Dead Wood as an Element Enriching Biodiversity of Forest Ecosystems: A Case Study Based on Mites from the Suborder Uropodina (Acari: Parasitiformes)
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Uropodina Mite Communities Inhabiting Litter, Soil and Dead Wood Merocenoses in Examined Forest Ecosystems
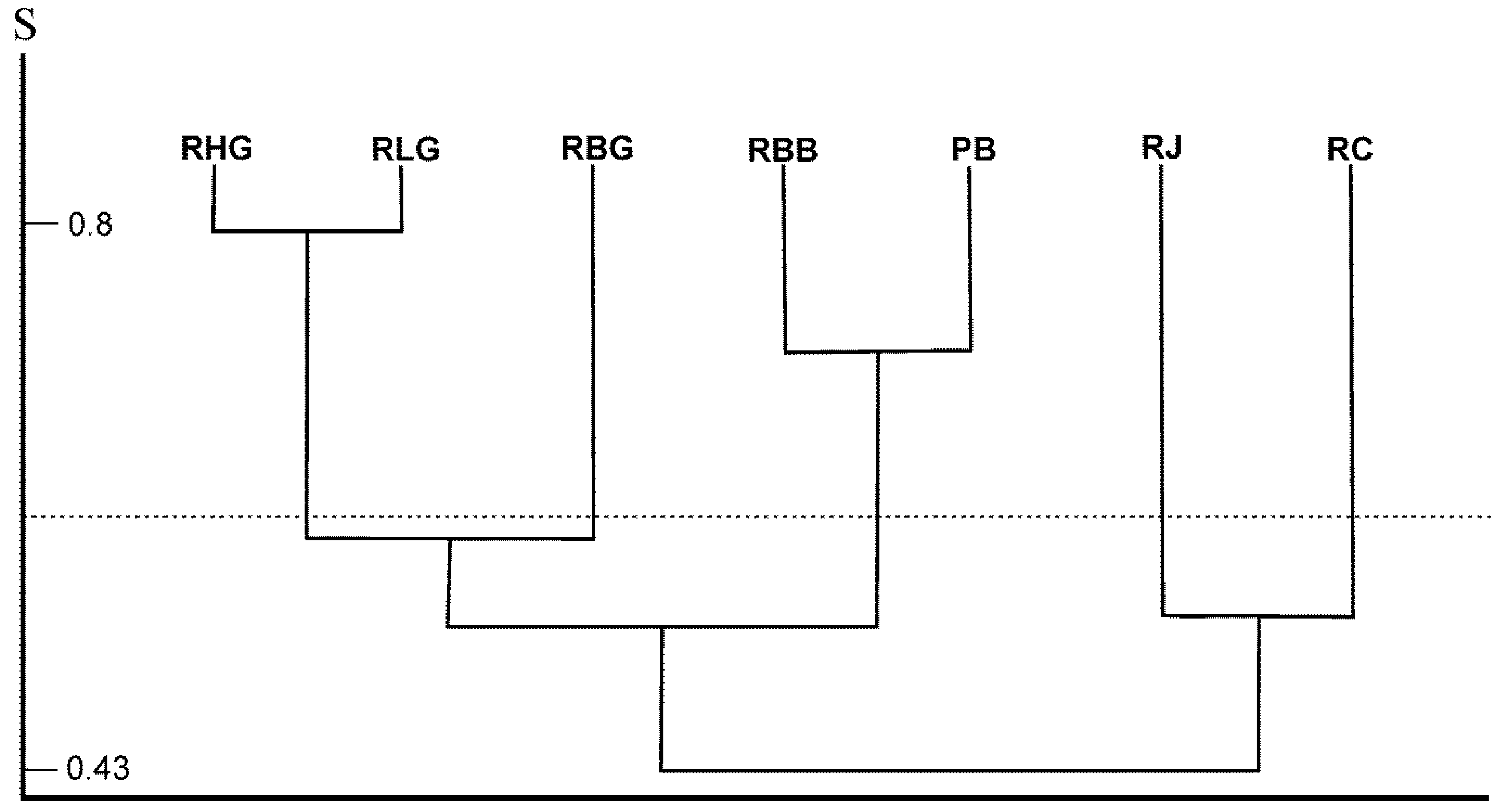
3.2. Differences in Species Composition in Dead Wood Merocenoses in Examined Locations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| Area | PB | RC | RJ | RL | RBB | RBG | RHG | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | D | F | D | F | D | F | D | F | D | F | D | F | D | F |
| T. aegrota | D2 | F3 | D1 | F2 | D2 | F3 | D2 | F4 | D2 | F3 | D2 | F2 | D2 | F4 |
| O. ovalis | D5 | F4 | D4 | F3 | D5 | F5 | D5 | F5 | D5 | F5 | D5 | F4 | D5 | F5 |
| O. minima | D1 | F1 | D1 | F2 | D1 | F2 | D1 | F3 | D2 | F2 | D2 | F2 | D1 | F2 |
| U. tecta | D1 | F2 | D1 | F2 | D1 | F1 | D1 | F1 | D1 | F3 | D3 | F2 | D1 | F2 |
| T. pauperior | D1 | F1 | D1 | F1 | D1 | F2 | D1 | F2 | D1 | F1 | D1 | F2 | ||
| D. woelkei | D1 | F1 | D1 | F1 | D1 | F2 | D3 | F2 | D1 | F2 | D2 | F2 | D2 | F2 |
| P. pulchella | D3 | F2 | D2 | F2 | D1 | F1 | D1 | F1 | D3 | F2 | D2 | F1 | D3 | F3 |
| U. pannonica | D1 | F1 | D1 | F1 | D1 | F1 | ||||||||
| D. perforatus | D1 | F1 | D1 | F1 | D2 | F1 | D1 | F1 | D1 | F1 | D1 | F1 | ||
| D. carinatus | D4 | F2 | D2 | F2 | D4 | F4 | D3 | F3 | D1 | F2 | D4 | F2 | D1 | F2 |
| Pseudouropoda spp. | D1 | F1 | D1 | F1 | D1 | F1 | D1 | F1 | D1 | F1 | ||||
| D. arcuatus | D2 | F2 | D1 | F2 | D1 | F1 | D1 | F1 | D1 | F1 | ||||
| A. infirmus | D1 | F1 | D1 | F1 | D1 | F1 | D1 | F1 | D1 | F1 | D1 | F1 | ||
| T.elegans | D1 | F1 | D2 | F2 | D1 | F1 | D1 | F1 | D1 | F1 | ||||
| C. rafalskii | D1 | F1 | D1 | F1 | D1 | F1 | ||||||||
| P. rackei | D1 | F1 | D1 | F1 | ||||||||||
| L. orbicularis | D1 | F1 | D1 | F1 | D1 | F1 | D1 | F1 | ||||||
| T. lamda | D1 | F1 | ||||||||||||
| C. insularis | D1 | F1 | D1 | F1 | ||||||||||
| P. sansonei | D1 | F1 | D1 | F1 | D1 | F1 | ||||||||
| O. karawaiewi | D1 | F1 | ||||||||||||
| D. inermis | D1 | F1 | ||||||||||||
| U. obovata | D1 | F1 | D1 | F1 | D1 | F1 | D1 | F1 | ||||||
| P. cylindricus | ||||||||||||||
| N. splendida | D1 | F1 | ||||||||||||
| D. baloghi | D1 | F1 | D3 | F2 | D1 | F1 | ||||||||
| P. pyriformis | D1 | F1 | D4 | F1 | D1 | F1 | ||||||||
| P. patavinus | D1 | F1 | D1 | F1 | D1 | F1 | ||||||||
| O. obscurasimilis | D1 | F1 | ||||||||||||
| D. modesta | D1 | F1 | ||||||||||||
| I. penicillata | D1 | F1 | ||||||||||||
| O. misella | ||||||||||||||
| O. kargi | D1 | F1 | ||||||||||||
| U. hamulifera | D1 | F1 | ||||||||||||
| U. paradoxa | D1 | F1 | D1 | F1 | ||||||||||
| N. breviunguiculata | D1 | F1 | D1 | F1 | ||||||||||
| P. tuberosa | D1 | F1 | ||||||||||||
| T. polytricha | D1 | F1 | ||||||||||||
| T. bipilis | D1 | F1 | ||||||||||||
| T. coccinea | D1 | F1 | ||||||||||||
| U. fumicola | ||||||||||||||
| U. conspicua | D1 | F1 | ||||||||||||
| O. stammeri | D1 | F1 | ||||||||||||
| O. franzi | D1 | F1 | ||||||||||||
| Oplitis sp. | ||||||||||||||
| U. ipidis | D1 | F1 | ||||||||||||
| U. baloghi | D1 | F1 | ||||||||||||
| U. similiobovata | D1 | F1 | ||||||||||||
| U. marginata | D1 | F1 | ||||||||||||
| N. stylifera | D1 | F1 | ||||||||||||
| D. cordieri | D1 | F1 | ||||||||||||
| D. septentrionalis | D1 | F1 | ||||||||||||
| D. sublaevis | ||||||||||||||
| Uropoda sp. | D1 | F1 | ||||||||||||
| Number of specimens | 2053 | 2826 | 847 | 1439 | 1241 | 1169 | 2081 | |||||||
| Number of species | 29 | 33 | 14 | 13 | 18 | 17 | 16 | |||||||
References
- Huhta, V.; Siira-Pietikäinen, A.; Penttinen, R. Importance of dead wood for soil mite (Acarina) communities in boreal old-growth forests. Soil Org. 2012, 84, 499–512. [Google Scholar]
- Błoszyk, J.; Napierała, A.; Markowicz-Rucińska, M. Zróżnicowanie zgrupowań Uropodina (Acari: Mesostigmata) wybranych rezerwatów Wielkopolski. Parki nar. Rez. Przyr. 2003, 22, 285–298. [Google Scholar]
- Napierała, A.; Błoszyk, J. Unstable microhabitats (merocenoses) as specific habitats of Uropodina mites (Acari: Mesostigmata). Exp. Appl. Acarol. 2013, 60, 163–180. [Google Scholar] [CrossRef] [Green Version]
- Napierała, A.; Konwerski, S.; Gutowski, J.M.; Błoszyk, J. Species diversity of Uropodina communities (Acari: Parasitiformes) in soil and selected microhabitats in the Białowieża Primeval Forest. In Mites (Acari) of the Białowieża Primeval Forest, 1st ed.; Błoszyk, J., Napierała, A., Eds.; Kontekst: Poznań, Poland, 2020; pp. 11–60. [Google Scholar]
- Niedbała, W.; Błoszyk, J.; Gutowski, J.M.; Konwerski, S.Z.; Napierała, A. A characteristic of communites of ptyctimous mites (Acari: Acariformes: Oribatida) in the Białowieża Primeval Forest, Central Europe. In Mites (Acari) of the Białowieża Primeval Forest, 1st ed.; Błoszyk, J., Napierała, A., Eds.; Kontekst: Poznań, Poland, 2020; pp. 61–127. [Google Scholar]
- Wielkoobszarowa inwentaryzacja stanu lasów w Polsce. Available online: https://www.bdl.lasy.gov.pl/portal/Media/Default/Publikacje/WISL2016_2020.pdf (accessed on 31 July 2021).
- Siitonen, J. Microhabitats. In Biodiversity in Dead Wood, 1st ed.; Stokland, J.N., Siitonen, J., Jonsson, B.G., Eds.; Cambridge University Press: Cambridge, UK, 2012; pp. 150–182. [Google Scholar]
- Siitonen, J.; Stokland, J.N. Tree size. In Biodiversity in Dead Wood, 1st ed.; Stokland, J.N., Siitonen, J., Jonsson, B.G., Eds.; Cambridge University Press: Cambridge, UK, 2012; pp. 183–193. [Google Scholar]
- Stokland, J.N.; Siitonen, J. Species diversity of saproxylic organisms. In Biodiversity in Dead Wood, 1st ed.; Stokland, J.N., Siitonen, J., Jonsson, B.G., Eds.; Cambridge University Press: Cambridge, UK, 2012; pp. 248–274. [Google Scholar]
- Gutowski, J.M.; Bobiec, A.; Pawlaczyk, P.; Zub, K. Drugie Życie Drzewa; WWF: Warszawa, Poland, 2004; 245p. [Google Scholar]
- Stokland, J.N.; Siitonen, J.; Bengt, G.J. Dead wood in natural forest. In Biodiversity in Dead Wood, 1st ed.; Cambridge University Press: Cambridge, UK, 2012; 301p. [Google Scholar]
- Ulyshen, M.D. Saproxylic Insects: Diversity, Ecology and Conservation, 1st ed.; Springer: Athens, GA, USA, 2018; 904p. [Google Scholar]
- Siitonen, J. Decaying wood and saproxylic Coleoptera in two old spruce forests: A comparison based on two sampling methods. Ann. Zool. Fenn. 1994, 31, 89–95. [Google Scholar]
- Tikkanen, O.-P.; Martikainen, P.; Hyvärinen, E.; Junninen, K.; Kouki, J. Red-listed boreal forest species of Finland: Associations with forest structure, tree species, and decaying wood. Ann. Zool. Fenn. 2006, 43, 373–383. [Google Scholar]
- Andersson, L.I.; Hytteborn, H. Bryophytes and decaying wood: A comparison between managed and natural forest. Holarctic Ecol. 1991, 14, 121–130. [Google Scholar] [CrossRef]
- Lindblad, I. Wood-inhabiting fungi on fallen logs of Norway spruce: Relations to forest management and substrate quality. Nord. J. Bot. 1998, 18, 243–255. [Google Scholar] [CrossRef]
- Siepel, H.; de Ruiter-Dijkman, E.M. Feeding guilds of oribatid mites based on their carbohydrase activities. Soil Biol. Biochem. 1993, 25, 1491–1498. [Google Scholar] [CrossRef]
- Erdmann, G.; Otte, V.; Langel, R.; Scheu, S.; Maraun, M. The trophic structure of bark-living oribatidmite communities analysed with stable isotopes (15N, 13C) indicates strong niche differentiation. Exp. Appl. Acarol. 2007, 41, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y. Predation by manure-inhabiting mesostigmatids (Acarina: Mesostigmata) on some free-living nematodes. Appl. Ent. Zool. 1971, 6, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Karg, W. Acari (Acarina) Milben, Unterordnung Parasitiformes (Anactinochaeta). Uropodina Kramer, Schildkrötenmilben. In Die Tierwelt Deutschlands und der Angrenzenden Meeresteile; Senglaub, K., Hannemann, H.-J., Schumann, H., Eds.; Teil.–Gustav Fischer Verlag: Jena, Thuringia, 1989; Volume 67, pp. 1–203. [Google Scholar]
- Karg, W. Acari (Acarina) Milben, Parasitiformes (Anactinochaeta) Cohors Gamasina Leach: Raubmilben. In Zoologisches Museum Berlin, 1st ed.; Dahl, F., Ed.; Die Tierwelt Deutschlands und der angrenzenden Meeresteilem, 59; Teil.–Gustav Fischer Verlag: Jena, Thuringia, 1993; pp. 1–523. [Google Scholar]
- Koehler, H. Mesostigmata (Gamasida, Uropodina), efficient predators in agroecosysytems. Agric. Ecosyst. Environ. 1997, 62, 105–117. [Google Scholar] [CrossRef]
- Koehler, H. Predatory mites (Gamasida, Mesostigmata). Agric. Ecosyst. Environ. 1999, 74, 395–410. [Google Scholar] [CrossRef]
- Faasch, H. Beitrag zur Biologie der einheimischen Uropodiden Uroobovella marginata (C. L. Koch 1839) und Uropoda orbicularis (O. F. Müller 1776) und experimentelle Analyse ihres Phoresieverhaltens. Zool. Jb. Syst. Bd. 1967, 94, 521–608. [Google Scholar]
- El-Banhawy, E.M.; El-Borolossy, M.A.; El-Sawaf, B.M.; Afia, S.I. Biological aspects and feeding behaviour of the predacious soil mite Nenteria hypotrichus (Uropodina: Uropodidae). Acarology 1997, 38, 356–360. [Google Scholar]
- Athias-Binche, F. Etude quantitative des Uropodides (Acariens: Anactinotriches) d’un arbre mort de la Hetraie de La Massane. 1. Caracteres generaux du peuplement. Vie Et Mileu 1977, 27, 157–175. [Google Scholar]
- Athias-Binche, F. Etude quantitative des Uropodides (Acariens: Anactinotriches) d’un arbre mort de la Hetraie de La Massane. 2. Elemenet demographiques d’une population d’Allodinychus flagelliger (Berlese, 1910). Vie Et Mileu 1979, 28-29, 35–60. [Google Scholar]
- Błoszyk, J. Fauna Uropodina (Acari: Mesostigmata) spróchniałych pni drzew i dziupli w Polsce. Zesz. Probl. Post. Nauk Roln. 1990, 373, 217–235. [Google Scholar]
- Athias-Binche, F. La Phoresie Chez les Acariens. Aspects Adapttifs et Evolutifs; Presses de l’Universite de Perpignan: Perpignan, France, 1994; 179p. [Google Scholar]
- Błoszyk, J. Geograficzne i Ekologiczne Zróżnicowanie Zgrupowań Roztoczy z Kohoryty Uropodina (Acari: Mesostigmata) w Polsce: Uropodina Lasów Grądowych (Carpinion betuli); Kontekst: Poznań, Poland, 1999; 245p. [Google Scholar]
- Bajerlein, D.; Błoszyk, J. Two cases of hyperphoresy in mesostigmatic mites (Acari: Gamasida; Uropodidae, Macrochelidae). Biol. Lett. 2003, 40, 135–136. [Google Scholar]
- Bajerlein, D.; Błoszyk, J. Phoresy of Uropoda orbicularis (Acari: Mesostigmata) by beetles (Coleoptera) associated with catlle dung in Poland. Eur. J. Entomol. 2004, 101, 185–188. [Google Scholar] [CrossRef]
- Błoszyk, J.; Klimczak, J.; Leśniewska, M. Phoretic relationships between Uropodina (Acari: Mesostigmata) and centipedes (Chilopoda) as an example of evolutionary adaptation of mites to temporary microhabitats. Eur. J. Entomol. 2006, 103, 699–707. [Google Scholar] [CrossRef] [Green Version]
- Napierała, A.; Książkiewicz, Z.; Leśniewska, M.; Gwiazdowicz, D.J.; Mądra, A.; Błoszyk, J. Phoretic relationships between uropodid mites (Acari: Mesostigmata) and centipedes (Chilopoda) in urban agglomeration areas. Int. J. Acarol. 2015, 41, 250–258. [Google Scholar] [CrossRef]
- Konwerski, S.; Gutowski, J.M.; Książkiewicz-Parulska, Z.; Błoszyk, J. Repeatability of the phoretic relationships between mites of the genus Trichouropoda Berlese (Parasitiformes: Uropodina) and longhorn beetles of the genus Tetropium Kirby (Coleoptera: Cerambycidae) in Białowieża Primeval Forest, Central Europe. Int. J. Acarol. 2017, 43, 612–621. [Google Scholar] [CrossRef]
- Konwerski, S.; Gutowski, J.; Błoszyk, J. Analysis of the phoretic relationships between mites of the genus Trichouropoda Berlese (Parasitiformes: Uropodina) and the longhorn beetle Plagionotus detritus (Linnaeus). Int. J. Acarol. 2019, 45, 29–40. [Google Scholar] [CrossRef]
- Konwerski, S.; Gutowski, J.; Błoszyk, J. Patterns of Distribution of Phoretic Deutonymphs of Uropodina on Longhorn Beetles in Białowieża Primeval Forest, Central Europe. Diversity 2020, 12, 239. [Google Scholar] [CrossRef]
- Faliński, J.B. Vegetation Dynamics in Temperate Lowland Primeval Forest. Ecological Studies in Białowieża Forest, 1st ed.; Dr. W. Junk Publishers: Dordrecht, The Netherlands, 1986; pp. 1–537. [Google Scholar]
- Bobiec, A.; Burgt, H.; Meijer, K.; Zuyderduyn, C.; Haga, J.; Vlaanderen, B. Rich deciduous forests in Białowieża as a dynamic mosaic of developmental phases: Premises for nature conservation and restoration management. Forest Ecol. Manag. 2000, 130, 159–175. [Google Scholar] [CrossRef]
- Kujawa, A.; Orczewska, A.; Falkowski, M.; Blicharska, M.; Bohdan, A.; Buchholz, L.; Chylarecki, P.; Gutowski, J.M.; Latałowa, M.; Mysłajek, R.W.; et al. The Białowieża Forest—A UNESCO Natural Heritage Site—Protection priorities. For. Res. Pap. 2016, 77, 302–323. [Google Scholar] [CrossRef] [Green Version]
- Jaroszewicz, B.; Cholewińska, O.; Gutowski, J.M.; Samojlik, T.; Zimny, M.; Latałowa, M. Białowieża Forest – a relic of the high naturalness of European forests. Forests 2019, 10, 849. [Google Scholar] [CrossRef] [Green Version]
- Myczkowski, S. Zespoły leśne rezerwatu cisowego “Wierzchlas”. Ochrona Przyrody 1961, 27, 91–107. [Google Scholar]
- Boiński, M. Szata roślinna Borów Tucholskich; PWN: Warszawa, Poland, 1985; 107p. [Google Scholar]
- Błoszyk, J.; Krysiak, D. Uropodina (Acari: Mesostigmata) rezerwatu “Cisy Staropolskie im. Leona Wyczółkowskiego” w Wierzchlesie. Park. Nar. Rez. Przy. 2000, 19, 115–121. [Google Scholar]
- Napierała, A.; Labijak, B.; Skwierczyński, F.; Konwerski, S.; Błoszyk, J. Influence of habitat type and natural disturbances on uropodine mite communities (Acari: Mesostigmata: Uropodina) in oak hornbeam forests in Central Europe. Int. J. Acarol. 2014, 41, 41–52. [Google Scholar] [CrossRef]
- Błoszyk, J.; Markowicz, M.; Labijak, B.; Skwierczyński, F.; Napierała, A. Microgeographic diversity of Uropodina (Acari: Mesostigmata) communities in dead wood and tree hollows. Redia 2015, 98, 3–12. [Google Scholar]
- Błoszyk, J.; Napierała, A. Community structure of mesofauna in the light of qualitative and quantitative research on soil mites. Eur. J. Biol. Res. 2018, 8, 252–262. [Google Scholar]
- Hirschmann, W.; Zirngiebl-Nicol, I. Gangsystematic der Parasitiformes. Acarol. Schr. Für Vgl. Milbenkunde 1961, 4, 1–41. [Google Scholar]
- Błoszyk, J. Uropodina Polski (Acari: Mesostigmata). Ph.D. Thesis, Adam Mickiewicz University, Poznań, Poland, 1983; p. 543. [Google Scholar]
- Karg, W. Acari (Acarina) Milben, Unterordnung Parasitiformes (Anactinochaeta). Uropodina Kramer Schildkrötenmilben. Tierwelt Deutsch. 1989, 67, 1–203. [Google Scholar]
- Mašan, P. Mites of the cohort Uropodina (Acarina, Mesostigmata) in Slovakia, 1st ed.; Annotationes zoologicae et botanicae: Bratislava, Slovakia, 2001; 320p. [Google Scholar]
- Magurran, A.E. Measuring Biological Diversity; Blackwell Publishing: Oxford, UK, 2004; 256p. [Google Scholar]
- Napierała, A.; Książkiewicz-Parulska, Z.; Błoszyk, J. A Red List of mites from the suborder Uropodina (Acari: Parasitiformes) in Poland. Exp. Appl. Acarol. 2018, 75, 467–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuberski, Ł.; Paluch, R.; Kraszewski, B.; Zin, E.; Stereńczak, K. Zasoby Martwego Drewna w Puszczy Białowieskiej na Podstawie Aktualnej Inwentaryzacji na Stałych Powierzchniach Badawczych, Instytut Badawczy Leśnictwa. Available online: http://www.forbiosensing.pl/documents/20182/48898/LK+ref/e3c721de-6bcc-408b-830c-568d72e2d835 (accessed on 4 August 2021).
- Napierała, A.; Błoszyk, J. The maturity index for Uropodina (Acari: Mesostigmata) communities as an indicator of human-caused disturbance in selected forest complexes of Poland. Exp. Appl. Acarol. 2021, 83, 475–491. [Google Scholar] [CrossRef] [PubMed]
- Bongers, T. The maturity index: An ecological measure of environmental disturbance based on nematodes species composition. Oecologia 1990, 83, 14–19. [Google Scholar] [CrossRef]
- Napierała, A. Struktura Zgrupowań i Rozkład Przestrzenny Uropodina (Acari: Mesostigmata) w Wybranych Kompleksach Leśnych Wielkopolski. Ph.D. Thesis, Adam Mickiewicz University, Poznań, Poland, 2008. [Google Scholar]
- Skubała, P.; Duras, M. Do decaying logs represent habitat islands? Oribatid mite communities in dead wood. Ann. Zool. 2008, 58, 453–466. [Google Scholar] [CrossRef]
- Lindo, Z.; Winchester, N.N. A comparison of microarthropod assemblages with emphasis on oribatid mites in canopy suspended soil and forest floors associated with ancient western red cedar trees. Pedobiologia 2006, 50, 31–41. [Google Scholar] [CrossRef]
- Siira-Pietikäinen, A.; Penttinen, R.; Huhta, V. Oribatid mites (Acari: Oribatida) in boreal forest floor and decaying wood. Pedobiologia 2008, 52, 111–118. [Google Scholar] [CrossRef]
- Déchêne, A.D.; Buddle, C.M. Decomposing logs increase oribatid mite assemblage diversity in mixedwood boreal forest. Biodivers. Conserv. 2010, 19, 237–256. [Google Scholar] [CrossRef]

| Area | PB | RC | RJ | RL | RBB | RBG | RHG |
|---|---|---|---|---|---|---|---|
| Location | northern-east Poland, 52°46′ N 23°52′ E | northern-west Poland, 53°30′ N 18°07′ E | western Poland, 52°29′ N 16°16′ E | western Poland, 52°28′ N 16°14′ E | western Poland, 52°28′ N 16°28′ E | western Poland, 52°28′ N 16°29′ E | western Poland, 52°27′ N 16°31′ E |
| Legal status | national park, strict nature reserve and unprotected areas | nature reserve | nature reserve | nature reserve | nature reserve | nature reserve | nature reserve |
| Establishment year | 1932 | 1827 | 1959 | 1959 | 1959 | 1959 | 1959 |
| Age of tree stand [years] | >150 | >150 | >150 | <100 | ~100 | >100 | >80 |
| Area [ha] | 10,517 | 113.61 | 4.02 | 7.32 | 15.15 | 6.71 | 14.73 |
| Data collection period | 1961–2018 | 1992–2019 | 1978–2000 | 1979–2000 | 2002–2004 | 2002–2004 | 2002–2004 |
| Number of samples | 249 | 979 | 3489 | 2101 | 200 | 285 | 226 |
| Dead wood [m3/ ha] | 131 | 118 | 47 | 47 | 39 | 39 | 39 |
| % of dead wood in reference area | 83 | 75 | 30 | 30 | 78 | 78 | 78 |
| References | [4] | [47] | [45,47] | [45,47] | [46] | [46] | [46] |
| Figures | S1 | S2 | S3 | S4 | S5 | S6 | S7 |
| Area | PB | RC | RJ | RL | RBB | RBG | RHG | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of Habitat | S | DW | S | DW | S | DW | S | DW | S | DW | S | DW | S | DW |
| Number of Samples | 92 | 157 | 800 | 179 | 3434 | 55 | 2051 | 50 | 100 | 100 | 95 | 190 | 124 | 102 |
| Trachytes aegrota (Koch, 1841) | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Oodinychus ovalis (Koch, 1839) * | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Olodiscus minima (Kramer, 1882) | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| Urodiaspis tecta (Kramer, 1876) | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| Trachytes pauperior (Berlese, 1914) | + | + | + | + | + | + | + | + | + | + | + | + | ||
| Dinychus woelkei Hirschmann et Zirngiebl-Nicol, 1969 | + | + | + | + | + | + | + | + | + | + | ||||
| Pulchellaobovella pulchella (Berlese, 1904) * | + | + | + | + | + | + | + | + | + | + | ||||
| Urodiaspis pannonica Willmann, 1952 | + | + | + | + | + | + | + | + | + | + | ||||
| Dinychus perforatus Kramer, 1882 | + | + | + | + | + | + | + | + | + | + | ||||
| Dinychus carinatus Berlese, 1903 | + | + | + | + | + | + | + | + | + | + | ||||
| Pseudouropoda spp. | + | + | + | + | + | + | + | |||||||
| Dinychus arcuatus (Trägårdh, 1922) | + | + | + | + | + | + | + | |||||||
| Apionoseius infirmus Berlese, 1887 * | + | + | + | + | + | + | + | |||||||
| Trematurella elegans (Kramer, 1882) | + | + | + | + | + | + | + | |||||||
| Cilliba rafalskii Błoszyk, Stachowiak et Halliday 2006 | + | + | + | + | + | + | ||||||||
| Phaulodiaspis rackei (Oudemans, 1912) | + | + | + | + | + | + | ||||||||
| Leiodinychus orbicularis (Koch, 1839) * | + | + | + | + | + | + | ||||||||
| Trachytes lamda (Berlese, 1903) | + | + | + | + | + | |||||||||
| Cilliba insularis Willmann, 1938 | + | + | + | + | + | |||||||||
| Polyaspis sansonei Berlese, 1916 | + | + | + | + | + | |||||||||
| Oodinychus karawaiewi (Berlese, 1903) * | + | + | + | + | ||||||||||
| Dinychus inermis (Koch, 1841) | + | + | + | + | ||||||||||
| Uroobovella obovata (Canestrini et Berlese, 1884) * | + | + | + | + | ||||||||||
| Polyaspinus cylindricus Berlese, 1916 | + | + | + | |||||||||||
| Neodiscopoma splendida (Kramer, 1882) | + | + | + | |||||||||||
| Discourella baloghi Hirschmann et Zirngiebl-Nicol, 1969 | + | + | + | |||||||||||
| Uroobovella pyriformis (Berlese, 1920) * | + | + | + | |||||||||||
| Polyaspis patavinus Berlese, 1881 | + | + | + | |||||||||||
| Oodinychus obscurasimilis (Hirschmann et Zirngiebl-Nicol, 1961) | + | + | ||||||||||||
| Discourella modesta (Leonardi, 1889) | + | + | ||||||||||||
| Ipiduropoda penicillata (Hirschmann et Zirngiebl-Nicol, 1961) | + | + | ||||||||||||
| Olodiscus misella (Berlese, 1916) | + | + | ||||||||||||
| Olodiscus kargi (Hirschamann et Zirngiebl-Nicol, 1969) | + | + | ||||||||||||
| Uropolyaspis hamulifera Berlese, 1904 * | + | + | ||||||||||||
| Uroplitella paradoxa (Canestrini et Berlese, 1884) | + | + | ||||||||||||
| Nenteria breviunguiculata (Willmann, 1949) * | + | + | ||||||||||||
| Pseudouropoda tuberosa (Hirschmann et Zirngiebl-Nicol, 1961) * | + | |||||||||||||
| Trichouropoda polytricha (Vitzthum, 1923) * | + | |||||||||||||
| Trichouropoda bipilis (Vitzthum, 1920) | + | |||||||||||||
| Trachyuropoda coccinea (Michael, 1891) | + | |||||||||||||
| Uropoda fumicola Hirschmann et Zirngiebl-Nicol, 1969 | + | |||||||||||||
| Uroplitella conspicua Berlese, 1903 | + | |||||||||||||
| Oplitis stammeri (Hirschmann et Zirgiebl-Nicol, 1961) | + | |||||||||||||
| Oplitis franzi Hirschmann et Zirngiebl-Nicol, 1969 | + | |||||||||||||
| Oplitis sp. | + | |||||||||||||
| Uroobovella ipidis Hirschmann et Zirngiebl-Nicol, 1962 | + | |||||||||||||
| Uroobovella baloghi Hirschmann et Zirngiebl-Nicol, 1962 | + | |||||||||||||
| Uroobovella similiobovata Hirschmann et Zirgiebl-Nicol, 1962 | + | |||||||||||||
| Uroobovella marginata (Koch, 1829) | + | |||||||||||||
| Nenteria stylifera (Berlese, 1904) | + | |||||||||||||
| Dinychura cordieri (Berlese, 1916) | + | |||||||||||||
| Dinychus septentrionalis (Trägårdh, 1943) | + | |||||||||||||
| Dinychus sublaevis (Trägårdh, 1943) | + | |||||||||||||
| Uropoda sp. | + | |||||||||||||
| Number of species in S or DW | 24 | 29 | 24 | 33 | 13 | 14 | 12 | 13 | 8 | 18 | 6 | 17 | 10 | 16 |
| Total number of species in the community | 37 | 37 | 19 | 18 | 19 | 19 | 18 | |||||||
| Habitat/Microhabitat | PB | RC | RJ | RLG | RBB | RBG | RHG | Ave ± SD |
|---|---|---|---|---|---|---|---|---|
| soil | 64.9 | 65.8 | 68.4 | 66.7 | 42.1 | 35.3 | 55.6 | 57.0 ± 13.3 |
| soil/dead wood | 43.2 | 52.6 | 42.1 | 38.9 | 36.8 | 35.3 | 44.4 | 41.9 ± 5.8 |
| dead wood | 35.1 | 34.2 | 31.6 | 33.3 | 57.9 | 64.7 | 44.4 | 43.0 ± 13.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Błoszyk, J.; Rutkowski, T.; Napierała, A.; Konwerski, S.; Zacharyasiewicz, M. Dead Wood as an Element Enriching Biodiversity of Forest Ecosystems: A Case Study Based on Mites from the Suborder Uropodina (Acari: Parasitiformes). Diversity 2021, 13, 476. https://doi.org/10.3390/d13100476
Błoszyk J, Rutkowski T, Napierała A, Konwerski S, Zacharyasiewicz M. Dead Wood as an Element Enriching Biodiversity of Forest Ecosystems: A Case Study Based on Mites from the Suborder Uropodina (Acari: Parasitiformes). Diversity. 2021; 13(10):476. https://doi.org/10.3390/d13100476
Chicago/Turabian StyleBłoszyk, Jerzy, Tomasz Rutkowski, Agnieszka Napierała, Szymon Konwerski, and Michał Zacharyasiewicz. 2021. "Dead Wood as an Element Enriching Biodiversity of Forest Ecosystems: A Case Study Based on Mites from the Suborder Uropodina (Acari: Parasitiformes)" Diversity 13, no. 10: 476. https://doi.org/10.3390/d13100476
APA StyleBłoszyk, J., Rutkowski, T., Napierała, A., Konwerski, S., & Zacharyasiewicz, M. (2021). Dead Wood as an Element Enriching Biodiversity of Forest Ecosystems: A Case Study Based on Mites from the Suborder Uropodina (Acari: Parasitiformes). Diversity, 13(10), 476. https://doi.org/10.3390/d13100476








