Contrasting Patterns of Sensory Adaptation in Living and Extinct Flightless Birds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Moa
2.3. Kiwi
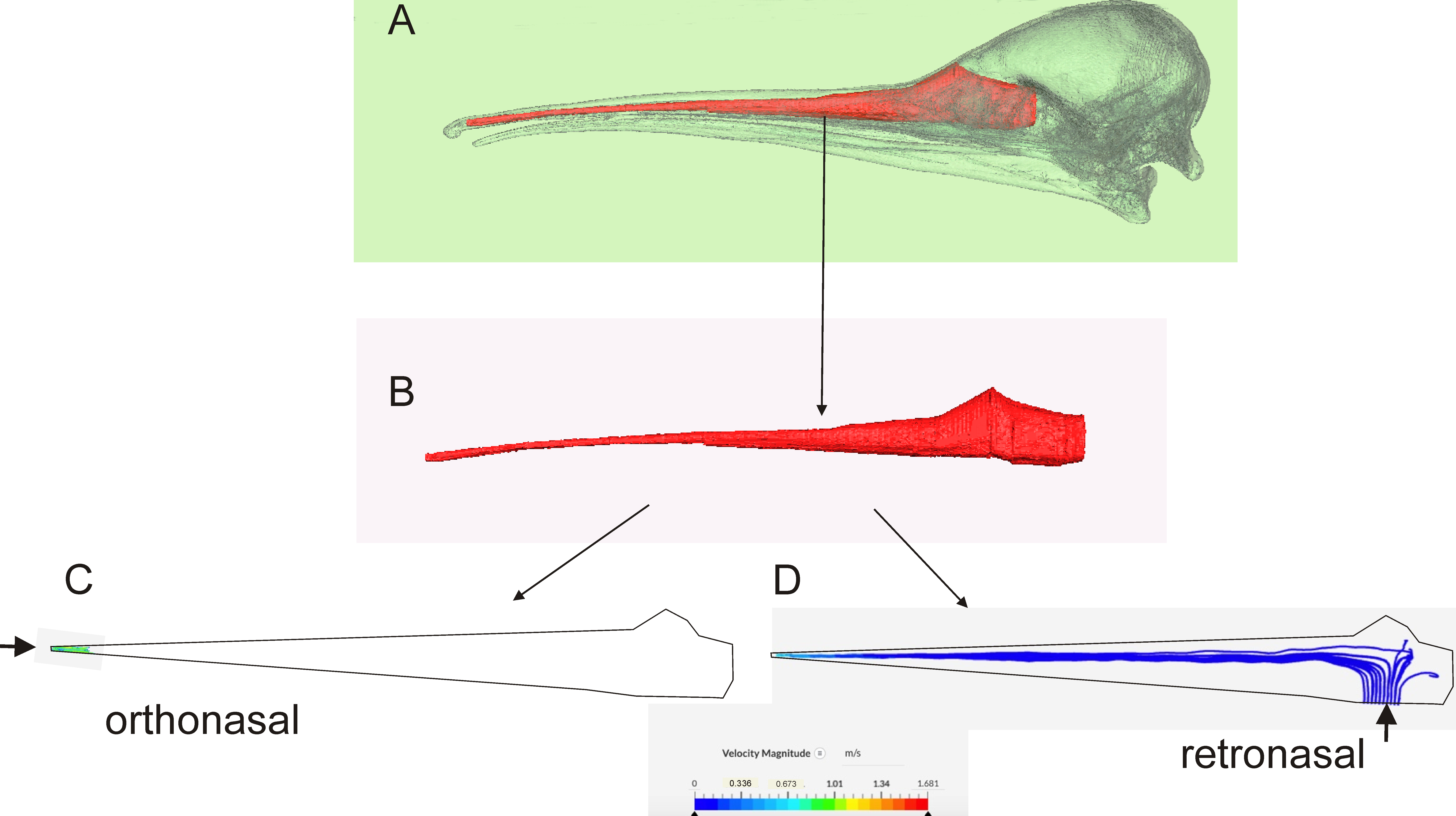
Computational Fluid Dynamics (CFD)
2.4. Comparative Material
2.5. Observation of Living Kiwi
2.6. Compliance Statement
2.7. Analyses
2.7.1. Vision
2.7.2. Hearing
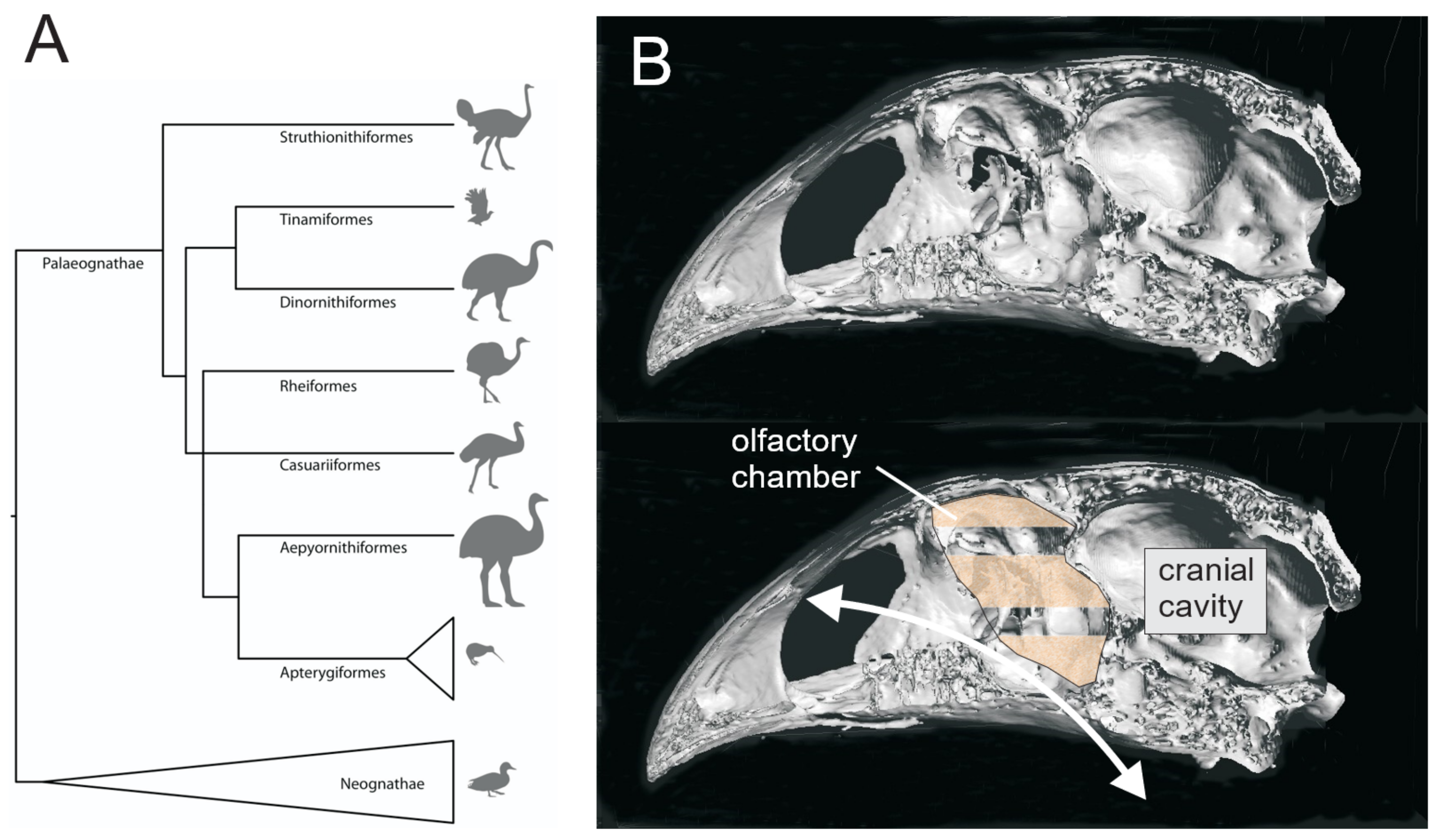
2.8. Phylogenetic Trees
3. Results
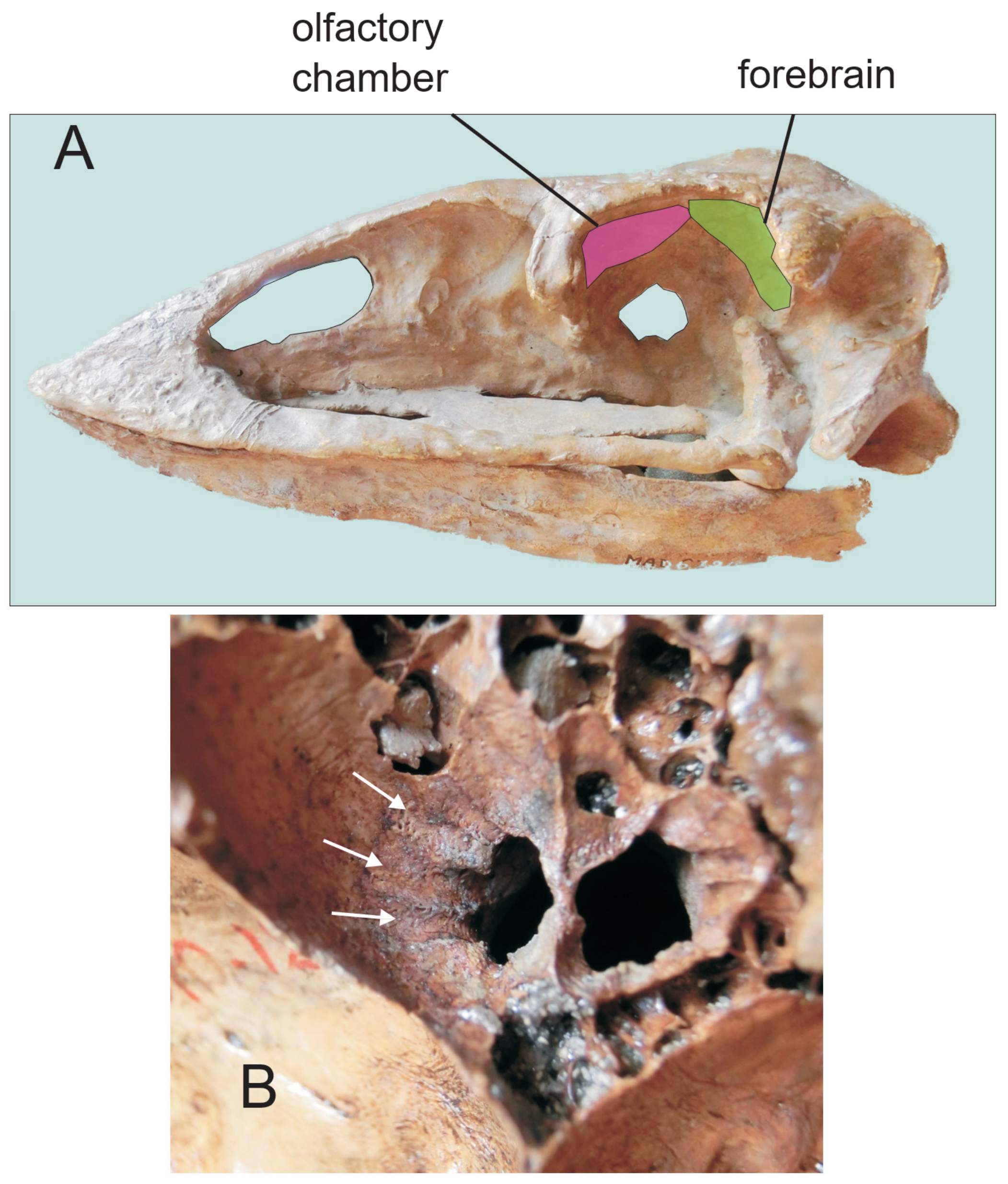
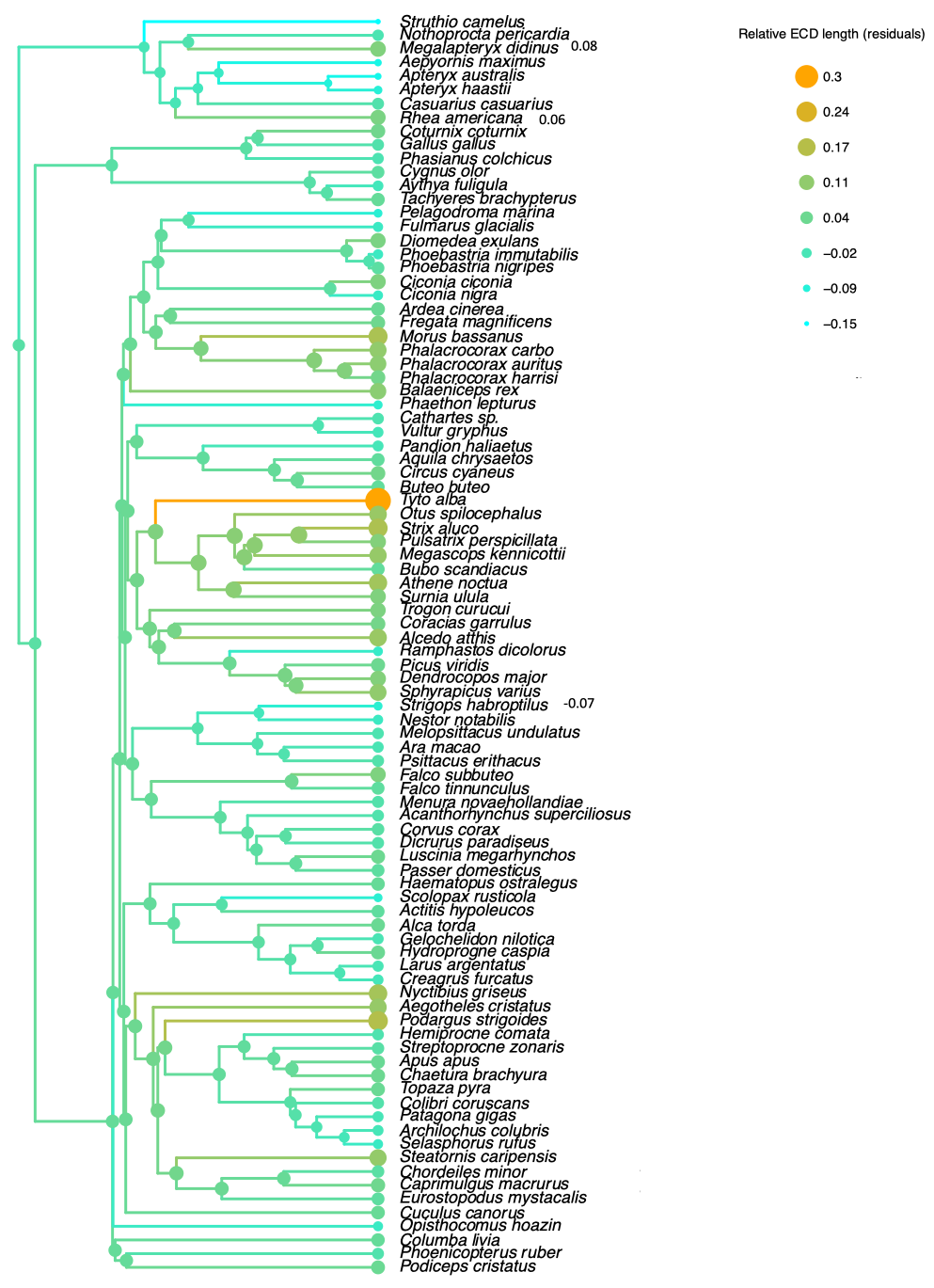
3.1. Olfaction
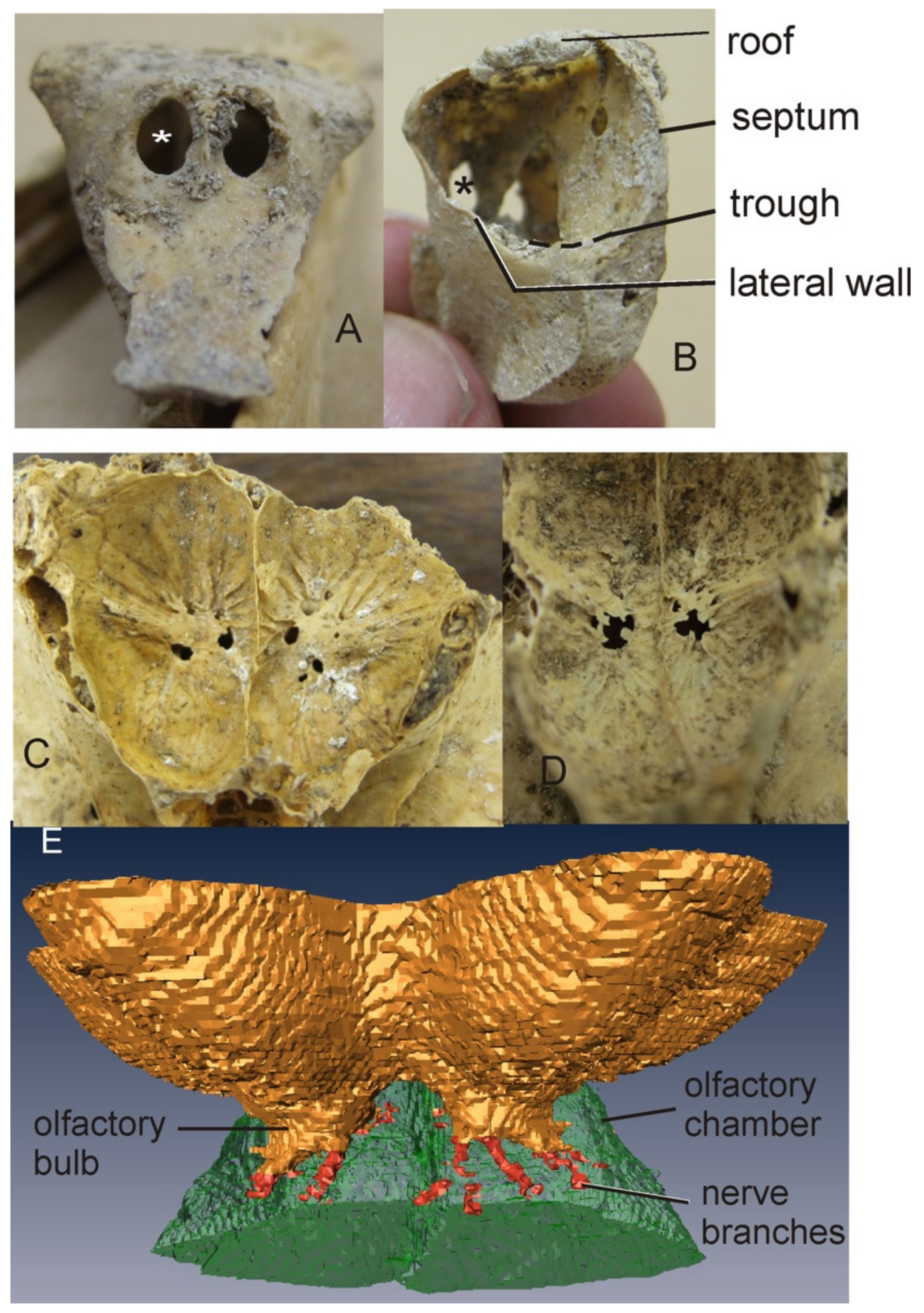
3.1.1. Moa
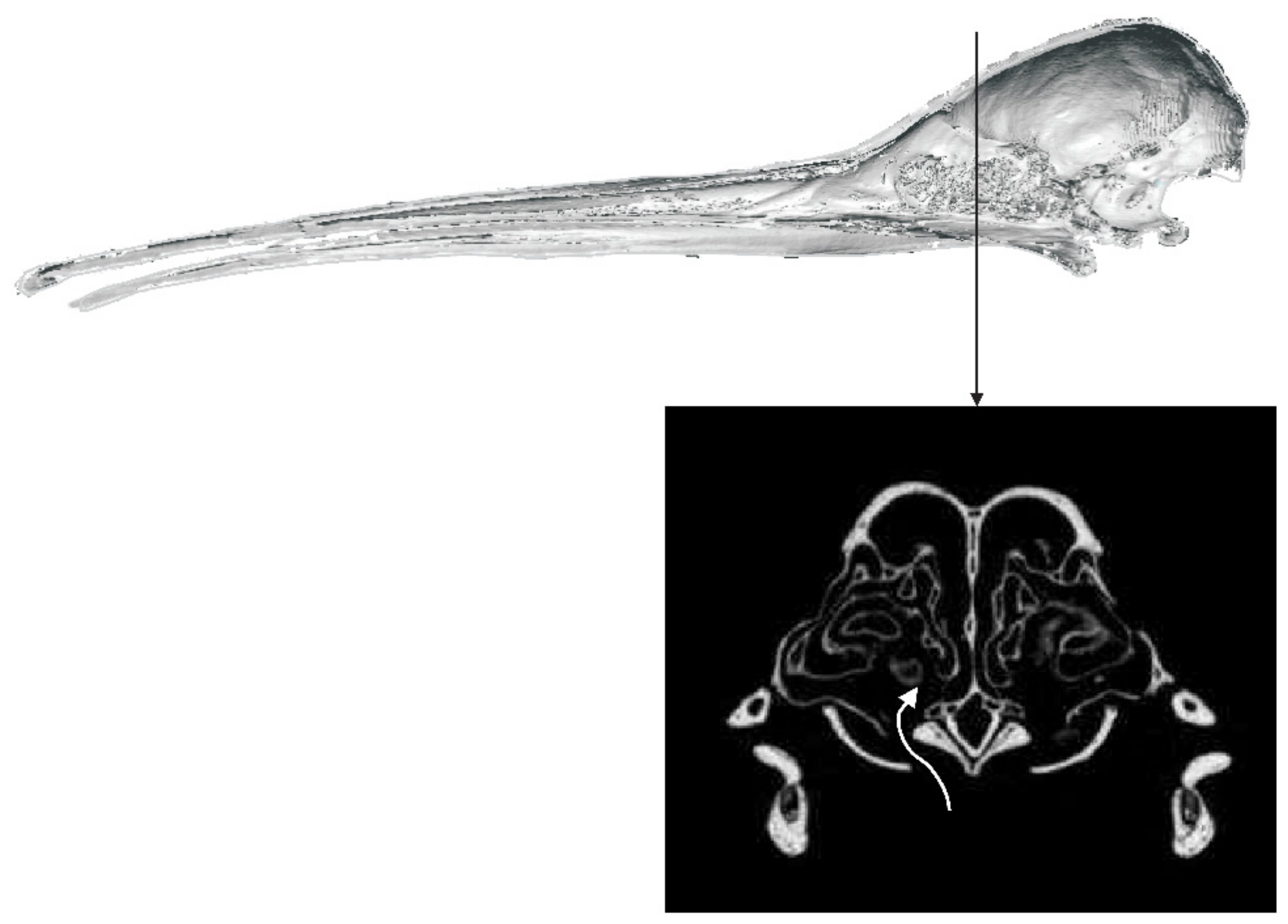
3.1.2. Kiwi
3.1.3. Aepyornithidae
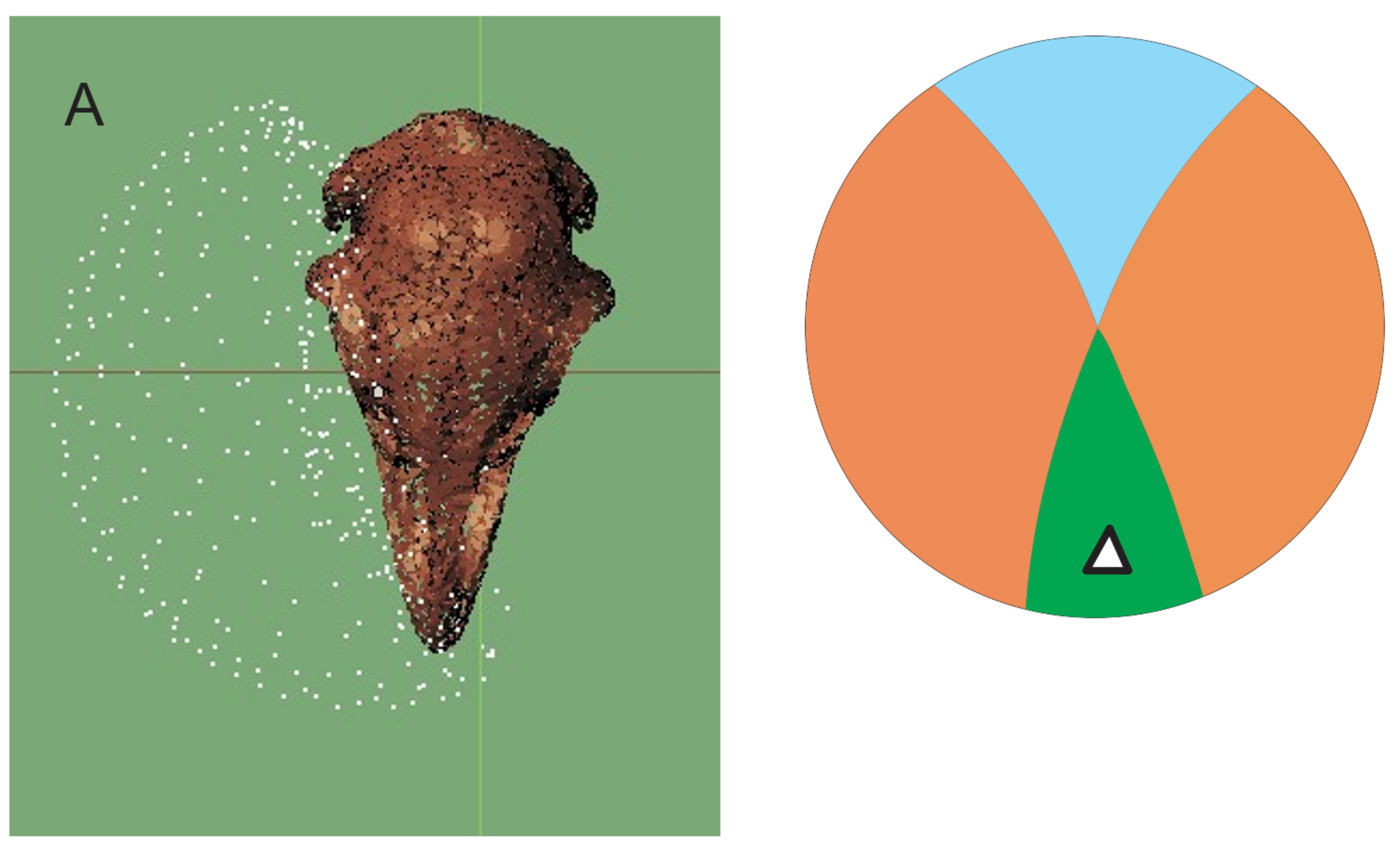
3.2. Vision
3.2.1. Moa
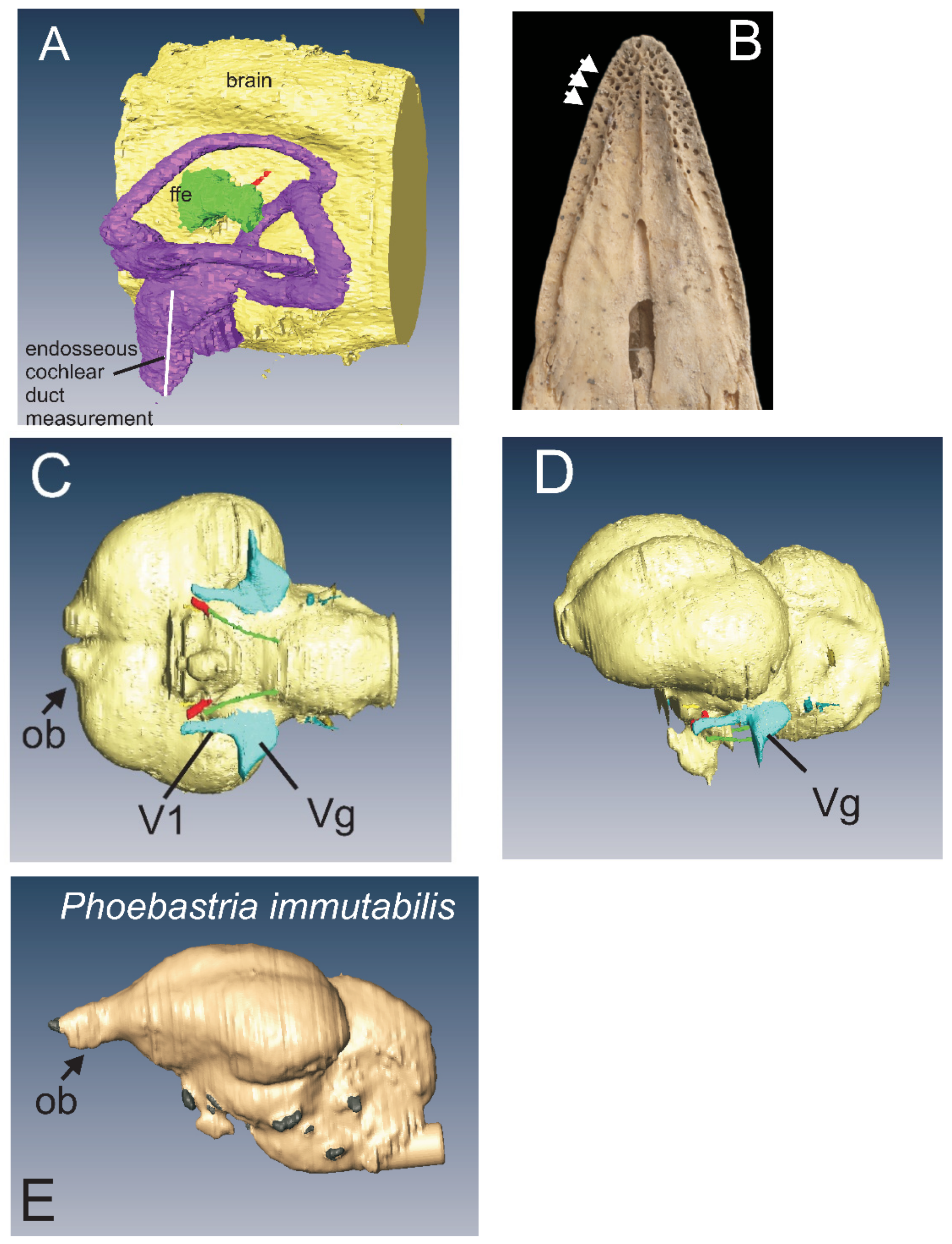
3.2.2. Kakapo
3.3. Hearing
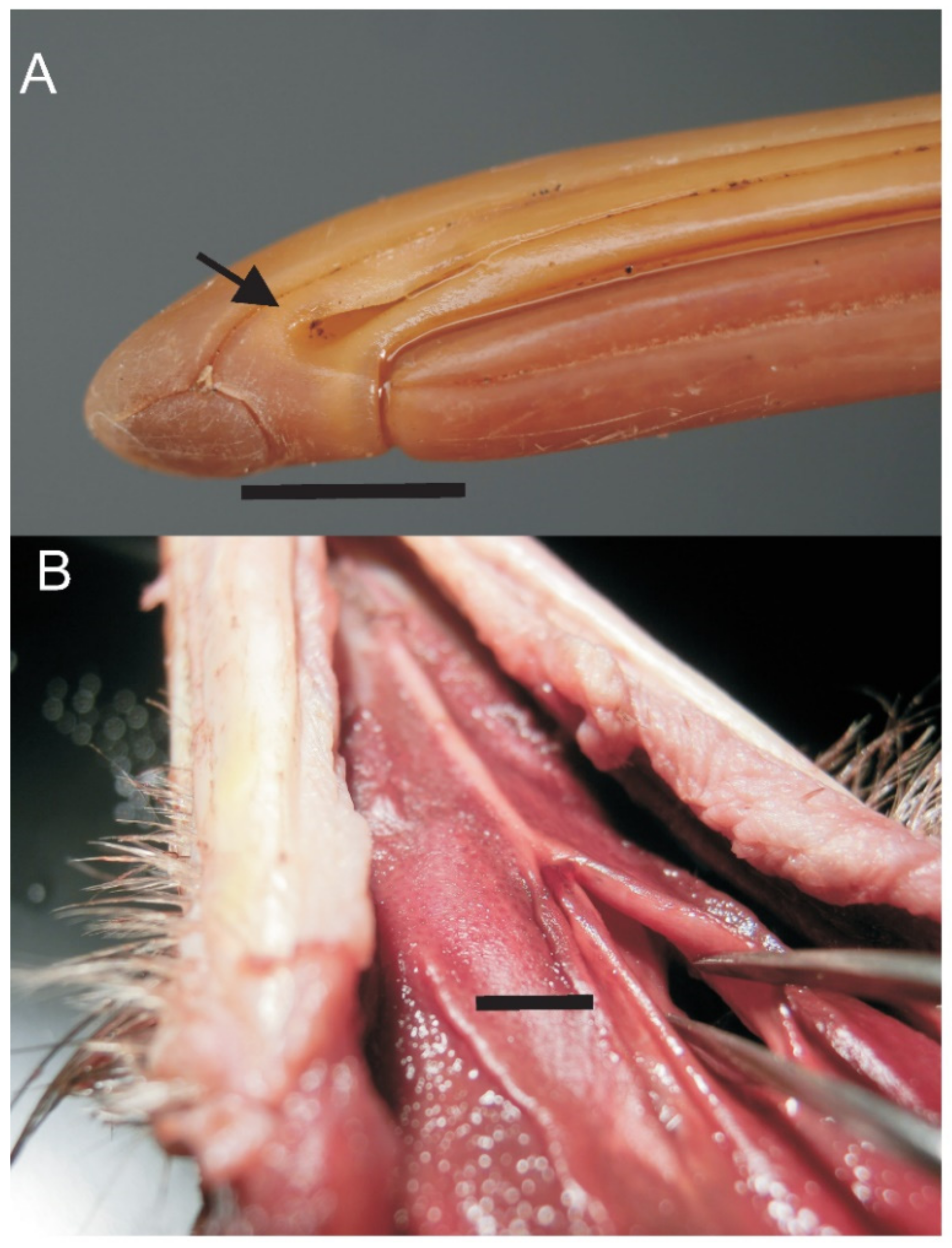
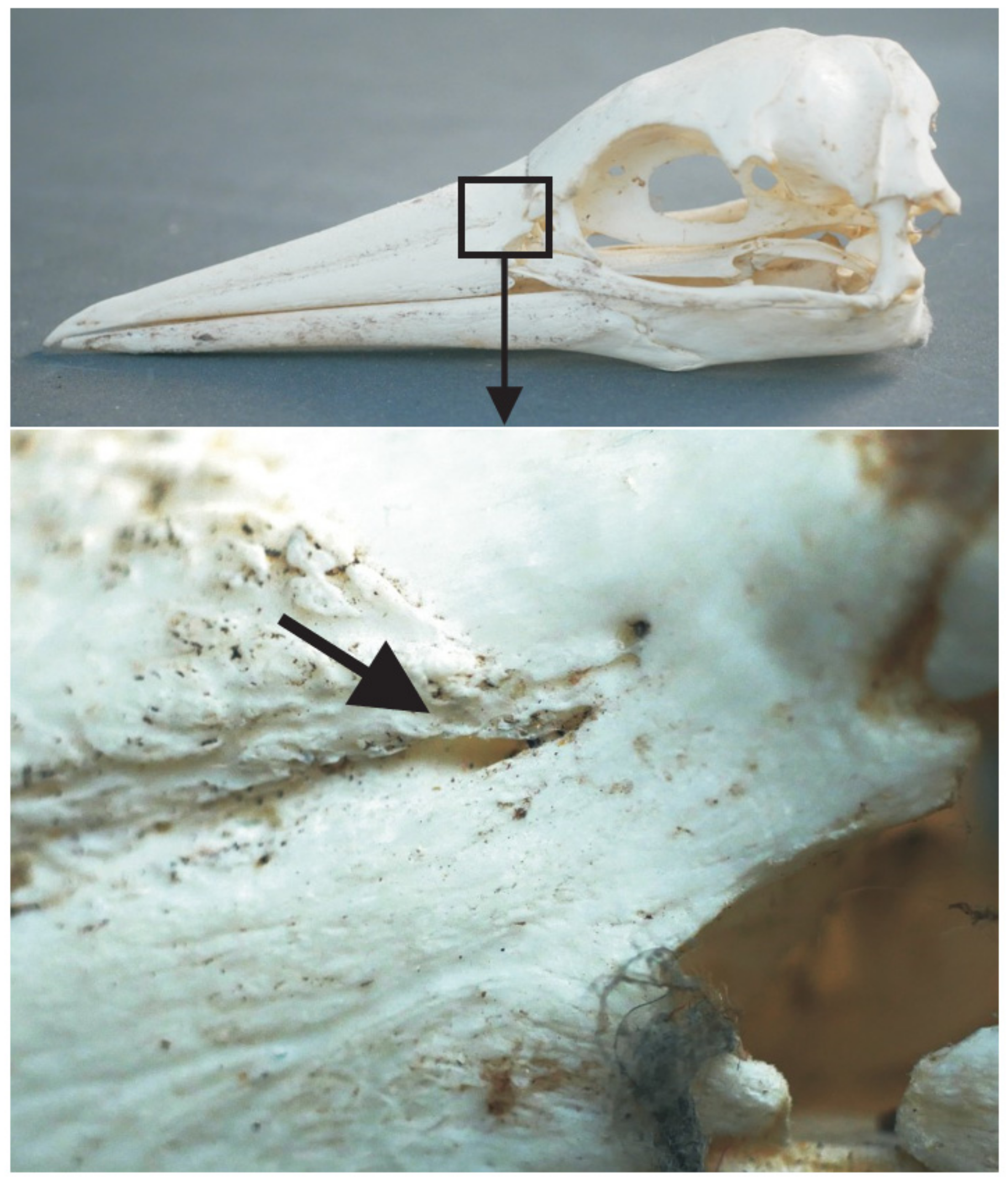
3.4. Bill Tip Sensory Organs
3.5. Comparative Material
3.5.1. Palaeognathae
3.5.2. Neognathae
4. Discussion
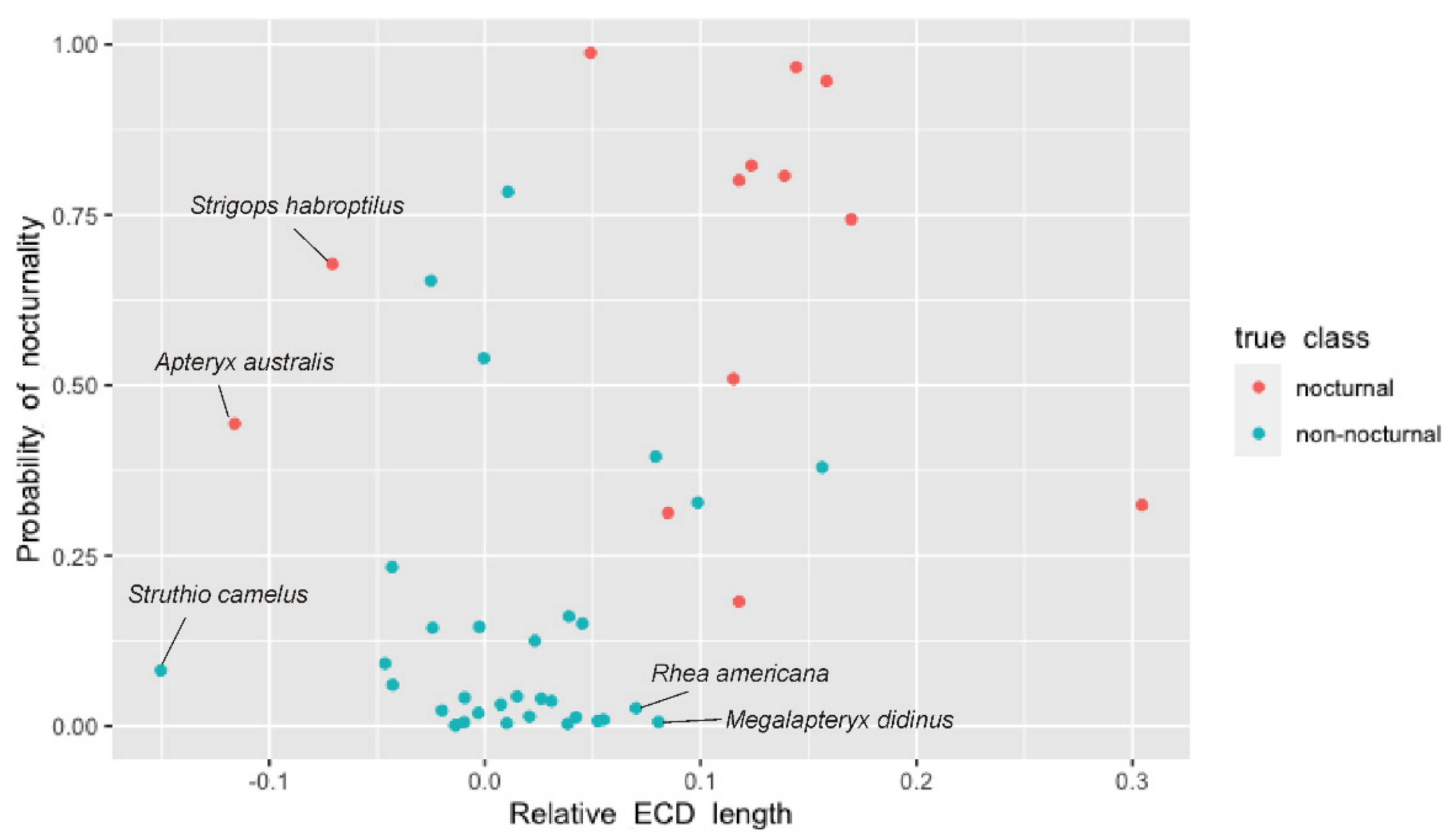
4.1. Olfaction and Sensory Systems in Kiwi
4.1.1. Retronasal Olfaction
4.1.2. Sniffing
4.2. Olfaction in Moa
4.3. Hearing in Moa
4.4. Vision in Moa
4.5. Bill-Tip Sensory Organ
4.6. Floccular Fossa Endocast
4.7. Kakapo
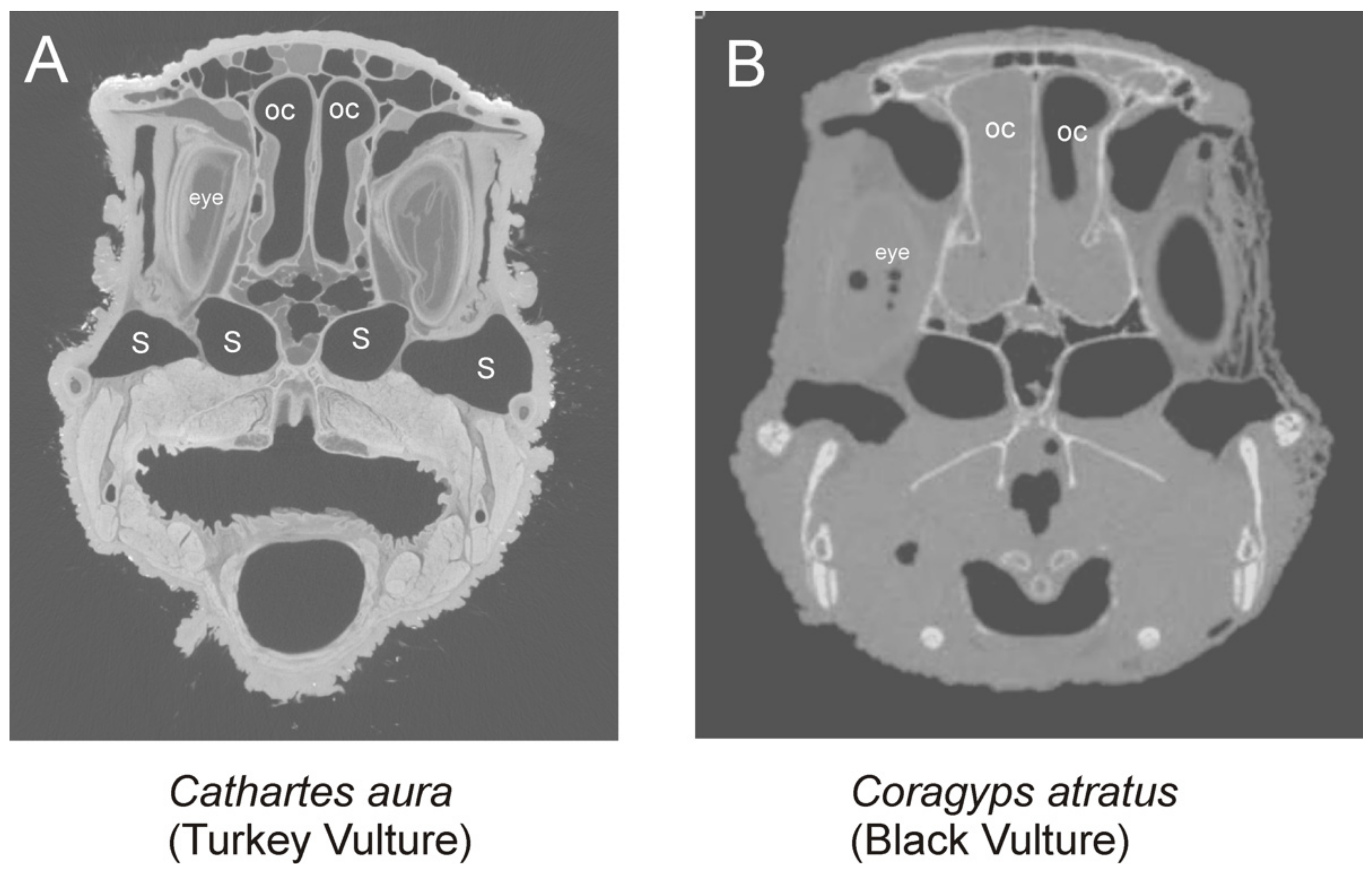
4.8. Aepyornithids
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations:
Appendix A
Material Examined
- Moa:
- Dinornis novaezealandiae: MNZ S37876; AIM: LB6400; LB6952; LB7870; LB6952; LB6400; LB6833; LB6401; LB6310; LB 6308; LB6432;
- Dinornis robustus: MNZ S28225
- Anomalopteryx didiformis MNZ S35274; MNZ S5795; AIM: LB5548; LB5545; LB5979; LB5596; LB5819; LB5796; LB5843; LB5465; LB5485; LB5504; LB5515; LB5519; LB5511; LB5552; LB5553; LB5555; LB5550; LB5593; LB5596; lb5627; LB5793; LB5620; LB5653; LB5684; LB5914; LB5798; LB5819;
- Emeus crassus MNZ S470; MNZ S792; AIM: LB6285;
- Euryapteryx curtus MNZ S30212; AIM LB6710; LB6637; LB6616; LB6666; LB6246; LB6251; LB6285;
- Pachyornis elephantopus AIM: LB5946
- Pachyornis geranoides AIM: LB6030; LB6020; LB6021; LB6024; LM6069;
- Pachyornis australis MNZ S27896
- Megalapteryx didinus MNZ S28206; MNZ S33763; MNZ S400; AIM: LB5904;
- Moa: CT scans: D. robustus MNZ S28225, A. didiformis MNZ S35274, E. crassus, MNZ S470, E. curtus, MNZ S30212, P. australis MNZ S27896, M.didinus, MNZ S28206. Pacific Radiology, Wellington (New Zealand), on a General Electric Discovery CT750 HD scanner, at 80 kV and 40 µA, and reconstructed as axial 0.3mm slices).
- P. elephantopus AIM LB5946; Mercy Radiology, Auckland. GE Discovery CT750, 120 kV, 150 mA, 0.625 mm slices.
- P. elephantopus MNHN 1875-602, from Dryad Digital Repository https://doi.org/10.5061/dryad.7519042 (accessed on 14 September 2021), C. Torres and J. Clarke
- M. didinus AIM LB5904, microCT scan (30.5.2011,Bioengineering Institute, University of Auckland; Skyscan 1172: 100 kV, 100 μA, reconstructed as 1626 slices, voxel size 34.6 μm, image size 1984 × 1984 pixels).
- Moa: MRI scan: M. didinus, MNZ S400 Siemens Magnetom Avanto 1.5 Tesla scanner with a Siemens 12 channel head matrix coil and B17 software. Performance per axis details were: maximum amplitude 33 mT/m, minimum rise time 264 microseconds from 0–33 mT/m, maximum slew rate 125 T/m/s.
- Kiwi:
- Apteryx mantelli: JVC 386, JVC 387; AIM LB7709; LB7289; LB2182; LB5540; LB7202; LB5539; LB9246; LB14145;
- Apteryx australis: AIM LB13427; LB2182
- Apteryx owenii: AIM LB9427; LB11246
- Kiwi: CT scan: Apteryx species—AMHN18456, http://digimorph.org/specimens/Apteryx_sp/ (accessed on 14 September 2021).
- Kiwi: MRI scan: Apteryx mantelli: Centre for Advanced MRI, University of Auckland. Siemens Magnetom Avanto 1–5T, gradient strength 40 (across) and 45 (along) mTm-1, maximum slew rate 200 Tm-1s-1 with a 4-channel wrist coil; 2D turbo spin echo with 0.4 mm in-plane resolution and 1 mm slice thickness; echo time/repetition time/flip angle/averages = 156 ms/5510 ms/1501/6—Jeremy Corfield
- Kiwi, histological series: ZSUT-SAJ78110. Apteryx australis. Serial sectioned head of hatchling
- Aepyornithidae:
- Aepyornis maximus MNHN 1910.12; Ae. ?hildebrandti MNHN MAD6724; Ae. ‘medius’ MNHN1911-27
- microCT scan: Ae. maximus MNHN 1910.12; 629 slices at voxel size 138 μm; Romain Allain and Ronan David
- Struthio camelus: JVC 343; AIM LB11730
- CT scan: L. Witmer lab: https://people.ohio.edu/witmerl/3D_ostrich.htm; https://youtu.be/gDQ8a0_oH6k (accessed on 14 September 2021).
- Dromaeus novaehollandiae: JVC355; AIM541
- CT scan, SAM39373,—Trevor Worthy
- Rhea americana TMM M-6721, from Dryad Digital Repository https://doi.org/10.5061/dryad.7519042 (accessed on 14 September 2021), C. Torres and J. Clarke
- Casuarius casuarius TMM M-12033, from Dryad Digital Repository https://doi.org/10.5061/dryad.7519042 (accessed on 14 September 2021), C. Torres and J. Clarke
- Rhea pennata: AIM LB1216
- Nothoprocta pericardia, UMNH 23838, from Dryad Digital Repository https://doi.org/10.5061/dryad.7519042, C. Torres and J. Clarke
- Morus serrator (Australasian gannet): JVC201
- Cathartes aura (Turkey Vulture); Morphosource 000125045, Jessie Maisano
- Coragyps atratus (Black Vulture); http://digimorph.org/specimens/Coragyps_atratus/ (accessed on 14 September 2021)—Tim Rowe
- Stripogs habroptilus (Kakapo): Morphosource 000158358, Roger Benson
- Pachyptila desolata: Morphosource 000167145, Jeff Zeyl
- Thalassarche chlororhynchos: Morphosource 000166936, Jeff Zeyl
- Fulmaris glacialis: Morphosource 000032762 Roger Benson
- Puffinus grisea: Morphosource 000166694, Jeff Zeyl
- Phoebastria immutabilis: http://digimorph.org/specimens/Diomedea_immutabilis/ (accessed on 14 September 2021)—Tim Rowe.
References
- Martin, G.R. The Sensory Ecology of Birds, 1st ed.; Oxford University Press: Oxford, UK, 2017; p. 296. [Google Scholar]
- Knoll, F.; Kawabe, S. Avian palaeoneurology: Reflections on the eve of its 200th anniversary. J. Anat. 2020, 236, 965–979. [Google Scholar] [CrossRef]
- Early, C.M.; Ridgely, R.C.; Witmer, L.M. Beyond Endocasts: Using Predicted Brain-Structure Volumes of Extinct Birds to As-sess Neuroanatomical and Behavioral Inferences. Diversity 2020, 12, 34. [Google Scholar] [CrossRef]
- Torres, C.R.; Clarke, J.A. Nocturnal giants: Evolution of the sensory ecology in elephant birds and other palaeognaths inferred from digital brain reconstructions. Proc. R. Soc. B 2018, 285, 20181540. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, T.; Segawa, T.; Mori, H.; Campos, P.; Hongoh, Y.; Endo, H.; Akiyoshi, A.; Kohno, N.; Nishida, S.; Wu, J.; et al. Phylogenomics and Morphology of Extinct Paleognaths Reveal the Origin and Evolution of the Ratites. Curr. Biol. 2017, 27, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Grealy, A.; Phillips, M.; Miller, G.; Gilbert, M.; Rouillard, J.-M.; Lambert, D.; Bunce, M.; Haile, J. Eggshell palaeogenomics: Palaeognath evolutionary history revealed through ancient nuclear and mitochondrial DNA from Madagascan elephant bird (Aepyornis sp.) eggshell. Mol. Phylogenetics Evol. 2017, 109, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.J.; Gibb, G.C.; Crimp, E.A.; Penny, D. Tinamous and Moa Flock Together: Mitochondrial Genome Sequence Analysis Reveals Independent Losses of Flight among Ratites. Syst. Biol. 2009, 59, 90–107. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, K.J.; Llamas, B.; Soubrier, J.; Rawlence, N.J.; Worthy, T.H.; Wood, J.R.; Lee, M.S.Y.; Cooper, A. Ancient DNA reveals elephant birds and kiwi are sister taxa and clarifies ratite bird evolution. Science 2014, 344, 898–900. [Google Scholar] [CrossRef]
- Corfield, J.R.; Gsell, A.C.; Brunton, D.; Heesy, C.P.; Hall, M.I.; Acosta, M.; Iwaniuk, A. Anatomical Specializations for Nocturnality in a Critically Endangered Parrot, the Kakapo (Strigops habroptilus). PLoS ONE 2011, 6, e22945. [Google Scholar] [CrossRef] [PubMed]
- Castro, I.; Cunningham, S.J.; Gsell, A.C.; Jaffe, K.; Cabrera, A.; Liendo, C. Olfaction in birds: A closer look at the Kiwi (Apter-ygidae). J. Avian Biol. 2010, 41, 213–218. [Google Scholar] [CrossRef]
- Owen, R. Memoirs on the Extinct Wingless Birds of New Zealand, with an Appendix on Those of England, Australia, New Foundland, Mauritius and Rodriquez; John van Voorst: London, UK, 1879. [Google Scholar]
- Corfield, J.; Eisthen, H.L.; Iwaniuk, A.N.; Parsons, S. Anatomical specialisations for enhanced olfactory sensitivity Kiwi, Ap-teryx mantelli. Brain Behav. Evol. 2014, 84, 214–226. [Google Scholar] [CrossRef]
- Bang, B. Functional Anatomy of the Olfactory System in 23 Orders of Birds. Acta Anat. 1971, 79 (Suppl. 1), 1–76. [Google Scholar] [CrossRef] [PubMed]
- Hansford, J.P.; Turvey, S.T. Unexpected diversity within the extinct elephant birds (Aves: Aepyornithidae) and a new identity for the world’s largest bird. R. Soc. Open Sci. 2018, 5, 181295. [Google Scholar] [CrossRef] [PubMed]
- Worthy, T.H.; Holdaway, R.N. The Lost World of Moa; Indiana University Press: Bloomington, IN, USA, 2002. [Google Scholar]
- Owen, R. On Dinornis (part XXIII): Containing a description of the head and feet, with their dried integuments, of an indi-vidual of the species Dinornis didinus, Owen. Trans. Zool. Soc. Lond. 1883, 11, 257–261. [Google Scholar]
- Parker, T.J. On the cranial osteology, classification and phylogeny of Dinornithidae. Transactions of the Zoological Society of London. 1895, 8, 373–428. [Google Scholar] [CrossRef][Green Version]
- Ashwell, K.; Scofield, R. Big Birds and Their Brains: Paleoneurology of the New Zealand Moa. Brain Behav. Evol. 2007, 71, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Corfield, J.R.; Wild, J.; Hauber, M.E.; Parsons, S.; Kubke, M.F. Evolution of Brain Size in the Palaeognath Lineage, with an Emphasis on New Zealand Ratites. Brain Behav. Evol. 2007, 71, 87–99. [Google Scholar] [CrossRef]
- Corfield, J.R.; Price, K.; Iwaniuk, A.N.; Gutiérrez-Ibáñez, C.; Birkhead, T.; Wylie, D.R. Diversity in olfactory bulb size in birds reflects allometry, ecology, and phylogeny. Front. Neuroanat. 2015, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Zelenitsky, D.K.; Therrien, F.; Ridgely, R.C.; McGee, A.R.; Witmer, L. Evolution of olfaction in non-avian theropod dinosaurs and birds. Proc. R. Soc. B. 2011, 278, 3625–3634. [Google Scholar] [CrossRef]
- Atkinson, I.A.E.; Greenwood, R.M. Relationships between moas and plants. N. Z. J. Ecol. 1989, 12, 67–96. [Google Scholar]
- Worthy, T.H. Aspects of the biology of two moa species (Aves: Dinornithiformes). N. Z. J. Archaeol. 1989, 11, 77–86. [Google Scholar]
- Vickers-Rich, P.; Trusler, P.; Rowley, M.J.; Cooper, A.; Chambers, G.K.; Bock, W.J.; Millener, P.; Worthy, T.H.; Yaldwyn, J.C. Morphology, Myology, Collagen and DNA of a Mummified Uplandmoa, Megalapteryx Didinus (Aves: Dinornithiformes) from New Zealand. Tuhinga Rec. Mus. New Zealand Te Papa Tongarewa 1995, 4, 1–26. [Google Scholar]
- Rawlence, N.; Wood, J.; Scofield, R.; Fraser, C.; Tennyson, A.; Wood, J.; Scofield, R. Soft-tissue specimens from pre-European extinct birds of New Zealand. J. R. Soc. N. Z. 2013, 43, 154–181. [Google Scholar] [CrossRef]
- Attard, M.R.G.; Wilson, L.A.B.; Worthy, T.H.; Scofield, R.P.; Johnston, P.; Parr, W.H.C.; Wroe, S.R. Moa diet fits the bill: Virtual reconstruction incorporating mummified remains and prediction of biomechanical performance in avian giants. Proc. R. Soc. B 2016, 283, 20152043. [Google Scholar] [CrossRef]
- Simscale. Available online: https://www.simscale.com (accessed on 14 September 2021).
- Autodesk Meshmixer. Available online: https://www.meshmixer.com (accessed on 14 September 2021).
- Brackenbury, J.H. Lung-Air-Sac Anatomy and Respiratory Pressures in the Bird. J. Exp. Biol. 1972, 57, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Beale, G. A radiological study of the kiwi (Apteryx australis mantelli). J. R. Soc. N. Z. 1985, 15, 187–200. [Google Scholar] [CrossRef]
- Morphosource. Available online: https://www.morphosource.org (accessed on 14 September 2021).
- Rowe, T.B. Digimorph. Available online: http://digimorph.org/index.phtml (accessed on 14 September 2021).
- Goswami, A. Phenome10K: A Free Online Repository for 3-D Scans of Biological and Palaeontological Specimens. Available online: www.phenome10k.org. (accessed on 14 September 2021).
- Witmer, L.M. WitmerLab. Available online: https://people.ohio.edu/witmerl/projects.htm (accessed on 14 September 2021).
- Community, B.O. Blender-A 3D Modelling and Rendering Package. Available online: http://www.blender.org. (accessed on 14 September 2021).
- Choiniere, J.N.; Neenan, J.M.; Schmitz, L.; Ford, D.P.; Chapelle, K.E.J.; Balanoff, A.M.; Sipla, J.S.; Georgi, J.A.; Walsh, S.A.; Norell, M.A.; et al. Evolution of vision and hearing modalities in theropod dinosaurs. Science 2021, 372, 610–613. [Google Scholar] [CrossRef]
- Schmitz, L.; Motani, R. Morphological differences between the eyeballs of nocturnal and diurnal amniotes revisited from op-tical perspectives of visual environments. Vis. Res. 2010, 50, 936–946. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; Team, R.C. Nlme: Linear and Nonlinear Mixed Effects Models. 2014. Available online: http://CRAN.R-project.org/package=nlme (accessed on 30 October 2020).
- Paradis, E.; Schliep, K. ape 5.0: An environment for modern phylogenetics and evolutionary analyses in R. Bioinform. 2018, 35, 526–528. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.A.; Barrett, P.M.; Milner, A.C.; Manley, G.; Witmer, L.M. Inner ear anatomy is a proxy for deducing auditory capa-bility and behaviour in reptiles and birds. Proc. R. Soc. B 2009, 276, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Hackett, S.J.; Kimball, R.T.; Reddy, S.; Bowie, R.C.K.; Braun, E.L.; Braun, M.J.; Chojnowski, J.L.; Cox, W.A.; Han, K.-L.; Harshman, J.; et al. A Phylogenomic Study of Birds Reveals Their Evolutionary History. Science 2008, 320, 1763–1768. [Google Scholar] [CrossRef]
- Jetz, W.; Thomas, G.; Joy, J.B.; Hartmann, K.; Mooers, A.O. The global diversity of birds in space and time. Nat. Cell Biol. 2012, 491, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Geneious Prime. Available online: http://www.geneious.com/ (accessed on 7 April 2020).
- Drummond, A.J.; Rambaut, A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 2007, 7, 214. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.-T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; Von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Jarvis, E.D.; Mirarab, S.; Aberer, A.J.; Li, B.; Houde, P.; Li, C.; Ho, S.Y.W.; Faircloth, B.C.; Nabholz, B.; Howard, J.T.; et al. Whole-genome analyses resolve early branches in the tree of life of modern birds. Science 2014, 346, 1320–1331. [Google Scholar] [CrossRef]
- Cloutier, A.; Sackton, T.B.; Grayson, P.; Clamp, M.; Baker, A.J.; Edwards, S.V. Whole-Genome Analyses Resolve the Phylogeny of Flightless Birds (Palaeognathae) in the Presence of an Empirical Anomaly Zone. Syst. Biol. 2019, 68, 937–955. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior Summarization in Bayesian Phylogenetics Using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Marples, B.J. The Structure and Development of the Nasal Glands of Birds. Proc. Zool. Soc. Lond. 1932, 102, 829–844. [Google Scholar] [CrossRef]
- Walsh, S.A.; Iwaniuk, A.; Knoll, M.A.; Bourdon, E.; Barrett, P.M.; Milner, A.C.; Nudds, R.L.; Abel, R.L.; Sterpaio, P.D. Avian Cerebellar Floccular Fossa Size Is Not a Proxy for Flying Ability in Birds. PLoS ONE 2013, 8, e67176. [Google Scholar] [CrossRef]
- Grigg, N.P.; Krilow, J.M.; Gutierrez-Ibanez, C.; Wylie, D.R.; Graves, G.R.; Iwaniuk, A.N. Anatomical evidence for scent guided foraging in the turkey vulture. Sci. Rep. 2017, 7, 17408. [Google Scholar] [CrossRef] [PubMed]
- Rowe, T.B.; Shepherd, G.M. Role of ortho-retronasal olfaction in mammalian cortical evolution. J. Comp. Neurol. 2016, 524, 471–495. [Google Scholar] [CrossRef] [PubMed]
- Bourke, J.M.; Porter, W.R.; Ridgely, R.C.; Lyson, T.R.; Schnachner, E.R.; Bell, P.R.; Witmer, L.M. Breathing life into dinosaurs: Tackling challenges of soft-tissue restoration and nasal airflowiin extinct species. Anat. Rec. 2014, 297, 2148–2186. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Dalton, P.; Yang, G.C.; Scherer, P.W. Numerical Modeling of Turbulent and Laminar Airflow and Odorant Transport during Sniffing in the Human and Rat Nose. Chem. Senses 2006, 31, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Huxley, T.H. On the Respiratory Organs of Apteryx. Proc. Zool. Soc. Lond. 1882, 50, 560–569. [Google Scholar] [CrossRef]
- Bang, B.G.; Wenzel, B.M. Nasal cavity and olfactory system. In Form and Function in Birds; King, A.S., McLelland, J., Eds.; Academic Press: London, UK, 1985; Volume 3, pp. 195–225. [Google Scholar]
- Müller, H. Die Morphologie und Entwicklung des Craniums von Rhea americana Linné. Z. Für Wissensschaftliche Zoologie 1961, 165, 221–319. [Google Scholar]
- Bellairs, A.D. The Early Development of the Interorbital Septum and the Fate of the Anterior Orbital Cartilages in Birds. J. Embryol. Exp. Morphol. 1958, 6, 68–85. [Google Scholar] [CrossRef]
- Hüppi, E.; Werneburg, I.; Sánchez-Villagra, M.R. Evolution and development of the bird chondrocranium. Front. Zool. 2021, 18, 21. [Google Scholar] [CrossRef]
- Parsons, T.S. Nasal Anatomy and the Phylogeny of Reptiles. Evolution 1959, 13, 175–187. [Google Scholar] [CrossRef]
- Ali, F.; Zelenitsky, D.K.; Therrien, F.; Weishampel, D.B. Homology of the “Ethmoid Complex” of Tyrannosaurids and its im-plications for reconstruction of the olfactory apparatus of non-avian theropods. J. Vertebr. Paleontol. 2008, 28, 123–133. [Google Scholar] [CrossRef]
- Parker, T.J., II. Observation on the anatomy and development of apteryx. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 1891, 182, 25–134. [Google Scholar] [CrossRef]
- Starck, D. Die endokraniale Morphologie der Ratiten, besonders der Apterygidae und Dinornithidae. Morphol. Jahrb. 1955, 96, 14–72. [Google Scholar]
- Wood, J.; Richardson, S.; McGlone, M.; Wilmshurst, J. The diets of moa (Aves: Dinornithiformes). N. Z. J. Ecol. 2020, 44, 3397. [Google Scholar] [CrossRef]
- Aidala, Z.; Huynen, L.; Brennan, P.L.R.; Musser, J.; Fidler, A.; Chong, N.; Capuska, G.E.M.; Andersen, M.G.; Tabala, A.; Lambert, D.; et al. Ultraviolet visual sensitivity in three avian lineages: Palaeognaths, parrots, and passerines. J. Comp. Physiol. A 2012, 198, 495–510. [Google Scholar] [CrossRef]
- McInerney, P.L.; Lee, M.S.Y.; Clement, A.M.; Worthy, T.H. The phylogenetic significance of the morphology of the syrinx, hyoid and larynx, of the southern cassowary, Casuarius casuarius (Aves, Palaeognathae). BMC Evol. Biol. 2019, 19, 1–18. [Google Scholar] [CrossRef]
- Pérez-Granados, C.; Schuchmann, K.-L. Vocalisations of the Greater Rhea (Rhea americana): An allegedly silent ratite. Bioacoustics 2021, 30, 564–574. [Google Scholar] [CrossRef]
- Oliver, W.R.B. The Moas of New Zealand and Australia; Dominion Museum, Bulletin 15: Wellington, New Zealand, 1949. [Google Scholar]
- Owen, R. On Dinornis (part XVI): Containing notices of the internal organs of some species, with a description of the brain and some nerves and muscles of the head of Apteryx australis. Trans. Zool. Soc. Lond. 1871, 7, 381–396. [Google Scholar] [CrossRef]
- Owen, R. On the Anatomy of Vertebrates; Longmans, Green: London, UK, 1866; Volume 2. [Google Scholar]
- Garcia, S.M.; Kopuchian, C.; Mindlin, G.B.; Fuxjager, M.J.; Tubaro, P.L.; Goller, F. Evolution of vocal diversity through mor-phological adaptation without vocal learning or complex neural control. Curr. Biol. 2017, 27, 2677–2683. [Google Scholar] [CrossRef]
- Leader-Williams, N.; Owen-Smith, R.N. Megaherbivores: The Influence of Very Large Body Size on Ecology. J. Anim. Ecol. 1990, 59, 381. [Google Scholar] [CrossRef]
- Martin, G.; Osorio, D.; Masland, D.; Albright, T.D. Vision in birds. In The Senses: A Comprehensive Reference; Masland, R.H., Al-bright, T.D., Dallos, P., Oertel, D., Firestein, S., Beauchamp, G.K., Bushnell, M.C., Basbaum, A., Kaas, J.H., Gardner, E.P., Eds.; Academic Press: Cambridge, MA, USA, 2008; Volume 1. [Google Scholar]
- Martin, G.R. What is binocular vision for? A birds’ eye view. J. Vis. 2009, 9, 14. [Google Scholar] [CrossRef]
- Tyrrell, L.P.; Fernández-Juricic, E. Avian binocular vision: It’s not just about what birds can see, it’s also about what they can’t. PLoS ONE 2017, 12, e0173235. [Google Scholar] [CrossRef]
- Froggatt, J.; Gill, B. Bill morphology reflects adaptation to a fibrous diet in the kākāpō (Strigops: Psittaciformes). N. Z. J. Zool. 2016, 43, 138–148. [Google Scholar] [CrossRef]
- Crole, M.R.; Soley, J. Comparative morphology, morphometry and distribution pattern of the trigeminal nerve branches supplying the bill tip in the ostrich (Struthio camelus) and emu (Dromaius novaehollandiae). Acta Zool. 2016, 97, 49–59. [Google Scholar] [CrossRef]
- Ferreira-Cardoso, S.; Araújo, R.; Martins, N.; Martins, G.; Walsh, S.; Martins, R.M.S.; Kardjilov, N.; Manke, I.; Hilger, A.; Castanhinha, R. Floccular fossa size is not a reliable proxy of ecology and behaviour in vertebrates. Sci. Rep. 2017, 7, 2005. [Google Scholar] [CrossRef] [PubMed]
- Early, C.M. Quantitative Assessments of Avian Endocasts as Tools for Inferring Neuroanatomical Traits and Potential Functional Capabilities; Ohio University: Athens, OH, USA, 2019. [Google Scholar]
- Johnston, P. Unpublished obeservations: Endocast Diversity in Moa. 2021. [Google Scholar]
- Hagelin, J.C. Observations on the olfactory ability of the Kakapo Strigops habroptilus, the critically endangered parrot of New Zealand. Ibis 2003, 146, 161–164. [Google Scholar] [CrossRef]
- Steiger, S.S.; Fidler, A.E.; Kempenaers, B. Evidence for increased olfactory receptor gene repertoire size in two nocturnal bird species with well-developed olfactory ability. BMC Evol. Biol. 2009, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- Wylie, D.R.; Gutiérrez-Ibáñez, C.; Iwaniuk, A.N. Integrating brain, behavior, and phylogeny to understand the evolution of sensory systems in birds. Front. Neurosci. 2015, 9, 281. [Google Scholar] [CrossRef]
- Selba, M.C.; Bryson, E.R.; Rosenberg, C.L.; Heng, H.G.; DeLeon, V.B. Selective breeding in domestic dogs: How selecting for a short face impacted canine neuroanatomy. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2021, 304, 101–115. [Google Scholar] [CrossRef]
- Kawabe, S.; Shimokawa, T.; Miki, H.; Matsuda, S.; Endo, H. Variation in avian brain shape: Relationship with size and orbital shape. J. Anat. 2013, 223, 495–508. [Google Scholar] [CrossRef]
- Weisbecker, V.; Rowe, T.; Wroe, S.; Macrini, T.E.; Garland, K.L.S.; Travouillon, K.J.; Black, K.; Archer, M.; Hand, S.J.; Berlin, J.C.; et al. Global elongation and high shape flexibility as an evolutionary hypothesis of accommodating mammalian brains into skulls. Evolution 2021, 75, 625–640. [Google Scholar] [CrossRef]
- Benson, R.B.J.; Starmer-Jones, E.; Close, R.A.; Walsh, S.A. Comparative analysis of vestibular ecomorphology in birds. J. Anat. 2017, 231, 990–1018. [Google Scholar] [CrossRef] [PubMed]
- Isler, K.; van Shaik, C. Costs of encephalization: The energy trade-off hypothesis tested on birds. J. Hum. Evol. 2006, 51, 228–243. [Google Scholar] [CrossRef] [PubMed]
- Wright, N.A.; Steadman, D.W.; Witt, C.C. Predictable evolution toward flightlessness in volant island birds. Proc. Natl. Acad. Sci. USA 2016, 113, 4765–4770. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sinha, A.; André, A.A.; Spötl, C.; Vonhof, H.B.; Meunier, A.; Kathayat, G.; Duan, P.; Voarintsoa, N.R.G.; Ning, Y.; et al. A multimillenial climatic context for the megafaunal extinctions in Madagascar and Mascarene Islands. Sci. Adv. 2020, 6, 42. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnston, P.; Mitchell, K.J. Contrasting Patterns of Sensory Adaptation in Living and Extinct Flightless Birds. Diversity 2021, 13, 538. https://doi.org/10.3390/d13110538
Johnston P, Mitchell KJ. Contrasting Patterns of Sensory Adaptation in Living and Extinct Flightless Birds. Diversity. 2021; 13(11):538. https://doi.org/10.3390/d13110538
Chicago/Turabian StyleJohnston, Peter, and Kieren J. Mitchell. 2021. "Contrasting Patterns of Sensory Adaptation in Living and Extinct Flightless Birds" Diversity 13, no. 11: 538. https://doi.org/10.3390/d13110538
APA StyleJohnston, P., & Mitchell, K. J. (2021). Contrasting Patterns of Sensory Adaptation in Living and Extinct Flightless Birds. Diversity, 13(11), 538. https://doi.org/10.3390/d13110538





