Diversity of Ruderal Communities in Urban Environments—A Case Study from Serbia (SE Europe)
Abstract
:1. Introduction
2. Materials and Methods
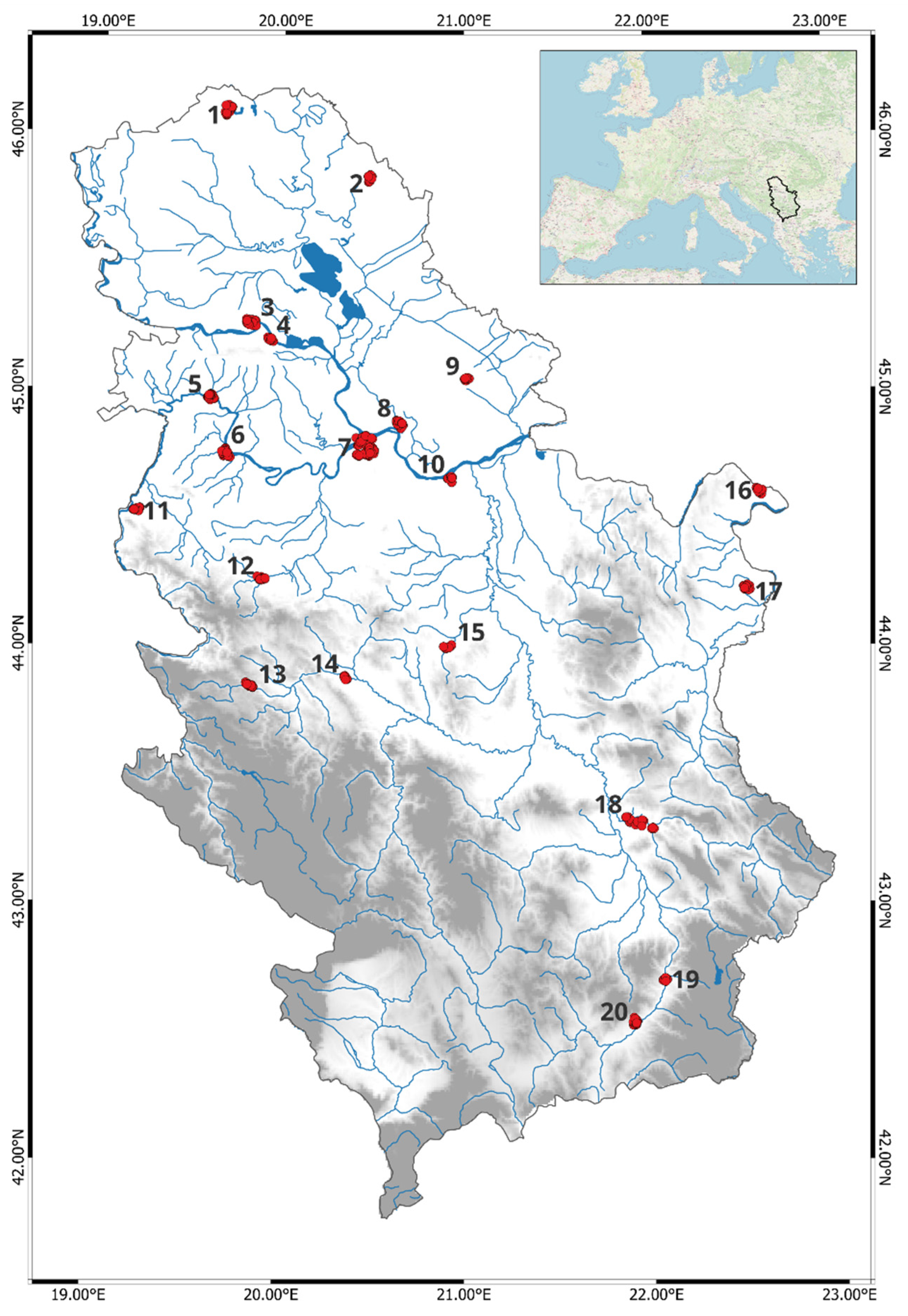
2.1. Study Area and Data Sampling
2.2. Data Analysis
3. Results and Discussion
3.1. Results of the Cluster and Ordination Analysis
3.2. General and Floristic Characteristics of Ruderal Communities
3.3. Characteristics of Plant Communities
3.4. Syntaxonomical Scheme
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Concepción, E.; Obrist, M.; Moretti, M.; Altermatt, F.; Baur, B.; Nobis, M. Impacts of urban sprawl on species richness of plants, butterflies, gastropods and birds: Not only built-up area matters. Urban. Ecosyst. 2016, 19, 225–242. [Google Scholar] [CrossRef]
- Lososová, Z.; Chytrý, M.; Kühn, I.; Hájek, O.; Horaková, V.; Pyšek, P.; Tichý, L. Patterns of plant traits in annual vegetation of man-made habitats in central Europe. Perspect. Plant. Ecol. Evol. Syst. 2006, 8, 69–81. [Google Scholar] [CrossRef]
- Šilc, U. Synanthropic vegetation: Pattern of various disturbances on life history traits. Acta Bot. Croat. 2010, 69, 215–227. [Google Scholar]
- Jovanović, S. Ekološka Studija Ruderalne Flore i Vegetacije Beograda [Ecological Study of Ruderal Flora and Vegetation in the City of Belgrade]; Faculty of Biology, University of Belgrade: Belgrade, Serbia, 1994. (In Serbian) [Google Scholar]
- Sukopp, H. Human-caused impact on preserved vegetation. Landsc. Urban Plan. 2004, 68, 347–355. [Google Scholar] [CrossRef]
- Šilc, U.; Košir, P. Synanthropic vegetation of the city of Kranj (central Slovenia). Hacquetia 2006, 5, 213–231. [Google Scholar]
- Rendeková, A. Overview of ruderal plant communities of Malacky city. Acta Bot. Univ. Comen. 2016, 51, 31–50. [Google Scholar]
- Altay, V.; Šilc, U.; Yarcı, C.; Kavgacı, A.; Čarni, A.; Ozturk, M. Urban vegetation of the Anatolian side of Istanbul. Phytocoenologia 2020, 50, 101–121. [Google Scholar] [CrossRef]
- Pyšek, P.; Chocholousková, Z.; Pyšek, A.; Jarošík, V.; Chytrý, M.; Tichý, L. Trends in species diversity and composition of urban vegetation over three decades. J. Veg. Sci. 2004, 15, 781–788. [Google Scholar] [CrossRef]
- Lososová, Z.; Simonová, D. Changes during the 20th century in species composition of synanthropic vegetation in Moravia (Czech Republic). Preslia 2008, 80, 291–305. [Google Scholar]
- Rendeková, A.; Mičieta, K. Changes in the representation of alien taxa in ruderal vegetation of an urban ecosystem over 50 years. A case study from Malacky city, Slovakia, Central Europe. Urban. Ecosyst. 2017, 20, 867–875. [Google Scholar] [CrossRef]
- Rendeková, A. Zmeny v spektre ruderálnych spoločenstiev Bratislavy po tridsiatich rokoch [Changes in spectrum of ruderal communities of Bratislava after thirty years]. Bull. Slov. Bot. Spoločn 2015, 37, 21–32. (In Slovakian) [Google Scholar]
- Vilà, M.; Pino, J.; Font, X. Regional assessment of plant invasions across different habitat types. J. Veg. Sci. 2007, 18, 35–42. [Google Scholar] [CrossRef]
- Sîrbu, C.; Oprea, A.; Samuil, C.; Tănase, C. Neophyte Invasion in Moldavia (Eastern Romania) in Different Habitat Types. Folia Geobot. 2012, 47, 215–229. [Google Scholar] [CrossRef]
- Medvecká, J.; Jarolímek, I.; Senko, D.; Svitok, M. Fifty years of plant invasion dynamics in Slovakia along a 2500 m altitudinal gradient. Biol. Invasions 2014, 16, 1627–1638. [Google Scholar] [CrossRef]
- Küzmič, F.; Šilc, U. Alien species in different habitat types of Slovenia: Analysis of vegetation database. Period. Biol. 2017, 119, 199–208. [Google Scholar] [CrossRef]
- Viciani, D.; Vidali, M.; Gigante, D.; Bolpagni, R.; Villani, M.; Acosta, A.; Adorni, M.; Aleffi, M.; Allegrezza, M.; Angiolini, C.; et al. A first checklist of the alien-dominated vegetation in Italy. Plant. Sociol. 2020, 57, 29–54. [Google Scholar] [CrossRef]
- Simonová, D.; Lososová, Z. Which factors determine plant invasions in man-made habitats in the Czech Republic? Perspect. Plant. Ecol. Evol. Syst. 2008, 10, 89–100. [Google Scholar] [CrossRef]
- Pyšek, P. Alien and native species in Central European urban floras: A quantitative comparison. J. Biogeogr. 1998, 25, 155–163. [Google Scholar] [CrossRef]
- Rendeková, A.; Miškovic, J.; Mičieta, K. Spoločenstvá inváznych neofytov zväzu Senecionion fluviatilis R. Tx. 1950 v ruderálnej vegetácii Bratislavy a ich biodiverzita [The communities of invasive neophytes from alliance Senecionion fluviatilis R. Tx. 1950 in the ruderal vegetation of Bratislava and their biodiversity]. Acta Univ. Matthiae Belii Ser. Environ. Manag. 2017, 19, 39–54. (In Slovakian) [Google Scholar] [CrossRef]
- Jovanović, S.; Glišić, M. An analysis of research into urban flora and vegetation in Southeast Europe. Acta Bot. Croat. 2021, 80, 74–81. [Google Scholar] [CrossRef]
- Rat, M.M.; Gavrilović, M.; Radak, B.; Bokić, B.; Jovanović, S.; Božin, B.; Boža, P.; Anačkov, G. Urban flora in the Southeast Europe and its correlation with urbanization. Urban. Ecosyst. 2017, 20, 811–822. [Google Scholar] [CrossRef]
- Ducić, V.; Radovanović, M. Klima Srbije [Climate of Serbia]; Zavod za Udžbenike i Nastavna Sredstva: Belgrade, Serbia, 2005. (In Serbian) [Google Scholar]
- Braun-Blanquet, J. Pflanzensoziologie: Grundzüge der Vegetationskunde [Phytosociology: Basics of Vegetation Science], 3rd ed.; Springer: Wien, Austria, 1964; (In German). [Google Scholar] [CrossRef]
- Dengler, J. Entwicklung und Bewertung Neuer Ansätze in der Pflanzensoziologie Unter Besonderer Berücksichtigung der Vegetationsklassifikation [Development and Evaluation of New Approaches in Phytosociology with Special Regard to Vegetation Classification]; Martina Galunder-Verlag: Nümbrecht, Germany, 2003; Volume 14. (In German) [Google Scholar]
- Chytry, M.; Otýpková, Z. Plot sizes used for phytosociological sampling of European vegetation. J. Veg. Sci. 2003, 14, 563–570. [Google Scholar] [CrossRef]
- McCune, B.; Mefford, M.J. PC-ORD. Multivariate Analysis of Ecological Data; Version 6.08; MjM Software: Gleneden Beach, OR, USA, 2011. [Google Scholar]
- Tichý, L. JUICE, software for vegetation classification. J. Veg. Sci. 2002, 13, 451–453. [Google Scholar] [CrossRef]
- Chytrý, M.; Tichý, L.; Holt, J.; Botta-Dukát, Z. Determination of diagnostic species with statistical fidelity measures. J. Veg. Sci. 2002, 13, 79–90. [Google Scholar] [CrossRef]
- Pignatti, S. Valori di Bioindicazione delle Piante Vascolari della Flora d’Italia [Indicator values of the vascular plants of the flora of Italy]. Braun-Blanquetia 2005, 39, 1–97. (In Italian) [Google Scholar]
- Šilc, U.; Vrbničanin, S.; Božić, D.; Čarni, A.; Dajić Stevanović, Z. Phytosociological alliances in the vegetation of arable fields in the northwestern Balkan Peninsula. Phytocoenologia 2008, 38, 241–254. [Google Scholar] [CrossRef]
- Šilc, U.; Vrbničanin, S.; Božić, D.; Čarni, A.; Dajić Stevanović, Z. Alien plant species and factors of invasiveness of anthropogenic vegetation in the Northwestern Balkans-A phytosociological approach. Cent. Eur. J. Biol. 2012, 7, 720–730. [Google Scholar] [CrossRef] [Green Version]
- Domina, G.; Galasso, G.; Bartolucci, F.; Guarino, R. Ellenberg Indicator Values for the vascular flora alien to Italy. Fl. Medit. 2018, 28, 53–61. [Google Scholar] [CrossRef]
- ter Braak, C.; Šmilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination (Version 5.0); Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- The Euro+Med Plantbase-The Information Resource for Euro-Mediterranean Plant Diversity. Available online: http://ww2.bgbm.org/EuroPlusMed/query.asp (accessed on 14 October 2021).
- Mucina, L.; Bültmann, H.; Dierßen, K.; Theurillat, J.-P.; Raus, T.; Čarni, A.; Šumberová, K.; Willner, W.; Dengler, J.; Gavilán García, R.; et al. Vegetation of Europe: Hierarchical floristic classification system of vascular plant, bryophyte, lichen, and algal communities. Appl. Veg. Sci. 2016, 19, 3–264. [Google Scholar] [CrossRef]
- QGIS: A Free and Open Source Geographic Information System. Available online: https://www.qgis.org/en/site/ (accessed on 14 October 2021).
- Cvijanović, D.; Lakušić, D.; Živković, M.; Novković, M.; Anđelković, A.; Pavlović, D.; Vukov, D.; Radulović, S. An overview of aquatic vegetation in Serbia. Tuexenia 2018, 38, 269–286. [Google Scholar] [CrossRef]
- Pellizzari, M. Cyperus-dominated vegetation in the eastern Po river. Plant. Sociol. 2020, 57, 1–16. [Google Scholar] [CrossRef]
- Lakušić, D.; Blaženčić, J.; Ranđelović, V.; Butorac, B.; Vukojičić, S.; Zlatković, B.; Jovanović, S.; Šinžar-Sekulić, J. Fitocenoze Srbije –Baza Podataka [Phytocoenosis of Serbia—Database]; Institute of Botany and Botanical Garden “Jevremovac”, Faculty of Biology, University of Belgrade: Belgrade, Serbia, 2005. (In Serbian) [Google Scholar]
- Šajinović, B. Ekološko-Fitocenološka Analiza Ruderalne Vegetacije Okoline Novog Sada [Ecological-Phytocoenological Analysis of Ruderal Vegetation in Novi Sad Surroundings]. Master’s Thesis, Faculty of science and mathematics, University of Belgrade, Belgrade, Serbia, 1968. (In Serbian). [Google Scholar]
- Milinčić, D. Ekološko-Fitogeografske Karakteristike Ruderalne Flore Kosovske Mitrovice [Ecological and Phytogeographical Characteristics of Ruderal Flora of Kosovska Mitrovica]. Master’s Thesis, Faculty of Biology, University of Belgrade, Belgrade, Serbia, 1998. (In Serbian). [Google Scholar]
- Ranđelović, V. Nitrofilna vegetacija klase Bidentetea tripartiti Tx., Lohm. et Prsg. 1950. u Leskovačkom polju [Nitrophilous vegetation of the class Bidentetea tripartiti Tx., Lohm. et Prsg. 1950. in Leskovačko polje]. Leskovački Zb. 1992, 3, 209–223. (In Serbian) [Google Scholar]
- Šumberová, K. MBA Bidention tripartitae. In Vegetace České republiky 3. Vodní a Mokřadní Vegetace [Vegetation of the Czech Republic 3. Aquatic and Wetland Vegetation]; Chytrý, M., Ed.; Academia: Prague, Czech Republic, 2011; pp. 349–371. (In Czech) [Google Scholar]
- Jarić, S.; Mitrović, M.; Vrbničanin, S.; Karadžić, B.; Đurđević, L.; Kostić, O.; Mačukanović-Jocić, M.; Gajić, G.; Pavlović, P. A contribution to studies of the ruderal vegetation of Southern Srem, Serbia. Arch. Biol. Sci. 2011, 63, 1181–1197. [Google Scholar] [CrossRef]
- Stanković, V. Ekološka Studija Invazivnih Biljnih Vrsta u Ramsarskim Područjima Vojvodine [Ecological Study of Invasive Plants in the Ramsar Sites of Vojvodina]. Ph.D. Thesis, Faculty of Biology, University of Belgrade, Belgrade, Serbia, 2018. (In Serbian). [Google Scholar]
- Goncharenko, I.; Yatsenko, H. Phytosociological study of the forest vegetation of Kyiv urban area (Ukraine). Hacquetia 2020, 19, 99–126. [Google Scholar] [CrossRef] [Green Version]
- Dziuba, T.; Melnik, R.; Shevera, M. A new association Phragmito australis-Amorphetum fruticosae ass. nova. prov. on the south of Ukraine. In Proceedings of the IX International Conference Anthropization and Environment of Rural Settlements, Flora and Vegetation, Kamyanets-Podilskiy & Boyany, Kyiv, Ukraine, 29 June–1 July 2010; M.G. Kholodny Institute of Botany, NAS of Ukraine: Kyiv, Ukraine, 2010; p. 25. [Google Scholar]
- Mucina, L. Galio-Urticetea. In Die Pflanzengesellschaften Österreichs, Anthropogene Vegetation [The Plant Communities of Austria, Anthropogenic Vegetation]; Mucina, L., Grabherr, G., Ellmauer, T., Eds.; Gustav Fischer Verlag: Jena, Germany, 1993; pp. 203–251. (In German) [Google Scholar]
- Lániková, D. XDE Aegopodion podagrariae Tüxen 1967. In Vegetace České republiky. 2. Ruderální, Plevelová, Skalní a Suťová Vegetace [Vegetation of the Czech Republic 2. Ruderal, Weed, Rock and Scree Vegetation]; Chytrý, M., Ed.; Academia: Prague, Czech Republic, 2009; pp. 348–375. (In Czech) [Google Scholar]
- Obratov-Petković, D.; Bjedov, I.; Skočajić, D.; Đunisijević-Bojović, D.; Đukić, M.; Grbić, M. Asteretum lanceolati: Xenospontaneous community on wet and riparian habitats. Bull. Fac. For. 2011, 103, 73–92. [Google Scholar] [CrossRef]
- Jovanović, S.; Hlavati-Širka, V.; Lakušić, D.; Jogan, N.; Nikolić, T.; Anastasiu, P.; Vladimirov, V.; Šinžar-Sekulić, J. Reynoutria niche modelling and protected area prioritization for restoration and protection from invasion: A Southeastern Europe case study. J. Nat. Conserv. 2018, 41, 1–15. [Google Scholar] [CrossRef]
- Dengler, J.; Eisenberg, M.; Schröder, J. Die grundwasserfernen Saumgesellschaften Nordostniedersachsens im europäischen Kontext–Teil II: Säume nährstoffreicher Standorte (Artemisietea vulgaris) und vergleichende Betrachtung der Saumgesellschaften insgesamt [The anhydromorphic forest edge communities of NE Lower Saxony (Germany) in an European context–Part II: Nutrient-rich sites (Artemisietea vulgaris) and comparative analysis of all forest edge communities]. Tuexenia 2007, 27, 91–136. (In German) [Google Scholar]
- Sanda, V.; Öllerer, K.; Burescu, P. Fitocenozele din România [The Phytocenoses in Romania]; Ars Docendi: Bucharest, Romania, 2008. (In Romanian) [Google Scholar]
- Stanković-Kalezić, R.; Jovanović, S.; Janjić, V.; Radivojević, L. Zajednica Arctio-Artemisietum vulgaris (Tx. 1942) Oberd. et al. 1967.: Najzastupljenija ruderalna zajednica na području Pančevačkog rita [Community Arctio-Artemisietum vulgaris (Tx. 1942) Oberd. et al. 1967.: The most widespread ruderal community at Pančevački rit]. Pestic. Fitomedicina 2009, 24, 113–121. (In Serbian) [Google Scholar] [CrossRef]
- Lániková, D. XCE Arction lappae Tüxen 1937. In Vegetace České Republiky. 2. Ruderální, Plevelová, Skalní a Suťová Vegetace [Vegetation of the Czech Republic 2. Ruderal, Weed, Rock and Scree Vegetation]; Chytrý, M., Ed.; Academia: Prague, Czech Republic, 2009; pp. 275–288. (In Czech) [Google Scholar]
- Stanković-Kalezić, R. Sinekološka i Floristička Studija Ruderalne Vegetacije na Području Pančevačkog Rita [A Synecological and Floristic Study of Ruderal Vegetation at Pancevacki rit]. Ph.D. Thesis, Faculty of Biology, University of Belgrade, Belgrade, Serbia, 2007. (In Serbian). [Google Scholar]
- Radulović, S. Vegetacija Ade Ciganlije [Vegetation of Ada Ciganlija]. Master’s Thesis, Faculty of Forestry, University of Belgrade, Belgrade, Serbia, 1982. (In Serbian). [Google Scholar]
- Slavnić, Ž. Pregled nitrofilne vegetacije Vojvodine [Overview of nitrophilous vegetation of Vojvodina]. Zb. Matice Srp. Za Prir. Nauke 1951, 1, 84–169. (In Serbian) [Google Scholar]
- Kojić, M.; Pejčinović, D. Korovska Flora i Vegetacija Kosova [Weed Flora and Vegetation of Kosovo]; Zavod za Udžbenike i Nastavna Sredstva SAP Kosova: Priština, Serbia, 1982. (In Serbian) [Google Scholar]
- Dancza, I. Syntaxonomic studies on the ruderal plant communities in Southwest Transdanubia (Hungary). Acta Bot. Hung. 2009, 51, 35–59. [Google Scholar] [CrossRef]
- Vassilev, K.; Nazarov, M.; Genova, B.; Grigorov, B.; Georgiev, S.; Velev, N. Syntaxonomical and Ecological Diversity of Class Artemisietea vulgaris Lohmeyer et al. in Tx. ex von Rochow 1951 in Bulgaria. Ecologia Balkanica 2021, 13, 177–196. [Google Scholar]
- Jovanović, S. Calystegio-Equisetetum telmateiae nova higrofilna ruderalna zajednica na području Beograda [Calystegio-Equisetetum telmateiae the new hygrophilous ruderal community in the city of Belgrade]. Acta Herbol. 1993, 2, 47–59. (In Serbian) [Google Scholar]
- Stanković-Kalezić, R.; Radivojević, L.; Jovanović, V.; Janjić, V.; Šantrić, L. Adventivna vrsta Asclepias syriaca L. na području Pančevačkog rita [Adventive species Asclepias siryaca L. at Pančevački rit]. Acta Herbol. 2008, 17, 95–103. (In Serbian) [Google Scholar]
- Lániková, D. XCB Dauco carotae-Melilotion Görs ex Rostański et Gutte 1971. In Vegetace České Republiky. 2. Ruderální, Plevelová, Skalní a Suťová Vegetace [Vegetation of the Czech Republic 2. Ruderal, Weed, Rock and Scree Vegetation]; Chytrý, M., Ed.; Academia: Prague, Czech Republic, 2009; pp. 226–258. (In Czech) [Google Scholar]
- Kojić, M.; Stanković-Kalezić, R.; Radivojević, L. Contribution to studies of the ruderal vegetation of eastern Srem II. Acta Herbol. 2004, 13, 75–82. [Google Scholar]
- Popov, M.; Konstantinović, B.; Nikolić, L. Ecological analysis of stands of ass. Asclepiadetum syriacae Lániková in Chytrý 2009 in Bačka region. Zb. Matice Srp. Za Prir. Nauke 2016, 131, 157–166. [Google Scholar] [CrossRef]
- Fanelli, G. Analisi fitosociologica dell’area metropolitana di Roma [Phytosociological analysis of the metropolitan area of Rome]. Braun-Blanquetia 2002, 27, 1–269. (In Italian) [Google Scholar]
- Carretero, J. Las comunidades vegetales de Conyza bonariensis, C. canadensis, C. sumatrensis y Aster squamatus en España [The plant communities of Conyza bonariensis, C. canadensis, C. sumatrensis and Aster squamatus in Spain]. Ecología 1994, 8, 193–202. (In Spanish) [Google Scholar]
- Mucina, L. Stellarietea mediae. In Die Pflanzengesellschaften Österreichs, Anthropogene Vegetation [The Plant Communities of Austria, Anthropogenic Vegetation]; Mucina, L., Grabherr, G., Ellmauer, T., Eds.; Gustav Fischer Verlag: Jena, Germany, 1993; pp. 110–168. (In German) [Google Scholar]
- Lososová, Z.; Otýpkova, Z.; Sádlo, J.; Lániková, D. Jednoletá vegetace polních plevelů a ruderálních stanovišť (Stellarietea mediae) [Annual vegetation of arable land and ruderal habitats]. In Vegetace České Republiky. 2. Ruderální, Plevelová, Skalní a Suťová Vegetace [Vegetation of the Czech Republic 2. Ruderal, Weed, Rock and Scree Vegetation]; Chytrý, M., Ed.; Academia: Prague, Czech Republic, 2009; pp. 73–205. (In Czech) [Google Scholar]
- Šilc, U. Odontito-Ambrosietum Jarolímek et al. 1997-A ruderal association new to Slovenia. Acta Bot. Croat. 2002, 61, 179–198. [Google Scholar]
- Pajazitaj, Q. Hordeetum murini Libbert, 1932-A ruderal association in Kosovo. Acta Agric. Slov. 2009, 93, 337–343. [Google Scholar]
- Velev, N. Arrhenatheretalia elatioris uncritical checklist of Europe. Phytol. Balc. 2018, 24, 99–147. [Google Scholar]
- Mucina, L. Artemisietea vulgaris. In Die Pflanzengesellschaften Österreichs, Anthropogene Vegetation [The Plant Communities of Austria, Anthropogenic Vegetation]; Mucina, L., Grabherr, G., Ellmauer, T., Eds.; Gustav Fischer Verlag: Jena, Germany, 1993; pp. 169–202. (In German) [Google Scholar]
- Simonová, D. Vegetation of trampled habitats in the Czech Republic: A formalized phytosociological classification. Phytocoenologia 2008, 38, 177–191. [Google Scholar] [CrossRef]
- Kojić, M.; Popović, R.; Karadžić, B. Sintaksonomski Pregled Vegetacije Srbije [Syntaxonomical Overview of Vegetation of Serbia]; Institut za biološka istraživanja “Siniša Stanković”: Belgrade, Serbia, 1998. (In Serbian) [Google Scholar]
- Tabašević, M.; Lakušić, D.; Kuzmanović, N.; Vukojičić, S.; Glišić, M.; Jovanović, S. Ruderal vegetation in Serbia–diversity and floristic composition. Bot. Serbica. 2021, 45, 251–261. [Google Scholar] [CrossRef]


| Cl. No. | Gr. | Association | No. of Rel. | No. of Cities | Avg. No. Sp./ Plot | Total No. of sp. | Shannon’s Equitability (EH) | Shannon-Wiener Index (H′) |
|---|---|---|---|---|---|---|---|---|
| 1 | A | Amaranthus tuberculatus (Bidention tripartitae) | 3 | 1 | 18.67 | 37 | 0.64 | 1.88 |
| 2 | A | Bidens frondosus-Persicaria lapathifolia (Bidention tripartitae) | 17 | 6 | 21.12 | 117 | 0.65 | 1.98 |
| 3 | A | Amorpha fruticosa (Rubo caesii-Amorphion fruticosae) | 21 | 7 | 17.19 | 116 | 0.58 | 1.65 |
| 4 | A | Asteretum lanceolati | 9 | 3 | 15 | 77 | 0.53 | 1.41 |
| 5 | B | Reynoutrietum japonicae | 21 | 4 | 14.19 | 92 | 0.56 | 1.48 |
| 6 | C | Arctietum lappae | 95 | 20 | 19.66 | 221 | 0.67 | 1.97 |
| 7 | C | Tanaceto vulgaris-Artemisietum vulgaris | 5 | 4 | 22 | 71 | 0.78 | 2.40 |
| 8 | C | Cichorietum intybi | 48 | 18 | 18.38 | 169 | 0.66 | 1.93 |
| 9 | C | Carduo acanthoidis-Onopordetum acanthii | 15 | 9 | 19.4 | 107 | 0.67 | 1.98 |
| 10 | C | Asclepiadetum syriacae | 7 | 2 | 21.86 | 78 | 0.67 | 2.08 |
| 11 | C | Erigeron sumatrensis (Eragrostietalia) | 4 | 3 | 23.25 | 57 | 0.69 | 2.17 |
| 12 | C | Sorghum halepense (Eragrostietalia) | 7 | 2 | 15.86 | 57 | 0.59 | 1.61 |
| 13 | C | Calystegio-Equisetetum telmateiae | 4 | 2 | 16.25 | 43 | 0.62 | 1.72 |
| 14 | C | Convolvulo arvensis-Elytrigietum repentis | 8 | 7 | 12.5 | 59 | 0.55 | 1.37 |
| 15 | C | Sambucetum ebuli | 95 | 17 | 13.75 | 178 | 0.59 | 1.51 |
| 16 | C | Poo compressae-Tussilaginetum farfarae | 2 | 2 | 18 | 31 | 0.52 | 1.51 |
| 17 | D | Cynodonto dactyli-Atriplicetum tataricae | 3 | 3 | 16.67 | 32 | 0.61 | 1.73 |
| 18 | D | Chenopodietum stricti | 65 | 18 | 17.68 | 179 | 0.66 | 1.88 |
| 19 | D | Ambrosietum artemisiifoliae | 60 | 17 | 18.05 | 188 | 0.65 | 1.88 |
| 20 | E | Hordeetummurini | 52 | 3 | 15.58 | 121 | 0.60 | 1.64 |
| 21 | E | Plantago lanceolata (Cynosurion cristati) | 6 | 2 | 16.83 | 49 | 0.66 | 1.85 |
| 22 | E | Malva sylvestris (Sisymbrion officinalis) | 10 | 2 | 19 | 72 | 0.66 | 1.94 |
| 23 | F | Cynodontetum dactyli | 14 | 5 | 9.79 | 35 | 0.51 | 1.16 |
| 24 | F | Lolietum perennis | 51 | 17 | 10.51 | 82 | 0.62 | 1.43 |
| 25 | F | Ochlopoa annua (Polygono-Coronopodion) | 5 | 2 | 9.4 | 20 | 0.62 | 1.39 |
| 26 | F | Polygonetum avicularis | 85 | 20 | 10.21 | 106 | 0.49 | 1.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabašević, M.; Jovanović, S.; Lakušić, D.; Vukojičić, S.; Kuzmanović, N. Diversity of Ruderal Communities in Urban Environments—A Case Study from Serbia (SE Europe). Diversity 2021, 13, 638. https://doi.org/10.3390/d13120638
Tabašević M, Jovanović S, Lakušić D, Vukojičić S, Kuzmanović N. Diversity of Ruderal Communities in Urban Environments—A Case Study from Serbia (SE Europe). Diversity. 2021; 13(12):638. https://doi.org/10.3390/d13120638
Chicago/Turabian StyleTabašević, Milena, Slobodan Jovanović, Dmitar Lakušić, Snežana Vukojičić, and Nevena Kuzmanović. 2021. "Diversity of Ruderal Communities in Urban Environments—A Case Study from Serbia (SE Europe)" Diversity 13, no. 12: 638. https://doi.org/10.3390/d13120638
APA StyleTabašević, M., Jovanović, S., Lakušić, D., Vukojičić, S., & Kuzmanović, N. (2021). Diversity of Ruderal Communities in Urban Environments—A Case Study from Serbia (SE Europe). Diversity, 13(12), 638. https://doi.org/10.3390/d13120638






