Leaf Elemental Concentrations, Stoichiometry, and Resorption in Guam’s Coastal Karst Forests
Abstract
:1. Introduction
2. Materials and Methods
3. Results
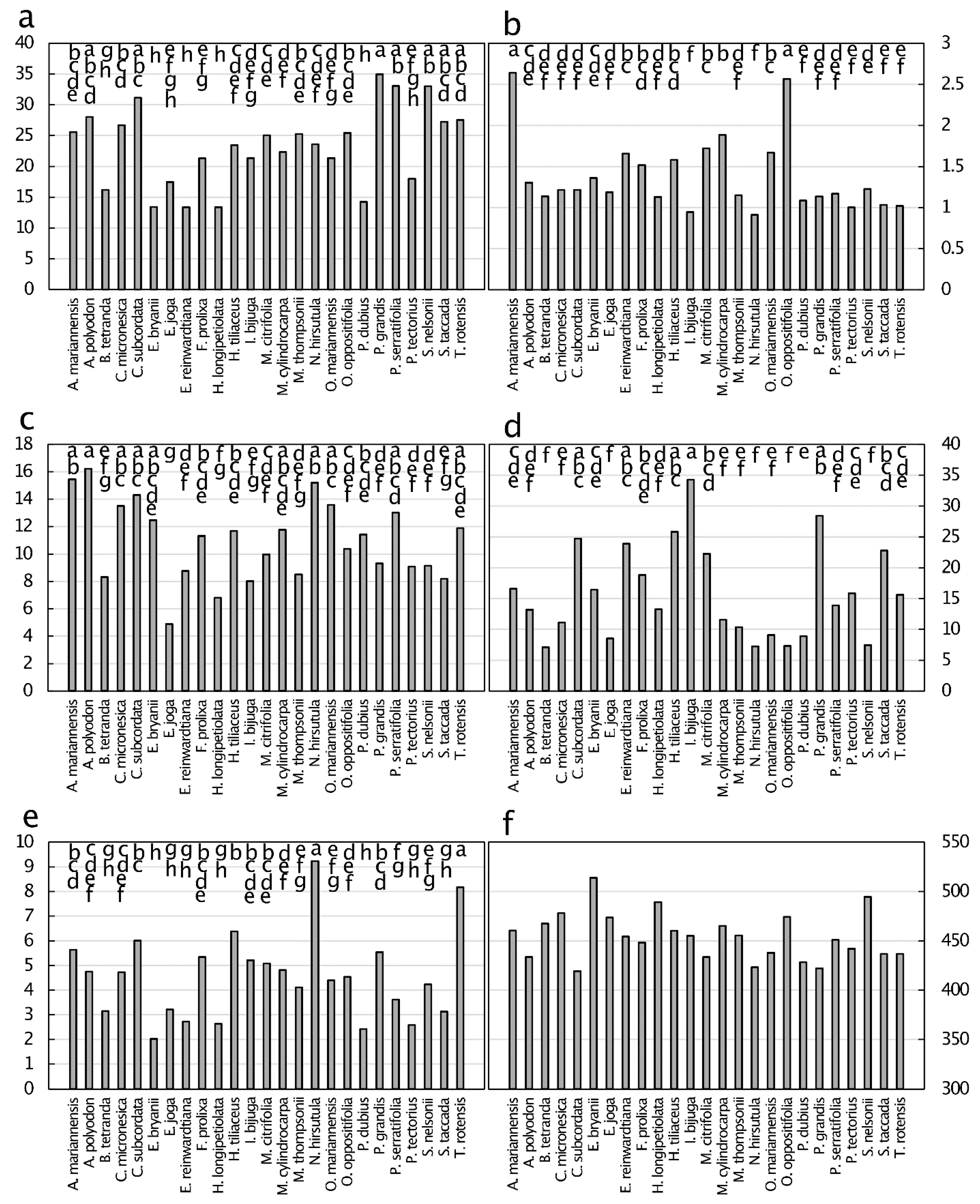
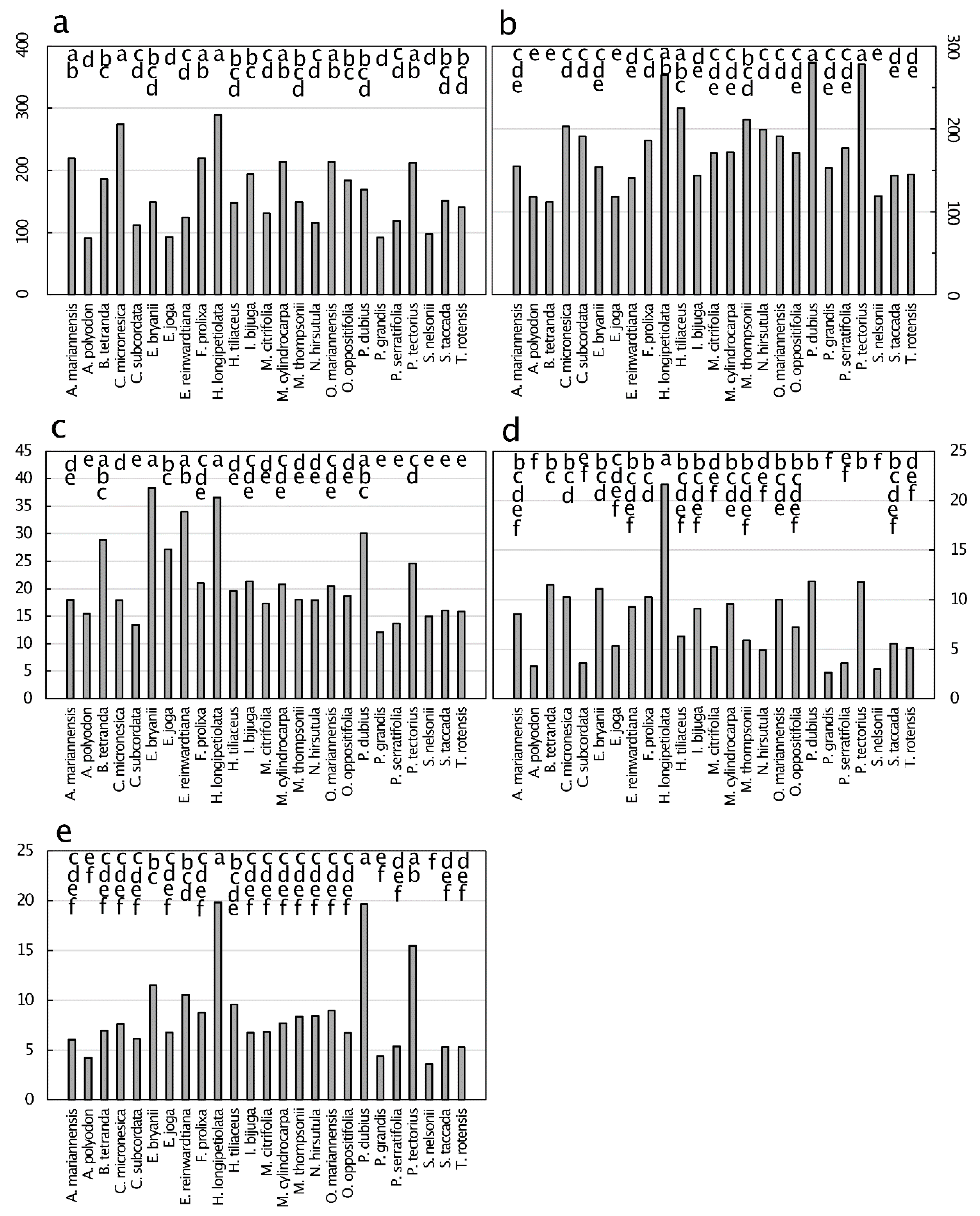
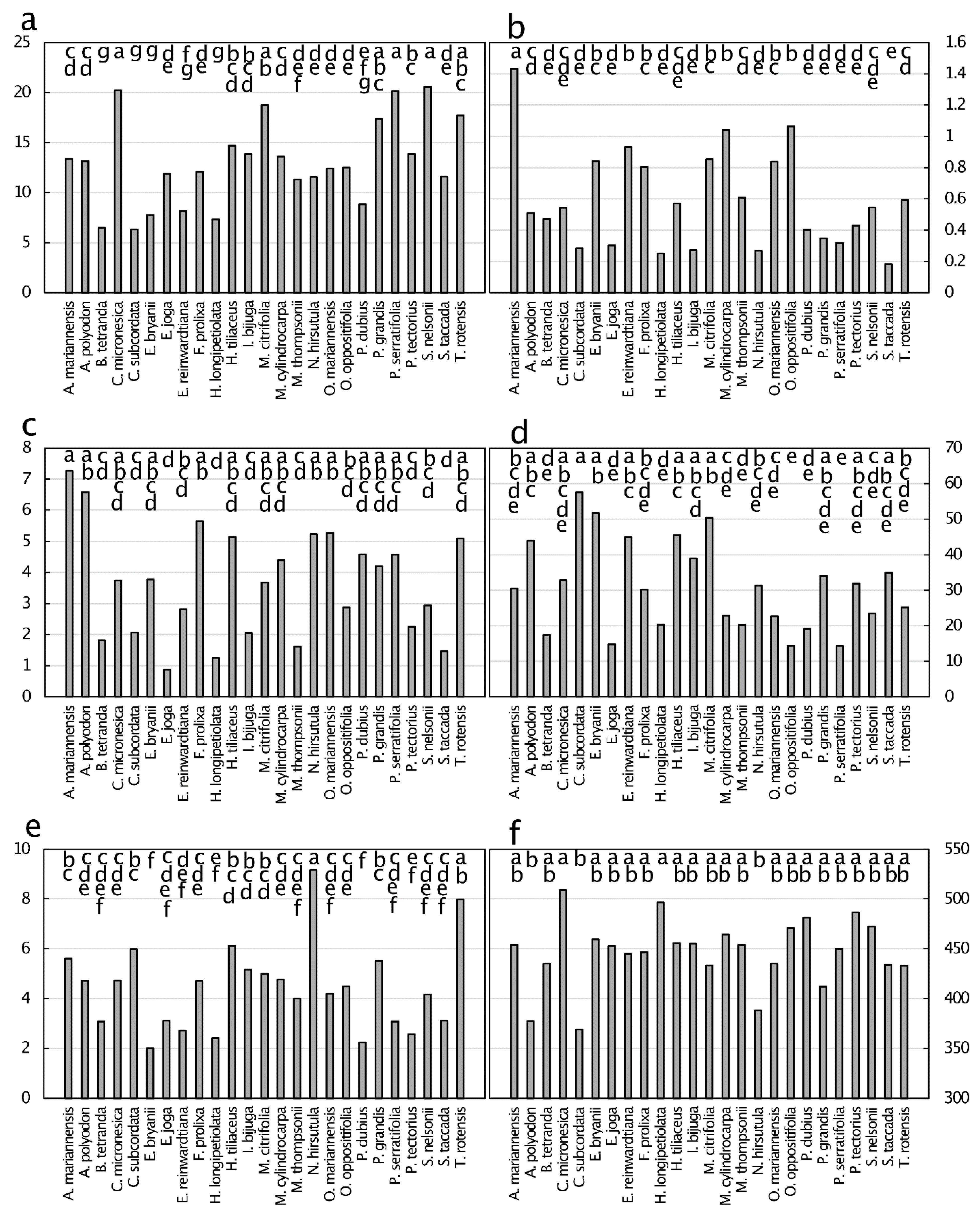
3.1. Green Leaf Element Concentrations
3.2. Green Leaf Stoichiometry and Nutrient Limitations
3.3. Leaf Stoichiometry Predicts Litter Quality
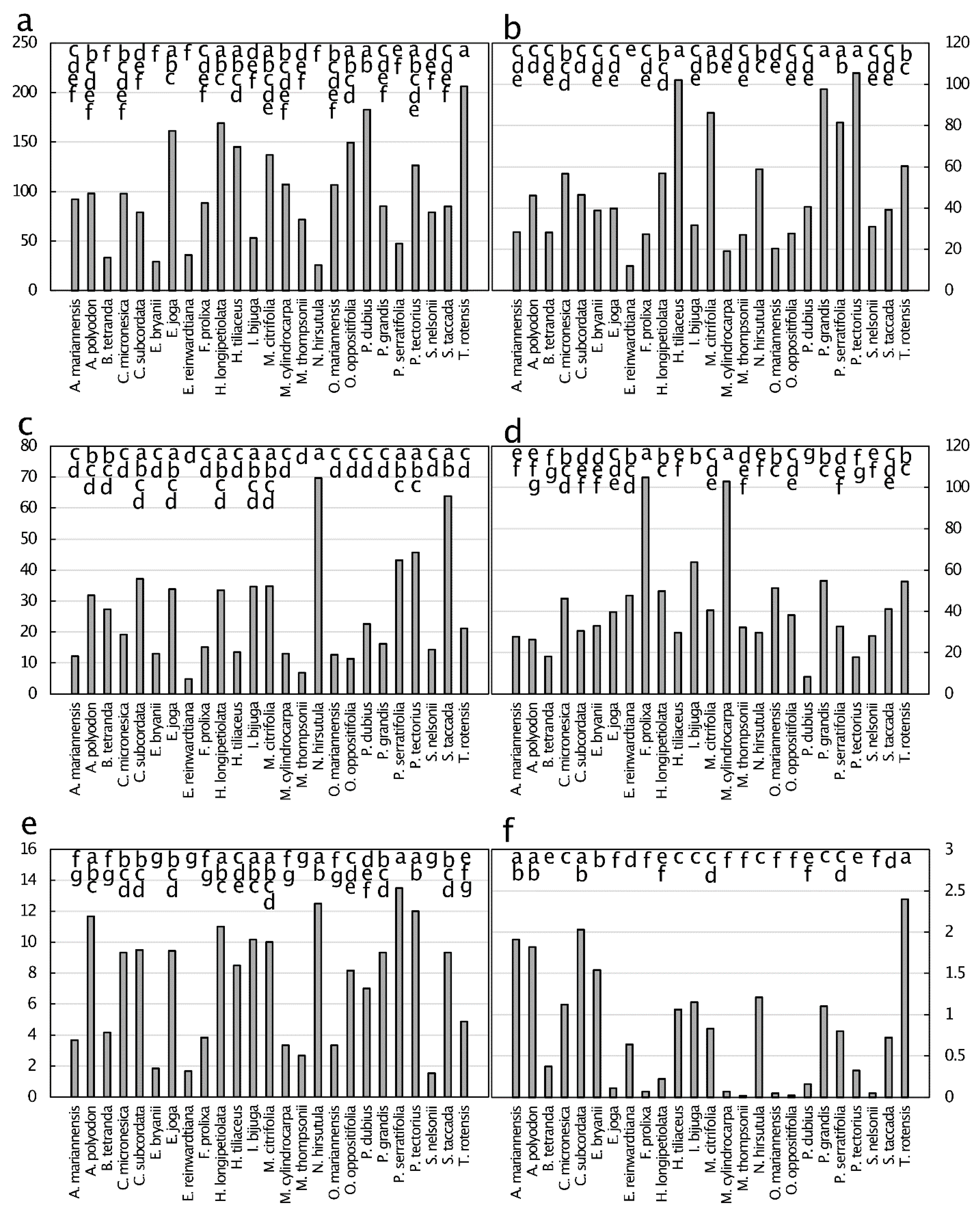
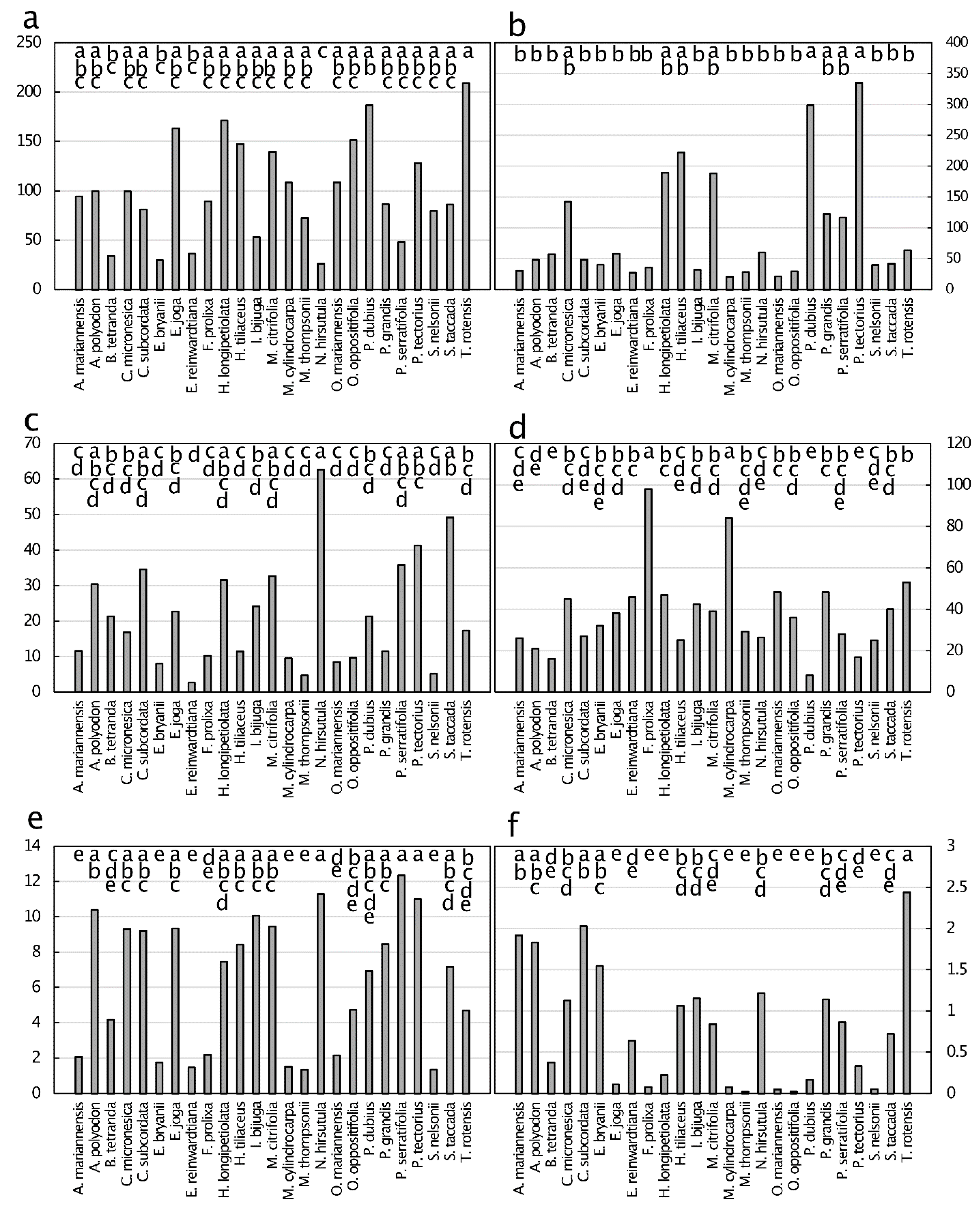
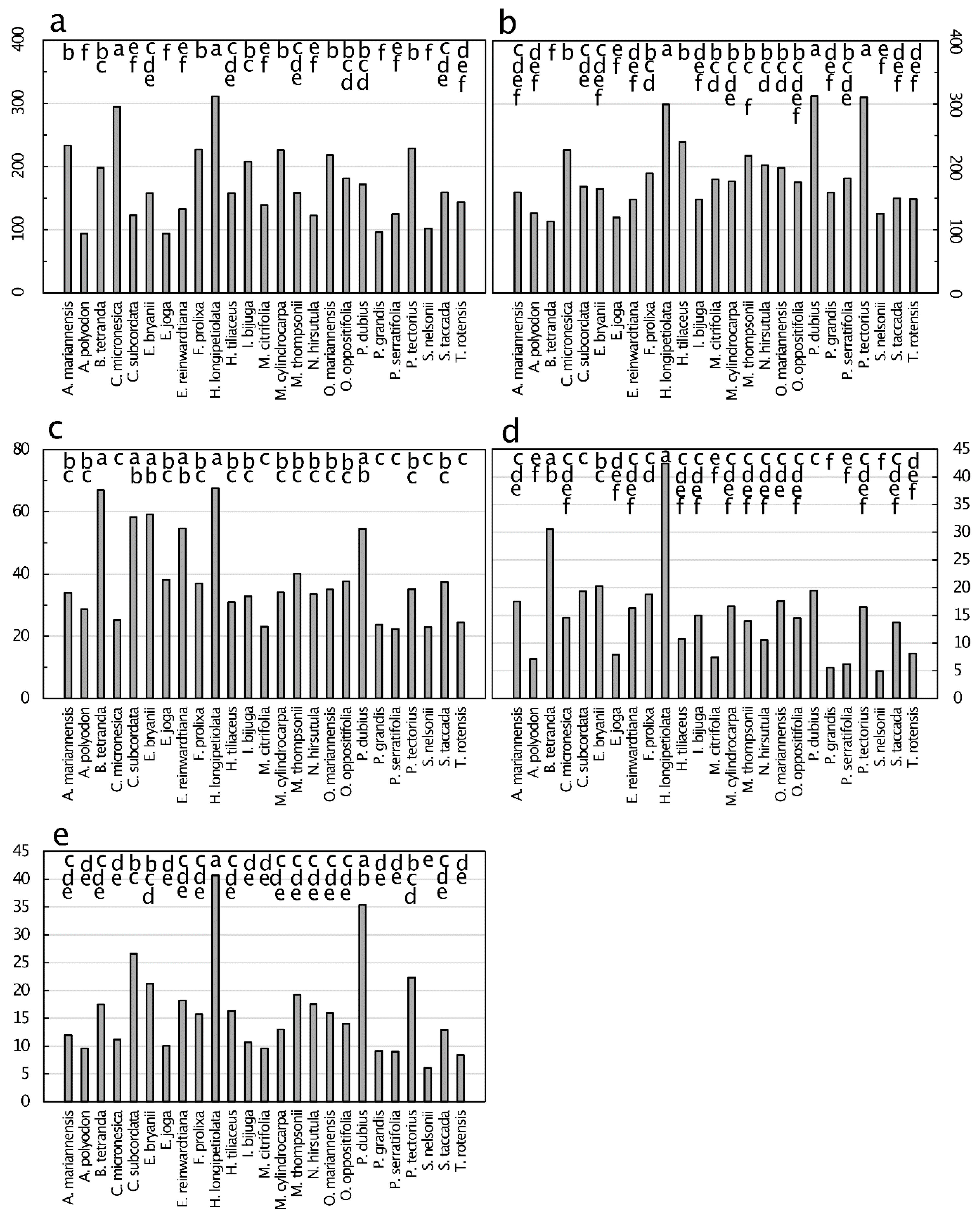
3.4. Senesced Leaf Element Concentrations
3.5. Senesced Leaf Stoichiometry and Litter Quality
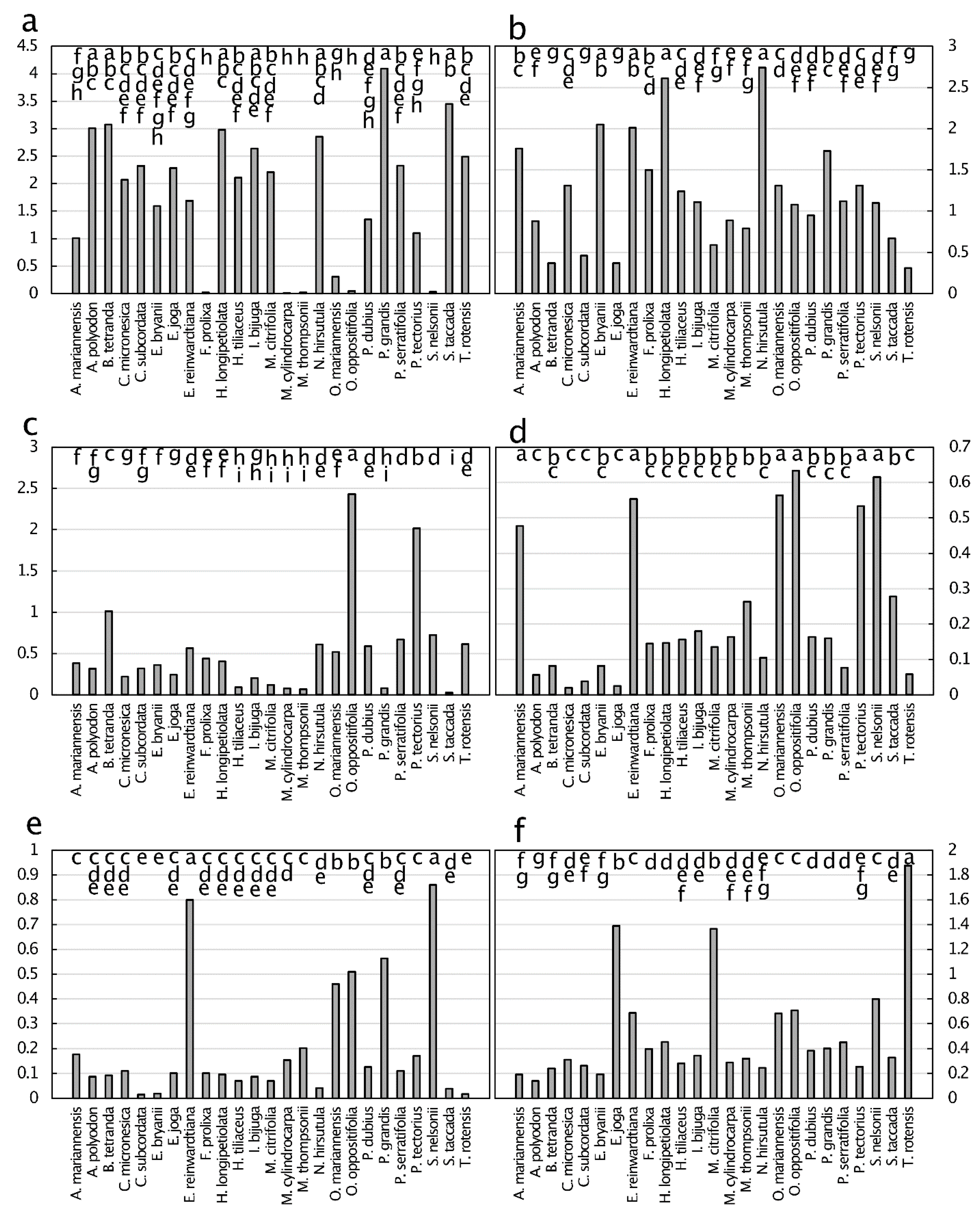
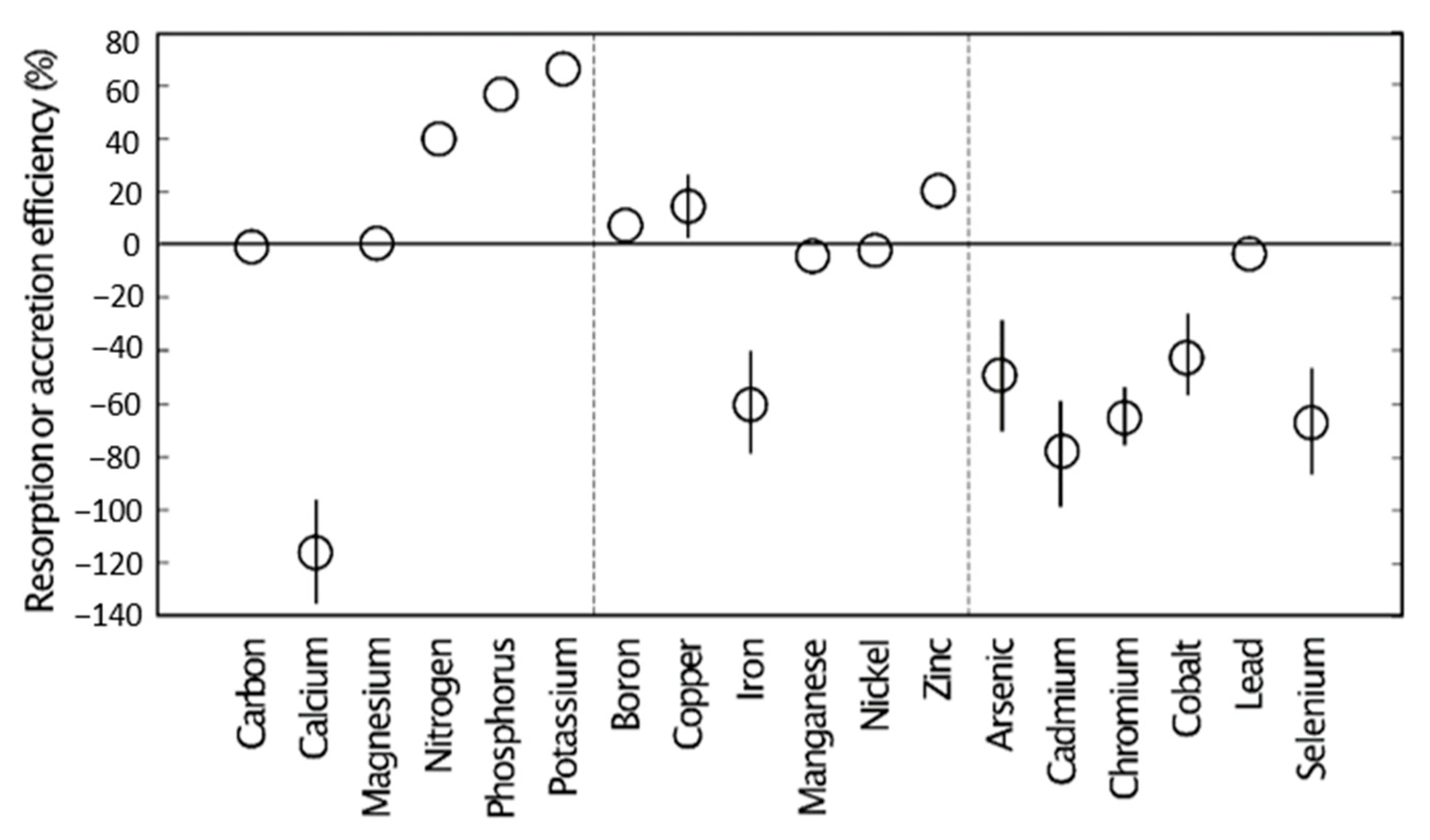
3.6. Resorption and Accretion
4. Discussion
4.1. Stoichiometry
4.2. Threatened Species Traits
4.3. Leaf Aging and Senenence Dynamics
4.4. Tropical Cyclones and Nutrient Cycling
4.5. Future Directions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Species | Family | Notes |
|---|---|---|
| Artocarpus mariannensis Trécul | Moraceae | Eudicot tree |
| Asplenium polyodon G. Forst. | Aspleniaceae | Fern |
| Bikkia tetrandra (L.f.) A. Rich. | Rubiaceae | Eudicot shrub |
| Cordia subcordata Lam. | Boraginaceae | Eudicot tree |
| Cycas micronesica K.D. Hill 1,2 | Cycadaceae | Gymnosperm tree |
| Elaeocarpus joga Merr. | Elaeocarpaceae | Eudicot tree |
| Eugenia bryanii Kaneh. 2 | Myrtaceae | Eudicot shrub |
| Eugenia reinwardtiana (Blume) A.Cunn. ex DC. | Myrtaceae | Eudicot tree |
| Ficus prolixa G. Forst. | Moraceae | Eudicot tree |
| Heritiera longipetiolata Kaneh. 2 | Malvaceae | Eudicot tree |
| Hibiscus tiliaceus L. | Malvaceae | Eudicot tree |
| Intsia bijuga (Colebr.) Kuntze 1 | Fabaceae | Eudicot tree, Caesalpinoideae |
| Macaranga thompsonii Merr. | Euphorbiaceae | Eudicot tree |
| Meiogyne cylindrocarpa (Burck) Heusden | Annonaceae | Eudicot tree |
| Morinda citrifolia L. | Rubiaceae | Eudicot tree |
| Nephrolepis hirsutula (G. Forst.) C. Presl | Nephrolepidaceae | Fern |
| Ochrosia mariannensis A.DC. | Apocynaceae | Eudicot tree |
| Ochrosia oppositifolia (Lam.) K. Schum. | Apocynaceae | Eudicot tree |
| Pandanus dubius Spreng. | Pandanaceae | Monocot tree |
| Pandanus tectorius Parkinson ex Du Roi | Pandanaceae | Monocot tree |
| Pisonia grandis R. Br. | Nyctaginaceae | Eudicot tree |
| Premna serratifolia L. | Lamiaceae | Eudicot tree |
| Scaevola taccada (Gaertn.) Roxb. | Goodeniaceae | Eudicot shrub |
| Serianthes nelsonii Merr. 1,2 | Fabaceae | Eudicot tree, Mimosoidae |
| Tabernaemontana rotensis (Kaneh.) P.T. Li 2 | Apocynaceae | Eudicot tree |
| Variable | Soil Trait |
|---|---|
| pH | 7.98 ± 0.14 |
| Calcium | 7.96 ± 0.19 mg·g−1 |
| Carbon | 91.42 ± 5.65 mg·g−1 |
| Copper | 6.29 ± 0.64 µg·g−1 |
| Iron | 32.77 ± 2.13 µg·g−1 |
| Magnesium | 884.25 ± 32.38 µg·g−1 |
| Manganese | 63.12 ± 5.15 µg·g−1 |
| Nitrogen | 8.03 ± 0.68 mg·g−1 |
| Phosphorus | 82.25 ± 7.36 µg·g−1 |
| Potassium | 596.63 ± 19.55 µg·g−1 |
| Zinc | 16.37 ± 3.24 µg·g−1 |
| Species | Nitrogen | Phosphorus | Potassium | Carbon 1 | Magnesium | Zinc | Copper | Boron |
|---|---|---|---|---|---|---|---|---|
| Aglaia mariannensis | 48 | 45 | 52 | 1 | 0.4 | 5 | 44 | 6 |
| Asplenium polyodon | 53 | 61 | 59 | 13 | 0.8 | 4 | 11 | 20 |
| Bikkia tetrandra | 59 | 58 | 78 | 7 | 2.3 | 22 | 1 | 12 |
| Cordia subcordata | 79 | 76 | 85 | 12 | 0.3 | 7 | 3 | 11 |
| Cycas micronesica | 24 | 53 | 73 | −6 | 0.4 | 12 | 1 | 3 |
| Elaeocarpus joga | 31 | 74 | 82 | 4 | 3.3 | 33 | 1 | 4 |
| Eugenia bryanii | 41 | 38 | 70 | 11 | 1.2 | 38 | 5 | 3 |
| Eugenia reinwardtiana | 38 | 42 | 66 | 2 | 0.7 | 45 | 13 | 3 |
| Ficus prolixa | 42 | 46 | 48 | 0 | 11.8 | 33 | 43 | 7 |
| Heritiera longipetiolata | 44 | 77 | 81 | −1 | 8.7 | 6 | 32 | 6 |
| Hibiscus tiliaceus | 37 | 62 | 56 | 1 | 4.2 | 15 | 1 | 15 |
| Intsia bijuga | 35 | 70 | 74 | 0 | 1.0 | 30 | 1 | 33 |
| Macaranga thompsonii | 54 | 47 | 81 | 0 | 2.7 | 32 | 51 | 10 |
| Meiogyne cylindrocarpa | 38 | 44 | 62 | 0 | 0.9 | 27 | 55 | 18 |
| Morinda citrifolia | 21 | 49 | 62 | 0 | 1.9 | 6 | 6 | 4 |
| Nephrolepis hirsutula | 50 | 70 | 65 | 8 | 0.7 | 10 | 10 | 11 |
| Ochrosia mariannensis | 41 | 49 | 61 | 0 | 4.7 | 33 | 36 | 6 |
| Ochrosia oppositifolia | 49 | 58 | 72 | 0 | 1.0 | 15 | 42 | 6 |
| Pandanus dubius | 36 | 63 | 59 | −12 | 7.8 | 6 | 1 | 4 |
| Pandanus tectorius | 23 | 57 | 74 | −10 | 0.8 | 10 | 8 | 5 |
| Pisonia grandis | 49 | 69 | 54 | 2 | 0.6 | 29 | 9 | 12 |
| Premna serratifolia | 37 | 73 | 64 | 0 | 14.7 | 17 | 9 | 14 |
| Scaevola taccada | 56 | 82 | 81 | 1 | 0.7 | 23 | 23 | 3 |
| Serianthes nelsonii | 37 | 55 | 67 | 5 | 1.6 | 64 | 12 | 11 |
| Tabernaemontana rotensis | 35 | 42 | 57 | 1 | 2.2 | 18 | 4 | 3 |
| PPI 2 | 73.2 | 53.7 | 43.5 | 99.7 | 97.8 | 93.3 | 99.7 | 92.5 |
| Species | Calcium | Iron | Manganese | Nickel | Lead | Arsenic | Cadmium | Chromium | Cobalt | Selenium |
|---|---|---|---|---|---|---|---|---|---|---|
| Aglaia mariannensis | 83 | 6 | 2.3 | 0.3 | 1.6 | 155 | 115 | 197 | 225 | 109 |
| Asplenium polyodon | 233 | 5 | 1.8 | 0.5 | 3.2 | 116 | 329 | 65 | 4 | 10 |
| Bikkia tetrandra | 147 | 102 | 1.6 | 0.9 | 1.3 | 12 | 22 | 162 | 9 | 49 |
| Cordia subcordata | 133 | 4 | 2.5 | 0.1 | 0.6 | 50 | 10 | 51 | 122 | 22 |
| Cycas micronesica | 195 | 151 | 1.7 | 0.3 | 0.9 | 156 | 250 | 103 | 92 | 95 |
| Elaeocarpus joga | 74 | 45 | 1.1 | 1.8 | 1.5 | 7 | 87 | 78 | 32 | 76 |
| Eugenia bryanii | 215 | 3 | 1.1 | 0.3 | 1.1 | 59 | 120 | 99 | 9 | 21 |
| Eugenia reinwardtiana | 88 | 126 | 0.9 | 0.1 | 0.9 | 55 | 86 | 107 | 4 | 40 |
| Ficus prolixa | 60 | 30 | 1.0 | 10.0 | 1.5 | 6 | 162 | 15 | 27 | 358 |
| Heritiera longipetiolata | 53 | 233 | 1.2 | 0.1 | 0.3 | 6 | 22 | 188 | 25 | 10 |
| Hibiscus tiliaceus | 76 | 117 | 1.6 | 0.3 | 1.3 | 87 | 107 | 121 | 171 | 28 |
| Intsia bijuga | 14 | 2 | 0.1 | 0.3 | 0.1 | 14 | 28 | 20 | 4 | 278 |
| Macaranga thompsonii | 95 | 4 | 1.0 | 5.0 | 1.7 | 2 | 10 | 2 | 4 | 11 |
| Meiogyne cylindrocarpa | 98 | 5 | 1.0 | 8.6 | 0.5 | 79 | 121 | 12 | 17 | 22 |
| Morinda citrifolia | 126 | 119 | 1.8 | 0.7 | 0.1 | 13 | 9 | 80 | 19 | 24 |
| Nephrolepis hirsutula | 334 | 2 | 0.5 | 0.5 | 0.9 | 3 | 8 | 11 | 10 | 15 |
| Ochrosia mariannensis | 150 | 5 | 1.5 | 0.7 | 0.5 | 130 | 10 | 4 | 11 | 39 |
| Ochrosia oppositifolia | 97 | 6 | 1.4 | 0.1 | 0.2 | 3 | 3 | 5 | 10 | 20 |
| Pandanus dubius | 116 | 194 | 2.1 | 3.8 | 0.1 | 119 | 35 | 60 | 28 | 94 |
| Pandanus tectorius | 101 | 217 | 1.3 | 3.1 | 0.3 | 55 | 22 | 116 | 15 | 136 |
| Pisonia grandis | 20 | 25 | 1.5 | 3.6 | 3.6 | 19 | 46 | 4 | 31 | 19 |
| Premna serratifolia | 4 | 43 | 1.7 | 7.5 | 1.1 | 10 | 250 | 22 | 94 | 16 |
| Scaevola taccada | 53 | 6 | 1.3 | 0.5 | 0.9 | 24 | 22 | 42 | 30 | 7 |
| Serianthes nelsonii | 216 | 27 | 0.6 | 6.0 | 0.3 | 13 | 38 | 6 | 6 | 121 |
| Tabernaemontana rotensis | 61 | 5 | 1.4 | 1.7 | 0.4 | 8 | 6 | 33 | 38 | 22 |
| PPI 1 | 98.8 | 99.3 | 95.7 | 99.5 | 97.8 | 98.5 | 99.2 | 99.7 | 98.3 | 98.2 |
References
- Lahner, B.; Gong, J.; Mahmoudian, M.; Smith, E.L.; Abid, K.B.; Rogers, E.E.; Guerinot, M.L.; Harper, J.F.; Ward, J.M.; McIntyre, L.; et al. Genomic scale profiling of nutrient and trace elements in Arabidopsis thaliana. Nat. Biotechnol. 2003, 21, 1215–1221. [Google Scholar] [CrossRef]
- Salt, E.D.; Baxter, I.; Lahner, B. Ionomics and the study of the plant ionome. Annu. Rev. Plant Biol. 2008, 59, 709–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, P.J.; Brown, P.H. Plant nutrition for sustainable development and global health. Ann. Bot. 2010, 105, 1073–1080. [Google Scholar] [CrossRef] [Green Version]
- Aerts, R.; Chapin, F.S., III. The mineral nutrition of wild plants revisited: A reevaluation of processes and patterns. Adv. Ecol. Res. 2000, 30, 1–67. [Google Scholar]
- Souri, M.K.; Hatamian, M. Aminochelates in plant nutrition: A review. J. Plant Nutr. 2019, 42, 67–78. [Google Scholar] [CrossRef]
- Ingestad, T. New concepts on soil fertility and plant nutrition as illustrated by research on forest trees and stands. Geoderma 1987, 40, 237–252. [Google Scholar] [CrossRef]
- Walworth, J.L.; Sumner, M.E. The diagnosis and recommendation integrated system (DRIS). Adv. Soil Sci. 1987, 6, 149–188. [Google Scholar]
- Sterner, R.W.; Elser, J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere; Princeton Univ. Press: Princeton, NJ, USA, 2002; p. 439. [Google Scholar]
- Körner, C. The grand challenges in functional plant ecology. Front. Plant Sci. 2011, 2, 1. [Google Scholar] [CrossRef] [Green Version]
- Sardans, J.; Janssens, I.A.; Ciais, P.; Obersteiner, M.; Peñuelas, J. Recent advances and future research in ecological stoichiometry. Perspect. Plant Ecol. Evol. Syst. 2021, 50, 125611. [Google Scholar] [CrossRef]
- Koerselman, W.; Meuleman, A.F.M. The vegetation N:P ratio: A new tool to detect the nature of nutrient limitation. J. Appl. Ecol. 1996, 33, 1441–1450. [Google Scholar] [CrossRef]
- Güsewell, S.; Koerselman, M. Variation in nitrogen and phosphorus concentrations of wetland plants. Perspect. Plant Ecol. 2002, 5, 37–61. [Google Scholar] [CrossRef]
- Olde Venterink, H.; Wassen, M.J.; Verkroost, A.W.M.; de Ruiter, P.C. Species richness-productivity patterns differ between N-, P- and K-limited wetlands. Ecology 2003, 84, 2191–2199. [Google Scholar] [CrossRef]
- Wang, M.; Moore, T.R. Carbon, nitrogen, phosphorus, and potassium stoichiometry in an ombrotrophic peatland reflects plant functional type. Ecosystems 2014, 17, 673–684. [Google Scholar] [CrossRef]
- Killingbeck, K.T. The terminological jungle revisited: Making a case for use of the term resorption. Oikos 1986, 46, 263–264. [Google Scholar] [CrossRef]
- Aerts, R. Nutrient resorption from senescing leaves of perennials: Are there general patterns? J. Ecol. 1996, 84, 597–608. [Google Scholar] [CrossRef]
- Killingbeck, K.T. Nutrients in senesced leaves: Key to search for potential resorption and resorption proficiency. Ecology 1996, 77, 1716–1727. [Google Scholar] [CrossRef]
- Freschet, G.T.; Cornelissen, J.H.C.; van Logtestijn, R.S.P.; Aerts, R. Substantial nutrient resorption from leaves, stems and roots in a subarctic flora: What is the link with other resource economics traits? New Phytol. 2010, 186, 879–889. [Google Scholar] [CrossRef]
- Deloso, B.E.; Krishnapillai, M.V.; Ferreras, U.F.; Lindström, A.J.; Calonje, M.; Marler, T.E. Chemical element concentrations of cycad leaves: Do we know enough? Horticulturae 2020, 6, 85. [Google Scholar] [CrossRef]
- Marler, T.E.; Lindström, A.J. Leaf nutrients of two Cycas L. species contrast among in situ and ex situ locations. J. Threat. Taxa 2020, 12, 16831–16839. [Google Scholar] [CrossRef]
- Marler, T.E.; Lawrence, J.H. Leaf and soil nutrient relations of Elaeocarpus joga Merr. in oceanic island calcareous soils. HortScience 2015, 50, 1644–1649. [Google Scholar] [CrossRef]
- Marler, T.E. Stem carbohydrates and adventitious root formation of Cycas micronesica following Aulacaspis yasumatsui infestation. HortScience 2018, 53, 1125–1128. [Google Scholar] [CrossRef] [Green Version]
- Marler, T.E.; Cruz, G.N. Source and sink relations mediate depletion of intrinsic cycad seed carbohydrates by Aulacaspis yasumatsui infestation. HortScience 2019, 54, 1712–1717. [Google Scholar] [CrossRef] [Green Version]
- Marler, T.E.; Cruz, G.N. Cycas micronesica stem carbohydrates decline following leaf and male cone growth events. Plants 2020, 9, 517. [Google Scholar] [CrossRef]
- Dumas, J.B.A. Procedes de L’analyse Organique. Ann. Chim. Phys. 1831, 47, 198–205. [Google Scholar]
- AOAC. Official Method 973. 18 fibre (acid detergent) and lignin in animal feed. In Official Methods of Analysis of AOAC International, 16th ed.; AOAC International: Arlington, VA, USA, 1997; pp. 28–29. [Google Scholar]
- Iiyama, K.; Wallis, A.F.A. Determination of lignin in herbaceous plants by an improved acetyl bromide procedure. J. Sci. Food Agric. 1990, 51, 145–161. [Google Scholar] [CrossRef]
- Young, F.J. Soil Survey of Territory of Guam; U.S. Dept. of Agric. Soil Conservation Service: Washington, DC, USA, 1988. [Google Scholar]
- Marler, T.E.; Krishnapillai, M.V. Cycas micronesica trees alter local soil traits. Forests 2018, 9, 565. [Google Scholar] [CrossRef] [Green Version]
- Marler, T.E. Late successional tree species in Guam create biogeochemical niches. Communic. Integr. Biol. 2019, 12, 86–90. [Google Scholar] [CrossRef] [Green Version]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; U.S. Dept. of Agric.: Washington, DC, USA, 1954; Volume 939. [Google Scholar]
- Berghage, R.D.; Krauskopf, D.M.; Warncke, D.D.; Widders, I. Micronutrient testing of plant growth media extractant, identification and evaluation. Commun. Soil Sci. Plant Anal. 1987, 18, 1089–1109. [Google Scholar] [CrossRef]
- Bahamonde, H.A.; Fernández, V.; Gyenge, J.; Mattenet, F.; Peri, P.L. Essential nutrient and trace element foliar resorption of two co-existing Nothofagus species grown under different environmental conditions in Southern Patagonia. Front. Plant Sci. 2019, 10, 1542. [Google Scholar] [CrossRef] [Green Version]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Cornelissen, J.H.; Falster, D.S.; Garnier, E.; Hikosaka, K.; Lamont, B.B.; Lee, W.; Oleksyn, J.; Osada, N.; et al. Assessing the generality of global leaf trait relationships. New Phytol. 2005, 166, 485–496. [Google Scholar] [CrossRef]
- Watanabe, T.; Broadley, M.R.; Jansen, S.; White, P.J.; Takada, J.; Satake, K.; Takamatsu, T.; Tuah, S.J.; Osaki, M. Evolutionary control of leaf element composition in plants. New Phytol. 2007, 174, 516–523. [Google Scholar] [CrossRef]
- Xing, K.; Niinemets, Ü.; Rengel, Z.; Onoda, Y.; Xia, J.; Chen, H.Y.H.; Zhao, M.; Han, W.; Li, H. Global patterns of leaf construction traits and their covariation along climate and soil environmental gradients. New Phytol. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- White, P.; George, T.; Dupuy, L.; Karley, A.; Valentine, T.; Wiesel, L.; Wishart, J. Root traits for infertile soils. Front. Plant Sci. 2013, 4, 193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neugebauer, K.; Broadley, M.R.; El-Serehy, H.A.; George, T.S.; McNicol, J.W.; Moraes, M.F.; White, P.J. Variation in the angiosperm ionome. Physiol. Plant. 2018, 163, 306–322. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Huang, Y.; Wu, F.; Liu, W.; Ning, Y.; Huang, Z.; Tang, S.; Liang, Y. Plant adaptability in karst regions. J. Plant Res. 2021, 134, 889–906. [Google Scholar] [CrossRef]
- Güsewell, S. N:P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef]
- Hooper, D.U.; Adair, E.C.; Cardinale, B.J.; Byrnes, J.E.; Hungate, B.A.; Matulich, K.L.; Gonzalez, A.; Duffy, J.E.; Gamfeldt, L.; O’Connor, M.I. A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 2012, 486, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Sardans, J.; Rivas-Ubach, A.; Peñuelas, J. The C: N: P stoichiometry of organisms and ecosystems in a changing world: A review and perspectives. Perspect. Plant Ecol. Evol. Syst. 2012, 14, 33–47. [Google Scholar] [CrossRef]
- Sala, O.E.; Chapin, F.S.; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef]
- Wu, T.; Qu, C.; Li, Y.; Li, X.; Zhou, G.; Liu, S.; Chu, G.; Meng, Z.; Lie, Z.; Liu, J. Warming effects on leaf nutrients and plant growth in tropical forests. Plant Ecol. 2019, 220, 663–674. [Google Scholar] [CrossRef]
- Reich, P.B.; Oleksyn, J. Global patterns of plant leaf N and P in relation to temperature and latitude. Proc. Natl. Acad. Sci. USA 2004, 101, 11001–11006. [Google Scholar] [CrossRef] [Green Version]
- Reich, P.B.; Oleksyn, J.; Modrzynski, J.; Mrozinski, P.; Hobbie, S.E.; Eissenstat, D.M.; Chorover, J.; Chadwick, O.A.; Hale, C.M.; Tjoelker, M.G. Linking litter calcium, earthworms and soil properties: A common garden test with 14 tree species. Ecol. Lett. 2005, 8, 811–818. [Google Scholar] [CrossRef]
- Tian, D.; Yan, Z.; Niklas, K.J.; Han, W.; Kattge, J.; Reich, P.B.; Luo, Y.; Chen, Y.; Tang, Z.; Hu, H.; et al. Global leaf nitrogen and phosphorus stoichiometry and their scaling exponent. Natl. Sci. Rev. 2018, 5, 728–739. [Google Scholar] [CrossRef] [Green Version]
- Lovett, G.M.; Arthur, M.A.; Crowley, K.F. Effects of calcium on the rate and extent of litter decomposition in a northern hardwood forest. Ecosystems 2016, 19, 87–97. [Google Scholar] [CrossRef]
- Joly, F.-X.; Milcu, A.; Scherer-Lorenzen, M.; Jean, L.-K.; Bussotti, F.; Dawud, S.M.; Müller, S.; Pollastrini, M.; Raulund-Rasmussen, K.; Vesterdal, L.; et al. Tree species diversity affects decomposition through modified micro-environmental conditions across European forests. New Phytol. 2017, 214, 1281–1293. [Google Scholar] [CrossRef] [Green Version]
- Gillespie, L.M.; Hättenschwiler, S.; Milcu, A.; Wambsganss, J.; Shihan, A.; Fromin, N. Tree species mixing affects soil microbial functioning indirectly via root and litter traits and soil parameters in European forests. Funct. Ecol. 2021, in press. [Google Scholar] [CrossRef]
- McGroddy, M.E.; Daufresne, T.; Hedin, L.O. Scaling of C:N:P stoichiometry in forests worldwide: Implications of terrestrial Redfield-type ratios. Ecology 2004, 85, 2390–2401. [Google Scholar] [CrossRef]
- Elser, J.J.; Fagan, W.F.; Denno, R.F.; Dobberfuhl, D.R.; Folarin, A.; Huberty, A.; Interlandi, S.; Kilham, S.S.; McCauley, E.; Schulz, K.L.; et al. Nutritional constraints in terrestrial and freshwater food webs. Nature 2000, 408, 578–580. [Google Scholar] [CrossRef] [PubMed]
- Enríquez, S.C.M.D.; Duarte, C.M.; Sand-Jensen, K. Patterns in decomposition rates among photosynthetic organisms: The importance of detritus C: N: P content. Oecologia 1993, 94, 457–471. [Google Scholar] [CrossRef]
- Aerts, R. Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: A triangular relationship. Oikos 1997, 79, 439–449. [Google Scholar] [CrossRef]
- Cornwell, W.K.; Cornelissen, J.H.; Amatangelo, K.; Dorrepaal, E.; Eviner, V.T.; Godoy, O.; Hobbie, S.E.; Hoorens, B.; Kurokawa, H.; Pérez-Harguindeguy, N.; et al. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol. Lett. 2008, 11, 1065–1071. [Google Scholar] [CrossRef]
- Semchenko, M.; Xue, P.; Leigh, T. Functional diversity and identity of plant genotypes regulate rhizodeposition and soil microbial activity. New Phytol. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Tian, D.; Han, W.; Tang, Z.; Fang, J. An assessment on the uncertainty of the nitrogen to phosphorus ratio as a threshold for nutrient limitation in plants. Ann. Bot. 2017, 120, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Du, E.; Terrer, C.; Pellegrini, A.F.; Ahlström, A.; van Lissa, C.J.; Zhao, X.; Xia, N.; Wu, X.; Jackson, R.B. Global patterns of terrestrial nitrogen and phosphorus limitation. Nat. Geosci. 2020, 13, 221–226. [Google Scholar] [CrossRef]
- United States Fish and Wildlife Service. Determination of endangered status for Serianthes nelsonii Merr. (Hayun lagu or Tronkon Guafi). Fed. Regist. 1987, 52, 4907–4910. [Google Scholar]
- United States Fish & Wildlife Service. Endangered and threatened wildlife and plants; endangered status for 16 species and threatened status for 7 species in Micronesia. Fed. Regist. 2015, 80, 59424–59497. [Google Scholar]
- United States Fish and Wildlife Service. Recovery Plan for Serianthes Nelsonii; USFWS: Portland, OR, USA, 1994. [Google Scholar]
- Marler, T.E.; Cascasan, A.N. Number of emerged seedlings and seedling longevity of the non-recruiting, critically endangered Håyun lågu tree Serianthes nelsonii Merr. (Fabales: Leguminosae) are influenced by month of emergence. J. Threat. Taxa 2015, 7, 8221–8225. [Google Scholar] [CrossRef] [Green Version]
- Marler, T.; Musser, C. Potential stressors leading to seedling mortality in the endemic Håyun lågu tree (Serianthes nelsonii Merr.) in the island of Guam. Trop. Conserv. Sci. 2015, 8, 738–744. [Google Scholar] [CrossRef] [Green Version]
- Marler, T.E.; Cruz, G.N. Extreme wind events influence seed rain and seedling dynamics of Guam’s Serianthes nelsonii Merr. Trop. Conserv. Sci. 2019, 12, 1–6. [Google Scholar] [CrossRef]
- Knops, J.M.H.; Bradley, K.L.; Wedin, D.A. Mechanisms of plant species impacts on ecosystem nitrogen cycling. Ecol. Lett. 2002, 5, 454–466. [Google Scholar] [CrossRef] [Green Version]
- Cong, W.F.; van Ruijven, J.; Mommer, L.; De Deyn, G.B.; Berendse, F.; Hoffland, E. Plant species richness promotes soil carbon and nitrogen stocks in grasslands without legumes. J. Ecol. 2014, 102, 1163–1170. [Google Scholar] [CrossRef]
- Semchenko, M.; Saar, S.; Lepik, A. Intraspecific genetic diversity modulates plant–soil feedback and nutrient cycling. New Phytol. 2017, 216, 90–98. [Google Scholar] [CrossRef] [Green Version]
- Lawton, J.H.; Brown, V.K. Redundancy in ecosystems. In Biodiversity and Ecosystem Function; Schulze, E.D., Mooney, H.A., Eds.; Springer: Berlin, Germany, 1993; pp. 255–270. [Google Scholar]
- Mouchet, M.A.; Villéger, S.; Mason, N.W.H.; Mouillot, D. Functional diversity measures: An overview of their redundancy and their ability to discriminate community assembly rules. Funct. Ecol. 2010, 24, 867–876. [Google Scholar] [CrossRef]
- Ricotta, C.; de Bello, F.; Moretti, M.; Caccianiga, M.; Cerabolini, B.E.; Pavoine, S. Measuring the functional redundancy of biological communities: A quantitative guide. Methods Ecol. Evol. 2016, 7, 1386–1395. [Google Scholar] [CrossRef] [Green Version]
- Biggs, C.R.; Yeager, L.A.; Bolser, D.G.; Bonsell, C.; Dichiera, A.M.; Hou, Z.; Keyser, S.R.; Khursigara, A.J.; Lu, K.; Muth, A.F.; et al. Does functional redundancy affect ecological stability and resilience? A review and meta-analysis. Ecosphere 2020, 11, e03184. [Google Scholar] [CrossRef]
- Doak, D.F.; Bigger, D.; Harding, E.K.; Marvier, M.A.; O’Malley, R.E.; Thomson, D. The statistical inevitability of stability–diversity relationships in community ecology. Am. Nat. 1998, 151, 264–276. [Google Scholar] [CrossRef]
- Walker, B. Biodiversity and ecological redundancy. Conserv. Biol. 1992, 6, 18–23. [Google Scholar] [CrossRef]
- Wiles, G.; Williams, E. Serianthes nelsonii . IUCN Red List Threat. Species 2017. [Google Scholar] [CrossRef]
- Donnegan, J.A.; Butler, S.L.; Grabowiecki, W.; Hiserote, B.A.; Limtiaco, D. Guam’s Forest Resources, 2002; Resource Bulletin PNW-RB-243; U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 2004. [Google Scholar]
- Marler, T.E. Cycad aulacaspis scale invades the Mariana Islands. Mem. N. Y. Bot. Gard. 2012, 106, 20–35. [Google Scholar]
- Marler, T.E.; Krishnapillai, M.V. Longitude, forest fragmentation, and plant size influence Cycas micronesica mortality following island insect invasions. Diversity 2020, 12, 194. [Google Scholar] [CrossRef]
- Marler, T.E.; Lindström, A.J. The value of research to selling the conservation of threatened species: The case of Cycas micronesica (Cycadopsida: Cycadales: Cycadaceae). J. Threat. Taxa 2014, 6, 6523–6528. [Google Scholar] [CrossRef]
- Guo, Y.; Ren, G.; Zhang, K.; Li, Z.; Miao, Y.; Guo, H. Leaf senescence: Progression, regulation, and application. Mol. Hortic. 2021, 1, 5. [Google Scholar] [CrossRef]
- Zentgraf, U.; Andrade, A.G.; Doll, J. Editorial for special issue “leaf senescence” in plants. Plants 2021, 10, 1490. [Google Scholar] [CrossRef]
- Vergutz, L.; Manzoni, S.; Porporato, A.; Novais, R.F.; Jackson, R.B. Global resorption efficiencies and concentrations of carbon and nutrients in leaves of terrestrial plants. Ecol. Monogr. 2012, 82, 205–220. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Reed, S.C.; Lü, X.; Xiao, K.; Wang, K.; Li, D. Global resorption efficiencies of trace elements in leaves of terrestrial plants. Funct. Ecol. 2021, 35, 1596–1602. [Google Scholar] [CrossRef]
- Mediavilla, S.; González-Zurdo, P.; García-Ciudad, A.; Escudero, A. Morphological and chemical leaf composition of Mediterranean evergreen tree species according to leaf age. Trees 2011, 25, 669–677. [Google Scholar] [CrossRef]
- Kitajima, K.; Llorens, A.M.; Stefanescu, C.; Timchenko, M.V.; Lucas, P.W.; Wright, S.J. How cellulose-based leaf toughness and lamina density contribute to long leaf lifespans of shade-tolerant species. New Phytol. 2012, 195, 640–652. [Google Scholar] [CrossRef]
- Marler, T.E. Pacific island tropical cyclones are more frequent and globally relevant, yet less studied. Front. Environ. Sci. 2014, 2, 42. [Google Scholar] [CrossRef] [Green Version]
- Stone, B.C. America’s Asiatic flora: The plants of Guam. Amer. Sci. 1971, 59, 308–319. [Google Scholar]
- Marler, T.E. Perennial trees associating with nitrogen-fixing symbionts differ in leaf after-life nitrogen and carbon release. Nitrogen 2020, 1, 10. [Google Scholar] [CrossRef]
- Marler, T.E.; Dongol, N. Three invasive insects alter Cycas micronesica leaf chemistry and predict changes in biogeochemical cycling. Commun. Integr. Biol. 2016, 9, e1208324. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Reed, S.C.; Lü, X.; Xiao, K.; Wang, K.; Li, D. Coexistence of multiple leaf nutrient resorption strategies in a single ecosystem. Sci. Total Environ. 2021, 772, 144951. [Google Scholar] [CrossRef] [PubMed]
- Marler, T.E. Reciprocal garden study reveals acute spatial-edaphic adaptation for Cycas micronesica. Diversity 2021, 13, 237. [Google Scholar] [CrossRef]
- Waring, B.G. A meta-analysis of climatic and chemical controls on leaf litter decay rates in tropical forests. Ecosystems 2012, 15, 999–1009. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marler, T.E. Leaf Elemental Concentrations, Stoichiometry, and Resorption in Guam’s Coastal Karst Forests. Diversity 2021, 13, 545. https://doi.org/10.3390/d13110545
Marler TE. Leaf Elemental Concentrations, Stoichiometry, and Resorption in Guam’s Coastal Karst Forests. Diversity. 2021; 13(11):545. https://doi.org/10.3390/d13110545
Chicago/Turabian StyleMarler, Thomas E. 2021. "Leaf Elemental Concentrations, Stoichiometry, and Resorption in Guam’s Coastal Karst Forests" Diversity 13, no. 11: 545. https://doi.org/10.3390/d13110545






