of Suitable Pixels 2 | Estimate 3 | Standard Deviation 4 |
|---|---|---|---|
| 0.446 | 1766551 | 84,053 | 26,019 |
| 0.5 | 1398076 | 66,521 | 20,592 |
| 0.6 | 845964 | 40,251 | 12,460 |
| 0.7 | 452608 | 21,535 | 6666 |
| 0.75 | 313177 | 14,901 | 4613 |
| 0.8 | 204319 | 9722 | 3009 |
| 0.99 | 12 | <1 | 0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirk, D.A.; Karimi, S.; Maida, J.R.; Harvey, J.A.; Larsen, K.W.; Bishop, C.A. Using Ecological Niche Models for Population and Range Estimates of a Threatened Snake Species (Crotalus oreganus) in Canada. Diversity 2021, 13, 467. https://doi.org/10.3390/d13100467
Kirk DA, Karimi S, Maida JR, Harvey JA, Larsen KW, Bishop CA. Using Ecological Niche Models for Population and Range Estimates of a Threatened Snake Species (Crotalus oreganus) in Canada. Diversity. 2021; 13(10):467. https://doi.org/10.3390/d13100467
Chicago/Turabian StyleKirk, David Anthony, Sahebeh Karimi, Jared R. Maida, Jessica A. Harvey, Karl W. Larsen, and Christine A. Bishop. 2021. "Using Ecological Niche Models for Population and Range Estimates of a Threatened Snake Species (Crotalus oreganus) in Canada" Diversity 13, no. 10: 467. https://doi.org/10.3390/d13100467
APA StyleKirk, D. A., Karimi, S., Maida, J. R., Harvey, J. A., Larsen, K. W., & Bishop, C. A. (2021). Using Ecological Niche Models for Population and Range Estimates of a Threatened Snake Species (Crotalus oreganus) in Canada. Diversity, 13(10), 467. https://doi.org/10.3390/d13100467






