1. Introduction
The genus
Peniagone is the most species rich in the family Elpidiidae including 33 valid species according to WoRMS [
1]. The genus commonly occurs in all oceans except for the Arctic, with the highest species diversity in the Antarctic and the Pacific Ocean [
2]. The vertical distribution of
Peniagone ranges from 220 to 8660 m, with most records at the lower bathyal and abyssal depths. The genus is characterized by a high diversity of morphological features such as form and size of its velum, the position and size of its tube feet and the configuration of the tentacle crown. These features are of particular interest for questions on colonization and adaptation to the deep-sea environments since in
Peniagone species they are related to the swimming mode and/or adaptations to balancing of the body on substrate, in the presence of the near-bottom currents [
3].
The deep-sea northwest Pacific echinoderm fauna is one of the most species rich [
4]. Data on the northwest Pacific deep-sea holothurians began accumulating since the HMS
Challenger, USFS
Albatross and the local Japanese cruises, from 1875 to the early 20th century [
5,
6,
7]. The Zoological Institute in Leningrad (now St. Petersburg, Russia) has conducted numerous cruises to the Bering Sea and to the Sea of Okhotsk, which resulted in a series of papers on the echinoderm taxonomy [
8,
9,
10,
11,
12]. Since 1949, the northwest Pacific has been explored by the Soviet RV
Vityaz expeditions which collected extensive material from the Kuril–Kamchatka, Aleutian, Japan and Izu–Bonine trenches and adjacent abyssal and bathyal areas. Based on these materials, several studies were published on Myriotrochidae [
13,
14],
Elpidia [
15],
Kolga [
16] and
Psychropotes [
17]. Despite relatively extensive historical explorations in the region, the recent deep-sea cruises KuramBio [
18], SokhoBio [
19] and KuramBio II [
20] collected many species and even genera new to science [
17,
21], most of them remaining undescribed.
So far, the northwest Pacific fauna of
Peniagone was not sufficiently explored. Among six species reported for the area,
P. dubia (Djakonov et Saveljeva),
P. japonica Ohshima and
P. mus Djakonov are known only from the original descriptions, all of them lacking information on the morphological characters presently used for species delimitation. Another species common to the area identified as
Peniagone cf.
incerta (Théel) in Mironov et al. [
4] requires further investigation due to identification uncertainty. Two more species,
P. purpurea Théel and
P. gracilis (Ludwig), reported by Gebruk [
22] are also in need of re-examination since some of their morphological features differ from those in the original descriptions. In the present study, we examine materials collected in recent expeditions to the northwest Pacific and re-examine some of earlier RV
Vityaz collections from this area. In particular, we re-describe two poorly known species,
Peniagone dubia and
P. mus, describe two species new to science,
P. minuta and
P. saveljevae and provide additional information on
P. vitrea Théel and
P. cf.
purpurea. (Figures 1–8). Molecular data were obtained for
P. mus,
P. saveljevae and
P. cf.
purpurea and used for phylogenetic analysis (Figures 9 and 10).
2. Materials and Methods
Specimens were collected during three German-Russian cruises: KuramBio (2012), SokhoBio (2015) and KuramBio II (2016). Additionally, the specimens obtained during the following cruises of the RV
Vityaz were re-examined: 8 (1951), 19 (1954), 22 (1955), 29 (1958), 39 (1966), 43 (1968), 45 (1969), 52 (1972) and 57 (1975). All specimens were collected using benthic trawls and mainly preserved in ethanol. Records of species with locality and sampling data are published through GBIF [
23].
Specimens were identified based on standard characters used for elpidiid holothurians [
24]. Features of external morphology were examined using a stereomicroscope; slide preparations of calcareous epidermal elements (ossicles) of dorsal and ventral sides were examined using a compound microscope Olympus BX43.
Abbreviations used for specimen repositories: IORAS, P.P. Shirshov Institute of Oceanology, Moscow, Russia; MIMB, Museum of the A.V. Zhirmunsky National Scientific Center of Marine Biology, Vladivostok, Russia; NHM, Natural History Museum, London, UK; NMNH, National Museum of Natural History, Washington, USA; NOCS, National Oceanography Centre, Southampton, UK; SGN, Senckenberg Research Institute and Natural History Museum, Frankfurt, Germany; ZIN, Zoological Institute, St. Petersburg, Russia; ZMBN, University Museum of Bergen, University of Bergen, Norway. Specimens from SokhoBio, KuramBio and KuramBio II cruises currently stored in IORAS will be later deposited at MIMB (SokhoBio and KuramBio) and SGN (KuramBio II).
Samples for molecular analyses were taken during the KuramBio, SokhoBio and KuramBio II cruises. Other sequences were obtained in GenBank and BOLD; GenBank Accession Numbers and BOLD Process ID are listed in
Tables S1 and S2. Laboratory work was performed in the DNA Lab of the University of Bergen, Norway. Fragments of cytochrome c oxidase subunit I (COI) and 16S ribosomal RNA (16S) were amplified and sequenced using the universal and specific echinoderm primers (
Table S1) [
25,
26,
27,
28,
29]. Genomic DNA was extracted with QuickExtract™ DNA Extraction Solution using the following protocol: 100 µL of QuickExtract solution was added to each sample air-dried from ethanol, incubated for 45 min at 65 °C, following 2 min at 98 °C. Amplification was performed with TaKaRa Ex Taq HS version kit (Takara BIO, Kusatsu, Japan) in a 25 μL reaction consisted of 1 µL of DNA extract, 17.35 µL of purified water, 2.5 µL of 10× Ex Taq buffer, 2 µL of dNTP mixture, 1 µL of each primer and 0.15 µL of TaKaRa Ex Taq HS. 16S fragment was amplified under the following conditions: 94 °C for 5 min; 35 cycles of 94 °C for 0.5 min, 50 °C for 1.5 min and 72 °C for 1 min; followed by final extension at 72 °C for 10 min. COI fragment was amplified using the following conditions: 94 °C for 12 min; 35 cycles of 94 °C for 35 s, 48 °C for 70 s and 72 °C for 55 s; followed by final extension at 72 °C for 10 min. The amplified products were purified and sequenced using the Sanger method by Macrogen Europe (Amsterdam, Netherlands).
Sequences of
Peniagone azorica von Marenzeller,
P. coccinea Rogacheva et Gebruk and
P. islandica Deichmann were obtained by C.A. from specimens collected on the ECOMAR cruise in 2010 [
30]. DNA was extracted using QIAGEN DNeasy extraction kit following the manufacturers protocol. The PCR mix contained 0.2 μM of each forward and reverse primer (
Table S1), 1× QIAGEN Multiplex PCR Master Mix and 1 μg template DNA in a final 20 μL reaction volume. The PCR amplification conditions were as follows: 94 °C for 15 min, followed by 35 cycles at 94 °C for 30 s, 50 °C (for 16S) and 48 °C (for COI) for 90 s, 72 °C for 60 s, and a final extension at 60 °C for 10 min.
Sequences were manually edited and assembled in
Geneious v.7 software and then aligned in MEGA 7 [
31] using MUSCLE algorithm.
PartitionFinder2 [
32] was used for selecting best-fit partitioning schemes and models of nucleotide evolution. The most appropriate models were HKY for COI codon positions 1 and 2, and K81 for COI codon position 3, and GTR + G for 16S. In the analysis based on concatenated COI and 16S data, GTR + I + G was most appropriate for all partitions. Phylogenetic trees were generated using Bayesian inference in
MrBayes v. 3.2 [
33] for single genes and for concatenated COI and 16S matrix. For each dataset, two independent analyses each of four chains were run for 10,000,000 generations. The trees were sampled every 100th generation and 25% trees discarded as burn-in. Sufficiency of run convergence was evaluated using
Tracer v.1.7.1. Genetic distances were calculated in MEGA 7 using Between Group Mean Distance algorithm.
3. Taxonomy
Order Elasipodida Théel, 1882
Family Elpidiidae Théel, 1882
Genus Peniagone Théel, 1882
3.1. Peniagone dubia (Djakonov et Saveljeva in Djakonov et al. 1958)
Elpidiogone dubia Djakonov et al., 1958: 361–363, Figures 2–4 [
11].
Peniagone dubia—Hansen, 1975: 144–145 [
24]; Gebruk, 1990 (in Russian): 102–103, Figure 40 [
22]; Mironov et al. 2018: 354 [
34].
Material examined. Syntype of Elpidiogone dubia ZIN 1/15626 (slide preparation of skin ossicles); RV Vityaz, St. 5637, 09.09.1966, 44° 29′ N, 149° 06′ E, 2685–3035 m, IORAS ECH01295 and ECH01118, numerous fragments; St. 6671, 23.06.1972, 40° 12′ N, 143° 35′ E, 2400–2720 m, IORAS ECH01476, 2 specimens and fragments of 70+ specimens.
Diagnosis (amended). Body width about ¼ of body length. Two pairs of free minute papillae behind velum. Tube feet four pairs free in the middle part of body, beginning at the level of the last pair of minute dorsal papillae; five-six pairs fused forming posterior lobe. Dorsal ossicles with arms 0.05–0.1 mm in length, curved to almost flat; two apophyses 0.02–0.1 mm in length, shorter or of same length with arms. Ventral ossicles with elongated central stem, arms 0.0–0.012 mm in length; small apophyses four rarely 2–3.
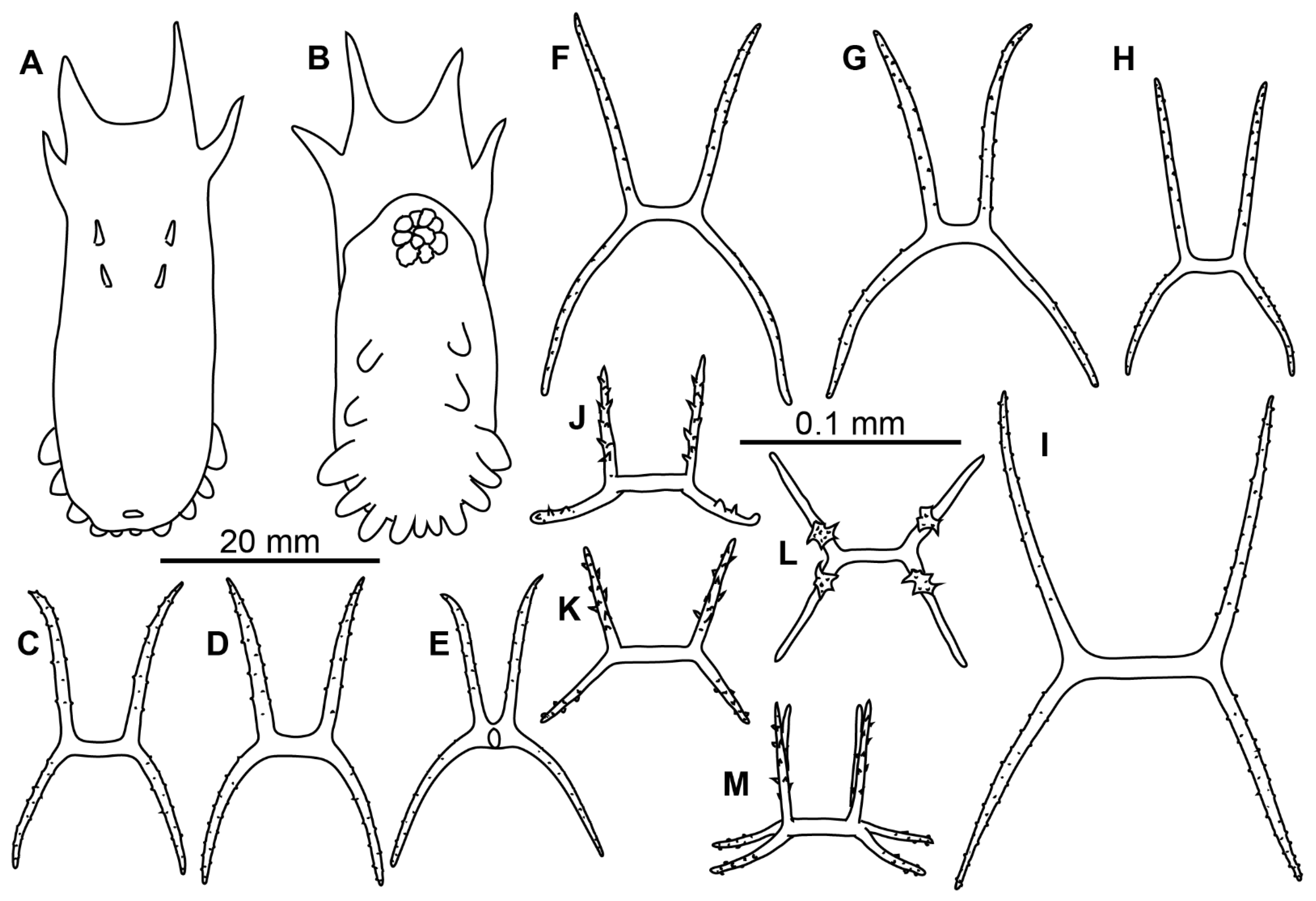
Description of material. Two preserved complete specimens were 27 mm long/6.5 mm wide and 29 mm long/7.2 mm wide. Both specimens were in poor condition, however main external morphological features can be ascertained. Body elongated (
Figure 1A,B). Dorsal velum composed of two pairs of papillae, first pair longer than second. Tentacles 10. Two anteriormost pairs of tube feet located in the anterior half of the body. Four anterior pairs of tube feet free, bigger than other pairs. Posterior tube feet decreased in size towards posterior end and partly fused. Dorsal ossicles (
Figure 2F–I) with arms curved to varying degree, may look down almost vertically; two apophyses shorter than arms or the same length with arms; arms 0.08–0.1 mm in length, apophyses 0.05–0.1 mm in length; smaller ossicles also present with arms about 0.05 mm and apophyses 0.02–0.03 mm; apophyses and arms with small spines. Ventral ossicles with elongated central stem, arms 0.0–0.012 mm in length; small apophyses usually four (one on each arm), but 2–3 apophyses also present.
Genetic data. No genetic data is available for this species.
Distribution. Type locality is in the southern part of the Sea of Okhotsk at depth 2850 m. New records are in the northwest Pacific at depths 2400–3015 m (Figure 11).
Remarks. Description of the species was based on six poorly preserved specimens deposited at the Zoological Institute, St. Petersburg (Russia). In the original description, details of dorsal and ventral ossicles were missing. However, ossicles were found during re-examination of the original slides of the bodywall skin of the syntype ZIN 1/15626 (
Figure 2A–E). Several ossicles were found with strongly curved arms that are characteristic of the dorsal skin in
Peniagone (
Figure 2A(left), B,D(right)). The species
P. dubia is well distinguished by small dorsal ossicles with slender arms and only two apophyses. Among the species of
Peniagone with ossicle arms ~0.1 mm in length, including
P. challengeri Théel,
P. lugubris Théel,
P. purpurea (Théel),
P. intermedia Ludwig,
P. papillata Hansen,
P. vedeli Hansen,
P. herouardi Gebruk, and
P. dubia, only in the latter the dominant dorsal ossicles bear one pair of apophyses. The arrangement of tube feet with four anteriormost pairs free and other pairs fused forming the posterior lobe is the unique feature of external morphology differing
P. dubia from the above listed species.
3.2. Peniagone minuta Kremenetskaia et Gebruk sp. nov.
Peniagone gracilis (Ludwig)—Gebruk, 1990 (in Russian): 96–97 (partim.), Figure 36 (1–12) [
22].
Holotype. Specimen 22 mm in length, IORAS ECH02166, RV Vityaz, St. 6085, 01.05.1969, Sigsbee Trawl, 50°48′6″ N, 173°29′0″ W, 6965–7000 m.
Paratypes. 12 specimens, 14–21 mm in length, IORAS ECH02170, collected at same station as the holotype.
Additional material. RV Vityaz, St. 3491, 07.10.1955, Sigsbee Trawl, 30°34′ N, 142°41.1′ E, 7305–7315 m, IORAS ECH01059, ECH01060, ECH01065, ECH01067, ECH01286, ECH01479, ~50 specimens; RV Vityaz, St. 6015, 25.04.1968, 26°51′ N, 165°32′ E, 5850 m, IORAS ECH01041, 2 specimens; St. 6085 (type locality), IORAS ECH01040, 84 specimens; St. 6144, 16.06.1969, Sigsbee Trawl, 51°42′5″ N, 168°5′0″ W, 7200 m, IORAS ECH01052, 28 specimens; St. 6145, 20.06.1969, Sigsbee Trawl, 51°1′19″ N, 174°35′5″ E, 7250 m, IORAS ECH01114, 5 specimens; St. 7404, 09.05.1975, Sigsbee Trawl, 29°14′ N, 142°30′ E, 6890–6770 m, IORAS ECH01050, 32 specimens; St. 7407, 11.05.1975, Galathea Trawl, 29°18′ N, 143° 15′ E, 6850–6740 m, IORAS ECH01054, 9 specimens.
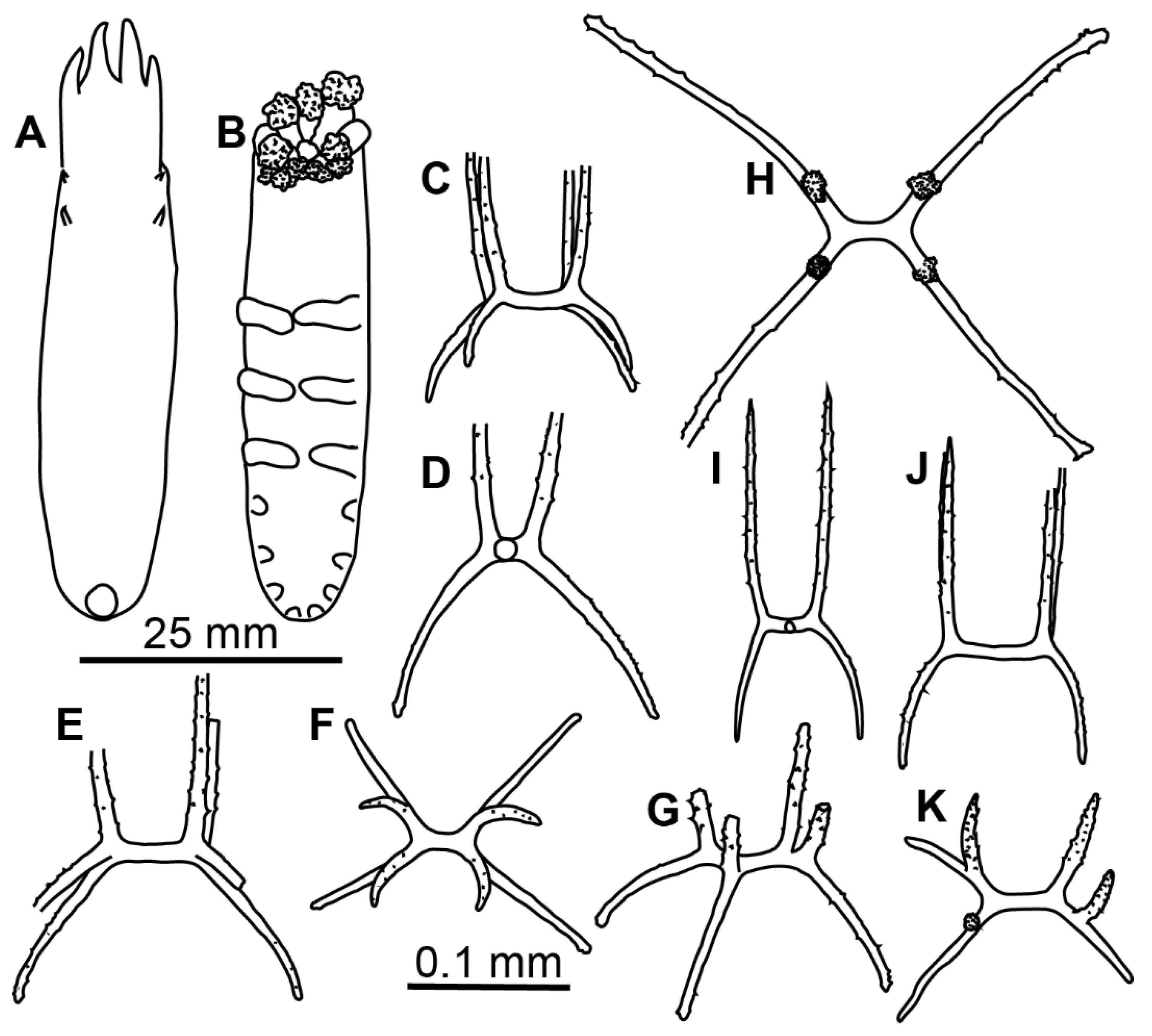
Diagnosis. Body oval, convex, body length up to 35 mm. Skin translucent, colorless, with dense ossicle layer. Tube feet 5–7 pairs, 3–4 anterior pairs bigger and 2–3 pairs smaller tube feet forming narrow posterior brim, anteriormost pair located at about a half of the body. Velum short, wide, two pairs of very small papillae behind velum. Dorsal ossicles with four spinous apophyses, apophyses slightly curved inside or straight, up to 0.35 mm long, from half to the same length as the arms; arms up to 0.4 mm, spinous proximally, curved downwardly and inside. Ventral ossicles with four apophyses up to 0.2 mm in length, arms curved downwardly, up to 0.35 mm.
Description of material. Specimens from St. 6085 were in the exceptionally good condition (
Figure 3E–G); other specimens in condition from poor to moderate. Body length from 11 to 24 mm (in type series) and up to 35 mm at other stations; body oval, convex on dorsum and sometime on ventrum (
Figure 1C,D and
Figure 3E–G). Ten tentacles; five anterior tentacles with a pair of long lobes on their aboral margin. Velum formed by two pairs of fully fused papillae, no free tips clearly visible in the preserved specimens. Dorsal papillae two pairs, very small, often lost. Tube feet located from about mid-body to the posterior end in most specimens although in some specimens anteriormost tube feet appeared in the anterior body half. Tube feet of four first pairs often the biggest in most of the specimens. Smaller posterior tube feet are often poorly visible, posterior brim narrow. Dorsal ossicles with arms mostly ~0.2 mm long, occasionally up to 0.4 mm, usually spaced widely, curved downwardly or occasionally almost straight, with small spines; apophyses four, spines more prominent and numerous than on arms, apophyses almost straight, mostly broken at their ends even in better preserved specimens, apophyses length up to 0.35 mm (
Figure 1E–L). Ventral ossicles similar to dorsal in shape, but smaller and slender; arms curved, up to 0.35 mm, apophyses four, up to 0.2 mm, spiny (
Figure 1M,N). Calcareous ring pieces with 9 pairs of arms, which can be subdivided. Polian vesicle single.
Genetic data. No genetic data is available for this species.
Etymology. The name minuta indicates the relatively small size of this species.
Distribution. Aleutian and Izu–Bonine trenches, at 6850–7315 m; one record in the northwest Pacific abyssal plain at 5850 m. Type locality in the Aleutian Trench, off Atka Island.
Remarks. Specimens collected at
Vityaz stations 3491, 6015, 6085, 6144, 6145, 7404 and 7407 were identified as
Peniagone gracilis (Ludwig) by Gebruk [
22] as well as the North Atlantic specimens from St. 390-3 of RV
Akademik Mstislav Keldysh. Re-examination of the syntypes of
Scotoanassa gracilis Ludwig NMNH 18178 and 18179 revealed remarkable differences in ossicle morphology: (i) ossicles in the syntypes were smaller than in
P. minuta, their arms rarely exceeded 0.2 mm in length; (ii) apophyses were usually longer than arms. Unfortunately, both syntypes were in poor condition lacking details of external morphology. According to Ludwig [
35], the syntypes were up to 68 mm long suggesting that
P. gracilis exceeds
P. minuta in body length.
Gebruk [
22] assigned
P. japonica Ohshima to
P. gracilis based on details of external and ossicle morphology in the original description [
7].
Peniagone japonica is known from three stations off Japan at 1142–1680 m and is characterized by body length 55 mm, eight pairs of tube feet, dorsal deposits with bent arms 0.12–0.2 mm long and four apophyses 0.7–0.12 mm long, and ventral ossicles of similar shape to those on dorsum, with arms 0.09–0.14 mm and apophyses 0.02–0.05 mm long. Although the shape of ossicles is similar in
P. minuta and
P. japonica, ossicles in
P. japonica are remarkably smaller and this distinguishes this species from
P. minuta.Peniagone minuta is characterized by the relatively small size, oval shape and a small number of tube feet, the features clearly distinguishing it from most of other species of the genus. Such a body shape and a small tube feet number are known in preferentially swimming species such as P. diaphana (Théel) and P. leander Pawson et Foel.
3.3. Peniagone mus Djakonov, 1952
Peniagone mus Djakonov, 1949: 68 [nomen nudum] [
36].
Peniagone mus Djakonov, 1952: 122–124, Figures 7–10 [
10].
Peniagone incerta (Théel)—Gebruk, 1990 (in Russian): 108–109 [partim.], Figure 42 (8–11) [
22].
Peniagone aff.
incerta—Mironov et al. 2019: 11–12 [
4].
Material examined. (In addition to material listed in Mironov et al. 2019 [
4]). Syntype slide preparation of ventral ossicles; RV
Vityaz, St. 956, 29.09.1951,
Sigsbee Trawl, 55°11′ N, 163°26′ E, 4810–4990 m, 50 specimens (IORAS ECH01101 and ECH01109); St. 3116, 28.08.1954,
Sigsbee Trawl, 51°53.1′ N, 161°49.6′ E, 5803–5853 m, IORAS ECH01949, 1 specimen; St. 3214, 24.10.1954,
Sigsbee Trawl, 38°10.9′ N, 143°56.2′ E, 6156–6207 m, IORAS ECH02008, 6 specimens; St. 4120, 05.11.1958,
Sigsbee Trawl, 53°37′ N, 159°40′ E, 6328–6296 m, IORAS ECH01287, 1 specimen; St. 5609, 23.07.1966,
Sigsbee Trawl, 46°06′ N, 153°18′ E, 6090–6235 m, IORAS ECH01243, 11 specimens; St. 5633, 06.09.1966,
Galathea Trawl, 44°7′ N, 149°34′ E, 6156–6117 m, ~300 specimens (IORAS ECH01227, ECH01230, ECH01248 and ECH01374[in formalin]); RV
Akademik M.A. Lavrentiev, cruise 71 (SokhoBio), St. 10-07, 29.07 2015, Epibenthic sledge, 46°06.0’–46°05.8′ N, 152°14.4’–152°14.6’ E, 4769–4798 m, 13 specimens; RV
Sonne, cruise 223 (KuramBio), St. 03-10, 05.08.2012, Agassiz Trawl, 47°14.27’–47°14.94′ N, 154°42.17’–154°43.18’ E, 4977–4986 m, 16 specimens (ID KB301, KB302); St. 11-10, 30.08.2012, Agassiz Trawl, 40°13.33’–40°12.53′ N, 148°06.48’–148°05.76’ E, 5348–5347 m, 1 specimen (ID KB1416); RV
Sonne, cruise 250 (KuramBio II), St. 18, 22.08.2016, Agassiz Trawl, 45°50.861′–45°51.954′ N, 153°49.568′–153°51.259′ E, 8200–8185 m, 3 specimens (ID KB2_428); St. 31, 28.08.2016, Agassiz Trawl, 45°56.688′–45°56.544′ N, 152°52.785′–152°54.667′ E, 6184.5–6220.6 m, IORAS ECH02146– ECH02148, ECH01808, ECH01812, ECH01813, 11 specimens; St. 86, 15.09.2016, Agassiz Trawl, 45°1.202’ N, 151°6.008’ E, 5572—5530 m, 6 specimens.
Diagnosis. Body elongated, approximately three times as long as wide. Colour in alcohol grey. Tube feet up to 10 pairs, present in the anterior 1/3 to 1/4 of the body, anteriormost five pairs free, others forming posterior lobe. Velum broad and short, formed by two pairs of papillae. Dorsal ossicles with strongly bent arms 0.1–0.3 mm long; apophyses two, rarely 1, 3 or 4, 0.09–0.2 mm long; apophyses and central stem thicker than arms. Ventral ossicles with arms 0.07–0.12 mm in length.
Material description. Body length up to 75 mm (
Figure 3A,B and
Figure 4A,B). Ten tentacles. Anus dorsal. Skin rough owing to the dense layer of ossicles. Velum varying in size, depending on contraction at preservation; velum did not cover the anterior end of the body and tentacles in most preserved specimens, suggesting its relatively small size in live specimens. Free tips of papillae short in preserved state, <5 mm long, lost in most of the specimens. Dorsal ossicles (
Figure 2J–L and
Figure 4C–H) with strongly bent arms, sometimes almost vertical, 0.08–0.25 mm long, rarely exceed 0.3 mm; apophyses usually two, rarely 3 or 4 and occasionally 1 apophysis can be found, apophyses almost vertical, 0.09–0.2 mm long in most ossicles. Apophyses usually slightly shorter than arms, although ossicles with much shorter apophyses also found (
Figure 2L). Arms and apophyses spinous, on apophyses spines more conspicuous. In bigger ossicles apophyses usually long and straight located close to each other. Ventrally flatten
Peniagone-type ossicles, with arms 0.07–0.12 mm in length, spines conspicuous; short spinous apophysis on each arm (
Figure 2M and
Figure 4I–K). Calcareous ring pieces with 9–10 pairs of arms, which can be subdivided. Polian vesicle single.
Distribution. Common in the Kuril–Kamchatka Trench area at abyssal and hadal depths, and also found in the Japan and Aleutian trenches (Figure 11). Depth range 4200–8200 m.
Remarks. Original description is based on three damaged specimens with upper layer of dorsal skin lacking, of which only two specimens are remain preserved. Djakonov [
36] at first mentioned this species without describing any taxonomic characters significant at the species level. In the later description [
10], details of external morphology and ventral ossicles were given. The dorsal ossicles illustrated in the original description on the Figure 10 apparently occur in the deeper skin layer and cannot be used for species identification. Our examined specimens were assigned to
P. mus based on unique features of ventral ossicles illustrated by Djakonov [
10].
Peniagone mus differs from other species of the genus by the type of dorsal ossicles with two apophyses clearly thicker than arms. Peniagone mus resembles P. incerta (Théel) in having five pairs of larger free tube feet, two of them located in the anterior body half and dorsal ossicles with nearly vertical apophyses straight and closely placed, usually two, rarely three or four. The re-examination of the syntype of Elpidia incerta Théel NHM 83.6.18.7 revealed remarkable variation in the morphology of dorsal ossicles in this species. Despite an obvious similarity in shape, ossicles of the syntype appeared significantly slender and smaller than in P. mus: arms and apophyses did not exceed 0.2 and 0.17 mm respectively, with most arms 0.1 mm in length.
Specimens from the northwest Pacific identified by Gebruk [
22] as
P. incerta were re-examined and found to belong to
P. mus (except for the specimens from stations 2144 and 2208, apparently lost).
3.4. Peniagone cf. purpurea (Théel, 1882)
Peniagone purpurea (Théel)—Gebruk, 1990 (in Russian): 111–113 (partim.), Figures 3–8 [
22].
Material examined. RV Vityaz, St. 5620, 15.08.1966, 44°48′ N, 156°33′ E, 5045–5005 m, IORAS ECH02013, 4 specimens; RV Sonne, cruise 223 (KuramBio), St. 01-12, 30.07.2012, 43°58.19′–43°57.81′ N, 157°19.11′–157°21.58′ E, 5422–5379 m, IORAS ECH01811, 1 specimen (ID KB113); St. 01-13, 31.07.2012, 43°58.36′–43°58.26′ N, 157°15.70′–157°14.60′ E, 5427–5425 m, IORAS ECH01810, 2 specimens (ID KB83, KB96); St. 06-09, 14.08.2012, 42°29.25′–42°28.32′ N, 154°00.05′–153°59.73′ E, 5293–5307 m, 1 specimen (ID KB2080).
Additional material. Syntypes of Elpidia purpurea Théel, NHM 83.6.18.4 and NHM 83.6.18.5 (slide preparation of dorsal ossicles).
Material description. Specimens 16–40 mm in length, condition from poor to moderated. Body close to oval, flattened (
Figure 5A,B). Ten big tentacles. Colour in alcohol from brownish to dark or black violet. Tube feet 7–9 pairs, missing in the anterior third of the body; first 2–3 pairs free, other tube feet forming posterior lobe. Velum large, long and wide, may exceed in length ½ of the body length; free ends of velum papillae long. Two pairs of small papillae on dorsum behind velum. Dorsal ossicles (
Figure 5F–I) with central stem of varying proportions—from 1/3 of arm length to the same length with arms or even slightly longer; arms straight or slightly curved, arm length 0.05–0.12 mm, usually up to 0.1 mm. Apophyses four, longer than arms, but not exceeding 0.1 mm in length, apophyses slightly curved outwardly (
Figure 5F,G), straight (
Figure 5H) or inwardly curved (
Figure 5I). Ventral ossicles (
Figure 5J–M) with arms curved outwards (
Figure 5J,L) or straight (
Figure 5K), arm length about 0.05 mm; apophyses four, longer than arms, about 0.07 mm in length. Arms and apophyses spinous both in dorsal and ventral ossicles. Calcareous ring pieces with 7 pairs of arms, which can be subdivided. Some of the arms are multiply branched at their ends. Polian vesicle single.
Distribution. Five records in the Kuril–Kamchatka Trench area and on abyssal plain east of the trench. Depth 5005–5427 m.
Remarks. By external morphology, examined specimens corresponded well to the original description of
Peniagone purpurea in colour, shape of the body, velum morphology, tube feet and dorsal papillae number and arrangement. Re-examination of the syntypes of
Elpidia purpurea NHM 83.6.18.4 and NHM 83.6.18.5 revealed some differences between them and our specimens in the dorsal ossicle morphology. In the syntypes, apophyses were curved outwardly in most of dorsal ossicles (
Figure 5C–E), whereas in the northwest Pacific specimens they were either straight or curved inward. Additionally, ossicles of the syntypes were smaller: arms up to 0.08 mm and apophyses up to 0.07 mm. However, ossicle size and shape of apophyses in the northwest Pacific specimens varied between the stations. Specimens from St. 5620 had the biggest ossicles with arms up to 0.12 mm and apophyses up to 0.1 mm. The apophyses in most ossicles were placed more widely and slightly curved inwardly, although few ossicles with apophyses curved outwardly were also present. In the northwest Pacific specimens from other stations the arm length of ossicles was <0.1 mm long and apophyses were straight or outwardly curved. The variation in dorsal ossicles of
P. purpurea requires further studies, therefore at present we identify our specimens as
Peniagone cf.
purpurea.
3.5. Peniagone saveljevae Kremenetskaia et Gebruk sp. nov.
Holotype. Specimen 60 mm, RV Akademik M.A. Lavrentiev, cruise 71 (SokhoBio), St. 01-11, 11.07.2015, Agassiz Trawl, 46°09.047′–46°08.738′ N, 146°00.789′–145°59.512′ E, 3305–3304 m. The specimen will be deposited at MIMB, currently stored at IORAS, Cat. Nr ECH02165.
Paratypes. 3 specimens 45–50 mm in length, collected at the same station as the holotype. Paratypes will be deposited at SGN, currently stored at IORAS, Cat. Nr ECH02164 and ECH02151.
Diagnosis. Body elongated, approximately 3–4 times as long as wide. Color in alcohol grey. Tube feet up to ~8 pairs, missing in the anterior 1/3 of the body. Velum broad and short, formed by two pairs of papillae. Two pairs of small papillae behind velum. Dorsal ossicles with curved arms up to 0.15 mm long; apophyses four, up to 0.12 mm long; apophyses slightly thicker than arms. Ventral ossicles with curved arms 0.07–0.1 mm in length and four apophyses longer than arms, up to 0.14 mm, or with more horizontal arms up to 0.2 mm.
Material description. Body strongly elongated (
Figure 3C,D and
Figure 6A,B). Skin rather thick, almost non-transparent in preserved state. Ambulacral appendages and tentacles are mostly lost, few tube feet are preserved in the paratype, IORAS ECH02151. In the latter only 7 pairs of tube feet are present, however some small posteriormost tube feet might have been lost. Arms and apophyses of dorsal ossicles spinous; spines on apophyses more numerous (
Figure 6C–G and
Figure 7A,B). Apophyses in dorsal ossicles of the same length with arms or slightly shorter (in ossicles with widely spaced arms); arms usually ~0.1 mm long. Apophyses always four, slightly curved inward. Ventral ossicles (
Figure 6I–K and
Figure 7C,D) usually of the same shape as dorsal but slenderer and smaller in size, with arms usually <0.1 mm long and apophyses slightly longer or equal in length with arms. Occasionally present flatten
Peniagone-type ossicles, with four short apophyses or sometime with no apophyses. Polian vesicle single.
Etymology. The species is named in honor of Tatyana Sergeyevna Saveljeva (1902–1982), a Soviet zoologist and expert on Holothuroidea.
Distribution. The species is known only from the type locality in the southern part of the Sea of Okhotsk.
Remarks. Peniagone saveljevae resembles P. dubia and P. mus in external morphology within the group of the northwest Pacific species, however it differs by the absence of tube feet in the anteriormost third or fourth of the body and small velum. The tube feet in the anterior half of the body is a common feature in Peniagone occurring in Peniagone islandica Deichmann, P. azorica von Marenzeller, P. willemoesi (Théel), P. herouardi Gebruk, P. vedeli Hansen and other species. This type of morphology suggests more epibenthic lifestyle. Peniagone saveljevae also differs by the dorsal ossicles with four apophyses and curved arms up to 0.15 mm long.
3.6. Peniagone vitrea Théel, 1882
Peniagone vitrea Théel, 1882: 50–52, Plates VII(7–9), XXXIV(17–18), XLIV(10) [
5]; Hansen, 1975: 148–150, Figure 70 [synonymy] [
24]; Gebruk, 1990 (in Russian): 93–94, Figure 34(4–9) [synonymy] [
22]; O’Loughlin, 1998: 504 [
37].
Peniagone vitrea var.
setosa Ludwig, 1893: 109; 1894: 105–108 [
38].
Peniagone setosa Ludwig—Clark, 1920: 136 [
39].
Material examined. RV Vityaz, St. 5603, 15.07.1966, Sigsbee Trawl, 46°22′ N, 153°3′ E, 3175–3250 m, IORAS ECH01201, 3 specimens; St. 5635, 08.09.1966, Galathea Trawl, 44°24′ N, 149°10′ E, 3900–4135 m, IORAS ECH00236, 14 specimens and fragments.
Genetic data. No genetic data is available for examined specimens.
Distribution. Type locality is off southern Chile at the depth 2652 m (1450 fathoms),
Challenger, St. 302. Other confirmed records are in the Eastern and Central Pacific, off Australia, and Antarctic (Figures 11 and 12). The new records are from the northwest Pacific, Kuril–Kamchatka Trench area. Depth range 1360–4507 m. There are also about ~30 records published in GBIF.org [
40] from the Eastern Pacific, off Australia and New Zealand identified by P.M. O’Loughlin, N. Davey and D. Pawson.
Remarks.
Peniagone vitrea currently has two junior synonyms:
P. vitrea var.
setosa Ludwig and
P. setosa Ludwig. Hansen [
24] has re-examined the type series of
P. vitrea and found pronounced variation in ossicle size among the types of the synonymized species and the specimens from the Central and East Pacific reported by Clark [
39] and from
Galathea St. 716. The biggest ossicles were in the
Galathea specimen with the arms reaching 0.7 mm in length whereas in the type specimens the arms were 0.3–0.5 mm long. In our specimens from the northwest Pacific the arms in the dorsal ossicles (
Figure 8C) attained 0.4 mm, with 2–4 apophyses, rather straight, longer than arms and spinous. External morphology of these specimens (
Figure 8A,B) corresponded well to that in the original description of the species. The body was strongly elongated, velum lobe broad and short, tube feet bordering from posterior half to one third of the body and forming broad posterior lobe.
3.7. Peniagone sp. “miniatura”
Material examined. RV Sonne, cruise 223 (KuramBio), St. 6-9, 14.08.2012, Agassiz Trawl, 42°29.25′–42°28.32′ N, 154°00.05′–153°59.73′ E, 5293–5307 m, IORAS ECH01809, 1 specimen.
Material description. Body 65 mm long, elongated, approximately three times as long as wide. Color in alcohol greyish. Dorsal velum long, may exceed in length 1/3 that of the body; papillae free at most of their length. Tube feet ~11 pairs, present in anterior 1/3 of the body; six pairs free, posterior five pairs closely placed; tube feet mostly lost, their arrangement can be determined based on ampullae position. Dorsal ossicles (
Figure 8D–F) rare, with arms almost straight or slightly curved, arm length 0.09–0.11 mm. Apophyses four (rarely three), shorter than arms, 0.05–0.06 mm in length. Arms and apophyses bare small spines. Central stem slightly shorter than apophyses or of similar length. Ventral ossicles (
Figure 8G) with straight arms 0.06–0.08 mm in length, arms slightly curved horizontally and/or vertically to varying degree; apophyses short, <½ of the arm length. Arms and apophyses spinous.
Genetic data. See
Table S1. Genetic data on both COI and 16S shows very small difference between
Peniagone sp. “miniatura” and
P. mus, up to 2.5%. It can be suggested that the observed morphological and genetic differences are related to an ongoing speciation process.
Remarks. This possibly new species has distinctive features of the ossicles, but in external morphology it is very similar to P. mus. Dorsal ossicles in Peniagone sp. “miniatura” are much smaller, mostly with four apophyses which do not form a dense layer in the skin.
4. Phylogeny
Peniagone was recovered monophyletic in both COI and 16S analyses as well as in concatenated dataset analysis with PP = 1.0 (
Figure S1). COI tree (
Figure 9) included six previously described species (
P. cf.
purpurea,
P. diaphana,
P. coccinea,
P. vignoni,
P. incerta and
P. islandica) that were either recovered monophyletic (PP = 1.00) or were represented by a single specimen.
Peniagone cf.
purpurea was one of the most basal species with COI p- distance to other
Peniagone species varying from 18.2 to 24.5%.
Peniagone mus was sister with
Peniagone sp. “miniatura” with PP = 0.99. The COI p-distance between these clades was 2.5%, whereas it varied from 5 to 26% between other species clades. The clade of
P. mus did not form sister relationships with the morphologically similar Antarctic species
P. incerta to which the specimens of
P. mus were previously assigned.
16S tree (
Figure S1) included six previously described species (
P. cf.
purpurea,
P. leander,
P diaphana,
P. azorica and
P. islandica) however was generally much less resolved. Furthermore,
P. azorica and
P. islandica were not recovered as monophyletic species. Similarly to the COI tree, a clade composed of
P. mus and
P. sp. “miniatura” was recovered with PP = 0.94. 16S p-distances showed 0.7% difference between
P. mus and
Peniagone sp. “miniatura”, whereas it varied from 0.4% to 12.8% between the other species.
Peniagone saveljevae was only present as a single specimen in 16S tree showing no close relationships to any of the other species in the analysis.
Analysis of the concatenated COI and 16S matrix included nine previously described species although as in 16S tree
P. islandica and
P. azorica did not form monophyletic clades. Nevertheless, the topology of the recovered tree was generally similar to the one from the COI analysis with slightly lower support in most of the nodes. Similar to the COI tree,
P. cf.
purpurea,
P. diaphana and two unidentified
Peniagone species (
Peniagone sp. YYH-2013 and
Peniagone sp. SIO-BIC E5608) appeared in the basal part of the tree and the rest of the species were split into two large sister clades A and B (PP = 0.89) (
Figure 10). Additionally,
P. leander lacking COI sequence data was recovered as sister to
P. diaphana (PP = 0.93) similar to the 16S tree (
Figure 10).
In all three trees, the basal-most species were reported from the North Pacific (
P. cf.
purpurea,
Peniagone spp.,
P. leander) and the North Atlantic (
P. diaphana), while the terminal clades (A and B) also included all the Antarctic species. The North Pacific species included in the present study (
P. cf.
purpurea,
P. mus and
P. saveljevae) did not show close relationship to each other appearing in the different larger clades on the phylogenetic trees. Occasionally swimming forms [
3] were mostly found within the terminal clades A and B (
Figure 9 and
Figure 10) although morphology of
Peniagone sp. YYH-2013 and
Peniagone sp. SIO-BIC E5608 specimens was not reported in the open sources and thus was not scored in the present study. Notably, two species with large velum and bright coloration,
P. cf.
purpurea and
P. coccinea did not form sister relationships on the obtained phylogenetic trees (
Figure 9 and
Figure 10).
5. Discussion
According to previously published records and our results, the northwest Pacific fauna of the genus
Peniagone includes at least seven species:
P. dubia,
P. japonica,
P. mus,
P. minuta,
P. cf.
purpurea,
P. saveljevae and
P. vitrea. The northwest Pacific
Peniagone sp. “miniatura” can also represent a separate species or a case of ongoing speciation within
P. mus. Although genetically it is very close to
P. mus, the differences in the ossicle morphology between the two potential species are remarkable. A similar case was described by Elgetany et al. [
41] as a grey zone of speciation in the two polychaete species,
Diopatra aciculata Knox et Cameron and
D. neapolitana Delle Chiaje. Although some statistical differences were found in several morphological characters, these species displayed low genetic distances: up to <0.0359 for COI data and <0.00028 for 16S.
All the examined species are not known outside the northwest Pacific except for
P. vitrea (
Figure 11 and
Figure 12). The latter is the example of a widespread species occurring widely in the Pacific with one record in the Pacific sector of the Antarctic. Morphological differences suggest separation of the northwest Pacific
Peniagone cf.
purpurea from
Peniagone purpurea described from the southern Indian Ocean. Some genetic evidence also point on that
P. purpurea might represent a species complex. Six sequences of
P. purpurea obtained from the Antarctic specimens are deposited in the BOLD database [
42]. According to the scheme of abyssal biogeography of Vinogradova [
43], these specimens were collected from one biogeographic subregion including the type locality of
P. purpurea. Although these sequences are stored privately and are not currently available for phylogenetic analysis, they do not cluster under the same Barcode Index Number (BIN) with the northwest Pacific
P. cf.
purpurea from the present study (BOLD: AEI4563) indirectly suggesting they might not be conspecific. Furthermore, the nearest neighbor BIN (BOLD: ABA3279) comprising these Antarctic specimens shows 7.53% p-distance in COI with the northwest Pacific bin which corresponds to the level of genetic distances between other
Peniagone species.
Most northwest Pacific species are distributed in a relatively narrow depth range (
Figure 11) although this can be partly explained by the low number of specimens collected from the deep sea. The shallowest species is
P. dubia inhabiting the lower bathyal (~2500–3000 m).
Peniagone saveljevae occurs in the upper abyssal (~3300 m) in the Sea of Okhotsk.
Peniagone vitrea in the northwest Pacific is known from the upper to mid-abyssal (~3200–4100 m) although it occurs in a slightly wider range in other areas (~1300–4500 m).
Peniagone cf.
purpurea is the lower abyssal species (~5300 m).
Peniagone mus and
P. minuta are the only two species occurring in the hadal in trenches.
Peniagone minuta was repeatedly found in the Aleutian and Izu–Bonine trenches as well as on the abyssal plain.
Peniagone mus is the deepest and the most eurybathic species (4200–8200 m) among the northwest Pacific
Peniagone. It is mainly known from the Kuril–Kamchatka Trench and adjacent abyssal areas, with single records in the Aleutian and Japan trenches.
Surprisingly,
Peniagone is absent in the relatively well studied Bering Sea. It can be explained by possible interspecies interactions. Rybakova et al. [
44] showed the abyssal community in the Bering Sea being dominated by another elpidiid species,
Kolga kamchatica Rogacheva. The latter may occupy the same trophic niche as
Peniagone replacing
Peniagone species in the Bering Sea.
No clear geographic affinity was revealed for the main clades within
Peniagone according to molecular data (
Figure 9 and
Figure 10). The northwest Pacific species appeared not closely related. Following more resolved COI tree (
Figure 9),
P. mus is the closest to the Antarctic species
Peniagone sp. ZMBN140277 and ZMBN140278 whereas west Pacific
P. cf.
purpurea and
Peniagone sp. YYH-2013 [unpublished], and east Pacific
Peniagone sp. SIO-BIC E5608 [
45] are placed distantly from all other species. The same pattern was revealed for the Atlantic species,
P. diaphana from northeast Atlantic [
45], and
P. coccinea and
P. islandica from the northern Mid-Atlantic Ridge. The Atlantic
P. azorica and
P. islandica are closely related species, which are almost indistinguishable by external morphological features, but differ in dorsal ossicle morphology [
3]. Analysis of their 16S, 18S, 28S rRNA and histone H3 sequences, showed clear separation of these species only in H3 data which may indicate ongoing speciation (CA, unpublished data). The Antarctic species, several of which were unidentified, were located only in terminal clades and absent in the basal part of the tree (
Figure 9 and
Figure 10). However, our data represent only a fraction of
Peniagone diversity and obtaining genetic data for other species worldwide may change the picture.
Rogacheva et al. [
3] described several adaptive forms within the genus
Peniagone with different swimming abilities. Occasionally swimming forms possess more tube feet bordering most of the body and small velum and tentacles which apparently facilitate “walking” mode of life. In more active swimmers the number of tube feet is reduced and velum is either large as in
P. coccinea, or reduced as in
P. diaphana,
P. leander and
P. minuta.
Forms that are proposed to be occasionally swimming, such as
P. islandica,
P. mus,
P. incerta and others, are present in terminal clades A and B (
Figure 9 and
Figure 10). However, they can potentially be also found among more basal taxa depending on the external morphology (yet unknown) in
Peniagone sp. SIO-BIC E5608 and
Peniagone sp. YYH-2013. More advanced swimmers bearing large velum and lacking tube feet in the anterior body half such as
P. diaphana and
P. cf.
purpurea occur in two separate clades suggesting their independent evolution. The species with almost completely reduced tube feet and very modified body shape presumably with predominantly pelagic life style,
P. diaphana and
P. leander, are closely related following our molecular data. Nevertheless, since the external morphology is not well described for some species in our analysis, and many species of the genus lack genetic data, the analysis of evolution of characters adaptive to swimming based on the obtained phylogenetic tree appears preliminary.