Involvement of a Fishing Community in the Eradication of the Introduced Cactus Mouse (Peromyscus eremicus cedrosensis) from San Benito Oeste Island, Mexico
Abstract
:1. Introduction
2. Materials and Methods
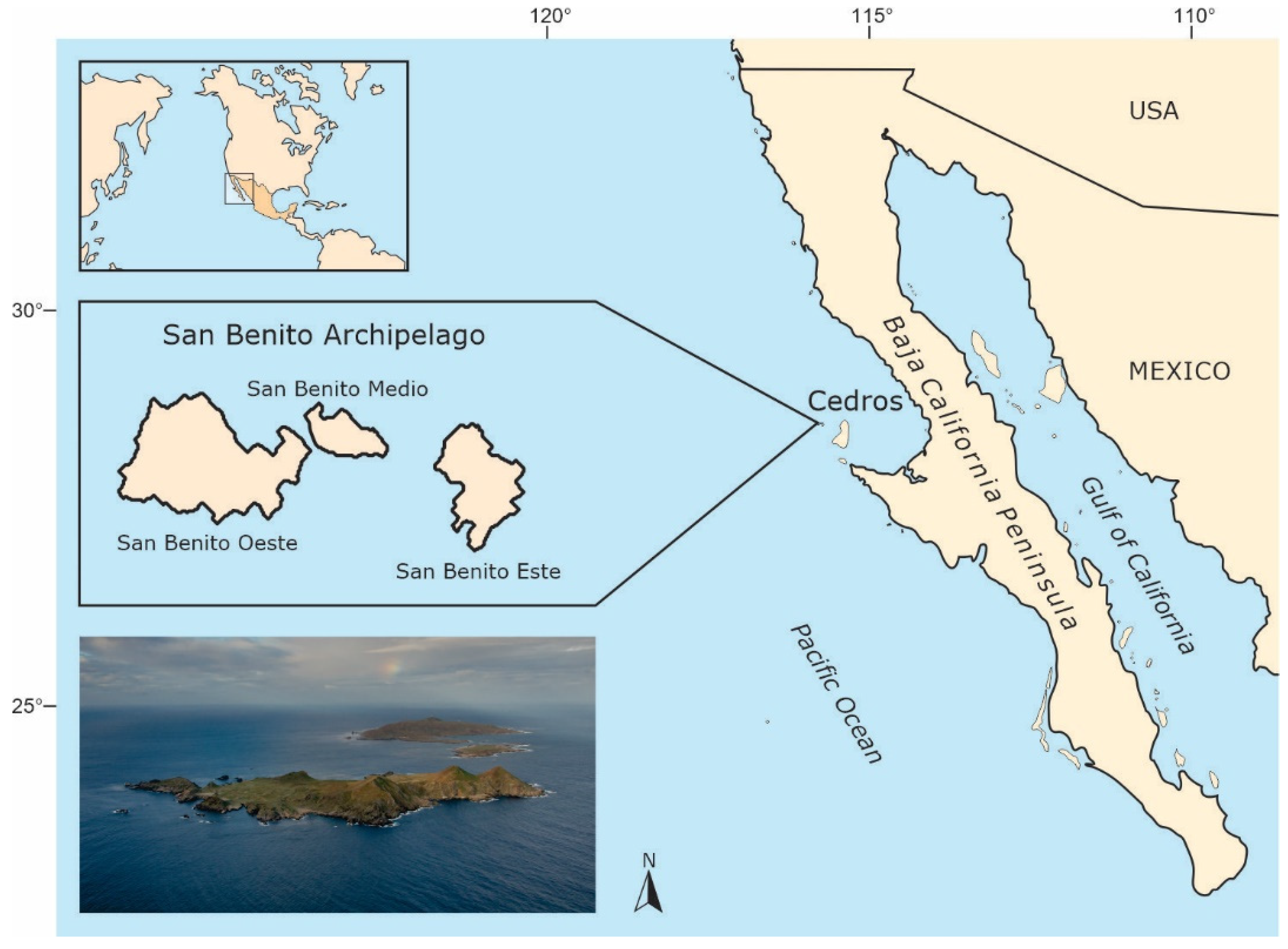
2.1. Study Site
2.2. PNA
2.3. Rodent Detection and Monitoring
2.4. Eradication Planning
2.5. Implementation
2.6. Confirmation
2.7. Wider Surveillance and Biosecurity
3. Results
3.1. Pre-Eradication Monitoring
3.2. Eradication Planning and Implementation
3.3. Confirmation
3.4. Wider Surveillance and Biosecurity
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mulder, C.P.H.; Anderson, W.B.; Towns, D.R.; Bellingham, P.J. (Eds.) Seabird Islands: Ecology, Invasion, and Restoration; Oxford University Press: New York, NY, USA, 2011. [Google Scholar]
- Provencher, J.F.; Borrelle, S.; Sherley, R.B.; Avery-Gomm, S.; Hodum, P.; Bond, A.; Major, H.L.; McCoy, K.D.; Crawford, R.; Merkel, F.; et al. Seabirds. In World Seas: An Environmental Evaluation, 2nd ed.; Sheppard, C., Ed.; Academic Press: New York, NY, USA, 2019; pp. 133–162. [Google Scholar]
- Spatz, D.R.; Newton, K.M.; Heinz, R.; Tershy, B.; Holmes, N.D.; Butchart, S.H.M.; Croll, D.A. The Biogeography of Globally Threatened Seabirds and Island Conservation Opportunities. Conserv. Biol. 2014, 28, 1282–1290. [Google Scholar] [CrossRef]
- Croxall, J.P.; Butchart, S.H.M.; Lascelles, B.; Stattersfield, A.J.; Sullivan, B.; Symes, A.; Taylor, P. Seabird conservation status, threats and priority actions: A global assessment. Bird Conserv. Int. 2012, 22, 1–34. [Google Scholar] [CrossRef] [Green Version]
- Dias, M.P.; Martin, R.; Pearmain, E.J.; Burfield, I.J.; Small, C.; Phillips, R.A.; Yates, O.; Lascelles, B.; Borboroglu, P.G.; Croxall, J.P. Threats to seabirds: A global assessment. Biol. Conserv. 2019, 237, 525–537. [Google Scholar] [CrossRef]
- Méndez Sánchez, F.; Bedolla Guzmán, Y.; Rojas Mayoral, E.; Aguirre-Muñoz, A.; Koleff, P.; Aguilar Vargas, A.; Álvarez Santana, F.; Arnaud, G.; Aztorga Ornelas, A.; Beltrán Morales, L.F.; et al. Population ecology of seabirds in Mexican Islands at the California Current System. bioRxiv 2021. [Google Scholar] [CrossRef]
- Wolf, S.; Keitt, B.; Aguirre-Muñoz, A.; Tershy, B.; Palacios, E.; Croll, D. Transboundary seabird conservation in an important North American marine ecoregion. Environ. Conserv. 2006, 33, 294–305. [Google Scholar] [CrossRef] [Green Version]
- Doherty, T.S.; Glen, A.S.; Nimmo, D.G.; Ritchie, E.G.; Dickman, C.R. Invasive predators and global biodiversity loss. Proc. Natl. Acad. Sci. USA 2016, 113, 11261–11265. [Google Scholar] [CrossRef] [Green Version]
- Bellard, C.; Cassey, P.; Blackburn, T.M. Alien species as a driver of recent extinctions. Biol. Lett. 2016, 12, 20150623. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Muñoz, A.; Bedolla-Guzmán, Y.; Hernández-Montoya, J.; Latofski-Robles, M.; Luna-Mendoza, L.; Méndez-Sánchez, F.; Ortiz-Alcaraz, A.; Rojas-Mayoral, E.; Samaniego-Herrera, A. The Conservation and Restoration of the Mexican Islands, a Successful Comprehensive and Collaborative Approach Relevant for Global Biodiversity. In Mexican Natural Resources Management and Biodiversity Conservation: Recent Case Studies; Ortega-Rubio, A., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 177–192. [Google Scholar]
- Aguirre-Muñoz, A.; Samaniego-Herrera, A.; Luna-Mendoza, L.; Ortiz-Alcaraz, A.; Rodríguez-Malagón, M.; Méndez-Sánchez, F.; Félix-Lizárraga, M.; Hernández-Montoya, J.C.; González-Gómez, R.; Torres-García, F.; et al. Island restoration in Mexico: Ecological outcomes after systematic eradications of invasive mammals. In Island Invasives: Eradication and Management, Proceedings of the International Conference on Island Invasives, Auckland, New Zealand, 8–12 February 2010; Veitch, C.R., Clout, M.N., Towns, D.R., Eds.; Occasional Paper of the IUCN Species Survival Commission No. 42; IUCN: Gland, Switzerland; CBB: Auckland, New Zealand, 2011; pp. 250–258. [Google Scholar]
- Howald, G.; Donlan, C.J.; Galván, J.P.; Russell, J.C.; Parkes, J.; Samaniego, A.; Wang, Y.; Veitch, D.; Genovesi, P.; Pascal, M.; et al. Invasive Rodent Eradication on Islands. Conserv. Biol. 2007, 21, 1258–1268. [Google Scholar] [CrossRef] [PubMed]
- Towns, D.R.; Broome, K.G. From small Maria to massive Campbell: Forty years of rat eradications from New Zealand islands. N. Z. J. Zool. 2003, 30, 377–398. [Google Scholar] [CrossRef]
- Harper, G.A.; Pahor, S.; Birch, D. The Lord Howe Island Rodent Eradication: Lessons Learnt from an Inhabited Island. In Proceedings of the 29th Vertebrate Pest Conference, Santa Barbara, CA, USA, 2–5 March 2020; p. 11. [Google Scholar]
- Glen, A.S.; Atkinson, R.; Campbell, K.J.; Hagen, E.; Holmes, N.D.; Keitt, B.S.; Parkes, J.P.; Saunders, A.; Sawyer, J.; Torres, H. Eradicating multiple invasive species on inhabited islands: The next big step in island restoration? Biol. Invasions 2013, 15, 2589–2603. [Google Scholar] [CrossRef]
- Brooke, M.d.L.; Bonnaud, E.; Dilley, B.J.; Flint, E.N.; Holmes, N.D.; Jones, H.P.; Provost, P.; Rocamora, G.; Ryan, P.G.; Surman, C.; et al. Seabird population changes following mammal eradications on islands. Anim. Conserv. 2018, 21, 3–12. [Google Scholar] [CrossRef]
- Holmes, N.D.; Spatz, D.R.; Oppel, S.; Tershy, B.; Croll, D.A.; Keitt, B.; Genovesi, P.; Burfield, I.J.; Will, D.J.; Bond, A.L.; et al. Globally important islands where eradicating invasive mammals will benefit highly threatened vertebrates. PLoS ONE 2019, 14, e0212128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbraud, C.; Delord, K.; Le Bouard, F.; Harivel, R.; Demay, J.; Chaigne, A.; Micol, T. Seabird population changes following mammal eradication at oceanic Saint-Paul Island, Indian Ocean. J. Nat. Conserv. 2021, 63, 126049. [Google Scholar] [CrossRef]
- Samaniego-Herrera, A.; Aguirre-Muñoz, A.; Bedolla-Guzmán, Y.; Cárdenas-Tapia, A.; Félix-Lizárraga, M.; Méndez-Sánchez, F.; Reina-Ponce, O.; Rojas-Mayoral, E.; Torres-García, F. Eradicating invasive rodents from wet and dry tropical islands in Mexico. Oryx 2017, 52, 559–570. [Google Scholar] [CrossRef]
- Samaniego-Herrera, A.; Aguirre-Muñoz, A.; Rodríguez-Malagón, M.; González-Gómez, R.; Torres-García, F.; Méndez-Sánchez, F.; Félix-Lizárraga, M.; Latofski-Robles, M. Rodent eradications on Mexican islands: Advances and challenges. In Island Invasives: Eradication and Management, Proceedings of the International Conference on Island Invasives, Auckland, New Zealand, 8–12 February 2010; Veitch, C.R., Clout, M.N., Towns, D.R., Eds.; Occasional Paper of the IUCN Species Survival Commission No. 42; IUCN: Gland, Switzerland; CBB: Auckland, New Zealand, 2011; pp. 350–355. [Google Scholar]
- Aguirre-Muñoz, A.; Samaniego-Herrera, A.; Luna-Mendoza, L.; Ortiz-Alcaraz, A.; Rodríguez-Malagón, M.; Félix-Lizárraga, M.; Méndez-Sánchez, F.; González-Gómez, R.; Torres-García, F.; Hernández-Montoya, J.C.; et al. Eradications of invasive mammals on islands in Mexico: The roles of history and the collaboration between government agencies, local communities and a non-government organisation. In Island Invasives: Eradication and Management, Proceedings of the International Conference on Island Invasives, Auckland, New Zealand, 8–12 February 2010; Veitch, C.R., Clout, M.N., Towns, D.R., Eds.; Occasional Paper of the IUCN Species Survival Commission No. 42; IUCN: Gland, Switzerland; CBB: Auckland, New Zealand, 2011; pp. 386–394. [Google Scholar]
- Aguirre-Muñoz, A.; Méndez-Sánchez, F. The New Baja California Pacific Islands Biosphere Reserve Sets a Conservation Benchmark: All Mexican Islands are Now Protected. FREMONTIA J. Calif. Native Plant Soc. 2017, 42, 27–31. [Google Scholar]
- Donlan, C.J.; Tershy, B.R.; Croll, D.A. Islands and Introduced Herbivores: Conservation Action as Ecosystem Experimentation. J. Appl. Ecol. 2002, 39, 235–246. [Google Scholar] [CrossRef]
- Sydeman, W.; Elliott, M. Developing the California Current Integrated Ecosystem Assessment, Module I: Select Time-Series of Ecosystem State. 2008. Available online: https://static1.squarespace.com/static/56a6b01dd8af105db2511b83/t/5760509d2eeb813699c0f046/1465929889374/IEA+Step+1+Rpt+Final.pdf (accessed on 25 October 2021).
- McClatchie, S. Regional Fisheries Oceanography of the California Current System: The CalCOFI Program; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- DOF. DECRETO por el que se declara Área Natural Protegida, con el Carácter de Reserva de la Biosfera, la Región Conocida como Islas del Pacífico de la Península de Baja California. 2016. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5464451&fecha=07/12/2016 (accessed on 20 October 2021).
- Junak, S.A.; Philbrick, R. Flowering plants of the San Benito Islands, Baja California, Mexico. In Proceedings of the Fifth California Islands Symposium, Santa Barbara, CA, USA, 29 March–1 April 1999; Browne, D.H., Chaney, H., Mitchell, K., Eds.; Santa Barbara Museum of Natural History: Santa Barbara, CA, USA, 2000; pp. 235–246. [Google Scholar]
- Heckel, G.; Ruiz Mar, G.; Schramm, Y.; Gorter, U. Atlas de Distribución y Abundancia de Mamíferos Marinos en México; Universidad Autónoma de Campeche, Instituto de Ecología, Pesquerías y Oceanografía del Golfo de México (EPOMEX): Campeche, Mexico, 2020. [Google Scholar]
- Samaniego Herrera, A.; Peralta García, A.; Aguirre Muñoz, A. (Eds.) Vertebrados de las Islas del Pacífico de Baja California: Guía de Campo; Grupo de Ecología y Conservación de Islas, A.C.: Ensenada, Mexico, 2007; p. 178. [Google Scholar]
- Wolf, S.; Phillips, C.; Zepeda-Dominguez, J.A.; Albores-Barajas, Y.; Martin, P. Breeding biology of Xantus’s Murrelet at the San Benito Islands, Baja California, México. Mar. Ornithol. 2005, 33, 123–129. [Google Scholar]
- Bedolla-Guzmán, Y.; Masello, J.F.; Aguirre-Muñoz, A.; Lavaniegos, B.E.; Quillfeldt, P. Breeding biology, chick growth, and diet of the Least Storm-Petrel Oceanodroma microsoma on Islas San Benito, Mexico. Mar. Ornithol. 2017, 45, 129–138. [Google Scholar]
- Whitworth, D.L.; Carter, H.R.; Palacios, E.; Gress, F. Breeding of Craveri’s Murrelet Synthliboramphus craveri at four islands off west-central baja california, México. Mar. Ornithol. 2018, 46, 117–124. [Google Scholar]
- Bedolla-Guzmán, Y.; Méndez-Sánchez, F.; Aguirre-Muñoz, A.; Félix-Lizárraga, M.; Fabila-Blanco, A.; Bravo-Hernández, E.; Hernández-Ríos, A.; Corrales-Sauceda, M.; Aguilar-Vargas, A.; Aztorga-Ornelas, A.; et al. Recovery and current status of seabirds on the Baja California Pacific Islands, Mexico, following restoration actions. In Island Invasives: Scaling up to Meet the Challenge; Veitch, C.R., Clout, M.N., Martin, A.R., Russell, J.C., West, C.J., Eds.; Occasional Paper SSC no. 62; IUCN: Gland, Switzerland, 2019; pp. 531–538. [Google Scholar]
- Bedolla-Guzmán, Y.; Masello, J.F.; Aguirre-Muñoz, A.; Lavaniegos, B.E.; Voigt, C.C.; Gómez-Gutiérrez, J.; Sánchez-Velasco, L.; Robinson, C.J.; Quillfeldt, P. Year-round niche segregation of three sympatric Hydrobates storm-petrels from Baja California Peninsula, Mexico, Eastern Pacific. Mar. Ecol. Prog. Ser. 2021, 664, 207–225. [Google Scholar] [CrossRef]
- Vidal, R.M.; Berlanga, H.; Del Coro Arizmendi, M. Important Bird Areas: Mexico. In Important Bird Areas Americas—Priority Sites for Biodiversity Conservation; BirdLife Conservation Series No. 16; BirdLife International: Quito, Ecuador, 2009. [Google Scholar]
- Ponce-Díaz, G.; Weisman, W.; McCay, B.J. Co-Responsability and Participation in Fisheries Management in Mexico: Lessons from Baja California Sur. Pesca Y Conserv. 2009, 1, 1–9. [Google Scholar]
- Méndez Sánchez, F.A. Co-Management and Small-Scale Fisheries in Mexico: The Case of a Fishers’ Cooperative in Cedros and San Benito Islands; The University of Auckland: Auckland, New Zealand, 2012. [Google Scholar]
- Phillips, B.; Bourillón, L.; Ramade, M. Case Study 2: The Baja California, Mexico, Lobster Fishery. In Seafood Ecolabelling: Principles and Practice; Ward, T., Phillips, B., Eds.; Blackwell Publishing Ltd.: Chichester, UK, 2008; pp. 259–268. [Google Scholar]
- Álvarez, P.; Delgado, C.; Espejel, I.; Seingier, G. Historia Ambiental del comanejo adaptativo en dos regiones pesqueras del noroeste mexicano. Relac. Estud. De Hist. Y Soc. 2018, 39, 41. [Google Scholar] [CrossRef] [Green Version]
- Mccay, B.J. Territorial Use Rights in Fisheries of the Northern Pacific Coast of Mexico. Bull. Marine Sci. 2017, 93, 69–81. [Google Scholar] [CrossRef]
- Krebs, C.J. Mammals. In Ecological Census Techniques: A Handbook, 2nd ed.; Sutherland, W.J., Ed.; Cambridge University Press: Cambridge, UK, 2006; pp. 351–369. [Google Scholar]
- Burgman, M.A.; Fox, J.C. Bias in species range estimates from minimum convex polygons: Implications for conservation and options for improved planning. Anim. Conserv. 2003, 6, 19–28. [Google Scholar] [CrossRef] [Green Version]
- GECI. Propuesta de Erradicación del Ratón Introducido en la isla San Benito Oeste, México; GECI: Ensenada, Mexico, 2009; p. 42. [Google Scholar]
- Bernard, H.R. Research Methods in Anthropology: Qualitative and Quantitative Approaches, 5th ed.; AltaMira Press: Lanham, MD, USA, 2011. [Google Scholar]
- Broome, K.G.; Cox, A.; Golding, C.; Cromarty, P.; Bell, P.; McLelland, P. Rat Eradication Using Aerial Baiting: Current Agreed Best Practice Used in New Zealand (Version 3.0); New Zealand Department of Conservation: Wellington, New Zeland, 2014. [Google Scholar]
- Rojas-Mayoral, E.; Méndez-Sánchez, F.; Rojas-Mayoral, B.; Aguirre-Muñoz, A. Improving the efficiency of aerial rodent eradications by means of the numerical estimation of rodenticide density. In Island Invasives: Scaling up to Meet the Challenge; Veitch, C.R., Clout, M.N., Martin, A.R., Russell, J.C., West, C.J., Eds.; Occasional Paper SSC no. 62; IUCN: Gland, Switzerland, 2019; pp. 47–50. [Google Scholar]
- Samaniego-Herrera, A.; Anderson, D.P.; Parkes, J.P.; Aguirre-Muñoz, A. Rapid assessment of rat eradication after aerial baiting. J. Appl. Ecol. 2013, 50, 1415–1421. [Google Scholar] [CrossRef]
- GECI. Introduced Mouse Eradication on San Benito Oeste Island, Mexico: 3rd Pre-Eradication Report; Grupo de Ecología y Conservación de Islas, A.C.: Ensenada, Mexico, 2010; p. 58. [Google Scholar]
- de Wit, L.A.; Croll, D.A.; Tershy, B.; Newton, K.M.; Spatz, D.R.; Holmes, N.D.; Kilpatrick, A.M. Estimating Burdens of Neglected Tropical Zoonotic Diseases on Islands with Introduced Mammals. Am. J. Trop. Med. Hyg. 2017, 96, 749–757. [Google Scholar] [CrossRef] [Green Version]
- Méndez-Sánchez, F.; Aguirre-Muñoz, A.; Bedolla-Guzmán, Y.; Cárdenas-Tapia, A.; Samaniego-Herrera, A. Mouse Eradication on San Benito Oeste Island, Mexico—Final Report; Grupo de Ecología y Conservación de Islas, A.C.: Ensenada, Mexico, 2016. [Google Scholar]
- Gleeson, D.; Prada, D. Population Genetic Structure of Savannah Sparrow from San Benito Islands, Mexico; Ecogene, Landcare Research: Lincoln, New Zealand, 2010; p. 5. [Google Scholar]
- Latofski-Robles, M.; Méndez-Sánchez, F.; Aguirre-Muñoz, A.; Jáuregui-García, C.; Koleff-Osorio, P.; González-Martínez, A.I.; Born-Schmidt, G.; Bernal-Stoopen, J.; Rendón-Hernández, E. Mexico’s island biosecurity programme: Collaborative formulation and implementation. In Island Invasives: Scaling up to Meet the Challenge; Veitch, C.R., Clout, M.N., Martin, A.R., Russell, J.C., West, C.J., Eds.; Occasional Paper SSC no. 62; IUCN: Gland, Switzerland, 2019; pp. 484–488. [Google Scholar]
- Méndez Sánchez, F.A.; Latofski Robles, M.; Garciadiego San Juan, M.d.M.; Marichal González, A.E. Comunicación y Bioseguridad Insular para la Gobernanza Ambiental de las Islas Mexicanas. In Hacia la Sostenibilidad en América Latina: Aportes Desde la Divulgación de la Ciencia; Mena-Young, M., Ed.; Red de Popularización de la Ciencia, la Tecnología y la Innovación en América Latina y el Caribe (RedPop) y Centro de Investigación en Comunicación (CICOM), Universidad de Costa Rica: San José, Costa Rica, 2019; pp. 81–101. [Google Scholar]
- Koleff, P.; Mendoza Alfaro, R.; Golubov, J.; González-Martínez, A.I.; Barrios-Caballero, Y.; De Jesús, S.D.J.; Ruiz-Utrilla, Z.P.; Méndez-Sánchez, F.; Latofski-Robles, M.; Garciadiego-San Juan, M.d.M.; et al. Invasive Alien Species in Mexico. In Invasive Alien Species; Wiley-Blackwell: Hoboken, NJ, USA, 2021; pp. 77–92. [Google Scholar]
- Aguirre Muñoz, A.; Latofski Robles, M.; Maldonado Flores, I.Y.; Marichal González, A.E. Litearatura, arte y Conservación Ambiental en las islas de México; Grupo de Ecología y Conservación de Islas, A.C.: Ensenada, Mexico, 2020; pp. 18–24. [Google Scholar]
- Born-Schmidt, G.; Servole, J.P.; Reyes-Gómez, V.; Hernández, E.R.; Chavira, E.A.; Barrientos, S.R.E. The Implementation of the Mexican Strategy on Invasive Species. In Invasive Alien Species; Wiley-Blackwell: Hoboken, NJ, USA, 2021; pp. 153–164. [Google Scholar]
- DIISE. The Database of Island Invasive Species Eradications. Available online: http://diise.islandconservation.org (accessed on 25 October 2021).
- Benkwitt, C.E.; Gunn, R.L.; Le Corre, M.; Carr, P.; Graham, N.A.J. Rat eradication restores nutrient subsidies from seabirds across terrestrial and marine ecosystems. Curr. Biol. 2021, 31, 2704–2711.e4. [Google Scholar] [CrossRef]
- Graham, N.A.J.; Wilson, S.K.; Carr, P.; Hoey, A.S.; Jennings, S.; MacNeil, M.A. Seabirds enhance coral reef productivity and functioning in the absence of invasive rats. Nature 2018, 559, 250–253. [Google Scholar] [CrossRef] [Green Version]
- Kurle, C.M.; Zilliacus, K.M.; Sparks, J.; Curl, J.; Bock, M.; Buckelew, S.; Williams, J.C.; Wolf, C.A.; Holmes, N.D.; Plissner, J.; et al. Indirect effects of invasive rat removal result in recovery of island rocky intertidal community structure. Sci. Rep. 2021, 11, 5395. [Google Scholar] [CrossRef]
- Reyes-Arriagada, R.; Schlatter, R.P.; Hodum, P.J.; Rozzi, R. Seabirds and Island Communities: Biodiversity Awareness as a Tool for the Conservation of Insular Species. In Seabirds and Songbirds; Mahala, G., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2015. [Google Scholar]
- McClelland, P.J.; Coote, R.; Trow, M.; Hutchins, P.; Nevins, H.M.; Adams, J.; Newman, J.; Moller, H. The Rakiura Titi Islands Restoration Project: Community action to eradicate Rattus rattus and Rattus exulans for ecological restoration and cultural wellbeing. In Island Invasives: Eradication and Management, Proceedings of the International Conference on Island Invasives, Auckland, New Zealand, 8–12 February 2010; Veitch, C.R., Clout, M.N., Towns, D.R., Eds.; Occasional Paper of the IUCN Species Survival Commission No. 42; IUCN: Gland, Switzerland; CBB: Auckland, New Zealand, 2011; pp. 451–454. [Google Scholar]
- Aguirre Muñoz, A.; Méndez Sánchez, F.; Latofski Robles, M.; Salizzoni Chávez, K. Mejoras en el marco legal para la conservación y restauración integral de las islas de México. In Reporte CESOP, Número 103, Febrero de 2017; Centro de Estudios Sociales y de Opinión Pública de la Cámara de Diputados: Mexico City, Mexico, 2017; pp. 19–23. [Google Scholar]
- Matos, J.; Little, A.; Broome, K.; Kennedy, E.; Méndez Sánchez, F.; Latofski-Robles, M.; Irvine, R.; Gill, C.; Espinoza, A.; Howald, G.; et al. Connecting island communities on a global scale: Case studies in island biosecurity. West. N. Am. Nat. 2018, 78, 959–972. [Google Scholar] [CrossRef]
- Millus, S.A.; Stapp, P. Interactions between seabirds and endemic deer mouse populations on Santa Barbara Island, California. Can. J. Zool. 2008, 86, 1031–1041. [Google Scholar] [CrossRef]
- Millus, S.A.; Stapp, P.; Martin, P. Experimental control of a native predator may improve breeding success of a threatened seabird in the California Channel Islands. Biol. Conserv. 2007, 138, 484–492. [Google Scholar] [CrossRef]
- Mallon, R. Naturalistic Approaches to Social Construction. Stanf. Encycl. Philos. 2019. Available online: https://plato.stanford.edu/archives/spr2019/entries/social-construction-naturalistic/ (accessed on 29 October 2021).



| Phase of Project | PNA Input | Technical Input |
|---|---|---|
| Detection of mice | Passive surveillance | PNA |
| Species identification | Transport and housing | GECI |
| Ecology of mice | Transport and housing | GECI |
| Feasibility and planning | PNA management board | GECI |
| Logistics | Transport and housing | GECI, SEMAR |
| Funding | In-kind support | GECI and external |
| Eradication | Ground baiting | GECI |
| Non-target mitigation | Transport and housing | GECI |
| Validation of absence | Transport and housing | GECI |
| Biosecurity | Active surveillance | GECI, CONANP |
| Item | Cost (USD) |
|---|---|
| Preparation and planning | 55,615.07 |
| Helicopter | 56,529.90 |
| Aerial bucket | 1 |
| Bait | 3191.30 2 |
| Boat expenses | 110,000.00 3 |
| Staff | 194,067.71 |
| Food, travel, fuel, materials | 165,403.15 |
| Lodging and air and ground transportation | 36,202.58 |
| Field equipment and materials | 38,046.44 |
| Total | 659,056.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Méndez Sánchez, F.; Aguirre-Muñoz, A.; Samaniego, A.; Bedolla Guzmán, Y.; Cárdenas Tapia, A.; Rojas Mayoral, E.; Latofski Robles, M.; Koleff, P.; Castellanos Vera, A.; Arnaud Franco, G.; et al. Involvement of a Fishing Community in the Eradication of the Introduced Cactus Mouse (Peromyscus eremicus cedrosensis) from San Benito Oeste Island, Mexico. Diversity 2021, 13, 588. https://doi.org/10.3390/d13110588
Méndez Sánchez F, Aguirre-Muñoz A, Samaniego A, Bedolla Guzmán Y, Cárdenas Tapia A, Rojas Mayoral E, Latofski Robles M, Koleff P, Castellanos Vera A, Arnaud Franco G, et al. Involvement of a Fishing Community in the Eradication of the Introduced Cactus Mouse (Peromyscus eremicus cedrosensis) from San Benito Oeste Island, Mexico. Diversity. 2021; 13(11):588. https://doi.org/10.3390/d13110588
Chicago/Turabian StyleMéndez Sánchez, Federico, Alfonso Aguirre-Muñoz, Araceli Samaniego, Yuliana Bedolla Guzmán, Ana Cárdenas Tapia, Evaristo Rojas Mayoral, Mariam Latofski Robles, Patricia Koleff, Aradit Castellanos Vera, Gustavo Arnaud Franco, and et al. 2021. "Involvement of a Fishing Community in the Eradication of the Introduced Cactus Mouse (Peromyscus eremicus cedrosensis) from San Benito Oeste Island, Mexico" Diversity 13, no. 11: 588. https://doi.org/10.3390/d13110588
APA StyleMéndez Sánchez, F., Aguirre-Muñoz, A., Samaniego, A., Bedolla Guzmán, Y., Cárdenas Tapia, A., Rojas Mayoral, E., Latofski Robles, M., Koleff, P., Castellanos Vera, A., Arnaud Franco, G., Beltrán Morales, L. F., & Ortega-Rubio, A. (2021). Involvement of a Fishing Community in the Eradication of the Introduced Cactus Mouse (Peromyscus eremicus cedrosensis) from San Benito Oeste Island, Mexico. Diversity, 13(11), 588. https://doi.org/10.3390/d13110588







