Trophic Ecology of Juvenile Southern King Crab Associated with Kelp Forest: Evidence of Cannibalism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Stomach Content Analysis
2.3. Stable Isotope Analysis
2.4. Trophic Position
2.5. Data Analysis
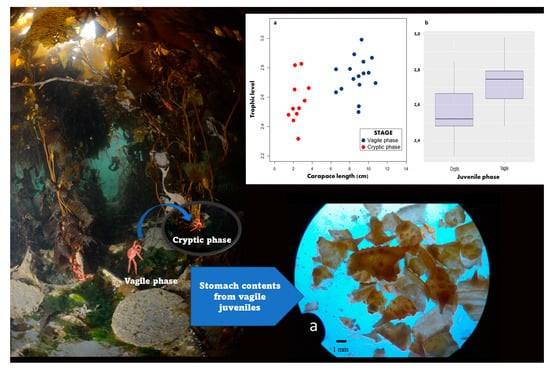
3. Results
3.1. Stomach Content Analysis
3.2. Trophic Niche Size and Overlap
3.3. Trophic Position
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Donaldson, W.E.; Byersdorfer, S.C. Biological Field Techniques for Lithodid Crabs; Alaska Sea Grant College Program—University of Alaska Fairbanks: Fairbanks, AK, USA, 2005; p. 76. [Google Scholar]
- Otto, R.S. A history of King crab fisheries with special reference to the north Pacific Ocean: Development, maturity and senescence. In King Crabs of the World: Biology and Fisheries Management; Stevens, B.G., Ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 81–138. [Google Scholar]
- Stevens, B.G.; Lovrich, G.A. King crabs of the world: Species and distributions. In King Crabs of the World: Biology and Fisheries Management; CRC Press: Boca Raton, FL, USA, 2014; pp. 1–30. [Google Scholar]
- Stevens, B.G.; Jewett, S.C. Growth, molting and feedings of king crabs. In King Crabs of the World: Biology and Fisheries Management; CRC Press: Boca Raton, FL, USA, 2014; pp. 315–361. [Google Scholar]
- Feder, H.M.; McCumby, K.; Paul, A.J. The food of post-larval king crab, Paralithodes camtschatica (Decapoda: Lithodidae), in Kachemak Bay, Alaska. Crust 1980, 39, 315–318. [Google Scholar] [CrossRef]
- Tarverdieva, M.I.; Zgurovsky, K.A. On food composition of the deep-water crab species Lithodes aequispina Benedict and Chionoecetes tanneri Rathbun in the Bering and Okhotsk seas. In International King Crab Symposium; University of Alaska Sea Grant Program: Anchorage, AK, USA, 1985; pp. 319–329. [Google Scholar]
- Vinuesa, J.H.; Varisco, M.A.; Balzi, P. Feeding strategy of early juvenile stages of the southern king crab Lithodes santolla in the San Jorge Gulf, Argentina. Rev. Biol. Mar. Oceanogr. 2013, 48, 353–363. [Google Scholar] [CrossRef] [Green Version]
- Brodersen, C.C.; Rounds, P.M.; Babcock, M.M. Diet influences cannibalism in laboratory-held juvenile red king crabs (Paralithodes camtschatica). In Proceedings of the International Symposium on King and Tanner Crabs, Anchorage, AK, USA, 28–30 November 1989; Alaska Sea Grant College Program Report: Anchorage, AK, USA, 1989; Volume 90, pp. 377–382. [Google Scholar]
- Stevens, B.G.; Swiney, K.M. Post-settlement effects of habitat type and predator size on cannibalism of glaucothoe and juveniles of red king crab Paralithodes camtschaticus. J. Exp. Mar. Biol. Ecol. 2005, 321, 1–11. [Google Scholar] [CrossRef]
- Sotelano, M.P.; Lovrich, G.A.; Tapella, R. Cannibalism among Lithodes santolla (Molina 1782) juveniles: Effect of stocking density, stage and molt condition. Aquac. Int. 2016, 24, 1025–1037. [Google Scholar] [CrossRef]
- Hines, A.H.; Lipcius, R.N.; Haddon, A.M. Population dynamics and habitat partitioning by size, sex, and molt stage of blue crabs Callinectes sapidus in a subestuary of central Chesapeake Bay. Mar. Ecol. Prog. Ser. 1987, 36, 55–64. [Google Scholar] [CrossRef]
- Araújo, M.S.L.C.; Barreto, A.V.; Negromonte, A.O.; Schwamborn, R. Population ecology of the blue crab Callinectes danae (Crustacea: Portunidae) in a Brazilian tropical estuary. An. Acad. Bras. Ciênc. 2012, 84, 129–138. [Google Scholar] [CrossRef]
- Stevens, B.G. (Ed.) Biology and ecology of juvenile king crabs. In King Crabs of the World: Biology and Fisheries Management; CRC Press: Boca Raton, FL, USA, 2014; Volume 636, pp. 261–284. [Google Scholar]
- Lovrich, G.A.; Tapella, F. Southern king crabs. In King Crabs of the World: Biology and Fisheries Management; Stevens, B.G., Ed.; CRC Press: Boca Raton, FL, USA, 2014; Volume 636, pp. 139–210. [Google Scholar]
- Tapella, F.; Lovrich, G.A. Asentamiento de estadios tempranos de las centollas Lithodes santolla y Paralomis granulosa (Decapoda: Lithodidae) en colectores artificiales pasivos en el Canal Beagle, Argentina. Invest. Mar. 2006, 34, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Cárdenas, C.A.; Cañete, J.I.; Oyarzún, S.; Mansilla, A. Podding of juvenile king crab Lithodes santolla (Molina, 1782) (Crustacea) in association with holdfasts of Macrocystis pyrifera (Linnaeus) C. Agardh, 1820. Invest. Mar. 2007, 35, 105–110. [Google Scholar] [CrossRef] [Green Version]
- Adami, M.L.; Gordillo, S. Structure and dynamics of the biota associated with Macrocystis pyrifera (Phaeophyta) from the Beagle Channel, Tierra del Fuego. Sci. Mar. 1999, 63, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Ríos, C.; Arntz, W.E.; Gerdes, D.; Mutschke, E.; Montiel, A. Spatial and temporal variability of the benthic assemblages associated to the holdfasts of the kelp Macrocystis pyrifera in the Straits of Magellan, Chile. Polar Biol. 2007, 31, 89–100. [Google Scholar] [CrossRef]
- Kaehler, S.; Pakhomov, E.A.; Kalin, R.M.; Davis, S. Trophic importance of kelp-derived suspended particulate matter in a through-flow sub-Antarctic system. Mar. Ecol. Prog. Ser. 2006, 316, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Calcagno, J.A.; Lovrich, G.A.; Thatje, S.; Nettelman, U.; Anger, K. First year growth in the lithodids Lithodes santolla and Paralomis granulosa reared at different temperatures. J. Sea Res. 2005, 54, 221–230. [Google Scholar] [CrossRef] [Green Version]
- Cárdenas, L.; Leclerc, J.; Bruning, P.; Garrido, I.; Détrée, C.; Figueroa, A.; Astorga, M.; Navarro, J.; Johnson, L.; Carlton, J.; et al. First mussel settlement observed in Antarctica reveals the potential for future invasions. Sci. Rep. 2020, 10, 5552. [Google Scholar] [CrossRef] [PubMed]
- Peterson, B.J.; Fry, B. Stable isotopes in ecosystem studies. Ann. Rev. Ecol. Syst 1987, 18, 293–320. [Google Scholar] [CrossRef]
- Hobson, K.A.; Clark, R.G. Assessing avian diets using stable isotopes II: Factors influencing diet-tissue fractionation. Condor 1992, 94, 189–197. [Google Scholar] [CrossRef]
- Duffy, D.; Jackson, S. Diet studies of seabirds: A review of methods. Col. Waterb. 1986, 9, 1–17. [Google Scholar] [CrossRef]
- DeNiro, M.; Epstein, S. Influence of diet on the distribution of carbon isotopes in animals. Geoch. Cosmochim. Act. 1978, 42, 495–506. [Google Scholar] [CrossRef]
- Bearhop, S.; Adams, C.E.; Waldron, S.; Fuller, R.A.; Macleod, H. Determining trophic niche width: A novel approach using stable isotope analysis. J. Anim. Ecol. 2004, 73, 1007–1012. [Google Scholar] [CrossRef] [Green Version]
- Fry, B. Using stable isotope tracers. In Stable Isotope Ecology; Springer: New York, NY, USA, 2006. [Google Scholar] [CrossRef]
- Palma, A.T.; Pardo, L.M.; Veas, R.I.; Cartes, C.; Silva, M.; Manriquez, K.; Diaz, A.; Muñoz, C.; Ojeda, F.P. Coastal brachyuran decapods: Settlement and recruitment under contrasting coastal geometry conditions. Mar. Ecol. Prog. Ser. 2006, 316, 139–153. [Google Scholar] [CrossRef] [Green Version]
- Brun, E. Food and feeding habits of Luidia ciliaris Echinodermata: Asteroidea. J. Mar. Biol. Ass. UK 1972, 52, 225–236. [Google Scholar] [CrossRef]
- Fratt, D.B.; Dearborn, J.H. Feeding biology of the Antarctic brittle star Ophionotus victoriae (Echinodermata: Ophiuroidea). Polar Biol. 1984, 3, 127–139. [Google Scholar] [CrossRef]
- Dearborn, J.H.; Ferrari, F.D.; Edwards, K.C. Can pelagic aggregations cause benthic satiation? Feeding biology of the Antarctic brittle star Astrotoma agassizzi (Echinodermata: Ophiuroidea). Biology Antarctic Seas XVII. Antarct. Res. Ser. 1986, 44, 1–28. [Google Scholar] [CrossRef]
- Hynes, H.B.N. The food of freshwater sticklebacks (Gasterosteus aculeatus and Pygosteus pungitius) with a review of methods used in studies of the food of fishes. J. Anim. Ecol. 1950, 19, 36–58. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Ogawa, N.; Ogura, N. Dynamics of particulate organic matter in the Tamagawa Estuary and inner Tokyo Bay. Estuar. Coast. Shelf Sci. 1997, 44, 263–273. [Google Scholar] [CrossRef]
- Lorrain, A.; Savoye, N.; Chauvaud, L.; Paulet, Y.-M.; Naulet, N. Decarbonation and preservation method for the analysis of organic C and N contents and stable isotope ratios of low-carbonated suspended particulate material. Anal. Chim. Acta 2003, 491, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Bunn, S.E.; Loneragan, N.R.; Kempster, M.A. Effects of acid washing on stable isotope ratios of C and N in penaeid shrimp and seagrass: Implications for food-web studies using multiple stable isotopes. Limnol. Oceanogr. 1995, 40, 622–625. [Google Scholar] [CrossRef] [Green Version]
- Bonde, A.L.; Jones, I.L. A practical introduction to stable-isotope analysis for seabird biologists: Approaches, cautions and caveats. Mar. Ornithol. 2009, 37, 183–188. [Google Scholar]
- Post, D. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 2002, 83, 703–718. [Google Scholar] [CrossRef]
- Iken, K.; Bluhm, B.; Dunton, K. Benthic food-web structure under differing water mass properties in the southern Chukchi Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2010, 57, 71–85. [Google Scholar] [CrossRef]
- McMeans, B.C.; Rooney, N.; Michel, T.A.; Fisk, A.T. Food web structure of a coastal Arctic marine ecosystem and implications for stability. Mar. Ecol. Prog. Ser. 2013, 482, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Quezada, R.C.; Jackson, A.; Hayden, B.; Kahilainen, K.; Lopes, C.; Harrod, C. tRophicPosition, an R package for the Bayesian estimation of trophic position from consumer stable isotope ratios. Met. Ecol. Evol. 2018, 9, 1592–1599. [Google Scholar] [CrossRef] [Green Version]
- Vander Zanden, M.J.; Rasmussen, J.B. Variation in δ15N and δ13C trophic fractionation; implications for aquatic food web studies. Limnol. Oceanogr. 2001, 46, 2061–2066. [Google Scholar] [CrossRef]
- Clarke, K.R.; Somerfield, P.J.; Chapman, M.G. On resemblance measures for ecological studies, including taxonomic dissimilarities and a zero-adjusted Bray-Curtis coefficient for denuded assemblages. J. Exp. Mar. Biol. Ecol. 2006, 330, 55–80. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER v7: User Manual/Tutorial Plymouth; PRIMER-E: Ivybridge, UK, 2015. [Google Scholar]
- Jackson, A.L.; Inger, R.; Parnell, A.C.; Bearhop, S. Comparing isotopic niche widths among and within communities: SIBER—Stable Isotope Bayesian Ellipses in R. J. Anim. Ecol. 2011, 80, 595–602. [Google Scholar] [CrossRef]
- Layman, C.A.; Arrington, A.; Montaña, C.G.; Post, D.M. Can stable isotope ratios provide for community-wide measures of trophic structure? Ecology 2007, 88, 42–48. [Google Scholar] [CrossRef]
- Swanson, H.K.; Lysy, M.; Power, M.; Stasko, A.D.; Johnson, J.D.; Reist, J.D. A new probabilistic method for quantifying n-dimensional ecological niches and niche overlap. Ecology 2015, 96, 318–324. [Google Scholar] [CrossRef]
- Meyer, R.; Lochner, S.; Melzer, R.R. Decapoda—Crabs, shrimps & lobsters. In Marine Benthic Fauna of Chilean Patagonia; Häussermann, V., Försterra, G., Eds.; Nature in Focus: Santiago de Chile, Chile, 2009; p. 1000. [Google Scholar]
- Borisov, R.R.; Epelbaum, A.B.; Kryakhova, N.V.; Tertitskaya, A.G.; Kovatcheva, N.P. Cannibalistic behavior in red king crabs reared under artificial conditions. Russ. J. Mar. Biol. 2007, 33, 227–231. [Google Scholar] [CrossRef]
- Daly, B.; Long, W.C. Inter-cohort cannibalism of early benthic phase blue king crabs (Paralithodes platypus): Alternate foraging strategies in different habitats lead to different functional responses. PLoS ONE 2014, 9, e88694. [Google Scholar] [CrossRef]
- Sotelano, M.P.; Lovrich, G.A.; Romero, M.C.; Tapella, F. Cannibalism during intermolt period in early stages of the Southern King Crab Lithodes santolla (Molina 1872): Effect of stage and predator-prey proportions. J. Exp. Mar. Biol. Ecol. 2012, 411, 52–58. [Google Scholar] [CrossRef]
- Comoglio, L.I.; Amin, O.A. Feeding habits of the false southern king crab Paralomis granulosa (Lithodidae) in the Beagle Channel, Tierra del Fuego, Argentina. Sci. Mar. 1999, 63, 361–366. [Google Scholar] [CrossRef] [Green Version]
- Takeshita, F.; Tamura, R. Optimal stocking density of juvenile red king crabs Paralithodes camtschaticus under cannibalism consideration. Fish. Sci. 2014, 80, 775–783. [Google Scholar] [CrossRef]
- Moksnes, P.-O. Self-regulating mechanisms in cannibalistic populations of juvenile shore crabs Carcinus maenas. Ecology 2004, 85, 1343–1354. Available online: www.jstor.org/stable/3450176 (accessed on 2 October 2021). [CrossRef] [Green Version]
- Pardo, L.M.; Palma, A.T.; Prieto, C.; Sepulveda, P.; Valdivia, I.; Ojeda, F.P. Processes regulating early post-settlement habitat use in a subtidal assemblage of brachyuran decapods. J. Exp. Mar. Biol. Ecol. 2007, 344, 10–22. [Google Scholar] [CrossRef]
- Stoner, A.W. Habitat-mediated survival of newly settled red king crab in the presence of a predatory fish: Role of habitat complexity and heterogeneity. J. Exp. Mar. Biol. Ecol. 2009, 382, 54–60. [Google Scholar] [CrossRef]
- Pirtle, J.L.; Eckert, G.L.; Stoner, A.W. Habitat structure influences the survival and predator-prey interactions of early juvenile red king crab Paralithodes camtschaticus. Mar. Ecol. Prog. Ser. 2012, 465, 169–184. [Google Scholar] [CrossRef] [Green Version]
- Long, W.C.; Whitefleet-Smith, L. Cannibalism in red king crab: Habitat, ontogeny, and the predator functional response. J. Exp. Mar. Biol. Ecol. 2013, 449, 142–148. [Google Scholar] [CrossRef]
- Tapella, F.; Sotelano, M.P.; Romero, M.C.; Lovrich, G.A. Experimental natural substrate preference of southern king crab Lithodes santolla larvae. J. Exp. Mar. Biol. Ecol. 2012, 411, 70–77. [Google Scholar] [CrossRef]
- Beck, M.W.; Heck, K.L.; Able, K.W.; Childers, D.L.; Eggleston, D.B.; Gillanders, B.M.; Halpern, B.; Hays, C.G.; Hoshino, K.; Minello, T.J. The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates: A better understanding of the habitats that serve as nurseries for marine species and the factors that create site-specific variability in nursery quality will improve conservation and management of these areas. Bioscience 2001, 51, 633–641. [Google Scholar]
- Krumhansl, K.A.; Okamoto, D.K.; Rassweiler, A.; Novak, M.; Bolton, J.J.; Cavanaugh, K.C.; Connell, S.D.; Johnson, C.R.; Konar, B.; Ling, S.D.; et al. Global patterns of kelp forest change over the past half-century. Proc. Natl. Acad. Sci. USA 2016, 113, 13785. [Google Scholar] [CrossRef] [Green Version]
- Newsome, S.D.; del Rio, C.M.; Bearhop, S.; Phillips, D. A niche for isotopic ecology. Front. Ecol. Envirom. 2007, 5, 429–436. [Google Scholar] [CrossRef]
- Saborowski, R.; Thatje, S.; Calcagno, J.A.; Lovrich, G.A.; Anger, K. Digestive enzymes in the ontogenetic stages of the southern king crab, Lithodes santolla. Mar. Biol. 2006, 149, 865–873. [Google Scholar] [CrossRef] [Green Version]
- Comoglio, L.I.; Lovrich, G.A.; Vinuesa, J.H. Feeding habits of southern king crab, Lithodes santolla, and false king crab, Paralomis granulosa in the Beagle Channel. In Proceedings of the International Symposium on King and Tanner Crabs, Anchorage, AK, USA, 28–30 November 1989; Alaska Sea Grant College Program Report: Anchorage, AK, USA, 1990; Volume 90, pp. 315–325. [Google Scholar]
- Fuhrmann, M.; Pedersen, T.; Nilssen, E. Trophic niche of the invasive red king crab Paralithodes camtschaticus in a benthic food web. Mar. Ecol. Prog. Ser. 2017, 565, 113–129. [Google Scholar] [CrossRef]
- Divine, L.M.; Bluhm, B.A.; Mueter, F.J.; Iken, K. Diet analysis of Alaska Arctic snow crabs (Chionoecetes opilio) using.stomach contents and δ13C and δ15N stable isotopes. Deep-Sea Res. II 2017, 135, 124–136. [Google Scholar] [CrossRef] [Green Version]
- Di Salvatore, P.; Sacristán, H.J.; Florentín, O.; Varisco, M.; Lovrich, G.A. Female reproductive output and potential recruitment of three fished southern king crab stocks from the Southern Atlantic Ocean. ICES J. Mar. Sci. 2021, 78, 2628–2692. [Google Scholar] [CrossRef]
- Molinet, C.; Olguín, A.; Gebauer, P.; Díaz, P.A.; Díaz, M.; Matamala, T.; Mora, P.; Paschke, K. Upswing and expansion of the southern king crab (Lithodes santolla) fishery in Northwest Patagonia: Drivers, trends and opportunities for management. Reg. Stud. Mar. Sci. 2020, 34, 101073. [Google Scholar] [CrossRef]
- Orensanz, J.M.; Ernst, B.; Armstrong, D.; Parma, A.M. Detecting early warnings of recruitment overfishing in male- only crab fisheries: An example from the snow crab fishery. In Fisheries Assessment and Management in Data-Limited Situations; Kruse, G.H., Gallucc, V.F., Hay, D.E., Perry, R.I., Peterman, R.M., Shirley, T.C., et al., Eds.; Alaska Sea Grant College Program: Fairbanks, AK, USA, 2005; pp. 267–287. [Google Scholar]
- Pardo, L.M.; Riveros, M.P.; Fuentes, J.P.; Pinochet, R.; Cárdenas, C.; Sainte-Marie, B. High fishing intensity reduces females’ sperm reserve and brood fecundity in a eubrachyuran crab subject to sex- and size-biased harvest. ICES J. Mar. Sci. 2017, 74, 2459–2469. [Google Scholar] [CrossRef]
- Stevens, B.G. Is it possible to enhance king crab populations in Alaska? In Alaska Crab Stock Enhancement and Rehabilitation: Workshop Proceedings, Kodiak, AK, USA, 14–16 March 2006; Alaska Sea Grant College Program; University of Alaska Fairbanks: Fairbanks, AK, USA, 2006; pp. 5–8. [Google Scholar]





| ITEMS | Av.Abund | Av.Abund | Av.Diss | Diss/SD | Contrib% | Cum.% |
|---|---|---|---|---|---|---|
| Vagile | Cryptic | |||||
| Crustaceans | 6.17 | 1.57 | 12 | 1.81 | 19.84 | 19.84 |
| Bivalves | 1.76 | 4.07 | 8.49 | 1.31 | 14.05 | 33.89 |
| Algae | 2.69 | 4.64 | 6.85 | 1.29 | 11.34 | 45.23 |
| Sediment | 3.61 | 2.55 | 6.65 | 1.18 | 10.99 | 56.22 |
| Hydrozoans | 1.62 | 2.07 | 5.02 | 1.14 | 8.3 | 64.52 |
| Echinoderms | 0.13 | 1.65 | 3.98 | 0.69 | 6.58 | 71.1 |
| Average Dissimilarity = 60.46 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pardo, L.M.; Andrade, C.; Zenteno-Devaud, L.; Garrido, B.; Rivera, C. Trophic Ecology of Juvenile Southern King Crab Associated with Kelp Forest: Evidence of Cannibalism. Diversity 2021, 13, 556. https://doi.org/10.3390/d13110556
Pardo LM, Andrade C, Zenteno-Devaud L, Garrido B, Rivera C. Trophic Ecology of Juvenile Southern King Crab Associated with Kelp Forest: Evidence of Cannibalism. Diversity. 2021; 13(11):556. https://doi.org/10.3390/d13110556
Chicago/Turabian StylePardo, Luis Miguel, Claudia Andrade, Lisette Zenteno-Devaud, Bastián Garrido, and Cristóbal Rivera. 2021. "Trophic Ecology of Juvenile Southern King Crab Associated with Kelp Forest: Evidence of Cannibalism" Diversity 13, no. 11: 556. https://doi.org/10.3390/d13110556
APA StylePardo, L. M., Andrade, C., Zenteno-Devaud, L., Garrido, B., & Rivera, C. (2021). Trophic Ecology of Juvenile Southern King Crab Associated with Kelp Forest: Evidence of Cannibalism. Diversity, 13(11), 556. https://doi.org/10.3390/d13110556