Lobster Distribution and Biodiversity on the Continental Shelf of Brazil: A Review
Abstract
:1. Introduction
2. Material and Methods
2.1. Lobster Site Description
2.2. Length Assessment
- (a)
- Males: TL = 32.8431 + 2.5197 * CL.
- (b)
- Females: TL = 36.3535 + 2.5402 * CL.
- (c)
- Total (both sexes): TL = 9.8740 + 2.9290 * CL.
- (d)
- Males: CL = −11.7974 + 0.3925 * TL.
- (e)
- Females: CL = −2.3439 + 0.3374 * TL
- (f)
- Total (both sexes): CL = −11.6569 + 0.3838 * TL.
2.3. Lobster Data Collection and Time Series
3. Results and Discussion
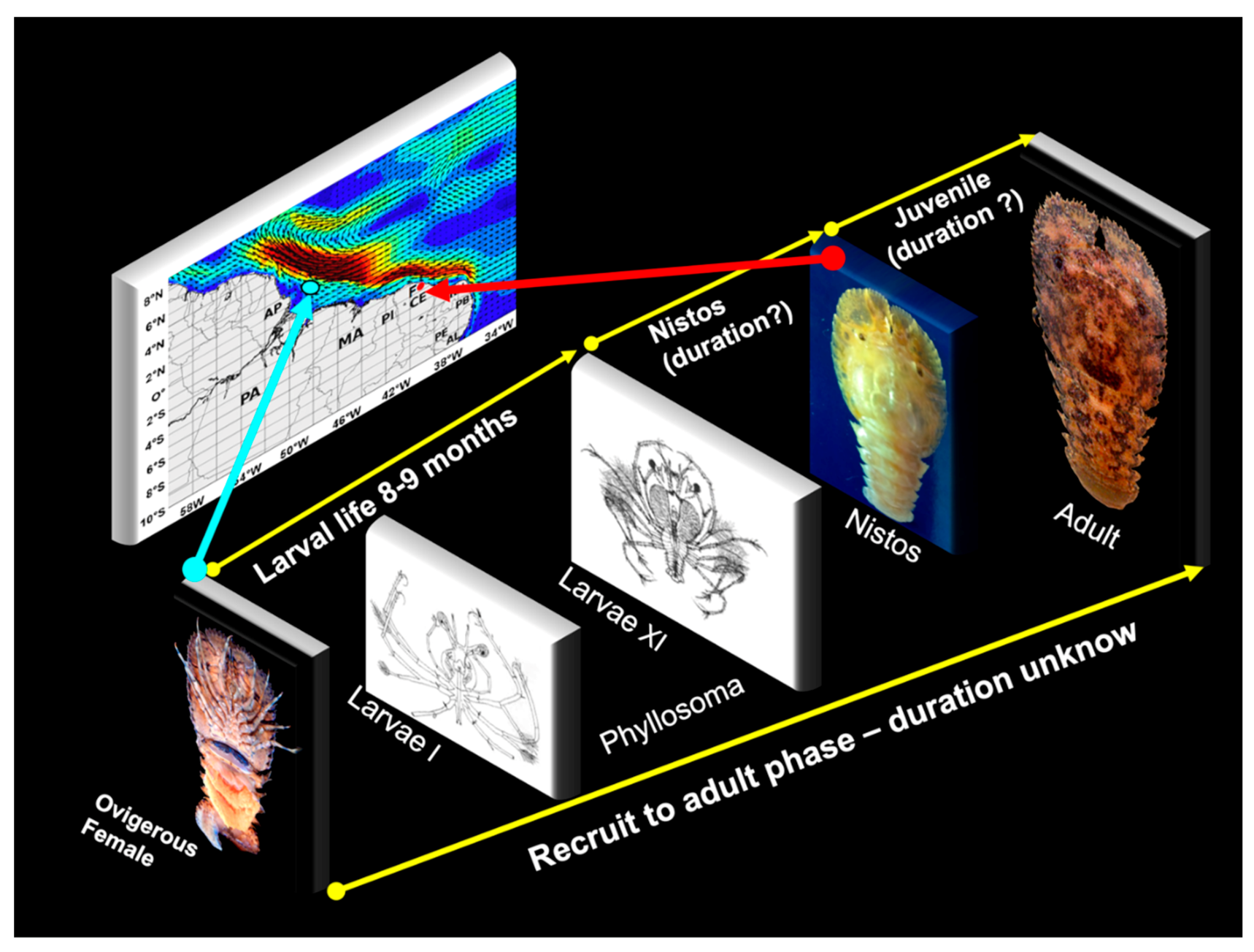
3.1. Connectivity of Spiny Lobsters (Panulirus spp.)
3.2. Lobster Distribution and Diversity
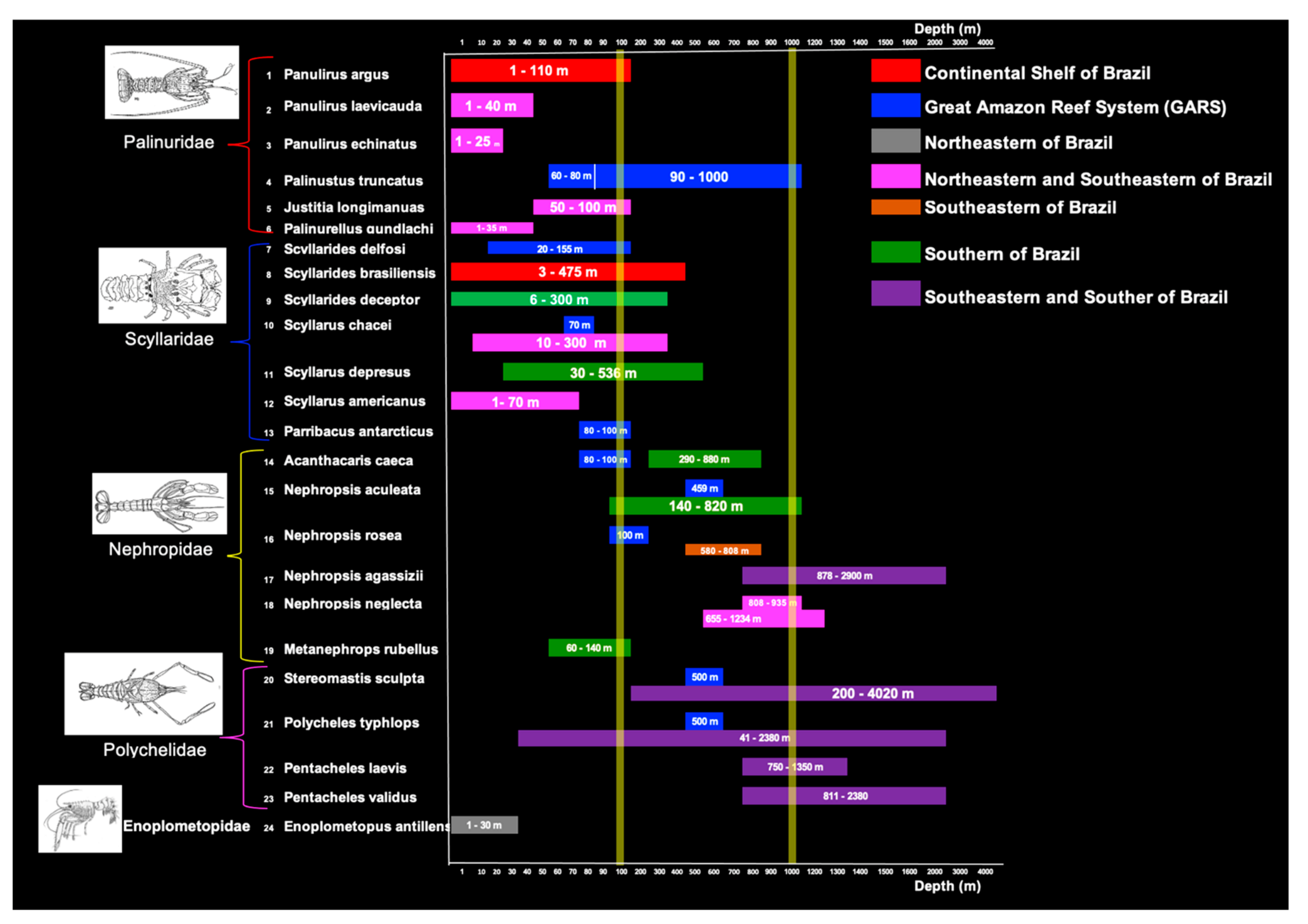
3.3. Bathymetric Distribution
3.4. Maximum Total Length (MTL) of Lobster Species
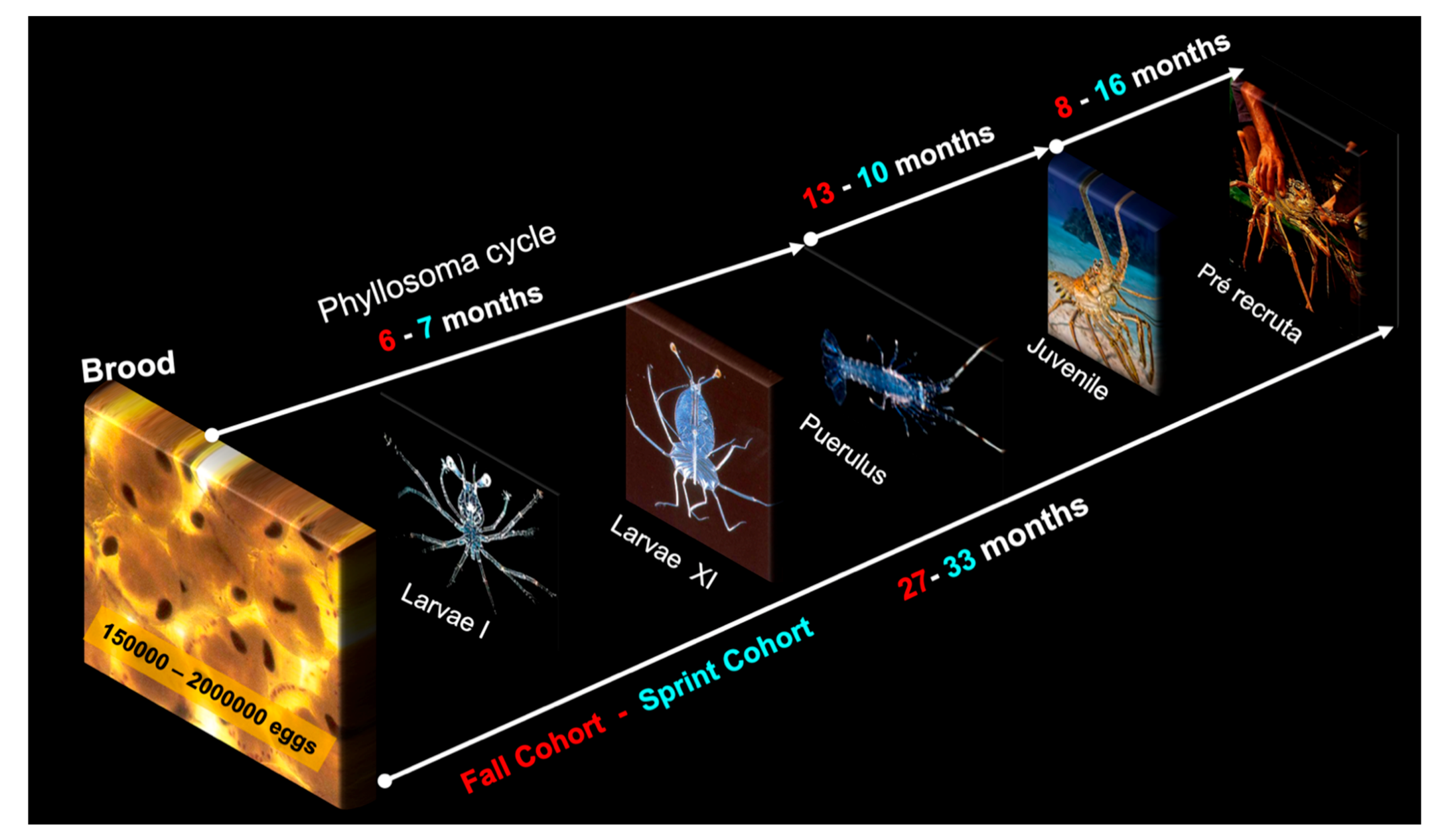
3.5. Life Cycle
3.6. Biological Controls
3.6.1. Predators
3.6.2. Feeding Behavior
3.6.3. Benthic Habitats and Length Assessment
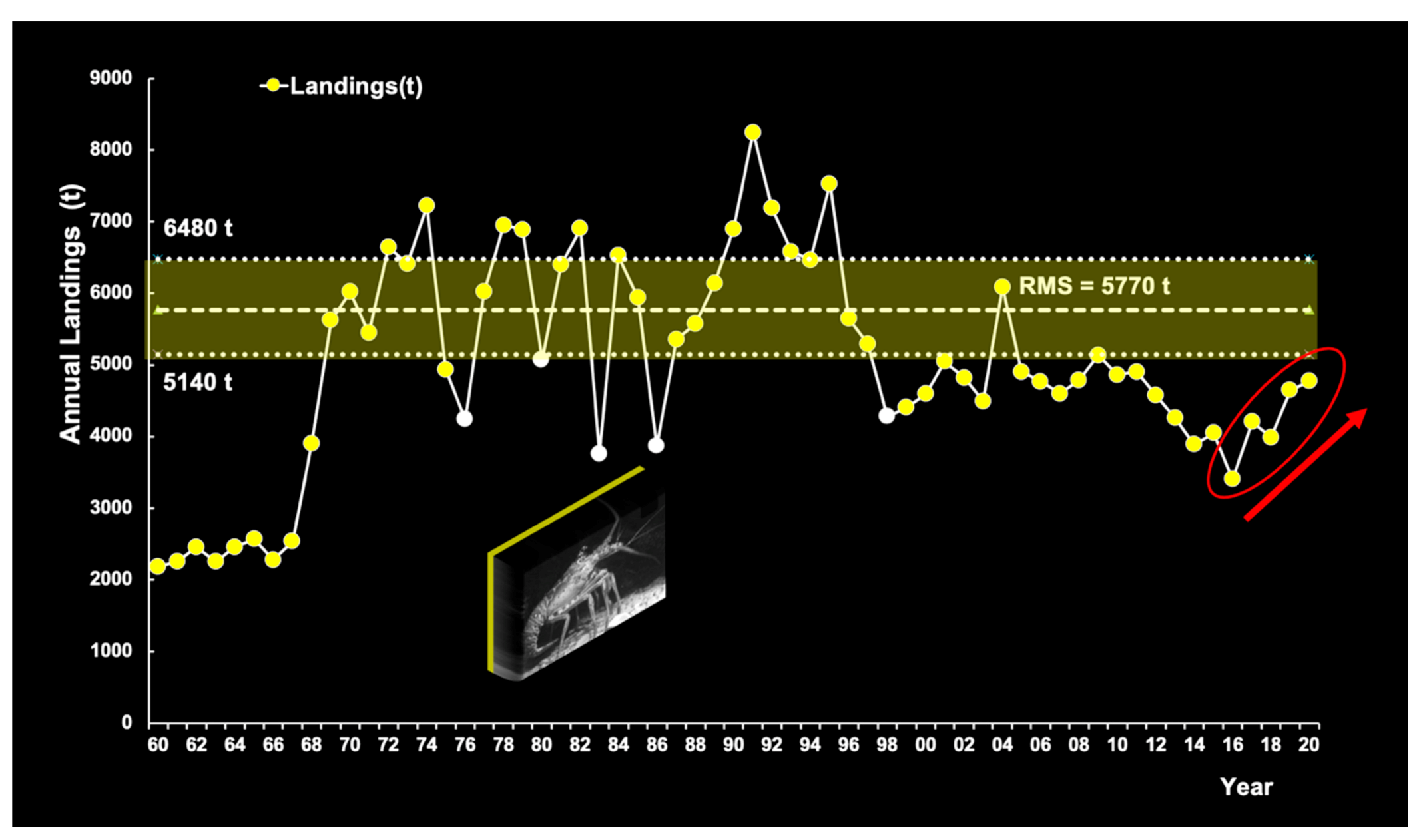
3.6.4. Commercial Fisheries
3.7. Predator-Prey Interactions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Provides a List of the Species Surveyed
| Palinuridae Latreille, 1802 (spiny lobsters) |
| 1. Brazilian form of Panulirus argus (Latreille, 1804). |
| 2. Panulirus laevicauda (Latreille, 1817). |
| 3. Panulirus echinatus Smith, 1869. |
| 4. Palinustus truncatus A. Milne-Edwards, 1880. |
| 5. Justitia longimanus (H. Milne Edwards, 1837). |
| 6. Palinurellus gundlachi von Martens, 1878. |
| Scyllaridae Latreille, 1825 (slipper lobsters) |
| 7. Scyllarides delfosi Holthuis, 1960. |
| 8. Scyllarides brasiliensis Rathbun, 1906. |
| 9. Scyllarides deceptor Holthuis, 1963. |
| 10. Scyllarus chacei Holthuis, 1960. |
| 11. Scyllarus depressus (S. I. Smith, 1881). |
| 12. Scyllarus americanus (S. I. Smith, 1869). |
| 13. Parribacus antarcticus (Lund, 1793). |
| Nephropidae Dana, 1852 (clawed lobsters) |
| 14. Acanthacaris caeca (A. Milne-Edwards, 1881). |
| 15. Nephropsis aculeata Smith, 1881. |
| 16. Nephropsis rosea Bate, 1888. |
| 17. Nephropsis agassizii A. Milne-Edwards, 1880. |
| 18. Nephropsis neglecta Holthuis, 1974. |
| 19. Metanephrops rubellus (Moreira, 1903). |
| Polychelidae Wood-Mason, 1874 (blind lobsters, primitive decapods) |
| 20. Stereomastis sculpta (Smith, 1880). |
| 21. Polycheles typhlops C. Heller, 1862. |
| 22. Pentacheles laevis Bate, 1878. |
| 23. Pentacheles validus A, Milne Edwards, 1880. |
| Enoplometopidae de Saint Laurent, 1988. |
| 24. Enoplometopus antillensis Lütken, 1865. |
Appendix B
| List of Species (Appendix A) | Authors (Photo Credits) |
| 1, 2, 3, 7, 8 | Cruz et al. [54]. |
| 4,13,14,16 | Silva et al. [55]. |
| 5,6 | Cruz et al. [56]. |
| 9 | Tavares et al. [36]. |
| 10 | Silva et al. [47]. |
| 11 | Puciarelly & Rego [57]. |
| 12 | No restrictions |
| 15,16 | Photo (live color) Poupin J. in Legal & Poupin [58]. |
| 17,18 | Photo (live color) Alves-Junior et al. [48]. |
| 19 | Photo (live color) Scarabino, F. [59]. |
| 20 | Photo (live color) Felder, D. [60]. |
| 21,22 | Photo (live color) Tin-Yam Chan/Muséum national d’Histoire naturelle” [24] |
| 23 | Ahyong & Shane [61]. |
| 24 | Holthuis [3]. |
| List of Species (Appendix A) | Line Drawing Credits |
| 4, 14, 17, 18, 19, 9, 25 | Holthuis [3]. |
| 5 | Cruz et al. [56]. |
| 15, 16, 20, 21 | Silva et al. [5]. |
| 12 | Adapted from Lavalli & Spanier [62]. |
References
- Alencar, C.A.G.; Tavares, L.S.; Cintra, I.H.A. Current State of lobster exports in Brazil Research. Soc. Dev. 2020, 9, e312985804. [Google Scholar] [CrossRef]
- Phillips, B.F.; Cobb, J.S.; George, C.R.W. General biology. In The Biology and Management of Lobsters; Cobb, J.S., Phillips, B.F., Eds.; Academic Press: New York, NY, USA, 1980; Volume 1, pp. 1–82. [Google Scholar]
- Holthuis, L.B. Marine lobsters of the world. An annotated and illustrated catalogue of marine lobsters known to date. In FAO Species Catalogue; FAO: Italy, Rome, 1991; Volume 13, pp. 1–292. [Google Scholar]
- Silva, K.C.A.; Cintra, I.H.A.; Ramos-Porto, M.; Viana, G.F.S.; Abrunhosa, F.A.; Cruz, R. Update on crustaceans known from the amazonian continental shelf and adjacent oceanic areas. Crustaceana 2020, 93, 687–701. [Google Scholar] [CrossRef]
- Silva, K.C.A.; Cruz, R.; Cintra, I.H.A.; Abrunhosa, F.A. Structure and diversity of the lobster community on the Amazon continental shelf. Crustaceana 2013, 86, 1084–1102. [Google Scholar] [CrossRef]
- Cruz, R.; Borda, C.A.; Santana, J.V.M.; Barreto, C.G.; Paiva, B.P.; Gaeta, J.C.; Torres, M.T.; Silva, J.L.S.; Cintra, I.H.A. Life cycle and connectivity of the spiny lobster, Panulirus spp.: Case studies from brazil and the wider caribbean (decapoda, achelata). Crustaceana 2021, 94, 603–645. [Google Scholar] [CrossRef]
- Cury, P.; Shannon, L.; Shin, Y.J. The functioning of marine ecosystems: A fisheries perspective. In Responsible Fisheries in the Marine Ecosystem; Sinclair, M., Valdimarsson, G., Eds.; FAO: Italy, Rome; CABI Publishing: Wallingford, UK, 2003; pp. 103–123. [Google Scholar]
- Cruz, R.; Teixeira, C.E.; Menezes, M.O.B.; Santana, J.V.M.; Neto, T.M.; Gaeta, J.C.; Freitas, P.P.; Silva, K.C.A.; Cinbtra, I.H.A. Large-scale oceanic circulation and larval recruitment of the spiny lobster Panulirus argus (Latreille, 1804). Crustaceana 2015, 88, 298–323. [Google Scholar] [CrossRef]
- Rebelo-Neto, J.E. Considerações sobre a pescaria do lagostim (Metanephrops rubellus) na região sudeste/sul do Brasil. CEPSUL—Documentos Técnicos 1986, 10, 1–33. [Google Scholar]
- Worm, B.; Barbier, E.B.; Beaumont, N.; Duffy, J.E.; Folke, C.; Halpern, B.S.; Jackson, J.B.C.; Lotze, H.K.; Micheli, F.; Palumbi, S.R.; et al. Impacts of Biodiversity Loss on Ocean Ecosystem Services. Science 2006, 314, 787–790. [Google Scholar] [CrossRef] [Green Version]
- Wineland, S.M.; Kistner, E.J.; Joern, A. Non-consumptive interactions between grasshoppers (Orthoptera: Acrididae). And wolf spiders (Lycosidae) produce trophic cascades in an old-field ecosystem. J. Orthoptera Res. 2015, 24, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Boudreau, S.A.; Worm, B. Ecological role of large benthic decapods in marine ecosystems: A review. Mar. Ecol. Prog. Ser. 2012, 469, 195–213. [Google Scholar] [CrossRef] [Green Version]
- Cruz, R.; Santana, J.V.M.; Barreto, C.G.; Borda, C.A.; Torres, M.T.; Gaeta, J.C.; Silva, J.L.S.; Saraiva, S.Z.R.; Salazar, I.O.; Cintra, I.H.A. Towards the rebuilding of spiny lobster stocks in Brazil: A review. Crustaceana 2020, 93, 957–983. [Google Scholar] [CrossRef]
- Fonteles-Filho, A.A. The state of the lobster fishery in northeast Brazil. In Spiny Lobster Management; Phillips, B.F., Kittaka, J., Eds.; Blackwell: Oxford, UK, 2000; pp. 121–134. [Google Scholar]
- Santana, J.V.M.; Neves, S.D.S.; Saraiva, S.Z.R.; Adams, C.; Cruz, R. Current Management and externalities in lobster fisheries exploitation on the continental shelf of Ceará, Brazil. Arq. Ciên. Mar. 2015, 48, 5–18. [Google Scholar]
- Nascimento, R.C. Impactos Sócio-Ambientais de Marambaias Para a Pesca da Lagosta: O Caso de Ponta Grossa Icapuí-Ce. Tese Mestrado, Universidade Federal do Ceará, Fortaleza, Brazil, 2006; 86p. [Google Scholar]
- Cruz, R.; Silva, K.C.A.; Neves, S.D.S.; Cintra, I.H.A. Impact of lobster size on catches and prediction of commercial spiny lobster landings in Brazil. Crustaceana 2013, 86, 1274–1290. [Google Scholar] [CrossRef]
- Moura, R.L.; Amado-Filho, G.M.; Moraes, F.C.; Brasileiro, P.S.; Salomon, P.S.; Mahiques, M.M.; Bastos, A.C.; Almeida, M.G.; Silva, J.M.; Araujo, B.F., Jr.; et al. An extensive reef system at the Amazon River mouth. Sci. Adv. 2016, 2, e1501252. [Google Scholar] [CrossRef] [Green Version]
- Coutinho, P.N.; Morais, J.O. Distribución de los sedimentos en la plataforma continental norte y nordeste del Brasil. Arq. Ciên. Mar. 1970, 10, 79–90. [Google Scholar]
- Foster, M.S. Rhodoliths: Between rocks and soft places. J. Phycol. 2001, 37, 659–667. [Google Scholar] [CrossRef]
- Amado-Filho, G.M.; Bahia, R.G.; Pereira-Filho, G.H.; Longo, L.L. South Atlantic rhodolith beds: Latitudinal distribution, species composition, structure and ecosystem functions, threats and conservation status. In Rhodolith/Maërl Beds: A Global Perspective; Riosmena-Rodríguez, R., Nelson, W., Aguirre, J., Eds.; Springer International Publishing (Coastal Research Library): London, UK, 2017; pp. 299–317. [Google Scholar]
- Mahiques, M.M.; Siegle, E.; Francini-Filho, R.B.; Thompson, F.L.; De Rezende, C.E.; Gomes, J.D.; Asp, N.E. Insights on the evolution of the living Great Amazon Reef System, equatorial West Atlantic. Sci. Rep. 2019, 9, 13699. [Google Scholar] [CrossRef]
- Cruz, R. Manual de Métodos de Muestreo para la Evaluación de las Poblaciones de Langosta Espinosa; FAO Documento Técnico de Pesca; FAO: Rome, Italy, 2002; pp. 1–43. [Google Scholar]
- Chan, T.Y. Updated checklist of the world’s marine lobsters. In Lobsters: Biology, Fisheries and Aquaculture; Radhakrishnan, E.V., Phillips, B.F., Achamveetil, G., Eds.; Springer: Singapore, 2019; pp. 35–64. [Google Scholar]
- Diggle, P. Time Series: A Bioestatistical Introduction; Oxford University Press: Oxford, UK, 1990; 272p. [Google Scholar]
- Rocha, L.A.; Craig, M.T.; Bowen, B.W. Phylogeography and the conservation of coral reef fishes. Coral Reefs 2007, 26, 501–512. [Google Scholar] [CrossRef]
- Giraldes, B.W.; Smyth, D.M. Recognizing Panulirus meripurpuratus sp. nov. (Decapoda: Palinuridae) in Brazil—Systematic and biogeographic overview of Panulirus species in the Atlantic Ocean. Zootaxa 2016, 4107, 353–366. [Google Scholar] [CrossRef] [Green Version]
- Sarver, S.K.; Silberman, J.D.; Walsh, P.J. Mitochondrial DNA sequence evidence supporting the recognition of two subspecies or species of the Florida spiny lobster Panulirus argus. J. Crustacean Biol. 1998, 18, 177–186. [Google Scholar] [CrossRef]
- Sarver, S.K.; Freshwater, D.W.; Walsh, P.J. The occurrence of the provisional Brazilian subspecies of spiny lobster (Panulirus argus westonii) in Florida waters. Fish. Bull. 2000, 98, 870–873. [Google Scholar]
- Briones-Fourzán, P.; Barradas-Ortiz, C.; Negrete-Soto, F.; Segura-García, I.; Lozano-Álvarez, E. Occurrence of Panulirus meripurpuratus and Panulirus laevicauda (Decapoda: Achelata: Palinuridae) in Bahía de la Ascensión, México. Lat. Am. J. Aquat. Res. 2019, 47, 694–698. [Google Scholar] [CrossRef] [Green Version]
- Knowlton, N. Molecular genetic analyses of species boundaries in the sea. Hydrobiologia 2000, 420, 73–90. [Google Scholar] [CrossRef]
- Fausto-Filho, J. Sobre a ocorrência de Palinustrus truncatus (H. Milne-Edwards, 1880), no litoral brasileiro e de Panulirus echinatus Smith 1860 no litoral do Estado do Ceará, Brasil (Crustácea, Decapoda, Palinuridae). Arq. Ciên. Mar. 1977, 17, 75–76. [Google Scholar]
- Santana, W.; Pinheiro, A.P.; Oliveira, J.E.L. Additional records of three Scyllarides species (Palinura: Scyllaridae) from Brazil, with the description of the fourth larval stage of Scyllarides species (Palinura: Scyllaridae) from Brazil, with the description of the fourth larval stage of Scyllarides aequinoctialis. Nauplius 2007, 15, 1–6. [Google Scholar]
- Melo, G.A.S. Manual de Identificação dos Crustácea Decapoda do Litoral Brasileiro: Anomura: Thalassinidea; Palinuridea e Astacidea: Cidade de São Paulo Plêiade: São Paulo, Brazil, 1999; 551p. [Google Scholar]
- Coelho, P.A.; Ramos-Porto, M. Sinopse de crustáceos decápodos brasileiros (famílias Scyllaridae, Palinuridae, Nephropidae, Parastacidae e Axiidae). An. Univ. Fed. Rural. Pernamb. 1985, 8, 38–47. [Google Scholar]
- Tavares, M.; Santana, W.; Pinheiro, A. On the type material of Scyllarides Deceptor, Holthuis, 1963 (Crustacea: Decapoda: Scyllaridae). Papéis Avulsos Zool. (Museo Zool. Univ. São Paulo) 2009, 49, 539–545. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, G.; Freire, A.S.; Bertuol, P.R.K. Reproductive biology of the, slipper lobster Scyllarides deceptor (Decapoda:Scyllaridae) along the southern Brazilian coast. J. Mar. Biol. Assoc. 2008, 88, 1433–1440. [Google Scholar] [CrossRef]
- Duarte, L.F.A.; Severino-Rodrigues, E.; Gasalla, M.A. Slipper lobster (Crustacea, Decapoda, Scyllaridae) fisheries off the southeastern coast of Brazil: I. Exploitation patterns between 23°00′ and 29°65′ S. Fish. Res. 2010, 102, 141–151. [Google Scholar] [CrossRef]
- Tavares, M. Scyllarus ramosae, new species, from the Brazilian continental slope, with notes on congeners occurring in the area (Decapoda: Scyllaridae). J. Crustacean Biol. 1997, 17, 716–724. [Google Scholar] [CrossRef]
- Tavares, C.R.; Young, P.S. Nephropidae (Crustacea, Decapoda) collected by the Revizee Score-Central Program from off Bahia to Rio de Janeiro states, Brazil. Arq. Mus. Nac. 2002, 60, 77–88. [Google Scholar]
- Schmitt, W. Crustacea Macrura and Anomura of Porto Rico and the Virgin Islands—Scientific Survey of Porto Rico and Virgin Islands. N. Y. Acad. Sci. 1935, 15, 125–262. [Google Scholar]
- Robertson, P.B. The early larval development of the Scyllarid lobster Scyllarides aequinoctialis (Lund) in the laboratory, with a revision of the larval characters of the genus. Deep Sea Res. 1969, 16, 557–586. [Google Scholar] [CrossRef]
- Dall’Occo, P.L.; Bento, R.T.; Melo, G.A.S. Range extensions for lobsters off the Brazilian Coast (Crustacea, Decapoda, Palinura, Astacidea). Biociências 2007, 15, 47–52. [Google Scholar]
- Dall’Occo, P.L. Taxonomia e Distribuição das Lagostas (Crustacea: Decapoda: Achelata e Polychelida) no Oceano Atlântico. Ph.D. Thesis, Universidade Estadual Paulista, Rio Claro, Brazil, 2010; 432p. [Google Scholar]
- Coelho, P.A.; Almeida, A.O.; Bezerra, L.E.A.; Souza-Filho, J.F. An updated checklist of decapod crustaceans (infraorders Astacidea, Thalassinidea, Polychelida, Palinura, and Anomura) from the northern and northeastern Brazilian coast. Zootaxa 2007, 1519, 1–16. [Google Scholar]
- Serejo, C.S.; Young, P.S.; Cardoso, I.C.; Tavares, C.; Rodrigues, C.; Almeida, T.C. Abundância, diversidade e zonação dos crustáceos no talude da costa central do Brasil (11°–22° S) coletados pelo programa REVIZEE\Score Central: Prospecção pesqueira. In Biodiversidade da Fauna Marinha Profunda na Costa Central Brasileira; Costa, P.A.S., Olavo, G., Martins, A.S., Eds.; Rio de Janeiro Museo Nacional: Cidade de Rio de Janeiro, Brazil, 2007; pp. 133–162. [Google Scholar]
- Silva, K.C.A.; Fransen, C.H.J.M.; Ramos-Porto, M.; Paiva, K.S.; Cintra, I.H.A.; Cruz, R. Report of Scyllarus chacei, Holthuis, 1960 (Decapoda, Scyllaridae) in Amapá state sontinental shelf of Brazil. Crustaceana 2012, 85, 1171–1177. [Google Scholar]
- Alves-Junior, F.A.; Araujo, M.S.L.C.; Souza-Filho, J.F. Distribution of two species of Nephropsis Wood-Mason, 1872 (Crustacea: Decapoda: Nephropidae) from northeastern Brazil. Zootaxa 2016, 4114, 090–094. [Google Scholar] [CrossRef]
- Tavares, M. Malacostraca-Eucarida. Nephropidae. In Catalog of Crustacea from Brazil; Young, P., Ed.; Museu Nacional: Rio de Janeiro, Brazil, 1999; pp. 377–378. [Google Scholar]
- Silva, K.C.A.; Cintra, I.H.A.; Ramos-Porto, M.; Viana, G.F.S. Lagostas capturadas durante pescarias experimentais para o programa Revizee/Norte. (Crustacea, Nephropoidea, Eryonoidea, Palinuroidea). Bol. Téc. Cient. Cepnor Belém 2003, 3, 21–35. [Google Scholar]
- Galil, B.S. Crustacea Decapoda: Review of the genera and species of the family Polychelidae Wood-Mason, 1874. In Résultats des Campagnes Musorstom; Crosnier, A., Ed.; Mémoires du Muséum National d’Histoire Naturelle: Paris, France, 2000; pp. 285–387. [Google Scholar]
- Gaeta, J.C.; Silva, M.B.; Godoy, T.; Cruz, R. Update on the lobster species from Rocas Atoll Marine Reserve, Brazil. Check List 2015, 11, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Severino-Rodrigues, E.; Hebling, N.J.; Melo, G.A.S.; Graça-Lopes, R. Biodiversidade no produto da pesca dirigida ao lagostim Metanephrops rubellus (Moreira,1903) no litoral do Estado de São Paulo, Brasil, com ênfase à carcinofauna. Inst. Pesca 2007, 33, 171–182. [Google Scholar]
- Cruz, R.; Conceição, R.N.L.; Marinho, R.A.; Barroso, J.C.; Holanda, J.S.; Félix, C.S.; Martins, M.E.O.; Santos, F.S.; Silva, K.C.A.; Furtado-Neto, M.A.A. Metodologias de amostragem para avaliação das populações de lagosta: Plataforma Continental do Brasil. Edição bilingüe português/espanhol). Colecao Habitat 2011, 6, 1–142. [Google Scholar]
- Silva, K.C.A.; Cintra, I.H.A.; Ramos-Porto, M.; Viana, G.F.S. Lagostas capturadas na plataforma continental do estado do Amapá (Crustacea, Nephropoidea, Palinuroidea). Bol. Téc. Cient. Cepnor Belém 2007, 7, 173–184. [Google Scholar] [CrossRef] [Green Version]
- Cruz, R.; Baisre, J.A.; Díaz, E.; Brito, R.; Blanco, W.; García, C.; Carrodeguas, C. Atlas Biológico-Pesquero de la Langosta en el Archipiélago Cubano; Revista Mar y Pesca: Ciudad Habana, Cuba, 1990; 125p. [Google Scholar]
- Puciarelly, P.; Rego, A.B. The Crustacean Collection, Museum National d’Histoire Naturelle. Paris, France. 2020. No Restrictions to Copy and Redistribute the Material in Any Medium or Format. Available online: https://creativecommons.org/licenses/by/4.0/deed.en (accessed on 15 August 2021).
- Legall, N.; Poupin, J. CRUSTA: Database of Crustacea (Decapoda and Stomatopoda), with Special Interest for Those Collected in French Overseas Territories. 2021. Available online: http://crustiesfroverseas.free.fr/ (accessed on 15 August 2021).
- Scarabino, F. Metanephrops rubellus (Moreira, 1903). (scampi, cigala, Uruguayan lobster). Photography of the body in dorsal view. Annotated catalogue and bibliography of marine and estuarine shrimps, lobsters, crabs and their allies (Crustacea: Decapoda) of Argentina and Uruguay (Southwestern Atlantic Ocean). In Frente Marítimo; 2019; Volume 26, 179p. [Google Scholar]
- Felder, D.L. The emergence of lobsters: Phylogenetic relationships, morphological evolution and divergence time comparisons of an ancient group (decapoda: Achelata, astacidea, glypheidea, polychelida). Syst. Biol. 2014, 63, 457–479. [Google Scholar]
- Ahyong, S.T.; Shan, T.Y. Polychelidae from the Bohol and Sulu seas collected by Panglao 2005 (Crustacea: Decapoda: Polychelidae). Raffles Bull. Zool. 2008, 19, 63–70. [Google Scholar]
- Lavalli, K.L.; Spanier, E. The biology and fisheries of the slipper lobster. In Crustaceans; Von, R., Ed.; CRC Press: Leiden, The Netherlands, 2007; 420p. [Google Scholar]
- Fischer, W. Lobsters: FAO Species Identification Sheets for Fishery Purposes; FAO: Rome, Italy, 1978; 1700p. [Google Scholar]
- Takeda, M. Crustaceans. In Crustaceans and Mollusks Trawled off Suriname and French Guiana; Takeda, M., Okutani, T., Eds.; Japan Marine Research Center: Tokyo, Japan, 1983; 354p. [Google Scholar]
- Fonteles-Filho, A.A. Population dynamics of spiny lobsters (Crustacea: Palinuridae) in Norhteast Brazil. Ciência Cultura 1992, 44, 192–196. [Google Scholar]
- Cruz, R.; Silva, K.C.A.; Gaeta, J.C.; Santana, J.V.M.; Cintra, I.H.A. Reproductive potential and stock recruitment of the Caribbean and Brazilian metapopulations of the spiny lobster, Panulirus argus (Latreille, 1804). Crustaceana 2014, 87, 1315–1337. [Google Scholar] [CrossRef]
- Cintra, I.H.A.; Martins, D.E.G.; Alves-Júnior, F.A.; Santos, W.C.R.; Valente, H.M.; Klautau, A.G.C.M.; Silva, K.C.A. Report of the sculptured slipper lobster Parribacus antarcticus (Lund, 1793) (Decapoda: Scyllaridae) on the Great Amazon Reef System, Pará, Brazil. Bol. Téc. Cient. Cepnor Belém 2003, 3, 21–35. [Google Scholar]
- Lipcius, R.N.; Herrnkind, W.F. Control and coordination of reproduction and molting in the spiny lobster Panulirus argus. Mar. Biol. 1987, 96, 207. [Google Scholar] [CrossRef]
- Cruz, R. Fecundidad y madurez sexual en la langosta comercial Panulirus argus (Latreille, 1804) Crustácea: Palinuridae en Cuba. Rev. Cubana Investig. Pesq. 1980, 5, 2–27. [Google Scholar]
- MacDiarmid, A.B.; Kittaka, J. Breeding. In Spiny Lobsters: Fisheries and Culture, 2nd ed.; Phillips, B.F., Kittaka, J., Eds.; Fishing News Books; Blackwell Science: Oxford, UK, 2000; pp. 485–507. [Google Scholar]
- Sekiguchi, H.; Booth, J.D.; Webber, W. Early Life Histories of Slipper Lobsters. In The Biology and Fisheries of the Slipper Lobster. Lavalli, K.L., Spanier, E., Eds.; CRC Press: Leiden, The Netherlands, 2007; pp. 69–90. [Google Scholar]
- Johnson, M.W. The phyllosoma larvae of slipper lobsters from the Hawaiian Islands and adjacent areas (Decapoda, Scyllaridae). Crustaceana 1971, 20, 77–103. [Google Scholar] [CrossRef]
- Palero, A.F.F.; Guerao, G.B.; Hall, M.C.; Chan, T.Y.D.; Clark, P.F.E. The ‘giant phyllosoma’ are larval stages of Parribacus antarcticus (Decapoda: Scyllaridae). Invertebr. Syst. 2014, 28, 258–276. [Google Scholar] [CrossRef]
- Buesa, R.J. Biologia de la langosta Panulirus argus, Latreille, 1804 (Crustacea, Decapoda, Reptantia) en Cuba; INPP/CIP: Ciudad Habana, Cuba, 1965; 228p. [Google Scholar]
- Munro, J.L. The Biology, Ecology, Exploitation and Management of Aribbean Reef Fishes: Crustaceans (Spiny Lobsters and Crabs); Zoology Department, University of the West Indies: Kingston, Jamaica, 1974; pp. 1–51. [Google Scholar]
- Aitken, K.A. Jamaica Spiny Lobster Investigations; FAO Fish. Rep.; FAO: Rome, Italy, 1977; pp. 11–22. [Google Scholar]
- Kanciruk, P. Ecology of juvenile and adult Palinuridae (spiny lobsters). In The Biology and Management of Lobster; Cobb, J.S., Phillips, B.F., Eds.; Academic Press: New York, NY, USA, 1980; Volume 2, Part 1; pp. 59–92. [Google Scholar]
- Cruz, R.; Brito, R.; Díaz, R.; Lalana, R. Ecología de la langosta (Panulirus argus) al SE de la Isla de la Juventud. II. Patrones de movimiento. Rev. Investig. Mar. 1986, 7, 19–35. [Google Scholar]
- Cruz, R.; Phillips, B.F. The artificial shelters (pesqueros) used for the spiny lobster (Panulirus argus) fisheries in Cuba. In Spiny Lobster Management: Fisheries and Culture, 2nd ed.; Phillips, B.F., Kittaka, J., Eds.; Blackwell: Oxford, UK, 2000; pp. 400–419. [Google Scholar]
- Joll, L.M. The Predation of Pot Caught Western Rock Lobster (Panulirus fongipes cygnus) by Octopus; Department of Fisheries and Wildlife: Washington, DC, USA, 1977; pp. 1–58. [Google Scholar]
- Ivo, C.T.C. Estudo Sobre la Biologia da Pesca do Pargo Lutjanus Purpureus Poey, No Nordeste Brasileiro, Arquivo de Ciências do Mar; Universidade Federal do Ceará (UFC): Fortaleza, Brazil, 1973; Part 2; pp. 113–116. [Google Scholar]
- Ivo, T.; Hanson, A.J. Aspectos da Biologia e Dinâmica Populacional do Pargo, Lutjanus purpureus Poey, no Norte e Nordeste do Brasil. Arq. Ciên. Mar, Fortaleza 1982, 22, 1–4. [Google Scholar]
- Pinheiro, R.; Silva, K.C.A. Dinâmica Alimentar do Pargo Lutjanus purpureus (Poey, 1875) Capturado na Plataforma Continental Amazônica. Bachelor’s Thesis, Universidade Federal Rural da Amazônia, Belém, Brazil, 2019; 37p. [Google Scholar]
- Silva, B.B.; Aragão, J.A.N.; Freire, J.L.; Lutz, I.A.F.; Sarmento, G.C.; Gomes, T.; Lima, W.P.G.; Santos, R.R.F. Documento Técnico Sobre a Situação Atual das Pescarias do Pargo na Região Norte do Brasil; Universidade Federal do Pará—Campus Bragança Instituto de Estudos Costeiros Laboratório de Bioecologia Pesqueira—LABIP: Ciudade de Bragança, Brazil, 2017; 132p. [Google Scholar]
- Monteiro-Filho, E.L.; Oliveira, L.V.; Monteiro, K.D.K.A.; Filla, G.F.; Quito, L.; Godoy, D.F. Guia ilustrado mamíferos marinhos-Brasil. In Projeto Boto-Cinza; Instituto de Pesquisas de Cananéia (IPeC): Ciudad de São Paulo, Brazil, 2013; 106p. [Google Scholar]
- Gupta, K.S.; Kizhakudan, S.J.; Kizhakudan, J.K.; Myousuf, K.S.S.; Raja, S. Preliminary observations on dominance of crustacean larvae in the diet of little tunny Euthynnus affinis (Cantor, 1849) caught off Chennai and Cuddalore coasts. Indian J. Fish 2014, 61, 40–44. [Google Scholar]
- Werth, A. Feeding in marine mammals. In Feeding. Chapter 16; Hampden-Sydney College: Sindey, Australia, 2000; pp. 487–526. [Google Scholar]
- Herrera, A.; Diaz-Iglesias, E.; Brito, R.; González, G.; Gotera, G.; Espinosa, J.; Ibarzábal, D. Alimentación natural de la langosta Panulirus argus en la región de los Indios (plataforma SW de Cuba) y su relación con el bentos. Rev. Investig. Mar. 1991, 12, 172–182. [Google Scholar]
- Cox, C.; Hunt, J.H.; Lyons, W.G.; Davis, G.E. Nocturnal foraging of the Caribbean spiny lobster (Panulirus argus) on offshore reefs of Florida, USA. Mar. Freshw. Res. 1997, 48, 671–680. [Google Scholar] [CrossRef]
- Lewis, J.B. The phyllosoma larvae of the spiny lobster Panulirus argus. Bull. Mar. Sci. 1951, 1, 78–103. [Google Scholar]
- Goldstein, J.S.; Matsuda, H.; Takenouchi, T. A description of the complete development of larval Caribbean spiny lobster, Panulirus argus (Latreille, 1804) in culture. J. Crust. Biol. 2008, 28, 306–327. [Google Scholar] [CrossRef]
- Greer, A.T.; Briseño-Avena, C.; Deary, A.L.; Cowen, R.K.; Herandez, F.J.; Graham, W.M. Associations between lobster phyllosoma and gelatinous zooplankton in relation to oceanographic properties in the northern Gulf of Mexico. Fish. Oceanogr. 2017, 26, 693–704. [Google Scholar] [CrossRef]
- Chow, S.; Suzuki, S.; Matsunaga, T.; Lavery, S.; Jeffs, A.G. Investigation on natural diets of larval marine animals using peptide nucleic-directed Polymerase Chain Reaction clamping. Mar. Biotechnol. 2010, 13, 305–313. [Google Scholar] [CrossRef] [PubMed]
- O’Rorke, R.; Lavery, S.; Chow, S.; Takeyama, H.; Tsai, P.; Beckley, L.E.; Thompson, P.A.; Waite, A.M.; Jeffs, A.G. Determining the diet of larvae of Western Rock Lobster (Panulirus Cygnus) using High-Throughput DNA Sequencing Techniques. PLoS ONE 2012, 7, e42757. [Google Scholar] [CrossRef] [PubMed]
- Phillips, B.F.; Mcwilliam, P.S. The pelagic phase of spiny lobster development. Can. J. Fish. Aquat. Sci. 1986, 43, 2153–2163. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Yang, C.-H.; Chan, T.Y.; Phillips, B.F. The final phyllosoma, nisto, and first juvenile stages of the slipper lobster Petrarctus brevicornis (Holthuis, 1946) (Decapoda: Achelata: Scyllaridae). J. Crustacean Biol. 2020, 40, 237–246. [Google Scholar] [CrossRef]
- Lalana, R.; Ortiz, M. Contenido estomacal de puérulos y post-puérulos de la langosta Panulirus argus en el Archipiélago de los Canarreos, Cuba. Rev. Investig. Mar. 1991, 12, 107–116. [Google Scholar]
- Francini-filho, R.B.; Asp, N.E.; Siegle, E.; Hocevar, J.; Lowyck, K.; D’Avila, N.; Vasconcelos, A.S.; Baitelo, R.; Rezende, C.E.; Omachi, C.Y.; et al. Perspectives on the Great Amazon Reef: Extension, biodiversity and threats. Front. Mar. Sci. 2018, 5, 103–123. [Google Scholar] [CrossRef]
- Muricy, G.; Moraes, F.C. Marine sponges of Pernambuco State, NE Brazil. Rev. Bras. Oceanogr. 1998, 46, 213–217. [Google Scholar] [CrossRef] [Green Version]
- Muricy, G.; Lopes, D.A.; Hajdu, E.; Carvalho, M.S.; Moraes, F.C.; Carla, M.K.; Pinheiro, M.U. Catalogue of Brazilian Porifera; Museo Nacional Livros Serie: Recife City, Brazil, 2011; Volume 46, 300p. [Google Scholar]
- Caddy, J.F. Why do assessments of demersal stocks largely ignore habitat? ICES J. Mar. Sci. 2014, 71, 2114–2126. [Google Scholar] [CrossRef] [Green Version]
- Igarashi, M.A. Note on photographic register and preliminary observations from puerulus to juvenile of Panulirus argus after settlement in Amansia sp. macroalgae in Brazil. Ciências Agrárias Londrina 2010, 31, 767–772. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, G. A Pesca da Lagosta Sapatera Scyllarides Deceptor HOLTHUIS, 1963 (CRUSTACEA: DECAPODA:SCYLLARIDAE) no sul do Brasil—Subsídios ao Conhecimiento da Biologia Reprodutiva e à Conservação da Espécie. Tese Apresentada como Requisito Parcial à Obtenção do grau de Mestre em Ciências Biológicas—Área de Concentração Zoologia; Programa de Pós-graduação em Zoologia, Setor de Ciências Biológicas da Universidade Federal do Paraná: Curitiba, Brazil, 2008; pp. 1–62. [Google Scholar]
- Linderman, K.; Lee, T.N.; Wilson, W.D.; Claro, R.; Jerald, A. Transport of Larvae Originating in Southwest Cuba and the Dry Tortugas: Evidence for Partial Retention in Grunts and Snappers; Gulf and Caribbean Fisheries Institute: Corpus Christi, TX, USA, 2001; pp. 732–747. [Google Scholar]
- Jeffs, A. Revealing the natural diet of the phyllosoma larvae of spiny lobster Bull. Fish. Res. Agen. 2007, 20, 9–13. [Google Scholar]
- Larrañeta, M.G. Ecología de la relación stock-reclutamiento en los peces marinos. Oceanides 1986, 11, 55–187. [Google Scholar]
- Doi, M.; Ohno, A.; Kohno, H.; Taki, Y.; Singhagraiwan, T. development of feeding ability in red snapper Lutjanus argentimaculatus early larvae. Fish. Sci. 1997, 63, 845–853. [Google Scholar] [CrossRef] [Green Version]
- Cruz, R.; Adriano, R. Regional and seasonal prediction of the Caribbean lobster (Panulirus argus) commercial catch in Cuba. Mar. Freshwat. Res. 2001, 52, 1633–1640. [Google Scholar] [CrossRef]
- Lang, J.M.; Benbow, M. Species Interactions and Competition. Nat. Educ. Knowl. 2013, 4, 1–8. [Google Scholar]






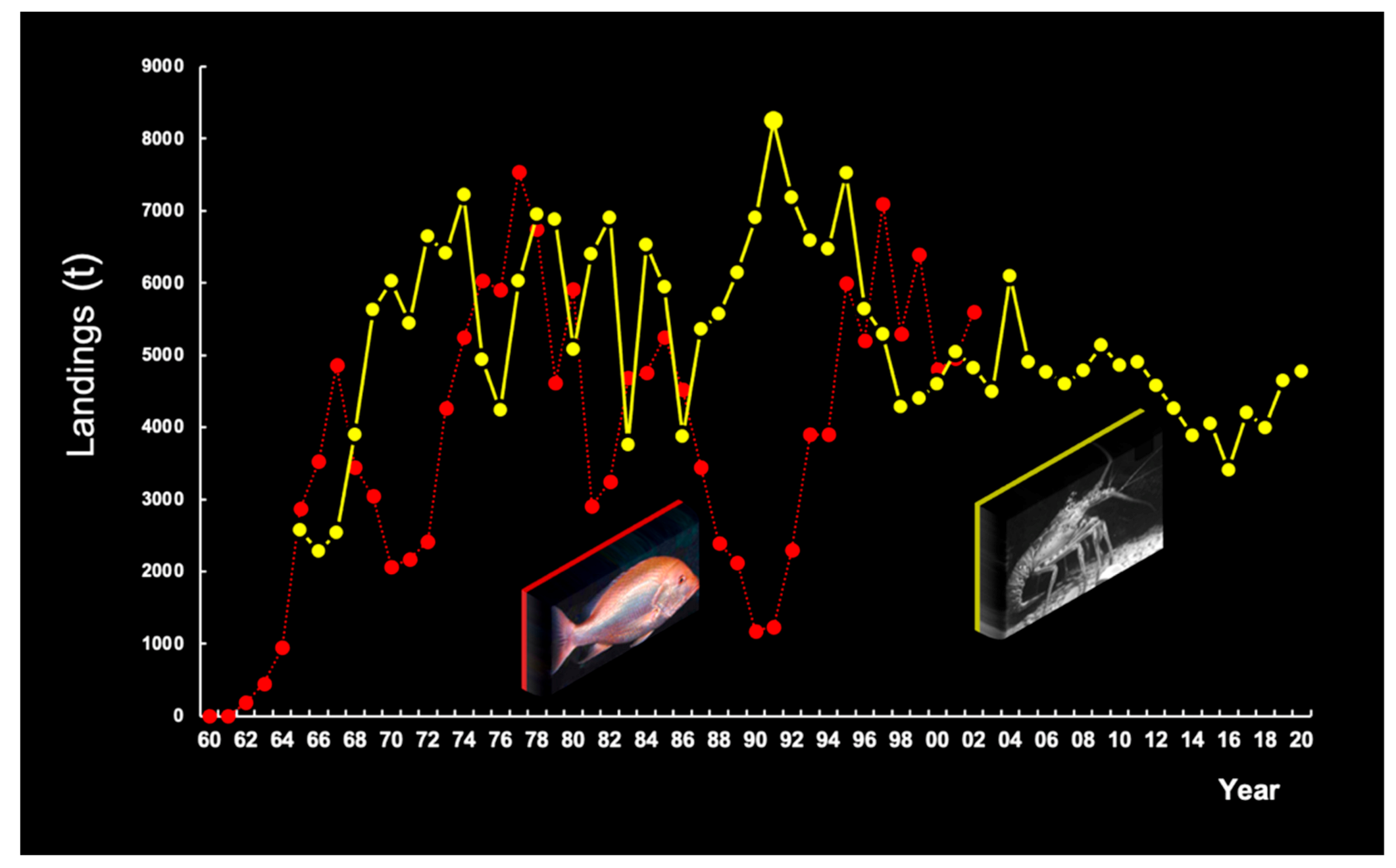
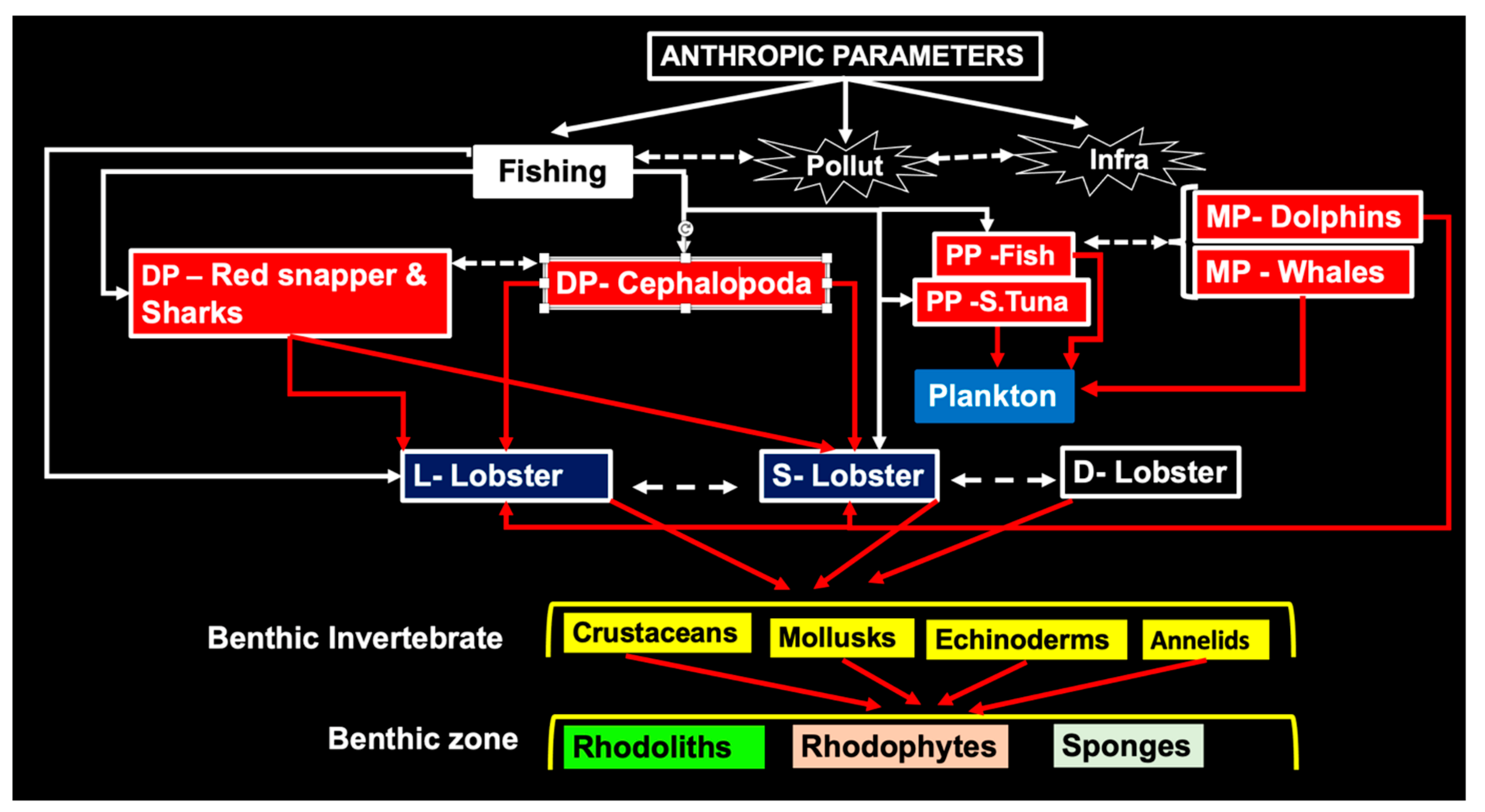
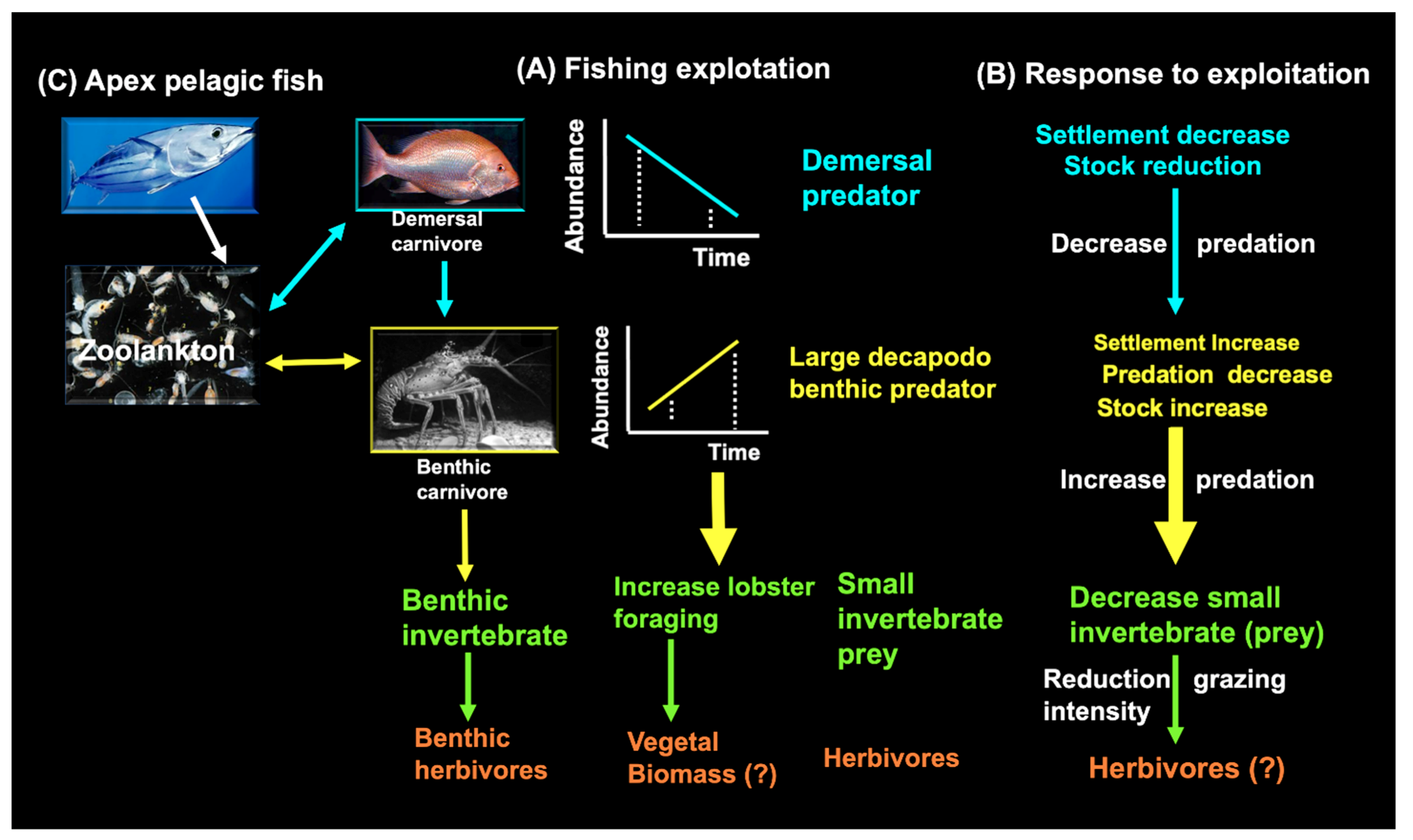
| Species | MTL (mm) | MCL (mm) | Wt (g) | Region | Depth (m) | Sex | Reference |
|---|---|---|---|---|---|---|---|
| 1- Brazilian form of Panulirus argus | 620 (a) | 233 | 3075 (b) | Br. GARS | 1–110 | M | Data base UFRA, Brazil |
| 802 (a) | 302 (b) | 6000 | Ber. | - | M | Internet (*) | |
| 2- Panulirus laevicauda | 352 (a) | 127 | Br. | 1–40 | M | Cruz et al. [13] | |
| 3- Panulirus echinatus | 328 (a) | 115 | Br. | 1–25 | M | Cruz et al. [13] | |
| 4- Palinustus truncatus | 118 | - | Br. | 60–1000 | F | Silva et al. [55] | |
| 5- Justitia longimanus | 150 | Br. | 50–100 | M | Dall’Occo [51] | ||
| 6- Palinurellus gundlachi | 150 | Cu. | 1–35 | Cruz et al. [15] | |||
| 7- Scyllarides delfosi | 234 | Br. GARS | 20–155 | M | Silva et al. [55] | ||
| 8- Scyllarides brasiliensis | 200 | - | Br. | 3–475 | - | Holthuis [3] | |
| 9- Scyllarides deceptor | 270 | 101 | Br. | 6–300 | F | Tavares et al. [16] | |
| 10- Scyllarus chacei | 30 | 14 | 12.4 | Br. GARS | 10–300 | F | Silva et al. [17] |
| 11- Scyllarus depressus | 147 | 27.7 | Br. | 30–536 | Puciarelli & Rego [18] | ||
| 12- Scyllarus americanus | 130 | 25.3 | Br. | 1–70 | No restrictions | ||
| 13- Parribacus antarcticus | 200 | Br. | 80–100 | Holthuis [3] | |||
| 14- Acanthacaris caeca | 300 | Br. GARS | 80–880 | Silva et al. [10] | |||
| 15- Nephropsis aculeata | 110 | Br. GARS | 140–820 | Silva et al. [10], Legal & Poupin [19] | |||
| 16- Nephropsis rosea | 145 | Br. GARS | 100–808 | Silva et al. [10] | |||
| 17- Nephropsis agassizii | 110 | Br. GARS | 878–2900 | F | Silva et al. [10] | ||
| 18- Nephropsis neglecta | 140 | Br. | 655–1234 | F | Alves–Júnior et al. [20] | ||
| 19- Metanephrops rubellus | 180 | Br. | 60–140 | M | Holthuis [3] | ||
| 20- Stereomastis sculpta | 63 | Br. GARS | 200–4020 | Silva et al. [10] | |||
| 21- Polycheles typhlops | 76 | Br. GARS | 41–2380 | Silva et al. (10] | |||
| 22- Pentacheles laevis | 52 | Br. | 750–1350 | Serejo et al. [53] | |||
| 23- Pentacheles validus | 47 | Br. | 811–2380 | Serejo et al. [53] | |||
| 24- Enoplometopus antillensis | 97 | Br. | 1–30 | Holthuis [3] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz, R.; T. Torres, M.; Santana, J.V.M.; Cintra, I.H.A. Lobster Distribution and Biodiversity on the Continental Shelf of Brazil: A Review. Diversity 2021, 13, 507. https://doi.org/10.3390/d13110507
Cruz R, T. Torres M, Santana JVM, Cintra IHA. Lobster Distribution and Biodiversity on the Continental Shelf of Brazil: A Review. Diversity. 2021; 13(11):507. https://doi.org/10.3390/d13110507
Chicago/Turabian StyleCruz, Raul, Marina T. Torres, João V. M. Santana, and Israel H. A. Cintra. 2021. "Lobster Distribution and Biodiversity on the Continental Shelf of Brazil: A Review" Diversity 13, no. 11: 507. https://doi.org/10.3390/d13110507
APA StyleCruz, R., T. Torres, M., Santana, J. V. M., & Cintra, I. H. A. (2021). Lobster Distribution and Biodiversity on the Continental Shelf of Brazil: A Review. Diversity, 13(11), 507. https://doi.org/10.3390/d13110507






