Abstract
Algae–bacteria interactions play an important role in water ecosystems. In this work, the BS2-15 algicidal strain was isolated from the bottom sediments of Lake Baikal and identified as Bacillus mycoides on the basis of 16S rDNA sequencing, its described ultrastructure, and biochemical properties. B. mycoides BS2-15 was demonstrated to have a strong algicidal effect against a freshwater diatom culture of Ulnaria acus, inhibiting its growth and increasing frustules fragility. By analyzing the impact of bacterial filtrate onto the cells of U. acus, we demonstrated that perhaps an algicidal compound is produced by bacteria independently in the presence of diatoms in a medium. Using methods of TUNEL and confocal microscopy, we revealed that the bacterial algicidal effect on the diatom cells results in DNA fragmentation, nucleus destruction, and neutral lipid accumulation. This phenomenon highlights the complexity of algae–bacteria interactions and their potential role in regulating water ecosystem microbial populations.
1. Introduction
Phytoplankton is essential for global carbon cycles [1], and diatom algae in particular have an organic carbon output comparable to that of land plants [2,3]. Primary producers in water ecosystems have complex relationships with bacteria [4]. Along with the search for algicidal bacteria useful in preventing toxic algal blooms [5], the mechanisms of algae-bacteria interactions have fundamental importance [6]; many bacterial strains have an algicidal effect on diatoms by releasing enzymes into the environment [7,8,9].
Bacillus spp. are Gram-positive bacteria widely distributed in the environment; this is likely because they form endospores resistant to different factors, such as drying out, UV radiation, and lack of nutrients [10,11]. Bacillus mycoides is characterized by its rhizoidal growth in agar medium [12]. In addition, B. mycoides, like many representatives of the genus Bacillus, are saprophytic organisms [13,14].
The nature of algicidal compounds produced by bacteria into the medium can be very diverse. There are many known algicidal secondary metabolites, which can be produced by bacteria of the genus Bacillus into the environment [15,16,17,18]. Moreover, it was demonstrated that B. mycoides negatively affect pathogenic fungi, as it emits toxic volatile compounds ammonia and dimethyl sulfide [19]. However, cytological manifestations of the algicidal effect have not been described. In this work, we studied the algicidal effect of B. mycoides BS2-15, a strain isolated from sediments of Lake Baikal.
The diatom Ulnaria acus (=Synedra acus) is a member of the dominant assemblage of recent Baikal phytoplankton, but its remains in the upper layer of bottom sediments and cannot be found in all regions of the lake because it is subject to considerable degradation, both in the water column and in the surface sediment layer [20]. The axenic monoclonal culture U. acus BK280 was applied as a model for multiple experimental studies [20,21,22,23,24,25,26].
Sediments of Lake Baikal were earlier found to contain bacteria involved in the destruction of diatom cells [9]. The bacteria B. mycoides BS2-15 and diatom U. acus were isolated from Lake Baikal. The aim of the study was to determine the effect of the algicidal impact of B. mycoides BS2-15 on U. acus BK280 and to reveal cytological manifestations of the diatom cells.
2. Materials and Methods
2.1. Diatom Culture
A culture of diatom microalgae U. acus was grown in March 2013 from a subglacial sample taken at Lake Baikal near Bolshye Koty settlement. Monoclonal diatom cultures were grown in Diatom medium (DM) [27] at 10 °C with illumination of 16 μmol/m2/s under a 12:12 day/light cycle. An axenic culture of U. acus BK280 was produced according to a published protocol [28]. The procedure involves filtration through a polycarbonate membrane with a pore size of 5 μm (to remove free bacteria), detergent treatment (20 μg/mL Triton X-100), cell treatment with the selected antibiotic (5 μg/mL ciprofloxacin, 18 h incubation), repeated filtration, and monoclonal culturing of diatom cells. Axenity of the culture was verified microscopically (after DAPI staining) and by molecular biological methods. The diatom cells were incubated and transferred to fresh DM media once every four weeks.
2.2. Isolation of Algicidal Bacteria
Sediment samples were taken using a benthic gravity corer aboard the research vessel ‘Vereschagin’. Sampling took place in southern Baikal (51°47′44″ N, 104°54′26″ E) in August 2010. The upper 10 cm of the sediment core was immediately sliced into 1 cm layers. The sediment from each layer (0.5 g) was mortared, placed in 100 mL of sterile distilled water and shaken for two hours. After settling for ten minutes, 1 mL of supernatant was spread on Petri dishes with tenfold diluted fish peptone agar (FPA/10) and cultivated under aerobic conditions at 25 °C for seven days. Pure bacterial cultures were isolated on the same medium using the streak inoculation technique. The morphological properties of the strains were examined using optical microscopy (Axiovert 200, Zeiss, Germany), and the physiological and biochemical properties and taxonomic identity were investigated as described previously [21].
2.3. Identification of Selected Bacterium
The identification of strain BS2-15 was based on its 16S ribosomal RNA sequence. A phenol-chloroform extraction protocol was applied for DNA purification, using lysozyme (50 mg/mL) and 10% SDS [20,29]. DNA was amplified using a PCR kit (Amplisens, Moscow, Russia) and universal eubacterial 16S rRNA primers: 27L (5′-AGAGTTTGATCATGGCTCAG-3′) and 1350R (5′-GACGGGCGGTGTGTACAAG-3′) in a BIS M-111 automatic thermocycler (BIS-N, Koltsovo, Russia) under the following conditions: 94 °C for 2 min; 94 °C for 30 s, 55 °C for 40 s, 72 °C for 50 s, 25 cycles; 72 °C for 2 min. The PCR products were analyzed in 1% agarose gel and purified with a CleanupStandard kit (Eurogen, Moscow, Russia). Sequencing was performed with a BigDye V3.1 Terminator Cycles Sequencing Kit and AmpliTaq DNA polymerase FS (Applied Biosystems, Waltham, USA) on an ABI 3130XL Genetic Analyser sequencer at the SB RAS Genomics Core Facility (Novosibirsk, Russia). Analysis of nucleotide sequences of the 16S ribosomal RNA gene was performed by searching reference databases (Genbank) for similar sequences using BLASTN [30]. MEGA 7.0 (Nearest-Neighbor-Interchange (NNI)) was used for phylogenetic analysis [31]. The 16S rDNA sequence of strain BS2-15 was deposited in the GenBank database under accession number MH638320.1.
2.4. Determination of Algicidal Activity
For all the experiments, the cells of BS2-15 from the medium FPA/10 were subcultured into 1% peptone solution and cultured at 25 °C for two days and nights until they reached an optical density of 0.9–1.0 at A600 nm. To determine the dose-dependency of the algicidal effect of bacteria, the cells of B. mycoides were added at dilutions 2, 10, 40, 60, 100, 200, 300 μL per mL of medium to the culture of U. acus at the exponential phase (for 7 days of culturing, 103 cells/mL) and were co-cultivated for 17 days. To further investigate the algicidal effect, the bacterial inoculum was added to the cells at a diluted 100 μL/mL. To determine if an algicidal agent is produced in the environment regardless of the presence of diatoms near bacteria, cell-free bacterial filtrate (100 μL/mL) ion obtained by filtration of bacterial culture through a sterile 0.20 μm filter (Whatman, Corning, USA), was added to the cells of U. acus. To determine whether direct interaction between bacteria and diatoms is necessary for an algicidal effect, bacterial cells washed from the medium were added to the cells at a dilution of 100 μL/mL; they were washed twice with DM medium with precipitation by centrifuge in a sterile test tube at 5000 rpm for 10 min. To exclude the suppressing effect of peptone on the growth of diatom algae, 1% sterile peptone solution was added to diatom culture diluted 100 μL/mL. The cultivation lasted nine days.
For all experiments, we used three biological and three technical replicates. Cells were counted every two days at the same time. An optical and epifluorescent microscope (Axiovert 200 Zeiss, Oberkochen, Germany) was used to enumerate diatom cells and identify bacteria, as previously described [20].
2.5. TUNNEL Assay
Fragmentation of genomic DNA was detected using dUTP end-labelling in situ (TUNEL). Axenic culture U. acus cultured with bacteria (100 μL/mL) for 24 h were used for this analysis. Cells were fixed with 3.7% formaldehyde solution (Sigma-Aldrich, St. Louis, MO, USA) with 0.1 M phosphate buffer (pH 7.4) for 15 min. After washing with the phosphate buffer, cells were permeabilized with 0.25% Triton™ X-100 (Sigma-Aldrich, St. Louis, USA). Detection of fragmented DNA was performed with a Click-iT™ TUNEL Alexa Fluor™ 488 Imaging Assay (Thermo Fischer Scientific, Waltham, MA, USA) according to the manufacturer’s protocol. The positive control sample was treated with DNase I (Thermo Fischer Scientific, USA) at room temperature for 30 min before the labeling reaction. Cell nuclei were stained with Hoechst 33,342 (Component F, Click-iT™ TUNEL Alexa Fluor™ 488 Imaging Assay). Prepared cells were placed in a ProLong® Gold Antifade Mountant (Thermo Fisher Scientific, USA). The samples were examined using a Zeiss LSM 710 confocal microscope (Zeiss, Oberkochen, Germany) with a Plan-Apochromat 63×/1.40 Oil DIC M27 objective. Hoescht 33,342 dye was excited at a wavelength of 405 nm and its emission registered between 410–483 nm. Click-iT™ TUNEL Alexa Fluor™ 488 dye was excited at 488 nm and was registered between 493–628 nm.
2.6. Lipid Droplets Analysis Using BODIPY
To determine lipid accumulation used diatom cells, on the seventh day of cultivation axenic culture and with bacterial culture, bacterial filtrate and peptone (all of the above was added to the culture of diatoms at a diluted 100 μL/mL). Diatoms cells were stained with BODIPY 493/503 (Thermo Fischer Scientific, Waltham, USA) by adding the dye to 500 μL of the cells suspension at a concentration of 2 μM and incubating it for 15 min. Then the cells were washed with 0.1 M phosphate buffer (pH 7.4) and fixed with 4% formaldehyde solution; subsequently, they were washed twice with 0.1 M phosphate buffer. Prepared cells were placed in a ProLong® Gold Antifade Mountant (Sigma-Aldrich, St. Louis, USA).
2.7. Fluorescent Microscopy
The cells of axenic culture, when cultured with bacteria and with peptone (both of which are added at a dilution of 100 μL/mL) were stained with the fluorescent dye Live Cell Labeling Kit (Abcam, Cambridge, UK). First 200 μL of the culture aliquot was added to a 96-well plate. The dye solution was added at a dilution of 0.5 μL/mL, and incubation was performed for 30 min at 10 °C with an illumination of 16 μmol/m2/s. To determine the proportion of living cells in the culture, the stained cells were counted among 100 randomly selected cells using epifluorescence microscope Axiovert 200 (Zeiss, Oberkochen, Germany) with a dark-blue light filter. Staining and counting were performed every two days for 9 days, all counts were performed in triplicate.
2.8. Transmission Electron Microscopy
Diatom cells were fixed with 2.5% glutaraldehyde (Sigma-Aldrich, St. Louis, USA) in 0.1 M phosphate buffer, pH 7.4, for 2 h and postfixed with 1% OsO4 (Sigma-Aldrich, St. Louis, USA)) in the same buffer for 2 h at room temperature. Thereafter, cells were dehydrated in an ascending ethanol series followed by 96% ethanol and acetone dehydrated with copper sulfate for 5 min each (all reagents were obtained from Reakhimkomplekt, Russia). Dehydrated samples were embedded with mixtures of Araldite 502 epoxy resin (Sigma-Aldrich, St. Louis, USA) and acetone and with pure Araldite, transferred to a new portion of Araldite supplemented with DMP-30 accelerator (Sigma-Aldrich, St. Louis, USA), and polymerized at 60 °C for three days. Ultrathin sections were prepared using an Ultracut R ultratome (Leica, Wetzlar, Germany) with an ULTRA 35 diamond knife (Diatom, Nidau, Switzerland), placed onto copper grids, and contrasted with uranyl acetate and lead citrate. TEM analysis was performed using a Leo 906 E microscope (Zeiss, Oberkochen, Germany) at an acceleration voltage of 80 kV. Microscopic images were taken with a MegaView II camera (Zeiss, Oberkochen, Germany) and processed using the MegaVision program.
2.9. Scanning Electron Microscopy
The cell suspension was dehydrated in an ascending ethanol series followed by 96% ethanol and then placed on SEM stubs. These plates were dried at room temperature and coated with gold. Microscopy was performed using a Quanta 200 scanning electron microscope (FEI Company, Hillsboro, OR, USA).
2.10. Statistical Analysis
Statistical assessment of the significance of the differences between retrievals was performed using the program Past 4 with a nonparametric Mann–Whitney U-test, taking into account the retrieval size and normality determined by the Shapiro-Wilk criterion. The differences were considered statistically significant at p < 0.05.
3. Results and Discussion
3.1. Isolation and Identification of Algicidal Bacterium
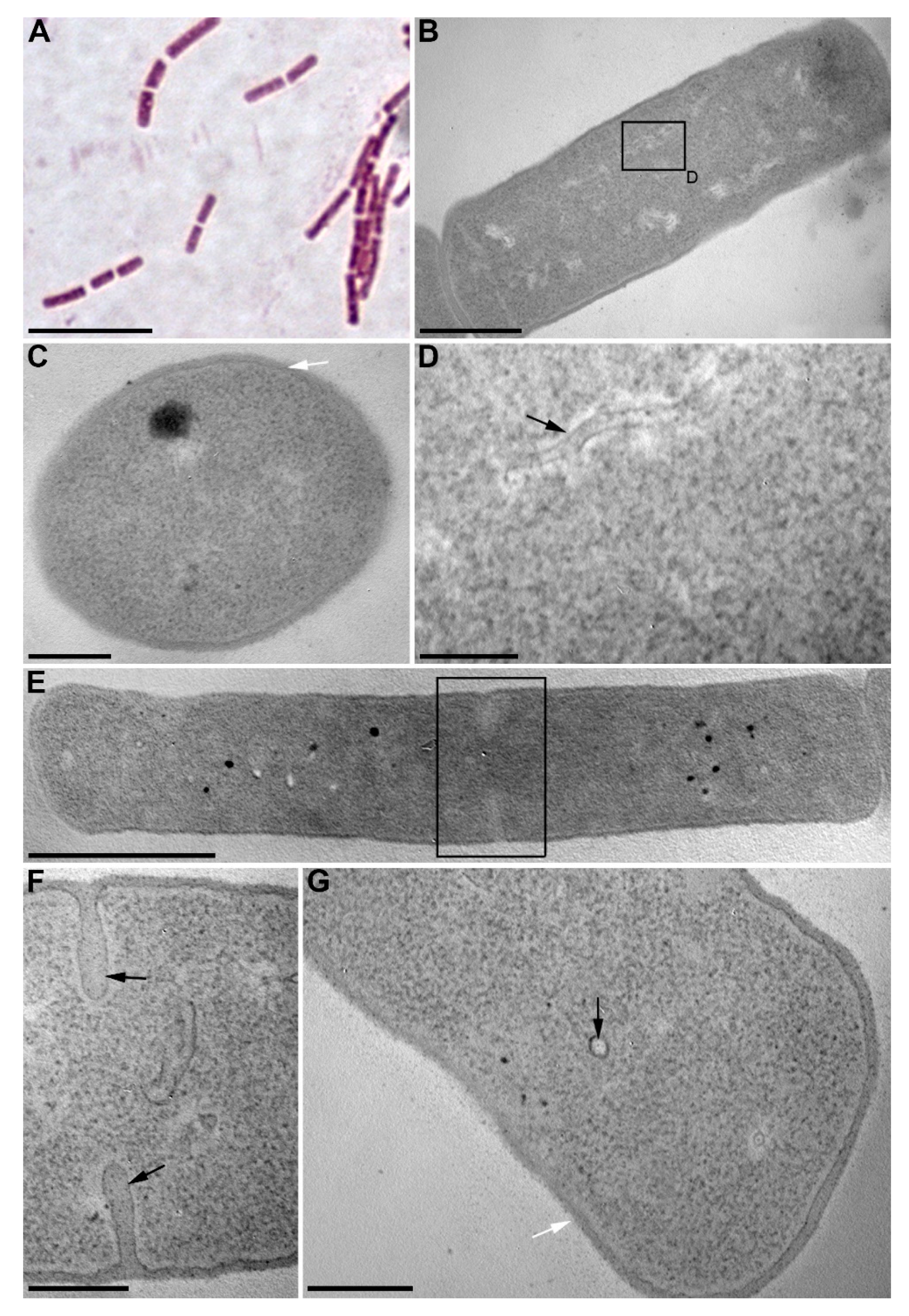
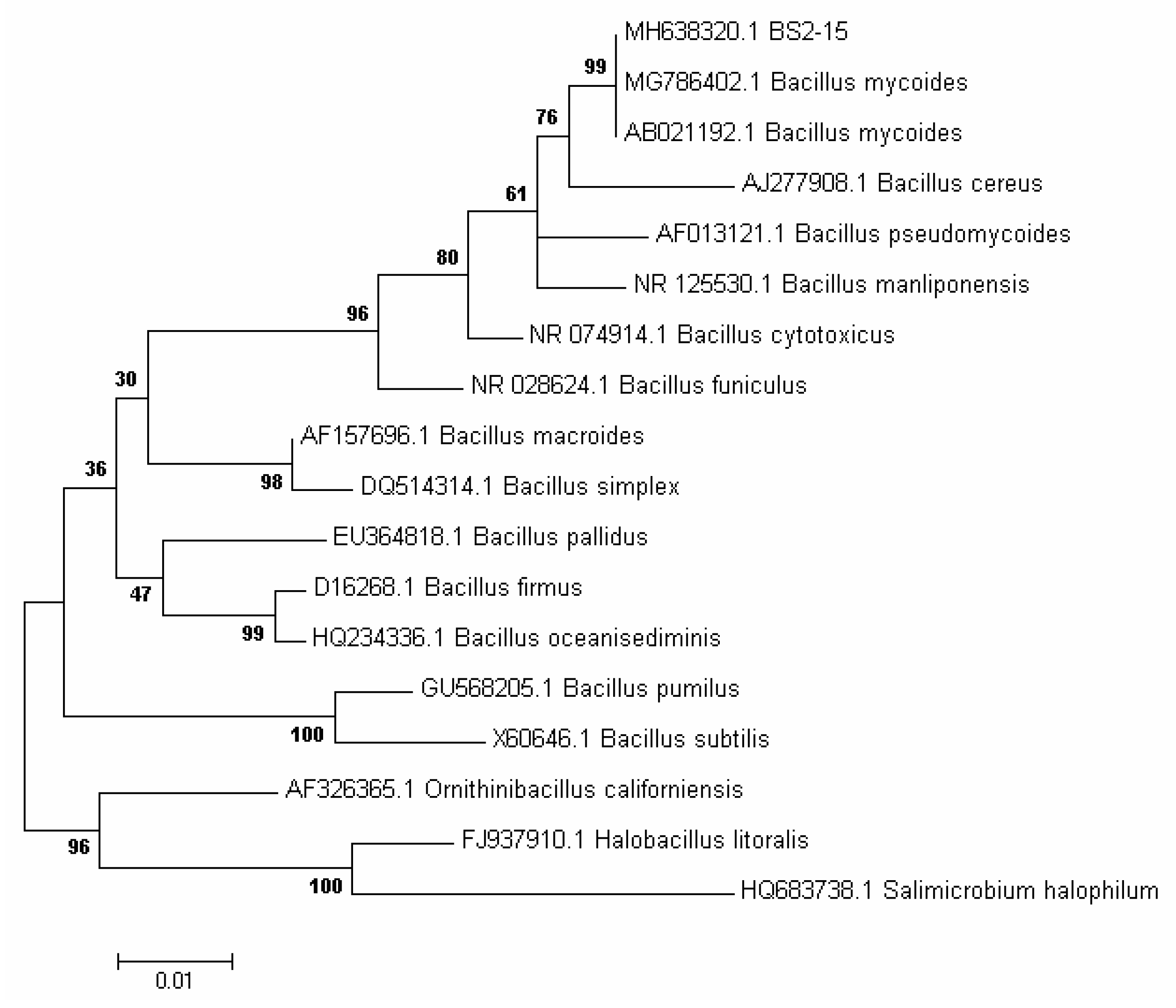
We isolated 40 bacterial strains from the bottom sediments of Lake Baikal and assessed for their algicidal effect on the diatom algae U. acus BK280. Eight strains depressed the growth of a monoclonal axenic culture of this alga. Strain BS2-15 (isolated from the upper layer of bottom sediments, 2 cm above surface sediments) showed the strongest algicidal effect and thus was selected for identification and further investigation of its effect on diatom nuclear DNA. On the basis of its morphologic and biochemical characteristics, it was classified as belonging to the genus Bacillus. The cells of this strain are rod-shaped (0.75–1.3) × (1.8–4.5) µm, Gram-positive, immobile, and form elliptic spores (Figure 1A, Table 1). Microphotographs of ultrathin sections revealed the inclusions, ribosomes, and electron-transparent areas within the cells, as well as long curving or circular structures in the cytoplasm and near septae forming in the dividing cells (Figure 1B–G). Strain BS2-15 showed morphological and biochemical similarity to B. mycoides and B. weihenstephanensis (Figure 2). Comparative analysis of the nucleotide sequences demonstrated that it is closest to a strain of B. mycoides (acc. no AB021192), with 99% similarity. On the basis of the phylogenetic analysis (Figure 2) and main characteristics of the species (Table 1), this strain was named Bacillus mycoides BS2-15.
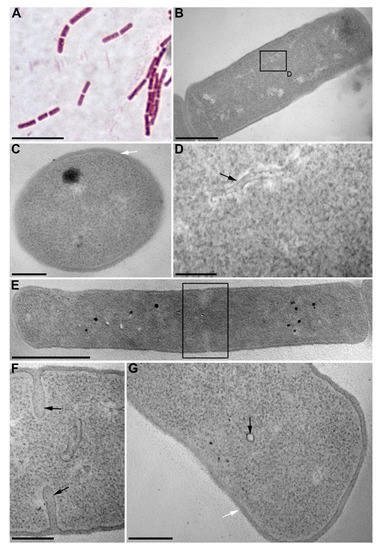
Figure 1.
Cells of B. mycoides BS2-15. (A) fluorescent microscopy; (B–G) transmission electron microscopy: (B) longitudinal section of a B. mycodies cell; (C) cross-section of a B. mycoides cell; (D) a fragment of Figure 1B showing the long curving structure in the cytoplasm; (E) a longitudinal section of a dividing cell; (F) a fragment of Figure 1E, septae are marked by the arrows; (G) a fragment of Figure 1E; black arrow marks a circular structure in the cytoplasm, white arrow marks the cell wall. Scale bars: (A) 5 µm; (B) 500 nm; (C,F,G) 200 nm; (D) 100 nm; (E) 1 µm.

Table 1.
Morphological and biochemical features of strain BS2-15.
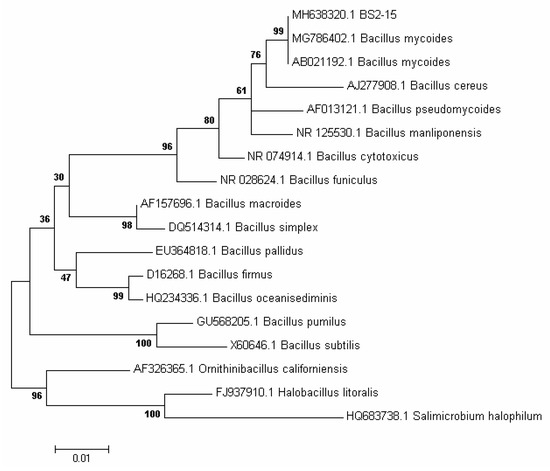
Figure 2.
A phylogenetic tree for the algicidal strain BS2-15 and its closest BLAST hits was constructed using the Neighbor-Joining method of MEGA software (version 7.0), based on 16 rRNA sequences. Bootstrap supports are shown at the nodes.
3.2. Algicidal Effect of B. mycoides BS2-15 on U. acus BK280
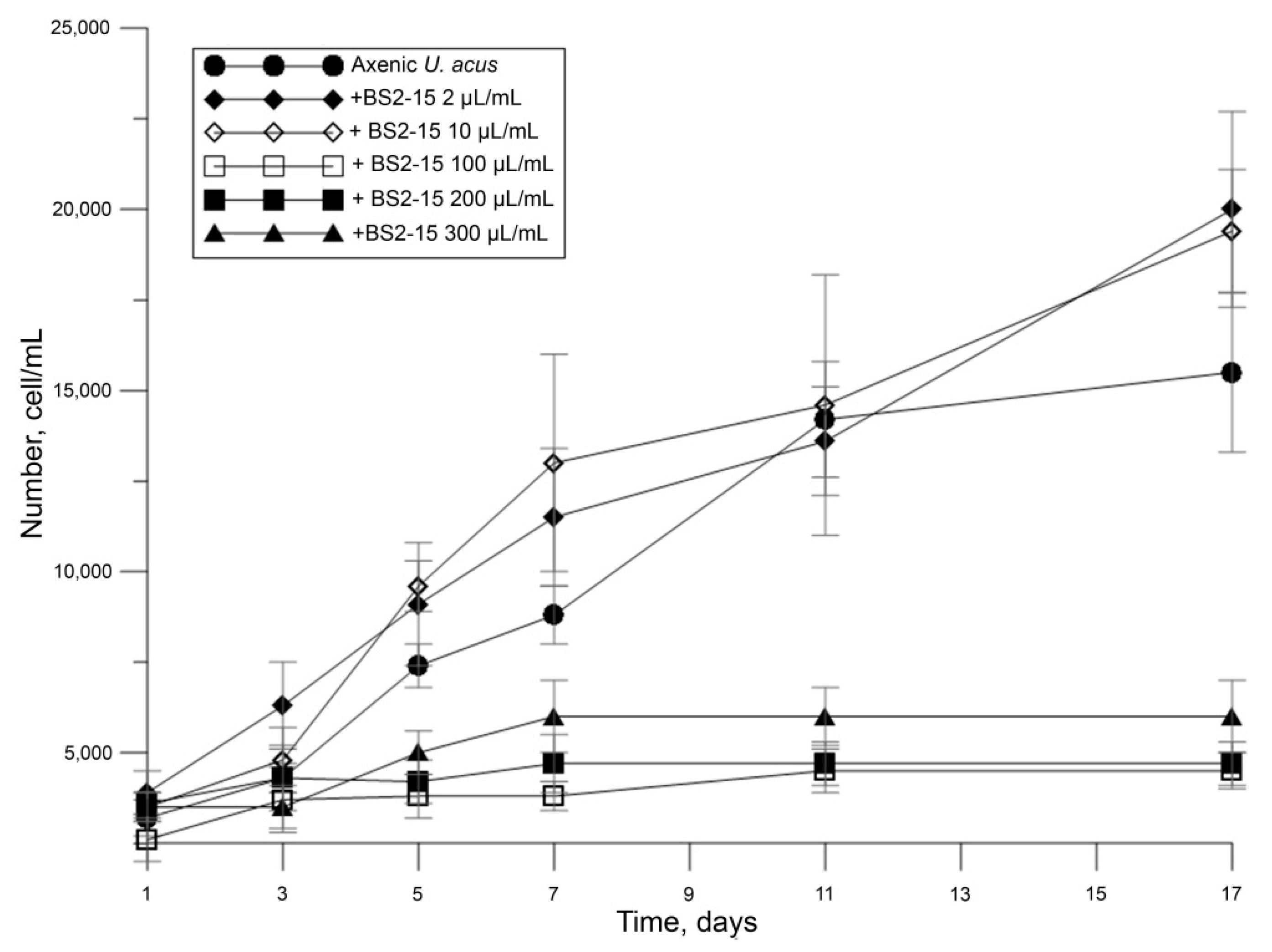
We demonstrated that different dilutions of the bacterial culture B. mycoides BS2-15 affect the diatom growth differently. A low density of added bacteria acted as a weak growth promoter of diatom culture (p = 0.01), whereas adding of bacteria at a high density (40–100 μL/mL) considerably decreased their growth (p < 0.01) (Figure 3).
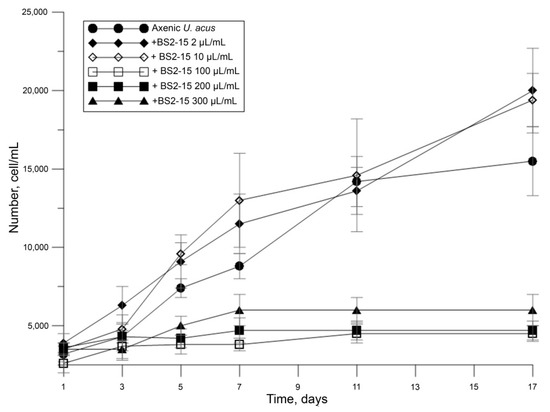
Figure 3.
Inhibition of U. acus BK280 growth after inoculation with different concentrations of B. mycoides BS2-15 bacterial culture (microliters from optical density of bacterial culture of 0.9–1.0 at A600 nm).
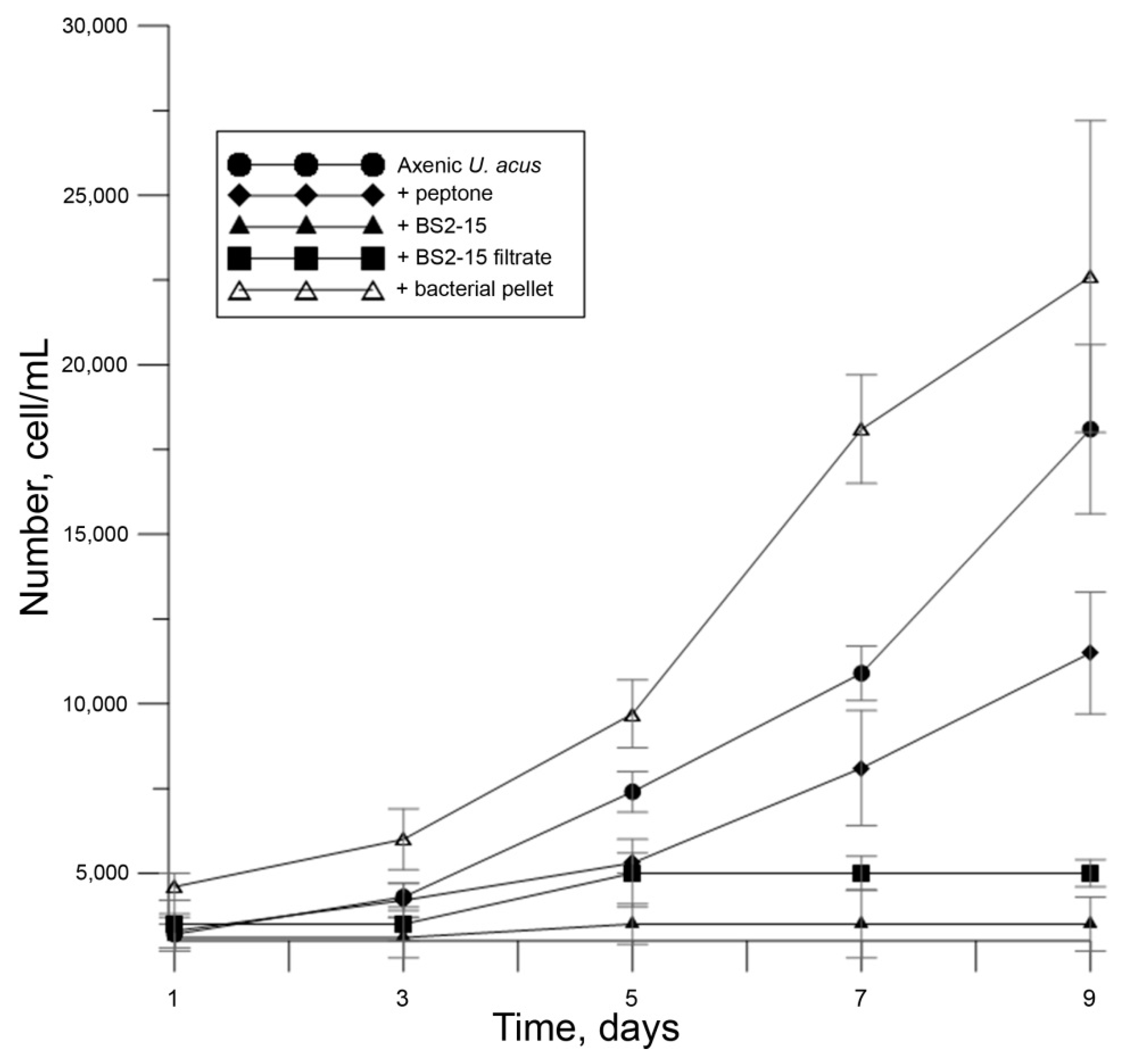
The bacterial cell suspension added to U. acus is cultured in a 1% peptone water; therefore, it is critical to study its influence on diatom growth because data suggest that it can suppress their growth and result in their death [32]. As suggested in [32], such a reaction may occur because peptone is a mixture of dissolved free amino acids (DFAA). In a natural environment, DFAA leakage can occur as a result of cell death and can hypothetically regulate the growth of diatoms. In this study, when peptone diluted to 100 μL/mL was added to U. acus, growth suppression was observed (p < 0.01) (Figure 4).
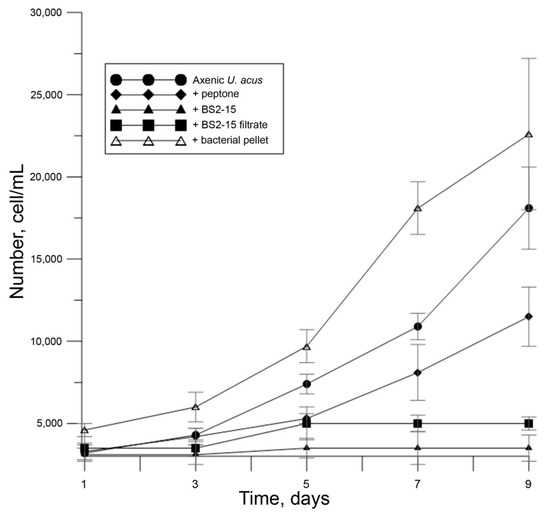
Figure 4.
Growth of U. acus BK280 during inoculation with 1% peptone solution (100 μL/mL), B. mycoides BS2-15 bacterial culture (100 μL/mL), bacterial filtrates (100 μL/mL) and bacterial pellet (bacterial culture washed from the 1% peptone solution, 100 μL/mL).
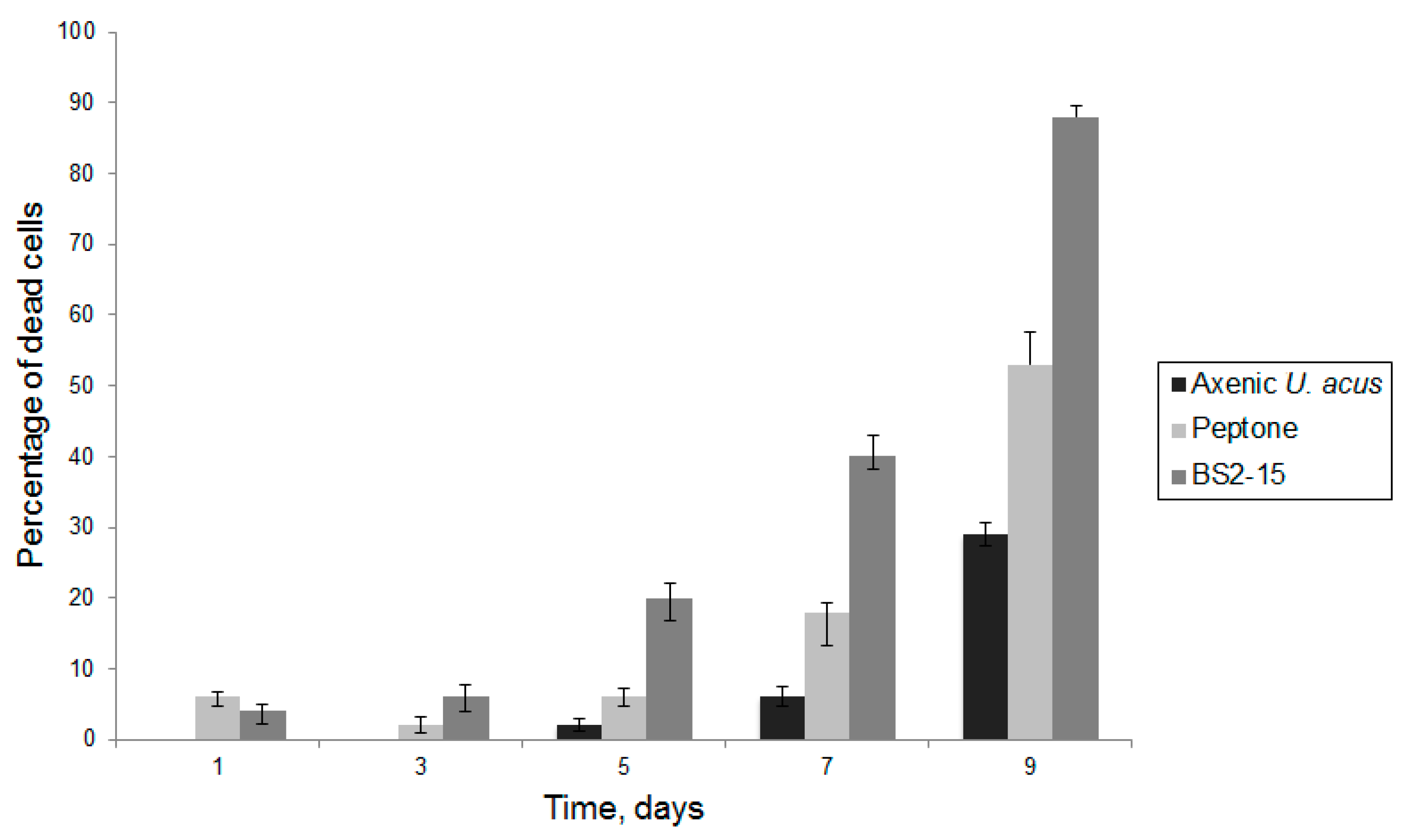
The assessment of the dynamics of cell mortality revealed that when culturing with 1% peptone solution, cell mortality increased compared with the control (p < 0.01); however, when culturing with bacterial culture, diatom cell mortality reached nearly 90% by the ninth day of culture and statistically significant differences were observed both with the axenic culture of diatoms and culture of diatoms cultivated with 1% peptone solution (p < 0.01) (Figure 5).
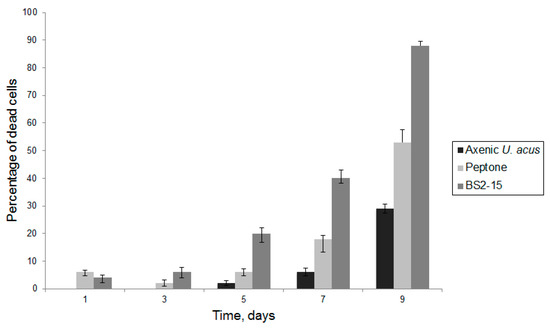
Figure 5.
Mortality of the cells of U. acus BK280 after inoculation with 1% peptone solution and B. mycoides BS2-15 bacterial culture.
It is known that bacteria can manifest an algicidal effect either through physical contact with algal cells [33] or by producing algicidal compounds into the environment [15].
To determine the source of the algicidal effect, we added the filtrate of bacteria as well as bacteria washed from culturing medium (bacterial pellet) to the diatom cells (Figure 4). Bacteria added at a dilution of 100 μL per mL of diatom culture but washed from the medium did not affect the growth of the U. acus. Bacterial filtrate resulted in suppression of diatom growth (p < 0.01). It can be concluded that the algicidal substance is produced by bacteria in the environment, regardless of the presence of diatoms.
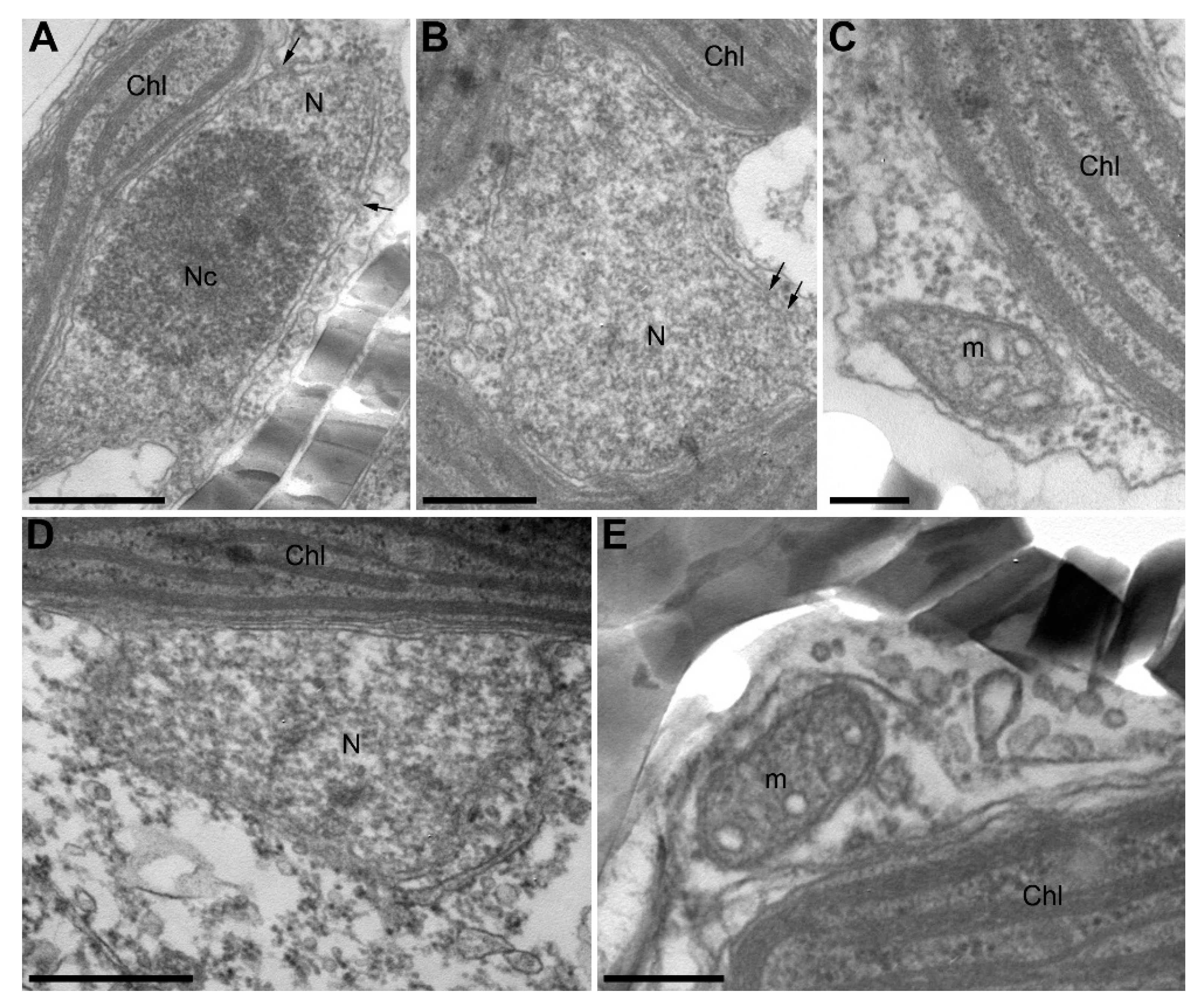
TEM has revealed that control cells had nuclei with large nucleoli, clearly visible envelopes, and nuclear pores (Figure 6A,B). The cross-sections of chloroplasts and mitochondria were similar to those observed previously (Figure 6A–C) [34]. Cells grown in the presence of B. mycoides also had normal chloroplasts and mitochondria similar to controls, but their nuclei often did not have clearly visible membranes on the side oriented to the cell center (Figure 6D,E). Even a series of slices from such cells did not contain clearly visible nuclear pores.
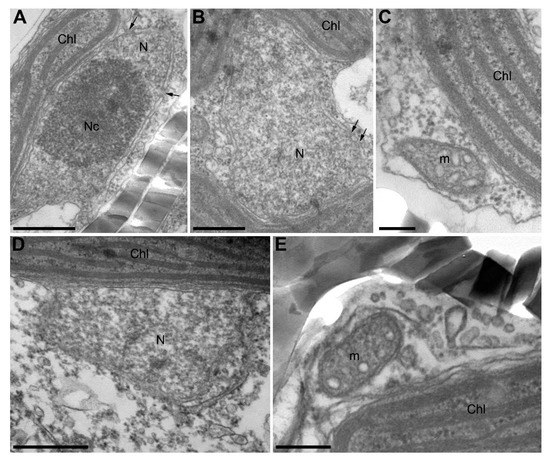
Figure 6.
U. acus cells in axenic culture (A–C) and after culturing with B. mycoides BS2-15 bacterial culture (D–E) (TEM). (A,B) cross-sections of nuclei in different cells, with arrows marking nuclear pores; (C) a cross-section of a mitochondrion and a part of a chloroplast in a native cell; (D) a damaged nucleus of an U. acus cell under algicidal effect; (E) a cross-section of a mitochondrion and a part of a chloroplast under algicidal effect. Abbreviations are: Chl—chloroplast; m—mitochondrion; N—nucleus; Nc—nucleolus. Scale bar: (A,B,D) 500 nm; (C,E) 200 nm.
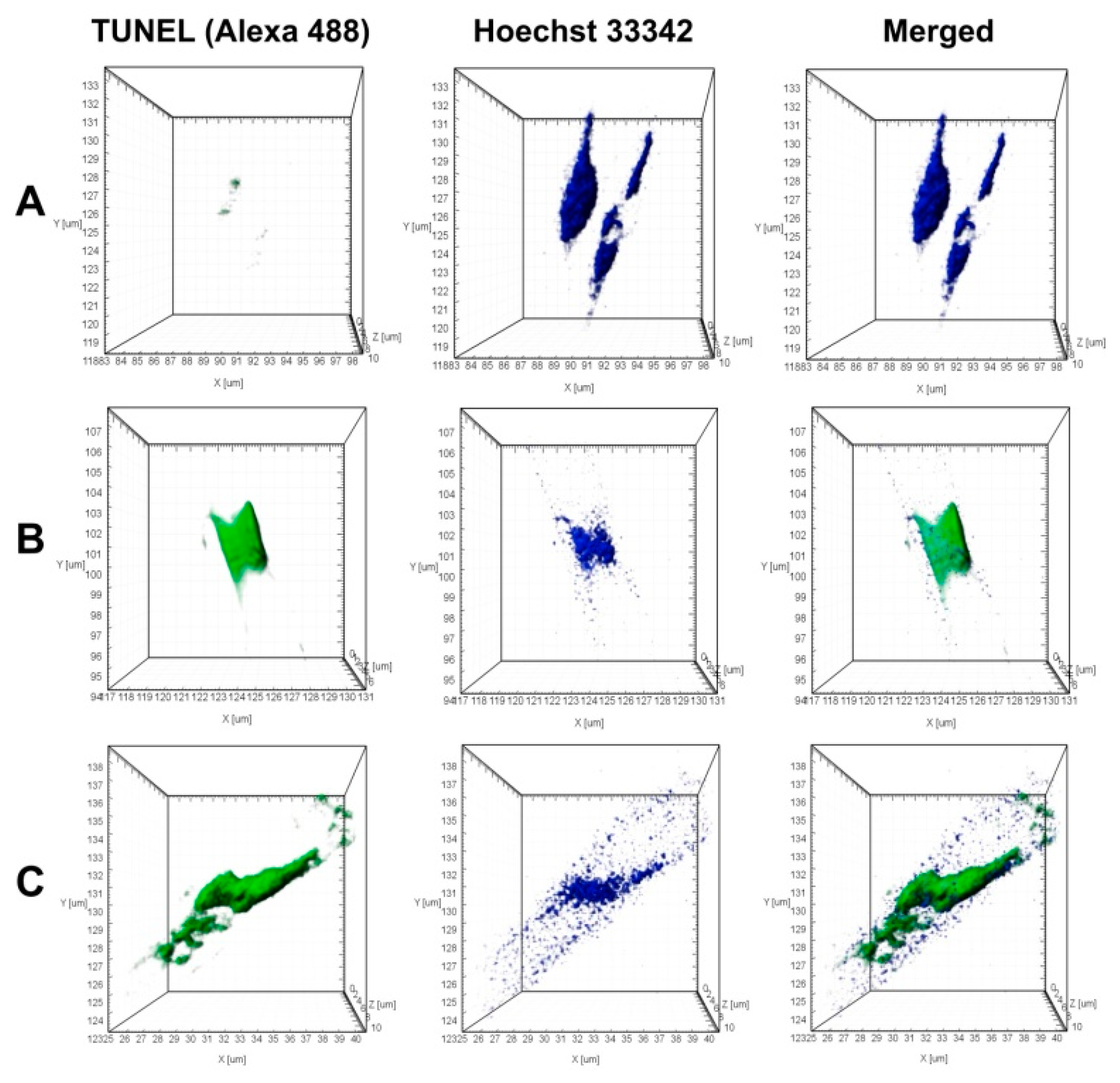
TUNEL-analysis demonstrated, that in the axenic culture, nucleus fragmentation did not occur (Figure 7A). To model the DNA lesions (positive control), the cells were permeabilized with DNAse I, which caused 90% of the nuclei to show DNA fragmentation (Figure 7B).
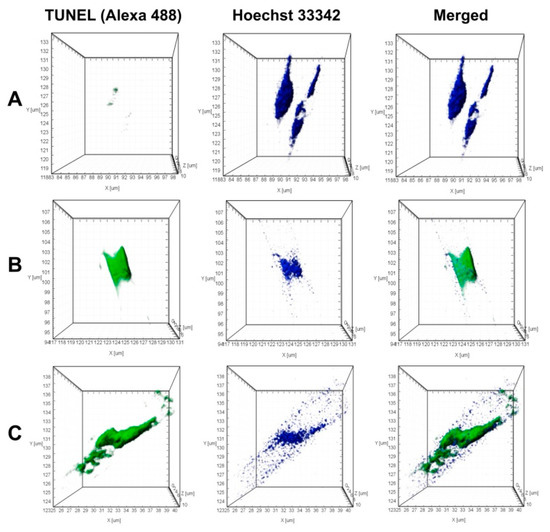
Figure 7.
Fragmentation of nuclear DNA (Click-iT™ TUNEL Alexa Fluor™ 488 Imaging Assay) in U. acus cells induced by culturing with B. mycoides BS2-15 bacterial culture. Staining for fragmented DNA (Click-iT™ TUNEL Alexa Fluor™ 488, green) and for nuclei (Hoechst 33342, blue); laser confocal microscopy; 3D reconstructions. (A) axenic U. acus; (B) TUNEL positive control (axenic U. acus treated with DNAse I to model DNA breakage); (C) experiment (culturing with B. mycoides BS2-15 bacterial culture for 24 h).
During co-cultivation of U. acus with B. mycoides BS2-15, numerous nuclei of diatom cells were already stained with Alexa Fluor™ 488 after 24 h, showing evidence of DNA fragmentation (Figure 7C). In addition, 5% of the nuclei appeared to contain both native and fragmented DNA. In such nuclei, separate regions were stained with the two dyes.
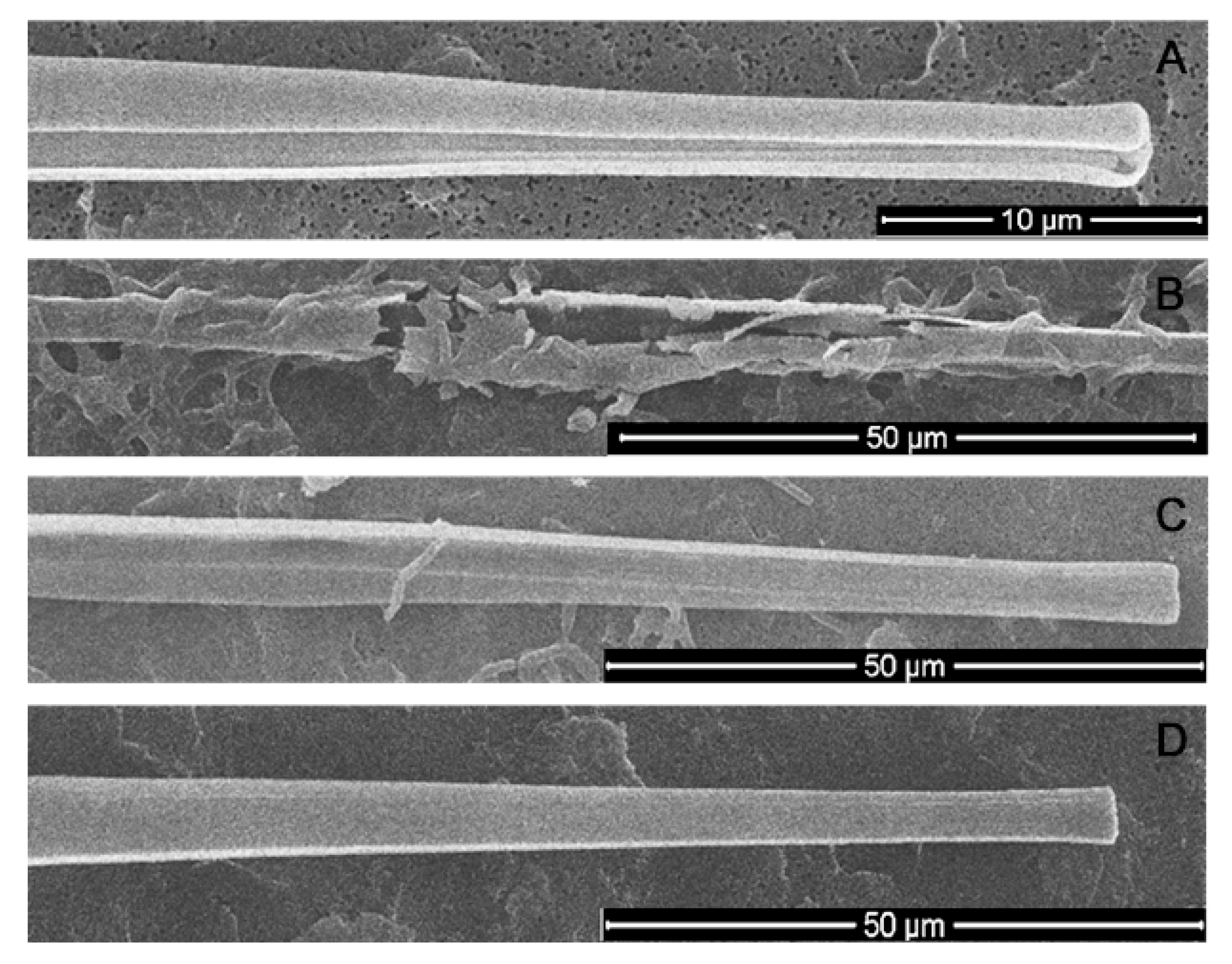
The cell valves of the axenic culture were intact (Figure 8A), as were the cell valves to which the bacterial filtrate was added (Figure 8D). When cultured with bacterial culture, many destroyed shells were encountered (Figure 8B), but also intact shells were preserved, but with B. mycoides cells attached to them (Figure 8C). It can be assumed that the bacteria consume the organic matter covering the valves of the diatoms, leading to increased fragility of the shells. Conversely, the algicidal effect is likely achieved by a complex of substances present in the environment and released by directly attached bacteria.
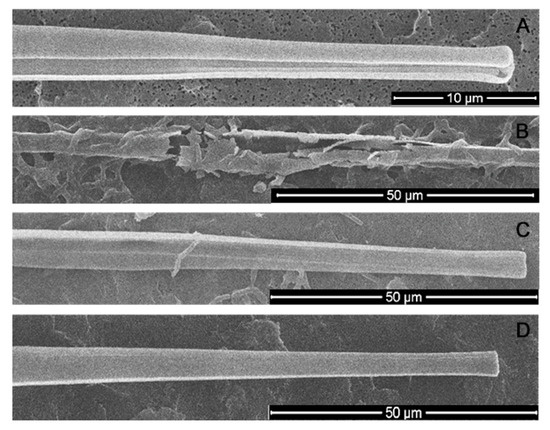
Figure 8.
The state of a silicon frustules of U. acus under different conditions. (A) axenic culture; (B,C) co-culturing with B. mycoides BS2-15 bacterial culture; (D) adding of bacterial filtrate to diatoms.
It was previously demonstrated that a large proportion of the near-bottom layer of Lake Baikal is composed just by U. acus and diverse bacteria [9]. Because of the vertical water exchange phenomenon, live diatoms can precipitate at the bottom. It was also observed that bacteria from bottom sediments can use the cell wall of U. acus as a carbon source [35], and this corresponds to the observed increase of frustules fragility after co-culturing.
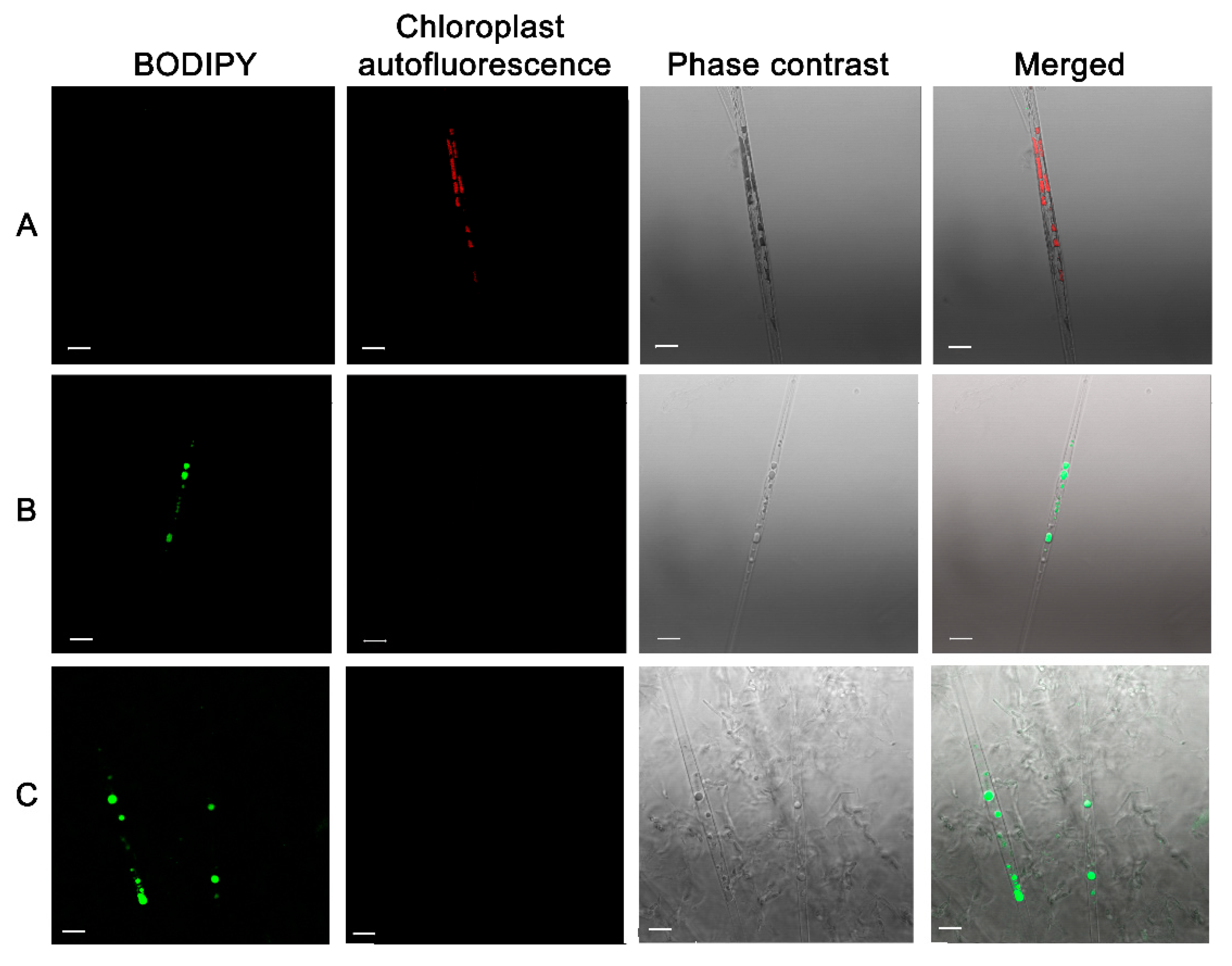
Stress conditions not only decrease the diatom growth rate but also result in lipid accumulation [36]. Axenic culture of diatoms contained whole chloroplasts and rare lipid droplets (Figure 9A). However, cells cultured with bacterial filtrate (Figure 9B) and bacterial cultures (Figure 9C) had no chloroplasts (indicating that the cells were dead) but contained numerous large and small lipid droplets.
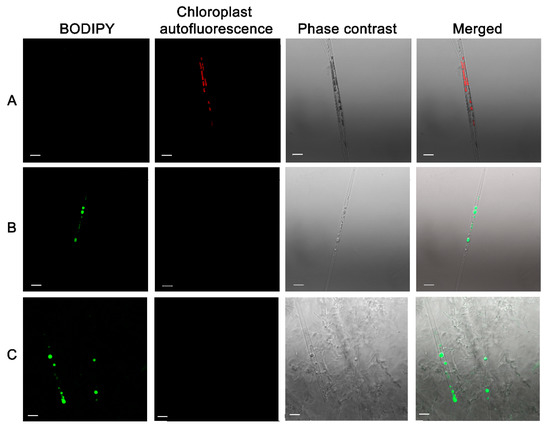
Figure 9.
Induction of lipids accumulation after culturing with bacterial culture B. mycoides BS2-15 and bacterial filtrate for 7 days. The cells were stained with BODIPY and observed with a confocal microscope. (A) axenic culture; (B) culture with added bacterial filtrate; (C) culture with added B. mycoides BS2-15 bacterial culture. The red marks autofluorescence of chloroplasts, dye BODIPY fluorescence with green. Scale bar: 10 µm.
It is known that nutrient deficiency in diatoms results in cell metabolism conversion to lipid accumulation [37]. We could suppose that diatoms cells compete with bacteria for nutrients, but the fact that lipids accumulated when diatoms were cultured with bacterial filtrate with the same result suggest that in this case, the mechanism of lipid accumulation is different. Also, the accumulation of elevated lipid results from the aging of the diatom culture and the effects of different chemicals [38,39].
4. Conclusions
Bacillus mycoides strain BS2-15 isolated from Lake Baikal bottom sediments produces a strong algicidal effect on the diatom algae Ulnaria acus. We revealed that algicidal effect does not depend on the presence of diatoms in a medium with bacteria, and bacterial pellets do not influence the growth of U. acus. We identified the fragmentation of the U. acus nucleus resulting from the co-culturing of bacteria and diatoms. Moreover, we demonstrated that DNA fragmentation at U. acus is observed after only 24 h of common culturing. The specific staining of these nuclei and decreased culture growth in the presence of B. mycoides BS2-15 suggests the presence of specific response mechanisms in diatom cells that are involved in the algicidal activity of the bacteria. This phenomenon highlights the complexity of algae-bacteria interactions, which can be involved in regulating microorganism populations in water ecosystems.
Author Contributions
Conceptualization, Y.Z.; methodology, Y.Z.; data preparation, Y.Z., Y.B., E.B., M.K., N.S. and I.K.; data analysis, Y.Z., Y.B. and E.B.; writing—original draft preparation, Y.B.; writing and editing of the manuscript E.B., Y.B. and Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
The investigation was done with financial support of the State Assignments of Limnological Institute Siberian Branch of Russian Academy of Sciences #0279-2021-0008 (121032300186-9).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The 16S rDNA sequence of strain BS2-15 was deposited in the GenBank database under accession number MH638320.1.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Geider, R.J.; Delucia, E.H.; Falkowski, P.G.; Finzi, A.C.; Grime, J.P.; Grace, J. Primary productivity of planet earth: Biological determinants and physical constraints in terrestrial and aquatic habitats. Glob. Chang. Biol. 2001, 7, 849–882. [Google Scholar] [CrossRef]
- Nelson, D.M.; Tréguer, P.; Brzezinski, M.A.; Leynaert, A.; Quéguiner, B. Production and dissolution of biogenic silica in the ocean: Revised global estimates, comparison with regional data and relationship to biogenic sedimentation. Glob. Biogeochem. Cycles 1995, 9, 359–372. [Google Scholar] [CrossRef]
- Ragueneau, O.; Tréguer, P.; Leynaert, A.; Anderson, R.F.; Brzezinski, M.A.; DeMaster, D.J. A review of the Si cycle in the modern ocean: Recent progress and missing gaps in the application of biogenic opal as a paleoproductivity proxy. Glob. Planet. Chang. 2000, 26, 317–365. [Google Scholar] [CrossRef]
- Amin, S.A.; Parker, M.S.; Armbrust, E.V. Interactions between diatoms and bacteria. Microbiol. Mol. Biol. Rev. 2012, 76, 667–684. [Google Scholar] [CrossRef]
- Mayali, X.; Azam, F. Algicidal bacteria in the sea and their impact on algal blooms. J. Eukaryot. Microbiol. 2004, 51, 139–144. [Google Scholar] [CrossRef]
- Ramanan, R.; Kim, B.H.; Cho, D.H.; Oh, H.M.; Kim, H.S. Algae-bacteria interactions: Evolution, ecology and emerging applications. Biotechnol. Adv. 2016, 34, 14–29. [Google Scholar] [CrossRef]
- Mitsutani, A.; Yamasaki, I.; Kitaguchi, H.; Kato, J.; Ueno, S.; Ishida, Y. Analysis of algicidal proteins of a diatom-lytic marine bacterium Pseudoalteromonas sp. strain A25 by two-dimensional electrophoresis. Phycologia 2001, 40, 286–291. [Google Scholar] [CrossRef]
- Kang, Y.H.; Kim, J.D.; Kim, B.H.; Kong, D.S.; Han, M.S. Isolation and characterization of a bio-agent antagonistic to diatom, Stephanodiscus hantzschii. J. Appl. Microbiol. 2005, 98, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Zakharova, Y.R.; Kurilkina, M.I.; Likhoshvay, Y.V.; Shishlyannikov, S.M.; Kalyuzhnaya, O.V.; Petrova, D.P. Effect of bacteria from the bottom water layer of Lake Baikal on degradation of diatoms. Paleontol. J. 2013, 47, 1030–1034. [Google Scholar] [CrossRef]
- Nicholson, W.L.; Munakata, N.; Horneck, G.; Melosh, H.J.; Setlow, P. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Biol. Rev. 2000, 64, 548–572. [Google Scholar] [CrossRef] [PubMed]
- Higgins, D.; Dworkin, J. Recent progress in Bacillus subtilis sporulation. FEMS Microbiol. Rev. 2012, 36, 131–148. [Google Scholar] [CrossRef]
- Di Franco, C.; Beccari, E.; Santini, T.; Pisaneschi, G.; Tecce, G. Colony shape as a genetic trait in the pattern-forming Bacillus mycoides. BMC Microbiol. 2002, 2, 33. [Google Scholar] [CrossRef]
- Sugiura, H.; Nagase, A.; Oiki, S.; Mikami, B.; Watanabe, D.; Hashimoto, W. Bacterial inducible expression of plant cell wall-binding protein YesO through conflict between Glycine max and saprophytic Bacillus subtilis. Sci. Rep. 2020, 10, 18691. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, L.K.; Jackson, M.A. Clarification of the Taxonomy of Bacillus mycoides. Microbiol. Soc. 1995, 45, 46–49. [Google Scholar] [CrossRef]
- Li, N.; Zhang, J.; Zhao, X.; Wang, P.; Tong, M.; Glibert, P.M. Allelopathic inhibition by the bacteria Bacillus cereus BE23 on growth and photosynthesis of the Macroalga Ulva prolifera. J. Mar. Sci. Eng. 2020, 8, 718. [Google Scholar] [CrossRef]
- Wu, L.; Wu, H.; Chen, L.; Xie, S.; Zang, H.; Borriss, R.; Gao, X. Bacilysin from Bacillus amyloliquefaciens FZB42 has specific bactericidal activity against harmful algal bloom species. AEM 2014, 80, 7512–7520. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, D.S.; Jeong, S.Y.; Lee, W.J.; Lee, M.S. Isolation and characterization of a marine algicidal bacterium against the harmful raphidophyceae Chattonella marina. J. Microbiol. 2009, 47, 9–18. [Google Scholar] [CrossRef]
- Hou, S.; Shu, W.; Tan, S.; Zhao, L.; Yin, P. Exploration of the antioxidant system and photosynthetic system of a marine algicidal Bacillus and its effect on four harmful algal bloom species. Can. J. Microbiol. 2015, 62, 49–59. [Google Scholar] [CrossRef]
- Huang, J.S.; Peng, Y.H.; Chung, K.R.; Huang, J.W. Suppressive efficacy of volatile compounds produced by Bacillus mycoides on damping-off pathogens of cabbage seedlings. J. Agric. Sci. 2018, 156, 795–809. [Google Scholar] [CrossRef]
- Zakharova, Y.R.; Galachyants, Y.P.; Kurilkina, M.I.; Likhoshvay, A.V.; Petrova, D.P.; Shishlyannikov, S.M.; Ravin, N.V.; Mardanov, A.V.; Beletsky, A.B.; Likhoshway, Y.V. The Structure of microbial community and degradation of diatoms in the deep near-bottom layer of Lake Baikal. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Zakharova, Y.R.; Adel’shin, R.V.; Parfenova, V.V.; Bedoshvili, Y.D.; Likhoshway, Y.V. Taxonomic characterization of the microorganisms associated with the cultivable diatom Synedra acus from Lake Baikal. Microbiology 2010, 79, 679–687. [Google Scholar] [CrossRef]
- Galachyants, Y.P.; Zakharova, Y.R.; Petrova, D.P.; Morozov, A.A.; Sidorov, I.A.; Marchenkov, A.M.; Logacheva, M.D.; Markelov, M.L.; Khabudaev, K.V.; Likhoshway, Y.V.; et al. Sequencing of the complete genome of an araphid pennate diatom Synedra acus subsp. radians from Lake Baikal. Dokl. Biochem. Biophys. 2015, 461, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Galachyants, Y.P.; Morozov, A.; Mardanov, A.V.; Beletsky, A.V.; Ravin, N.V.; Petrova, D.P.; Likhoshway, Y.V. Complete chloroplast genome sequence of freshwater araphid pennate diatom alga Synedra acus from Lake Baikal. Int. J. Biol. 2012, 4, 27–35. [Google Scholar] [CrossRef][Green Version]
- Galachyants, Y.P.; Zakharova, Y.R.; Volokitina, N.A.; Morozov, A.A.; Likhoshway, Y.V.; Grachev, M.A. De novo transcriptome assembly and analysis of the freshwater araphid diatom Fragilaria radians, Lake Baikal. Sci. Data 2019, 6. [Google Scholar] [CrossRef] [PubMed]
- Kharitonenko, K.V.; Bedoshvili, Y.D.; Likhoshway, Y.V. Changes in the micro- and nanostructure of siliceous frustule valves in the diatom Synedra acus under the effect of colchicine treatment at different stages of the cell cycle. J. Struct. Biol. 2015, 190, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Petrova, D.P.; Bedoshvili, Y.D.; Zakharova, Y.R.; Volokitina, N.A.; Likhoshway, Y.V.; Grachev, M.A. Changes in valve morphology of two pennate diatom species during long-term culture. Acta Biol. Sib. 2020, 6, 669–678. [Google Scholar] [CrossRef]
- Thompson, A.S.; Rhodes, J.C.; Pettman, I. Culture Collection of Algae and Protozoa: Catalogue of Strains; Titus Wilson and Son: Kendal, UK, 1988. [Google Scholar]
- Shishlyannikov, S.M.; Zakharova, Y.R.; Volokitina, N.A.; Mikhailov, I.S.; Petrova, D.P.; Likhoshway, Y.V. A procedure for establishing an axenic culture of the diatom Synedra acus subsp. radians (Kütz.) Skabibitsch. From Lake Baikal. Limnol. Oceanogr. Methods 2011, 9, 478–484. [Google Scholar] [CrossRef]
- Marmur, J. A procedure for the isolation of deoxyribonucleic acid from micro-organisms. J. Mol. Biol. 1961, 3, 208–218. [Google Scholar] [CrossRef]
- Johnson, M.; Zaretskaya, I.; Raytselis, Y.; Merezhuk, Y.; McGinnis, S.; Madden, T.L. NCBI BLAST: A better web interface. Nucleic Acids Res. 2008, 36, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Bruckner, C.G.; Rehm, C.; Grossart, H.P.; Kroth, P.G. Growth and release of extracellular organic compounds by benthic diatoms depend on interactions with bacteria. Environ. Microbiol. 2011, 13, 1052–1063. [Google Scholar] [CrossRef] [PubMed]
- Van Tol, H.M.; Amin, S.A.; Armbrust, E.V. Ubiquitous marine bacterium inhibits diatom cell division. ISME J. 2017, 11, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Bedoshvili, Y.D.; Popkova, T.P.; Likhoshway, Y.V. Chloroplast structure of diatoms of different classes. Cell Tissue Biol. 2009, 3, 297–310. [Google Scholar] [CrossRef]
- Pavlova, O.N.; Bukin, S.V.; Kostyreva, E.A.; Moskvin, V.I.; Manakov, A.Y.; Morozov, I.V.; Galachyants, Y.P.; Khabuev, A.V.; Zemskaya, T.I. Experimental transformation of organic matter by the microbial community from the bottom sediments of Akademichesky Ridge (Lake Baikal). Russ. Geol. Geophys. 2019, 60, 926–937. [Google Scholar] [CrossRef]
- Zulu, N.N.; Zienkiewicz, K.; Vollheyde, K.; Feussner, I. Current trends to comprehend lipid metabolism in diatoms. Prog. Lipid Res. 2018, 70. [Google Scholar] [CrossRef]
- Abida, H.; Dolch, L.-J.; Mei, C.; Villanova, V.; Conte, M.; Block, M.A.; Bastien, G.F.O.; Tirichine, L.; Bowler, C.; Rébeillé, F.; et al. Membrane glycerolipid remodeling triggered by nitrogen and phosphorus starvation in Phaeodactylum tricornutum. Plant Physiol. 2015, 167, 118–136. [Google Scholar] [CrossRef]
- Conte, M.; Lupette, J.; Seddiki, K.; Mei, C.; Dolch, L.-J.; Gros, V.; Barette, C.; Rébeillé, F.; Jouhet, J.; Maréchal, E. Screening for biologically annotated drugs that trigger triacylglycerol accumulation in the diatom Phaeodactylum. Plant Physiol. 2018, 177, 532–552. [Google Scholar] [CrossRef]
- Opute, F.I. Studies on fat accumulation in Nitzschia palea Kütz. Ann. Bot. 1974, 38, 889–902. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).