Is a High Abundance of Spring Diatoms in the Photic Zone of Lake Baikal in July 2019 Due to an Upwelling Event?
Abstract
:1. Introduction
2. Materials and Methods
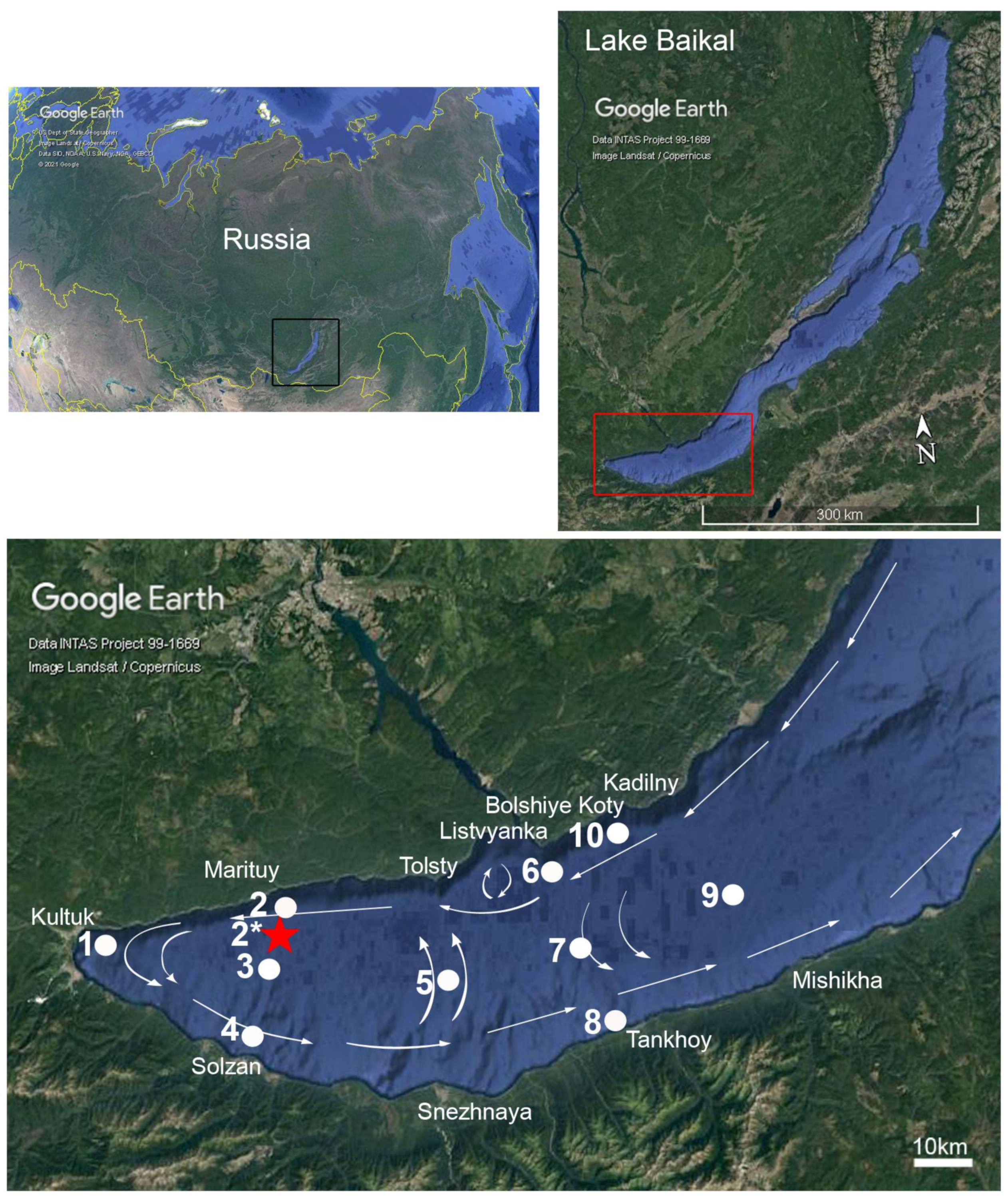
2.1. Study Area and Sampling
2.2. Enumeration of the Number of Microalgae and Cell Length Measurement
2.3. Determination of Silicon Assimilation Activity in Diatom Cells
2.4. Analysis of Environmental Parameters
2.5. The Bathymetry Map of the South Baikal Slope
3. Results and Discussion
3.1. Determination of the Species Composition of the Alga, Morphometric of Cells, and Analysis of the Absorption of Silicon by Cells
3.2. Lake Observations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Furnas, M.J. In situ growth rates of marine phytoplankton: Approaches to measurement, community and species growth rates. J. Plan. Res. 1990, 12, 1117–1151. [Google Scholar] [CrossRef]
- Winder, M.; Cloern, J.E. The annual cycles of phytoplankton biomass. Philos. Trans. R. Soc. 2010, 365, 3215–3226. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.L.; Izmest’eva, L.R.; Hampton, S.E.; Ozersky, T.; Shchapov, K.; Moore, M.V.; Shimaraeva, S.V.; Silow, E.A. The “Melosira years” of Lake Baikal: Winter environmental conditions at ice onset predict under-ice algal blooms in spring. Limnol. Oceanogr. 2015, 60, 1950–1964. [Google Scholar] [CrossRef]
- Scordo, F.; Chandra, S.; Suenaga, E.; Kelson, S.J.; Culpepper, J.; Scaf, L.; Tromboni, F.; Caldwell, T.J.; Seitz, C.; Fiorenza, J.E.; et al. Smoke from regional wildfires alters lake ecology. Sci. Rep. 2021, 11, 10922. [Google Scholar] [CrossRef] [PubMed]
- Izmest’eva, L.R.; Moore, M.V.; Hampton, S.E.; Silow, E.A. Seasonal dynamics of common phytoplankton in Lake Baikal. Proc. Russ. Acad. Sci. Cent. 2006, 8, 191–196. [Google Scholar]
- Pomazkina, G.V.; Belykh, O.I.; Domysheva, V.M.; Sakirko, M.V.; Gnatovsky, R.Y. Structure and dynamics of phytoplankton of Southern Baikal (Russia). Int. J. Algae 2010, 12, 64–79. [Google Scholar] [CrossRef]
- Popovskaya, G.I.; Usol’tseva, M.V.; Domysheva, V.M.; Sakirko, M.V.; Blinov, V.V.; Khodzher, T.V. The spring phytoplankton in the pelagic zone of Lake Baikal during 2007–2011. Geogr. Nat. Resour. 2015, 36, 253–262. [Google Scholar] [CrossRef]
- Popovskaya, G.I.; Genkal, S.I.; Likhoshway, Y.V. Diatoms of the Plankton of Lake Baikal. Atlas and Key, 2nd ed.; Trifonova, I.S., Grawford, R.M., Eds.; Nauka: Novosibirsk, Russia, 2016. [Google Scholar]
- Grachev, M.A. About the Current State of the Ecological System of Lake Baikal; Publishing House of SB RAS: Novosibirsk, Russia, 2002. (In Russian) [Google Scholar]
- Popovskaya, G.I. Ecological monitoring of phytoplankton in Lake Baikal. Aquat. Ecosyst. Health 2000, 3, 215–225. [Google Scholar] [CrossRef]
- Popovskaya, G.I.; Belykh, O.I. Stages of the study of autotrophic picoplankton of Lake Baikal. Hydrobiol. J. 2003, 39, 12–24. [Google Scholar] [CrossRef]
- Belykh, O.I.; Pomazkina, G.V.; Tikhonova, I.V.; Tomberg, I.V. Characteristics of Lake Baikal summer phytoplankton and autotrophic picoplankton. Int. J. Algae 2007, 9, 247–263. [Google Scholar] [CrossRef]
- Sherstyankin, P.P.; Kuimova, L.N.; Minenko, R.E. Dynamic conditions of frontogenesis and vortex genesis in Lake Baikal. Dokl. Akad. Nauk 1995, 345, 251–255. (In Russian) [Google Scholar]
- Shimaraev, M.N.; Troitskaya, E.S.; Blinov, V.V.; Ivanov, V.G.; Gnatovsky, R.Y. Upwellings in Lake Baikal. Dokl. Earth Sci. 2012, 442, 272–276. [Google Scholar] [CrossRef]
- Troitskaya, E.; Blinov, V.; Ivanov, V.; Zhdanov, A.; Gnatovsky, R.; Sutyrina, E.; Shimaraev, M. Cyclonic circulation and upwelling in Lake Baikal. Aquat. Sci. 2015, 77, 171–182. [Google Scholar] [CrossRef] [Green Version]
- Boyarinov, M.P.; Petrov, P.M. Thermal Regime Forming Processes of Deep Fresh Water Reservoirs; Nauka: Moscow, Russia, 1991. (In Russian) [Google Scholar]
- Shimaraev, M.N.; Verbolov, V.I.; Granin, N.G.; Sherstyankin, P.P. Physical Limnology of Lake Baikal: A Review; Shimaraev, M.N., Oruda, S., Eds.; Baikal International Center for Ecological Research: Irkutsk, Russia, 1994; p. 81. (In Russian) [Google Scholar]
- Shimaraev, M.N.; Gnatovskii, R.Y.; Blinov, V.V.; Ivanov, V.G. Renewal of deep waters of Lake Baikal revisited. Dokl. Earth Sci. 2011, 438, 652–655. [Google Scholar] [CrossRef]
- Piccolroaz, S.; Toffolon, M. The fate of Lake Baikal: How climate change may alter deep ventilation in the largest lake on Earth. Clim. Change 2018, 150, 181–194. [Google Scholar] [CrossRef] [Green Version]
- Roberts, D.C.; Egan, G.C.; Forrest, A.L.; Largier, J.L.; Bombardelli, F.A.; Laval, B.E.; Monismith, S.G.; Schladow, G. The setup and relaxation of spring upwelling in a deep, rotationally influenced lake. Limnol. Oceanogr. 2021, 66, 1168–1189. [Google Scholar] [CrossRef]
- Müller, B.; Maerki, M.; Schmid, M.; Vologina, E.G.; Wehrli, B.; Wüest, A.; Sturm, M. Internal carbon and nutrient cycling in Lake Baikal: Sedimentation, upwelling, and early diagenesis. Glob. Planet. Change 2005, 46, 101–124. [Google Scholar] [CrossRef]
- Moore, M.V.; Hampton, S.E.; Izmest’Eva, L.R.; Silow, E.A.; Peshkova, E.V.; Pavlov, B.K. Climate change and the world’s “sacred sea”—Lake Baikal, Siberia. BioScience 2009, 59, 405–417. [Google Scholar] [CrossRef] [Green Version]
- Verbolov, V.I. Currents and water exchange in Lake Baikal. Russ. Water Res. 1996, 23, 381–391. [Google Scholar]
- Utermöl, H. Zur Vervollkommnung der quatitativen Phytoplankton-Methodik: Mit 1 Tabelle und 15 abbildungen im Text und auf 1 Tafel. Int. Ver. Theor. Angew. Limnol. Mitt. 1958, 9, 1–38. [Google Scholar]
- Guseva, K.A. The methodology of phytoplankton accounting. Tr. Inst. Biol. Vodokhranilisch 1959, 2, 44–51. [Google Scholar]
- Sadchikov, A.P. Methods for Studying Freshwater Phytoplankton; Publishing House “University and School”: Moscow, Russia, 2003. (In Russian) [Google Scholar]
- Wiebe, P.H.; Benfield, M.C. From the Hensen net toward four-dimensional biological oceanography. Prog. Oceanogr. 2003, 56, 7–136. [Google Scholar] [CrossRef]
- Abakumov, V.A. Manual for Biological Monitoring of Freshwater Ecosystems; Gidrometeoizdat: St. Petersburg, Russia, 1992. (In Russian) [Google Scholar]
- Kuzmin, G.V. Phytoplankton. Species composition and abundance. In Techniques for Studying Biogeocenoses of Inland Water Bodies; Nauka: Moscow, Russia, 1975. (In Russian) [Google Scholar]
- Bedoshvili, Y.D.; Volokitina, N.A.; Marchenkov, A.M. Valve morphogenesis and silicon dynamics in the synchronized culture of Ulnaria danica. Limnol. Freshw. Biol. 2019, 5, 297–301. [Google Scholar] [CrossRef] [Green Version]
- Marsaglia, G.; Tsang, W.W.; Wang, J. Evaluating Kolmogorov’s distribution. J. Statist. Soft. 2003, 8, 1–4. [Google Scholar] [CrossRef]
- Descles, J.; Vartanian, M.; Harrak, A.E.; Quinet, M.; Bremond, N.; Sapriel, G.; Bibette, J.; Lopez, P.J. New tools for labeling silica in living diatoms. New Phytol. 2008, 177, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Bedoshvili, Y.; Gneusheva, K.; Popova, M.; Morozov, A.; Likhoshway, Y. Anomalies in the valve morphogenesis of the centric diatom alga Aulacoseira islandica caused by microtubule inhibitors. Biol. Open 2018, 7, bio035519. [Google Scholar] [CrossRef] [Green Version]
- ISO 7890-3:1988 Water quality—Determination of nitrate—Part 3: Spectrometric method using sulfosalicylic acid. 1988. Available online: https://www.iso.org/obp/ui/#iso:std:iso:7890:-3:ed-1:v1:en (accessed on 29 April 2021).
- Wetzel, R.G.; Likens, G.E. Limnological Analyses; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- ISO 6878:2004 Water Quality—Determination of Phosphorus—Ammonium Molybdate Spectrometric Method. 2004. Available online: https://www.iso.org/obp/ui/#iso:std:iso:6878:ed-2:v1:en (accessed on 29 April 2021).
- Khlystov, O.M.; Kononov, E.E.; Minami, H.; Khabuev, A.V.; Gubin, N.A.; Chenskii, A.G. New evidence on the relief of the southern underwater slope in the South Baikal Basin. Geogr. Nat. Resour. 2018, 39, 33–38. [Google Scholar] [CrossRef]
- Kononov, E.E.; Khlystov, O.M.; Kazakov, A.V.; Khabuev, A.V.; De Batist, M.; Naudts, L.; Minami, H. The lake floor morphology of the Southern Baikal rift basin as a result of Holocene and Late Pleistocene seismogenic and gravitational processes. Quat. Int. 2019, 524, 115–121. [Google Scholar] [CrossRef]
- Round, F.E.; Crawford, R.M.; Mann, D.G. Diatoms: Biology and Morphology of the Genera; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Podunay, Y.; Davidovich, N.A.; Kulikovskiy, S.; Gusev, E.S. Features of sexual reproduction and mating system of Ulnaria acus (Bacillariophyta). J. Sib. Fed. Univ.-Biol. 2018, 11, 75–87. (In Russian) [Google Scholar] [CrossRef] [Green Version]
- Saxton, M.A.; D’souza, N.A.; Bourbonniere, R.A.; McKay, R.M.L.; Wilhelm, S.W. Seasonal Si:C ratios in Lake Erie diatoms—Evidence of an active winter diatom community. J. Great Lakes Res. 2012, 38, 206–211. [Google Scholar] [CrossRef]
- Domysheva, V.M.; Pestunov, D.A.; Sakirko, M.V.; Shamrin, A.M.; Panchenko, M.V. Carbon dioxide, oxygen, and biogenic elements in subglacial water in the littoral zone of southern Baikal (2004–2016). Atmos. Ocean. Opt. 2017, 30, 277–283. [Google Scholar] [CrossRef]
- Khodzher, T.V.; Domysheva, V.M.; Sorokovikova, L.M.; Sakirko, M.V.; Tomberg, I.V. Current chemical composition of Lake Baikal water. Inland Waters 2017, 7, 250–258. [Google Scholar] [CrossRef]
- Mikhailov, I.S.; Zakharova, Y.R.; Bukin, Y.S.; Galachyants, Y.P.; Petrova, D.P.; Sakirko, M.V.; Likhoshway, Y.V. Co-occurrence networks among bacteria and microbial eukaryotes of Lake Baikal during a spring phytoplankton bloom. Microb. Ecol. 2019, 77, 96–109. [Google Scholar] [CrossRef]
- Zhdanov, A.A.; Granin, N.G.; Gnatovsky, R.Y.; Blinov, V.V. Horizontal macroturbulent exchange and dissipation rate of turbulent energy in the pelagic zone of Lake Baikal. Russ. Geogr. Nat. Res. 2009, 30, 30–34. [Google Scholar] [CrossRef]
- Tilstone, G.H.; Míguez, B.M.; Figueiras, F.G.; Fermín, E.G. Diatom dynamics in a coastal ecosystem affected by upwelling: Coupling between species succession, circulation and biogeochemical processes. Mar. Ecol. Prog. Ser. 2000, 205, 23–41. [Google Scholar] [CrossRef] [Green Version]
- Schnetzer, A.; Jones, B.H.; Schaffner, R.A.; Cetinic, I.; Fitzpatrick, E.; Miller, P.E.; Seubert, E.L.; Caron, D.A. Coastal upwelling linked to toxic Pseudo-nitzschia australis blooms in Los Angeles coastal waters, 2005–2007. J. Plankton Res. 2013, 35, 1080–1092. [Google Scholar] [CrossRef] [Green Version]
- Anglès, S.; Jordi, A.; Henrichs, D.W.; Campbell, L. Influence of coastal upwelling and river discharge on the phytoplankton community composition in the northwestern Gulf of Mexico. Prog. Oceanogr. 2019, 173, 26–36. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grachev, M.; Bukin, Y.; Blinov, V.; Khlystov, O.; Firsova, A.; Bashenkhaeva, M.; Kamshilo, O.; Titova, L.; Bairamova, E.; Bedoshvili, Y.; et al. Is a High Abundance of Spring Diatoms in the Photic Zone of Lake Baikal in July 2019 Due to an Upwelling Event? Diversity 2021, 13, 504. https://doi.org/10.3390/d13100504
Grachev M, Bukin Y, Blinov V, Khlystov O, Firsova A, Bashenkhaeva M, Kamshilo O, Titova L, Bairamova E, Bedoshvili Y, et al. Is a High Abundance of Spring Diatoms in the Photic Zone of Lake Baikal in July 2019 Due to an Upwelling Event? Diversity. 2021; 13(10):504. https://doi.org/10.3390/d13100504
Chicago/Turabian StyleGrachev, Mikhail, Yurij Bukin, Vadim Blinov, Oleg Khlystov, Alena Firsova, Maria Bashenkhaeva, Oxana Kamshilo, Lubov Titova, Elvira Bairamova, Yekaterina Bedoshvili, and et al. 2021. "Is a High Abundance of Spring Diatoms in the Photic Zone of Lake Baikal in July 2019 Due to an Upwelling Event?" Diversity 13, no. 10: 504. https://doi.org/10.3390/d13100504
APA StyleGrachev, M., Bukin, Y., Blinov, V., Khlystov, O., Firsova, A., Bashenkhaeva, M., Kamshilo, O., Titova, L., Bairamova, E., Bedoshvili, Y., Sakirko, M., & Zakharova, Y. (2021). Is a High Abundance of Spring Diatoms in the Photic Zone of Lake Baikal in July 2019 Due to an Upwelling Event? Diversity, 13(10), 504. https://doi.org/10.3390/d13100504







