Anthropogenic Food Utilization and Seasonal Difference in Diet of Cercopithecus lowei at a Community Protected Forest in Ghana
Abstract
:1. Introduction
2. Materials and Methods
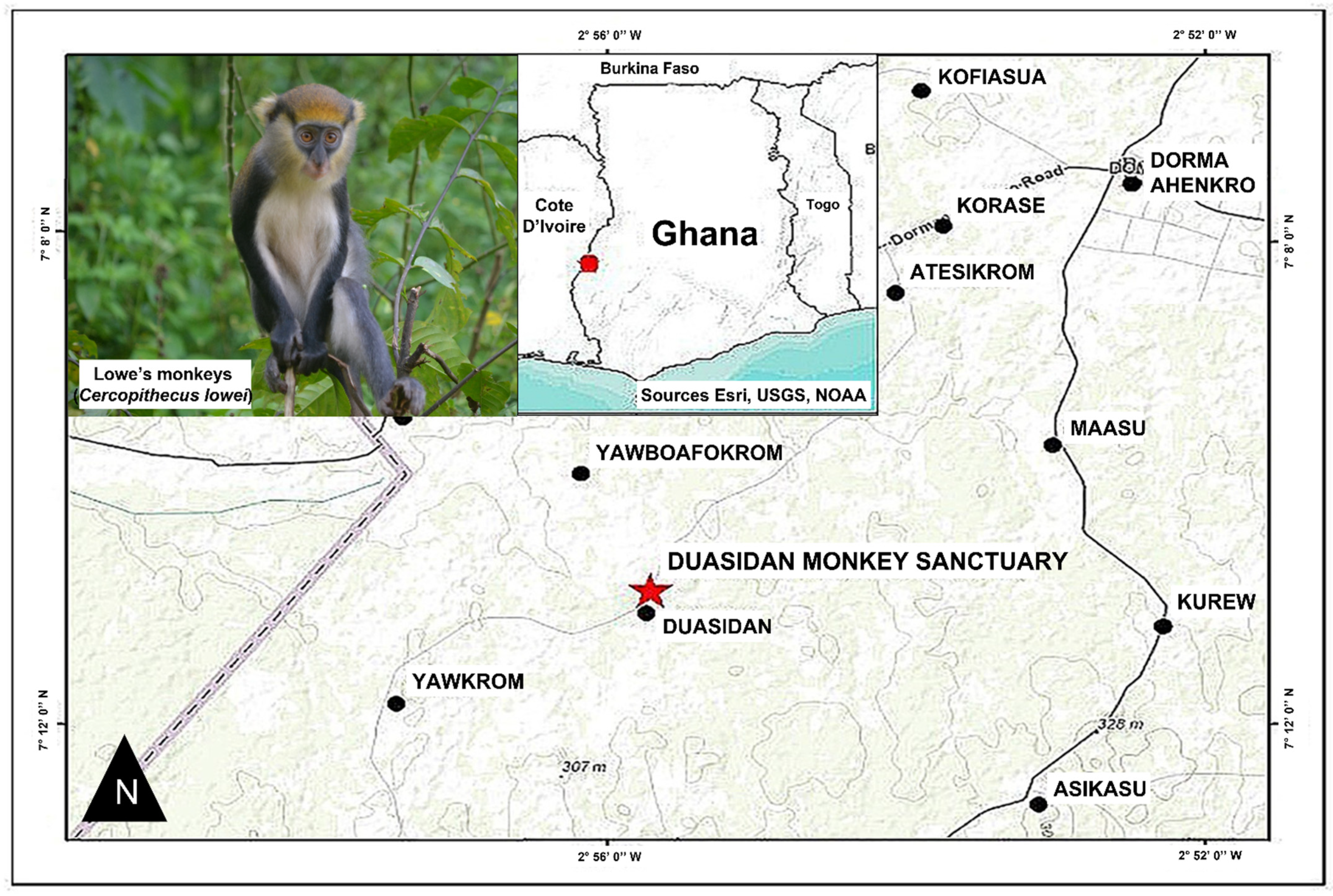
2.1. Study Area
2.2. Data Collection
2.3. Data Analysis
3. Results
3.1. Activity Budgets
3.2. Food Categories
3.2.1. Plants Consumed
3.2.2. Anthropogenic Food Type Consumed
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- White, T.C.R. The importance of a relative shortage of food in animal ecology. Oecologia 1978, 33, 71–86. [Google Scholar] [CrossRef]
- Hill, D.A. Seasonal variation in the feeding behavior and diet of Japanese macaques (Macaca fuscata yakui) in lowland forest of Yakushima. Am. J. Primatol. 1997, 43, 305–320. [Google Scholar] [CrossRef]
- Stone, A.I. Responses of squirrel monkeys to seasonal changes in food availability in an eastern Amazonian Forest. Am. J. Primatol. 2007, 69, 142–157. [Google Scholar] [CrossRef] [PubMed]
- Bateman, P.W.; Fleming, P.A. Big city life: Carnivores in urban environments. J. Zool. 2012, 287, 1–23. [Google Scholar] [CrossRef]
- McKinney, T. A classification system for describing anthropogenic influence on nonhuman primate populations. Am. J. Primatol. 2015, 77, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Altmann, J.; Muruthi, P. Differences in daily life between semiprovisioned and wild-feeding baboons. Am. J. Primatol. 1988, 15, 213–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLennan, M.R.; Spagnoletti, N.; Hockings, K. The Implications of Primate Behavioral Flexibility for Sustainable Human–Primate Coexistence in Anthropogenic Habitats. Int. J. Primatol. 2017, 38, 105–121. [Google Scholar] [CrossRef]
- Sengupta, A.; McConkey, K.R.; Radhakrishna, S. Primates, Provisioning and Plants: Impacts of Human Cultural Behaviours on Primate Ecological Functions. PLoS ONE 2015, 10, e0140961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKinney, T. The effects of provisioning and crop-raiding on the diet and foraging activities of human-commensal white-faced Capuchins (Cebus capucinus). Am. J. Primatol. 2010, 73, 439–448. [Google Scholar] [CrossRef]
- Lawson, B.; Robinson, R.A.; Toms, M.P.; Risely, K.; Macdonald, S.; Cunningham, A.A. Health hazards to wild birds and risk factors associated with anthropogenic food provisioning. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170091. [Google Scholar] [CrossRef]
- Plaza, P.I.; Lambertucci, S.A. How are garbage dumps impacting vertebrate demography, health, and conservation? Glob. Ecol. Conserv. 2017, 12, 9–20. [Google Scholar] [CrossRef]
- Baulu, J.; Everard, C.O.R.; Everard, J.D. Leptospires in vervet monkeys (Cercopithecus aethiops sabaeus) on barbados. J. Wildl. Dis. 1987, 23, 60–66. [Google Scholar] [CrossRef]
- Erdozain, G.; KuKanich, K.; Chapman, B.; Powell, D. Best Practices for Planning Events Encouraging Human-Animal Interactions. Zoonoses Public Health 2014, 62, 90–99. [Google Scholar] [CrossRef]
- Rogers, L.L. Black Bears, People, and Garbage Dumps in Minnesota. In Bearhuman Conflicts: Proceedings of a Symposium on Management Strategies; Bromley, M., Ed.; Northwest Territories Department of Renewable Resources, 1989; Available online: http://www.bearsmart.com/docs/Black_Bears_People_and_Garbarge_Dumps_in_Minnesota.pdf (accessed on 5 January 2020).
- Houston, D.C.; Mee, A.; McGrady, M. Why do condors and vultures eat junk? The implications for conservation. J. Raptor Res. 2007, 41, 235–238. [Google Scholar] [CrossRef]
- Saj, T.; Sicotte, P.; Paterson, J.D. Influence of Human Food Consumption on the Time Budget of Vervets. Int. J. Primatol. 1999, 20, 977–994. [Google Scholar] [CrossRef]
- Bino, G.; Dolev, A.; Yosha, D.; Guter, A.; King, R.; Saltz, D.; Kark, S. Abrupt spatial and numerical responses of overabundant foxes to a reduction in anthropogenic resources. J. Appl. Ecol. 2010, 47, 1262–1271. [Google Scholar] [CrossRef]
- Chamberlain, D.E.; Vickery, J.A.; Glue, D.E.; Robinson, R.A.; Conway, G.J.; Woodburn, R.J.W.; Cannon, A.R. Annual and seasonal trends in the use of garden feeders by birds in winter. Ibis 2005, 147, 563–575. [Google Scholar] [CrossRef]
- Higham, J.P.; Warren, Y.; Adanu, J.; Umaru, B.N.; MacLarnon, A.M.; Sommer, V.; Ross, C. Living on the edge: Life-history of olive baboons at Gashaka-Gumti National Park, Nigeria. Am. J. Primatol. 2009, 71, 293–304. [Google Scholar] [CrossRef]
- Strum, S.C. The Development of Primate Raiding: Implications for Management and Conservation. Int. J. Primatol. 2010, 31, 133–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, L.L. Effects of Food Supply and Kinship on Social Behavior, Movements, and Population Growth of Black Bears in Northeastern Minnesota. Wildl. Monogr. 1987, pp. 3–72. Available online: https://www.jstor.org/stable/3830545 (accessed on 5 January 2020).
- Stillfried, M.; Gras, P.; Busch, M.; Börner, K.; Kramer-Schadt, S.; Ortmann, S. Wild inside: Urban wild boar select natural, not anthropogenic food resources. PLoS ONE 2017, 12, e0175127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, L.L.; Kuehn, D.W.; Erickson, A.W.; Harger, E.M.; Verme, L.J.; Ozoga, J.J. Characteristics and management of black bears that feed in garbage dumps, campgrounds or residential areas. Bears Biol. Manag. 1976, 3, 169–175. [Google Scholar] [CrossRef]
- Wiafe, E.; Oates, J.F.; Gonedelé Bi, S.; Koné, I.; Matsuda Goodwin, R.; Osei, D. Cercopithecus lowei, The IUCN Red List of Threatened Species 2019: e.T136931A92373680. Available online: https://dx.doi.org/10.2305/IUCN.UK.2019-1.RLTS.T136931A92373680.en (accessed on 27 September 2021).
- Bourliere, F.; Hunkeler, C.; Bertrand, M. Ecology and behaviour of Lowe’s Guenon (Cercopithecus lowei) in Ivory Coast. In Old World Monkeys: Evolution, Systemaics and Behaviour; Napier, J.R., Napier, P.H., Eds.; Academic Press: London, UK, 1970; pp. 297–350. [Google Scholar]
- Kingdon, J. The Kingdon Field Guide to African Mammals; Academic Press: London, UK, 1997. [Google Scholar]
- Wiafe, E.D. Population studies of Lowe’s monkeys (Cercopithecus lowei Thomas, 1923) in Kakum Conservation Area, Ghana. J. Threat. Taxa 2016, 8, 8434–8442. [Google Scholar] [CrossRef] [Green Version]
- Barrett, L.; Dunbar, R.I.; Dunbar, P. Environmental influences on play behaviour in immature gelada baboons. Anim. Behav. 1992, 44, 111–115. [Google Scholar] [CrossRef]
- Norscia, I.; Carrai, V.; Borgognini-Tarli, S.M. Influence of Dry Season and Food Quality and Quantity on Behavior and Feeding Strategy of Propithecus verreauxi in Kirindy, Madagascar. Int. J. Primatol. 2006, 27, 1001–1022. [Google Scholar] [CrossRef]
- Biodiversity Monitoring Unit: Biodiversity Monitoring at Duasidan Monkey Sanctuary; Wildlife Division, Forestry Commission: Accra, Ghana, 2002.
- Sillero-Zubiri, C.; Switzer, D. Crop Raiding Primates: Searching for Alternative, Humane Ways to Resolve Conflict with Farmers in Africa; Wildlife Conservation Research Unit, Oxford University: Oxford, UK, 2001. [Google Scholar]
- Olaleru, F.; Ogunjemite, B.G.; Onadeko, A.B.; Egonmwan, R.I. Seasonal Availability and Nutrient Contents of Mona Monkey (Cercopithecus mona Schreber, 1774) Plant Diets in Lekki Conservation Centre, Nigeria. Munis Entomol. Zool. 2018, 13, 574–582. Available online: https://www.researchgate.net/publication/325880916 (accessed on 5 January 2020).
- Ejidike, B.N.; Salawu, A. Food and Feeding Habits of Mona Monkey Cercopithecus Mona in Ayede/Isan Forest Reserve, Ekiti State. Journal of Research in Forestry. Wildl. Environ. 2009, 1, 56–59. [Google Scholar]
- Mangama-Koumba, L.B.; Ella, G.W.E.; Akomo-Okoue, E.F.; Nguelet, F.L.M.; M’Batchi, B.; Mavoungou, J.F. Vegetarian diet in Guenon and Mangabey monkeys of Moukalaba-Doudou National Park, Gabon: Similarities and differences. Int. J. Biol. Chem. Sci. 2017, 10, 2435. [Google Scholar] [CrossRef] [Green Version]
- Ejidike, B.N.; Okosodo, E.F. Food and feeding habits of the thicktailed galago (Otelemur crassicaudatus) in Okomu National Park, Edo state. J. Fish. Int. 2007, 2, 231–233. [Google Scholar]
- Katlam, G.; Prasad, S.; Aggarwal, M.; Kumar, R. Trash on the menu: Patterns of animal visitation and foraging behaviour at garbage dumps. Curr. Sci. 2018, 115, 2322–2326. [Google Scholar] [CrossRef]
- Lee, P.C.; Brennan, E.J.; Else, J.G.; Altmann, J. Ecology and behavior of vervet monkeys in a tourist lodge habitat. In Primate Ecology and Conservation; Else, J.G., Lee, P.C., Eds.; Cambridge University Press: Cambridge, UK, 1986; pp. 229–235. [Google Scholar]
- Masau, J.M.; Strum, S.C. Response of wild baboon troops to incursion of agriculture at Gilgil, Kenya. Int. J. Primatol. 1984, 5, 364. [Google Scholar]
- Malik, I. Time budgets and activity patterns in free-ranging rhesus monkeys. In Primate Ecology and Conservation; Else, J.G., Lee, P.C., Eds.; Cambridge University Press: Cambrdige, UK, 1986; pp. 105–114. [Google Scholar]
- Pharis, R.P. Seasonal fluctuation in the foliage-moisture content of well-watered conifers. Bot. Gaz. 1967, 128, 179–185. [Google Scholar] [CrossRef]
- Milton, K. Micronutrient intakes of wild primates: Are humans different? Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2003, 136, 47–59. [Google Scholar] [CrossRef]
- Grueter, C.C.; Li, D.; Ren, B.; Wei, F.; Xiang, Z.; van Schaik, C.P. Fallback foods of temperate-living primates: A case study on snub-nosed monkeys. Am. J. Phys. Anthr. 2009, 140, 700–715. [Google Scholar] [CrossRef] [PubMed]
- Hanya, G.; Bernard, H. Fallback Foods of Red Leaf Monkeys (Presbytis rubicunda) in Danum Valley, Borneo. Int. J. Primatol. 2012, 33, 322–337. [Google Scholar] [CrossRef] [Green Version]
- Poulsen, J.R.; Clark, C.J.; Smith, T.B. Seasonal variation in the feeding ecology of the grey-cheeked mangabey (Lophocebus albigena) in Cameroon. Am. J. Primatol. 2001, 54, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Ndon, B.A. Some Morphological and Chemical Characteristics of Developing Fruits of Raphia hookeri. J. Exp. Bot. 1985, 36, 1817–1830. [Google Scholar] [CrossRef]
- Oluyori, A.; Dada, A.O.; Inyinbor, A. Phytochemical Analysis and Antioxidant Potential of Raphia hookeri leaf and Epicarp. Orient. J. Chem. 2018, 34, 2742–2746. [Google Scholar]
- Profizi, J.P. Swampy area transformations by exploitation of Raphia hookeri (Arecaceae) in southern Benin (West Africa). Hum. Ecol. 1988, 16, 87–94. [Google Scholar] [CrossRef]
- Udofia, S.I.; Oworen, U.I. Conservation status of Raphia hookeri Mann & Wendil in homegardens of Ika Local Government Area, Akwa Ibom State, Nigeria. Niger. J. Agric. Food Environ. 2018, 13, 170–175. [Google Scholar]
- Takenoshita, Y.; Ando, C.; Yamagiwa, J. Fruit phenology of the great ape habitat in the Moukalaba-Doudou National Park, Gabon. Afr. Study Monogr. 2008, 39, 23–39. [Google Scholar] [CrossRef]
- Bello, M.O.; Abdul-Hammed, M.; Ogunbeku, P. Nutrient and anti-nutrient phytochemicals in Ficus exasperata Vahl leaves. Int. J. Sci. Eng. Res. (IJSER) 2014, 5, 2177–2181. [Google Scholar]
- Bello, M.O.; Abdul-Hammed, M.; Adepoju, A.J.; Esan, O.A.; Tiamiyu, A.A. Nutritional Composition and fatty acids profile of Ficus exasperata fruit and fruit oil. J. Nat. Sci. Res. 2014, 4, 25–29. [Google Scholar]
- Oro, D.; Genovart, M.; Tavecchia, G.; Fowler, M.S.; Martínez-Abraín, A. Ecological and evolutionary implications of food subsidies from humans. Ecol. Lett. 2013, 16, 1501–1514. [Google Scholar] [CrossRef] [PubMed]
- Sabbatini, G.; Stammati, M.; Tavares, M.C.H.; Giuliani, M.V.; Visalberghi, E. Interactions between humans and capuchin monkeys (Cebus libidinosus) in the Parque Nacional de Brasília, Brazil. Appl. Anim. Behav. Sci. 2006, 97, 272–283. [Google Scholar] [CrossRef]
- Macdonald, D.W.; Boitani, L.; Barrasso, P. Foxes, wolves and conservation in the Abruzzo mountains. In The Red Fox; Springer: Dordrecht, The Netherlands, 1980; pp. 223–235. [Google Scholar] [CrossRef]
- Marty, P.R.; Balasubramaniam, K.N.; Kaburu, S.S.K.; Hubbard, J.; Beisner, B.; Bliss-Moreau, E.; Ruppert, N.; Arlet, M.E.; Sah, S.A.M.; Ismail, A.; et al. Individuals in urban dwelling primate species face unequal benefits associated with living in an anthropogenic environment. Primates 2019, 61, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, F.; Rasmussen, M.H.; Lusseau, D. Inferring activity budgets in wild animals to estimate the consequences of disturbances. Behav. Ecol. 2013, 24, 1415–1425. [Google Scholar] [CrossRef] [Green Version]
- Bejder, L. Linking Short and Long-Term Effects of Nature-Based Tourism on Cetaceans. Ph.D. Dissertation, Dalhousie University, Halifax, NS, Canada, 2005. [Google Scholar]
- Martina, A.; Gallarati, M. Use of a Garbage Dump by Some Mammal Species in the Majella Massif (Abruzzo, Italy). Hystrix Ital. J. Mammal. 1997, 9, pp. 1–2. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.616.1908andrep=rep1andtype (accessed on 5 January 2020).
- Conniff, R. Unnatural Balance: How Food Waste Impacts World’s Wildlife. Yale Environ. 2016. Yale Environment 360. Available online: https://e360.yale.edu/features/unnatural_balance_how_food_waste_impacts_world (accessed on 15 April 2020).
- Wiafe, E.D.; Arku, F.S. Victims’ Perspectives of Lowe’s Monkeys’ (Cercopithecus campbelli lowei) crop raiding events in Ghana: A case of Boabeng-Fiema Monkey Sanctuary. J. Biodivers. Environ. Sci. 2012, 2, 1–8. [Google Scholar]



| Species | Dry Season | Wet Season | ||
|---|---|---|---|---|
| Number of Feeding Observations | Preference Indices | Number of Feeding Observations | Preference Indices | |
| Bombax buonopozense | 2 | 0.39 * | - | - |
| Ceiba pentandra | 8 | 0.78 * | 13 | 1.26 * |
| Celtis milbraedii | 3 | 0.84 * | - | - |
| Cola gigantean | 5 | 2.44 ** | 7 | 3.42 ** |
| Dacryodes klaineana | 1 | 0.19 * | - | - |
| Discoglypremna caloneura | 4 | 1.56 * | 3 | 1.17 * |
| Ficus exasperata | 8 | 3.13 ** | 14 | 5.47 *** |
| Mangnifera indica | 7 | 2.27 ** | 6 | 1.95 ** |
| Milicia excelsa | 3 | 0.97 * | 2 | 0.65 * |
| Pycnanthus angolensis | 10 | 1.95 ** | 15 | 2.92 ** |
| Raphia hookeri | 9 | 1.75 * | 12 | 2.34 ** |
| Spondias mombin | 1 | 0.39 * | - | - |
| Terminalia ivorensis | 2 | 0.19 * | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bempah, G.; Lu, C.; Yi, Y. Anthropogenic Food Utilization and Seasonal Difference in Diet of Cercopithecus lowei at a Community Protected Forest in Ghana. Diversity 2021, 13, 610. https://doi.org/10.3390/d13120610
Bempah G, Lu C, Yi Y. Anthropogenic Food Utilization and Seasonal Difference in Diet of Cercopithecus lowei at a Community Protected Forest in Ghana. Diversity. 2021; 13(12):610. https://doi.org/10.3390/d13120610
Chicago/Turabian StyleBempah, Godfred, Changhu Lu, and Yoonjung Yi. 2021. "Anthropogenic Food Utilization and Seasonal Difference in Diet of Cercopithecus lowei at a Community Protected Forest in Ghana" Diversity 13, no. 12: 610. https://doi.org/10.3390/d13120610






