Abstract
Herbicide usage in rice fields over time may have a direct and indirect influence on the biodiversity of the fields. The impacts of herbicide usage on non-target organisms were assessed by examining the species richness and zooplankton density of two rice fields. One was 2.08 hectares in size and had been treated with pesticides during the sampling year (RF-PA). The second field, measuring 1.76 hectares, had received no pesticide treatment (RF-NPA). Every two weeks, zooplankton was quantitatively collected from ten sampling sites in each field. At each station, 20 L of measured water was filtered through a plankton net with a mesh size of 20 µm and preserved in 1% Lugol’s solution. The results revealed that RF-NPA and RF-PA had 112 and 88 species of zooplankton, respectively, with an abundance-based Jaccard index (Jabd) of 0.438. The total zooplankton density in RF-NPA was 24.4 ind./L, significantly higher than the 16.6 ind./L in RF-PA (p < 0.001). The Shannon-Wiener diversity index (H’) and evenness (J) were highest in RF-NPA at the second sampling (3.45 and 0.75, respectively). These results indicate that glyphosate application affects the diversity of species and density of zooplankton in rice fields.
1. Introduction
Insect infestation, weed competition, and fungal and bacterial pathogens are serious problems that lead to reduced rice production [1]. These problems are often solved by applying a wide range of pesticides to protect rice crops [2]. The quantity of pesticides used conforms to the number of pesticides imported into Thailand. Herbicides, insecticides, and fungicides are the main three pesticide imports [3]. In addition, it is a common practice to drain rice-field water into irrigation canals. From there, the water eventually flows into freshwater systems, causing rice fields and any surrounding water bodies to become severely contaminated with numerous types of pesticides [4]. In general, many pesticides are not only designed and developed to eradicate specific target pests, but also produce side effects on non-target organisms [5]. Thus, the contaminated water from rice fields can directly and indirectly affect small organisms as well as zooplankton [6].
Zooplankton play a critical role as a primary consumer in the food webs of an aquatic ecosystem [7]. Based on their community structures, rotifers, cladocerans, and some copepods are essential components of temporary habitats, and have a high diversity in temporary waters [8,9]. In the field of chemical monitoring, species richness and the density of zooplankton can be used as biological parameters that indicate the extent of substance contamination in aquatic environments [10,11]. The sensitivity of each zooplankton to chemicals appears to vary. For instance, some rotifers have been found to be more sensitive to pharmaceuticals than cladocerans [12]. Likewise, several cladoceran species have exhibited a significant increase in their populations after herbicide exposure (e.g., glifosato atanor and butachlor), whereas rotifers have demonstrated a reduction in only a small number of individuals [13,14]. The sensitivities of the developmental stages of copepods are also distinct: copepod nauplii have been found to be more sensitive to glyphosate herbicide than copepodites and the adult stages [15].
Previous investigations of the effects of herbicide application on zooplankton communities have been conducted in small areas of artificial microcosms. Rotifers appear to be more sensitive to pesticides than microcrustacean zooplankton, such as cladocerans or copepods [13,14,16]. Thus, we hypothesized that pesticide application might affect the community structure of zooplankton on the larger scale of a rice field. To evaluate this hypothesis, we compared the effects of pesticide application on the zooplankton assemblages of two rice fields. One had pesticides applied during the sampling year (RF-PA), while the other field had not received any pesticides during the year preceding the study (RF-NPA). We also compared environmental factors between the fields. The findings were used to assess the effects of pesticide application on the primary consumers in the rice field ecosystem.
2. Materials and Methods
2.1. Study Sites
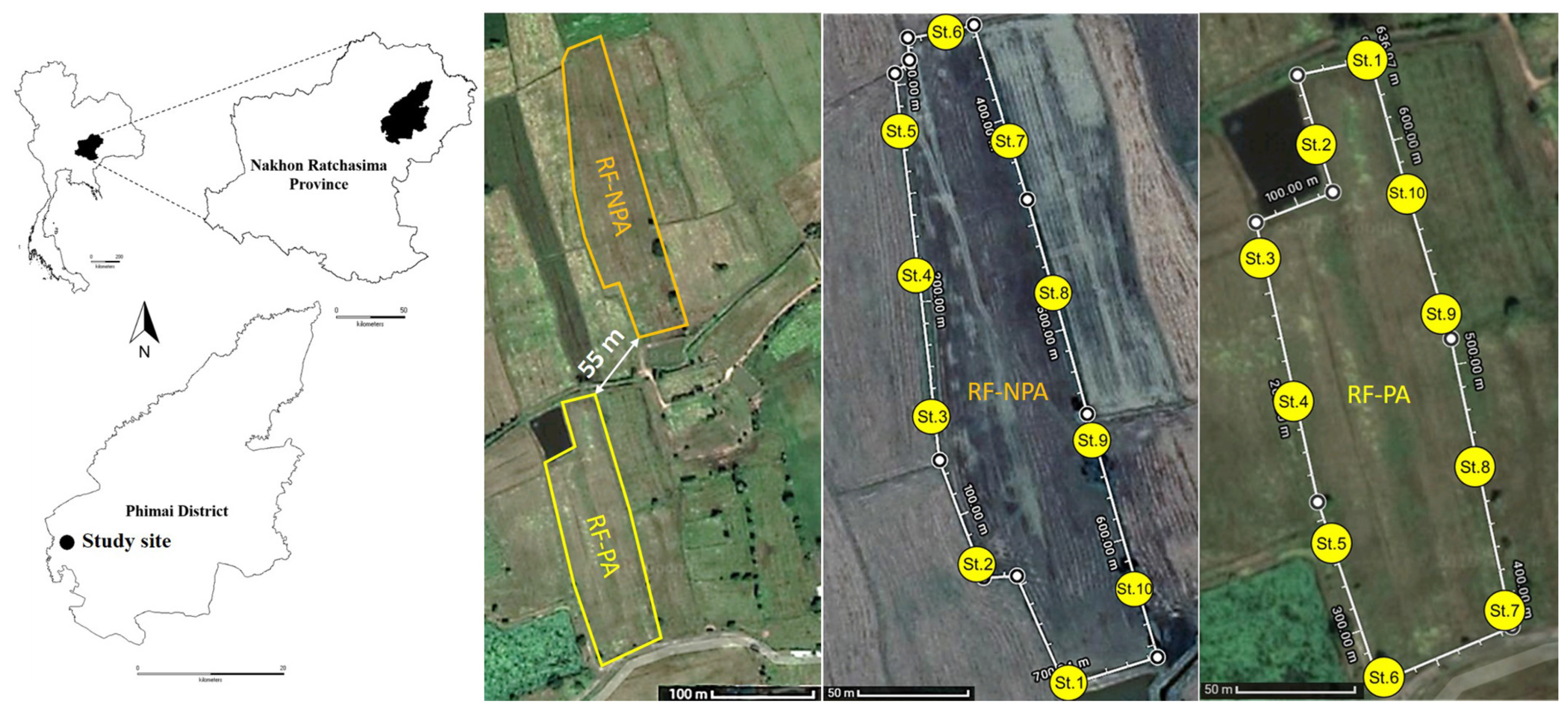
The two rice fields are situated in Ban Non Lukki, Than Lalot Subdistrict, Phimai District, Nakhon Ratchasima Province, in Northeastern Thailand. One was 1.76 ha in size, had no pesticides applied for at least one year before the sampling of this study (RF-NPA: “Rice Field Non-Pesticide Application”), and was located at 15°10′55.1″ N and 102°23′46.7″ E. The other field, located was at 15°10′45.0″ N and 102°23′46.1″ E, was 2.08 ha in size, and had pesticides applied during the sampling year (RF-PA: Rice Field Pesticide Application). These two rice fields are situated at an elevation of 146 m. RF-NPA is only about 55 m at its closest point to RF-PA (Figure 1). Both fields had historical pesticide and herbicide applications (viz., chlorpyrifos and glyphosate) for at least ten years. Rice (Oryza sativa L. cv. KDML 105) that had been planted prior to the field samplings developed into the reproductive phase at days 99 and 106 in RF-NPA and RF-PA, respectively. In the case of RF-PA, chlorpyrifos (an insecticide, 40% w/v) with a 0.48 L/ha application rate had been used at the seedling stage, and glyphosate (a herbicide, 48% w/v) with a 1.7 L/ha application rate, had been applied at the reproductive stage.
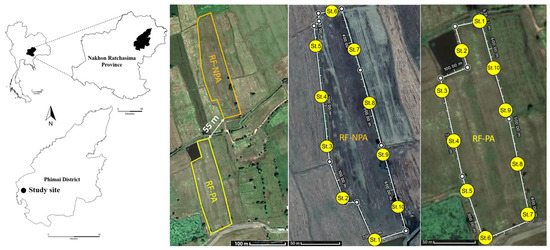
Figure 1.
Map of the study sites in Phimai District, Nakhon Ratchasima Province, showing the 10 sampling stations in RF-NPA and RF-PA.
2.2. Zooplankton Sampling and Identification
Zooplankton were quantitatively collected every two weeks from 10 sampling stations in each field. The first sampling (14 October 2018) was conducted one week after the application of glyphosate. After the second sampling period, the water in both rice fields dried out. At each sampling station, 20 L of sampled water were filtered through a plankton net (20 µm mesh size) before being preserved in 1% Lugol’s solution. All the zooplankton in the 40 samples were sorted, identified, and counted under a compound light microscope. Copepod specimens were dissected under a stereomicroscope using diluted glycerol as the mounting medium. Identification keys [17,18,19,20,21], as well as research papers (e.g., [22,23]), were used to identify the species.
2.3. Water Quality Measurement
Eight environmental variables were measured before collecting the water samples from the two fields. The water temperature (°C), electrical conductivity (EC; µS/cm), and total dissolved solids (TDS; mg/L) were measured with a multi-parameter meter, Hanna HI 98129, Hanna Instruments Inc., Woonsocket, RI, USA. The pH was measured with a pH meter, Index ID 1000, USA. Dissolved oxygen (DO; mg/L) and biochemical oxygen demand (BOD; mg/L) were determined by using the Winkler titration method [24]. Nitrate (mg/L) was analyzed colorimetrically with a spectrophotometer, Hach DR/2400, Hach Company, Loveland, CO, USA following the cadmium reduction method. Chlorophyll a content (µg/L) was determined by using an extracted-methanol method [25]. Pesticide residue in water (mg/L) was detected by using the gas chromatography-flame photometric detector (GC-FPD) method.
2.4. Data Analysis
The paired sample t-test and the independent-samples t-test were utilized to compare the differences in species richness, zooplankton density, and environmental variables of RF-NPA and RF-PA. The Spearman’s rank correlation analysis was used to determine the linear direction of the association between nitrate and chlorophyll a content. The data analyses were performed using IBM SPSS Statistics for Windows (version 26.0; IBM Corp., Armonk, NY, USA). In addition, the abundance-based Jaccard similarity index (Jabd) [26] was used to measure the similarity in the species compositions of the zooplankton of RF-NPA and RF-PA. The Shannon-Wiener diversity index (H′) [27] and Pielou’s evenness index (J) [28] were used to express species diversity and species-abundance distributions of zooplankton in the rice fields. In addition, the Sørensen−Dice index (Cs) [29] was applied to express the similarity in the species composition of the first and second samplings from each rice field. Additionally, the Dominance Candidate Index (DCi) [30] was used to ascertain the dominant species in each community. Furthermore, species accumulation [31] and the estimators of Chao, Chao2, Jacknife1, Jacknife2, and Bootstrap [32] were used to determine the number of species of zooplankton that could be found in each rice field. The calculations were performed with RStudio (version 3.6.1, RStudio, Boston, MA, USA).
3. Results
3.1. Environmental Factors of Rice Fields
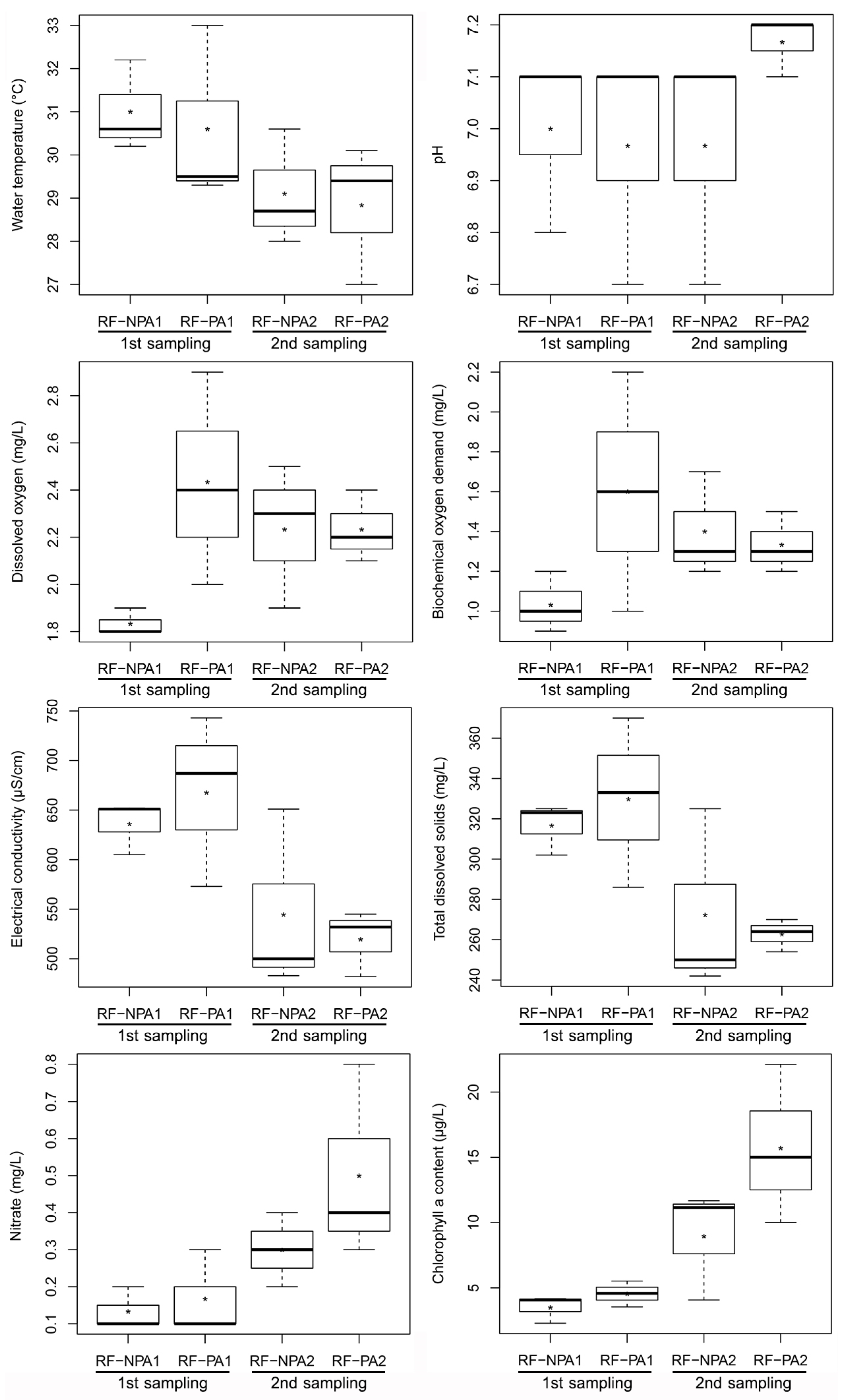
The independent samples t-test indicated that there were no significant differences between RF-NPA and RF-PA in terms of water environmental factors (p > 0.05) in both the first and second samplings. The results of the paired sample t-test showed that the first sampling of RF-PA revealed a significantly higher value of EC than the second sampling (t(2) = 4.671, p = 0.041) with 667.67 µS/cm and 519.67 µS/cm, respectively. In RF-NPA, the first sampling revealed a higher value of water temperature, EC, and TDS than the second sampling; on the other hand, DO, nitrate content, and chlorophyll a content had significantly lower values in the first sampling than in the second sampling (p < 0.05) (Figure 2). The dissolved oxygen of both RF-NPA and RF-PA did not exceed 3.0 mg/L in the first and second samples. Nitrate content had a positive relationship with chlorophyll a (r = 0.665; p = 0.018). The water depth in RF-PA was around 20 cm during the first measurement, and dropped during the second sampling (15 cm). The water in RF-NPA, on the other hand, was around 30 cm deeper than in RF-PA, and the water level remained rather consistent for both the first and second sampling periods. The presence of organophosphate pesticide was not detected in the water samples from either RF-NPA or RF-PA.
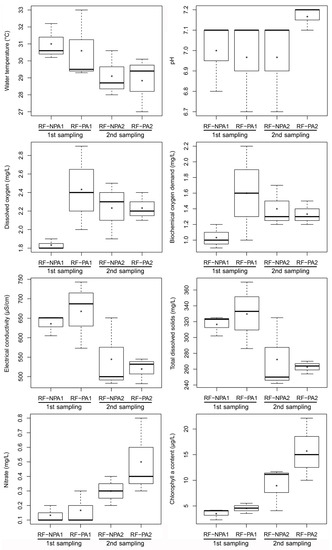
Figure 2.
Boxplot comparisons of the eight environmental parameters of RF-NPA and RF-PA. NPA1, PA1, NPA2, and PA2 represent the first sampling RF-NPA, the first sampling RF-PA, the second sampling of RF-NPA, and the second sampling of RF-PA, respectively.
3.2. Zooplankton Community Structure in RF-NPA and RF-PA
3.2.1. Species Composition
The species richness of the zooplankton of RF-NPA was higher than that of RF-PA (112 and 88 species, respectively) (Table 1). Both fields demonstrated a rather low similarity of species composition, achieving 0.434 with the abundance-based Jaccard similarity index (Jabd). Figure 3 depicts some of the zooplankton species found in the rice fields. A total of 112 species were found in RF-NPA, comprised of rotifers (79 species), cladocerans (21 species), and copepods (12 species). By comparison, 88 species, including 61 rotifers, 17 cladocerans, and 10 copepods, were found in RF-PA (Table 2). The total number of species in RF-NPA and RF-PA demonstrated a significant difference in the second sampling (p = 0.005). Based on relative richness, rotifers were the major component of zooplankton in both rice fields, with 70.5% in RF-NPA and 69.3% in RF-PA.

Table 1.
The list of zooplankton species in RF-NPA and RF-PA. The numbers 1, 2, 3, and 4 represent the first sampling RF-NPA, the second sampling RF-NPA, the first sampling RF-PA, and the second sampling RF-PA, respectively.
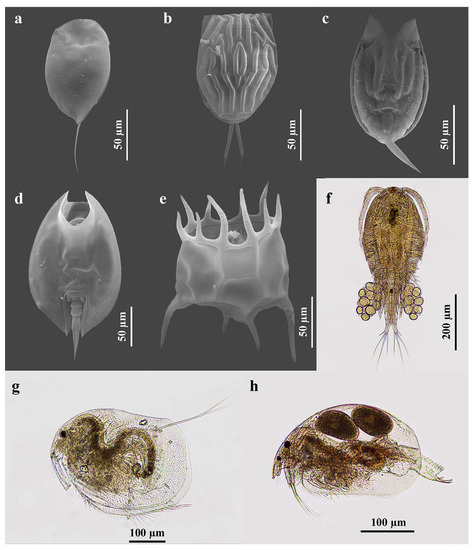
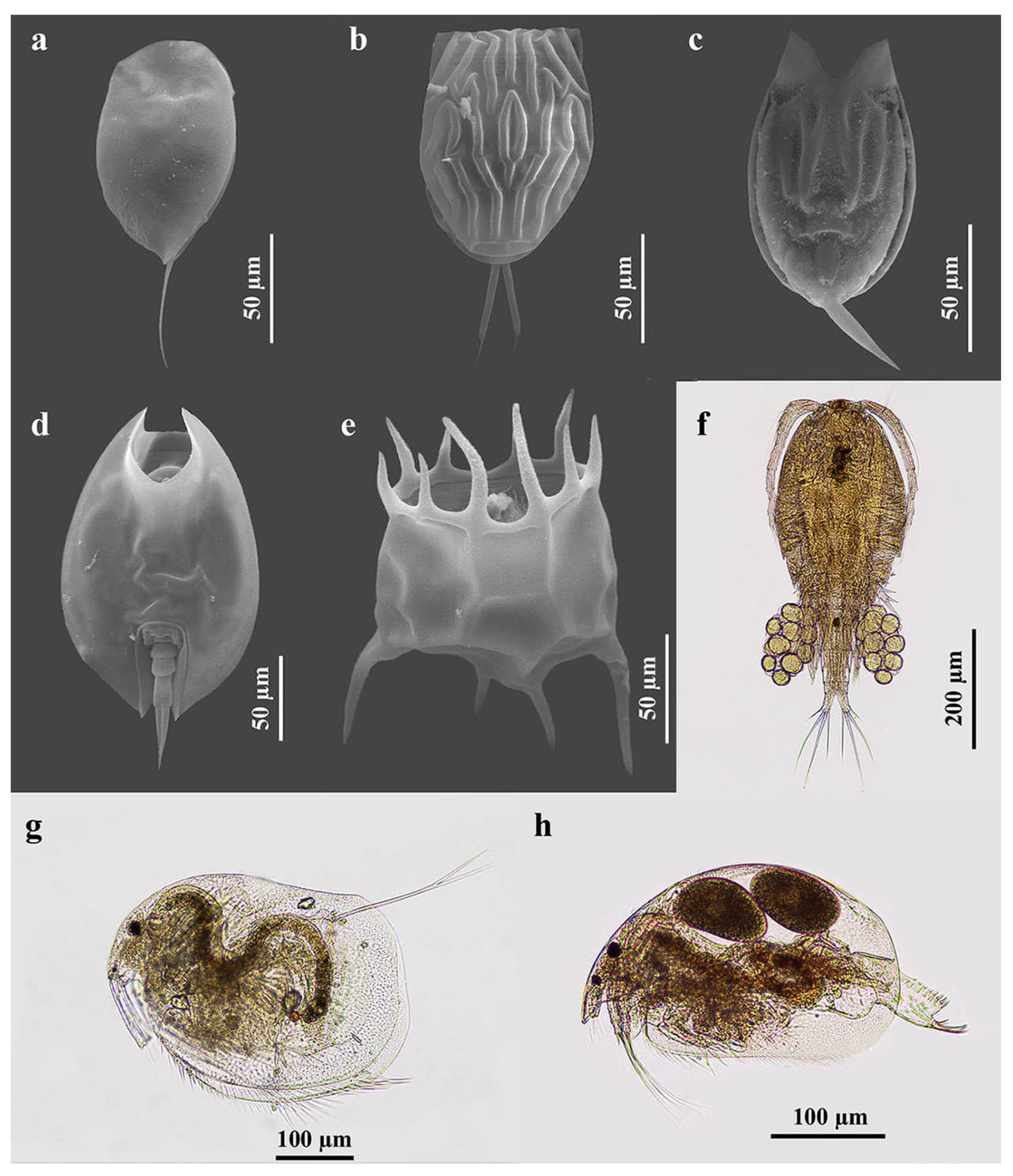
Figure 3.
Some zooplankton species that were found in the present study. (a) Colurella sanoamuangae Chittapun, Pholpunthin & Segers, 1999; (b) Lecane baimaii Sanoamuang & Savatenalinton, 1999; (c) Lecane hamata (Stokes, 1896); (d) Lepadella ovalis (Müller, 1786); (e) Plationus patulus (Müller, 1786); (f) Mesocyclops affinis Van de Velde, 1987; (g) Grimaldina brazzai Richard, 1892; and (h) Ovalona cambouei (Guerne & Richard, 1983).

Table 2.
Species richness, abundance (individuals), and diversity indices in the zooplankton community in RF-NPA and RF-PA on two occasions.
The total species richness of zooplankton in RF-PA showed a significant difference between the sampling times (p = 0.047). The overall species richness of the first and second samplings of RF-PA totaled 81 and 66 species, respectively. The similarity was 0.802. The total number of species in RF-NPA (97) was the same in the first and second samplings. The similarity index between the two samplings was 0.845.
The highest diversity index (H′ = 3.45) and evenness index (J = 0.75) were reported for RF-NPA in the second sampling. Conversely, the lowest diversity and evenness indices were recorded in the second sampling of RF-PA (3.09 and 0.70, respectively). Rotifers were the most diverse group in both rice fields, showing a diversity index of over 2.70. There was a high evenness index related to cladocerans, ranging from 0.73–0.76 (Table 2).
3.2.2. Species Accumulation and Estimate
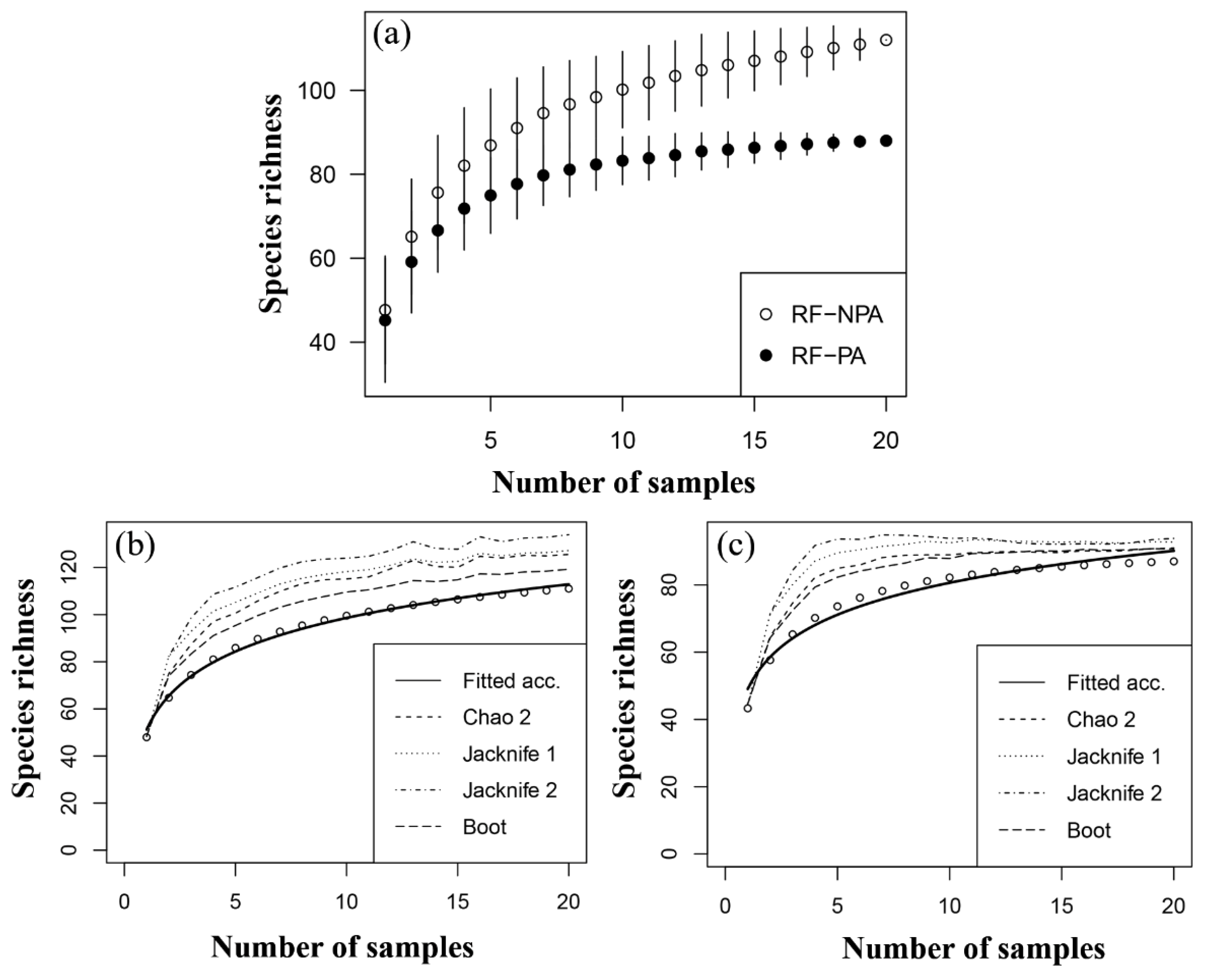
A species accumulation curve of zooplankton species richness in RF-NPA and RF-PA revealed a rise in the number of species in RF-NPA, but only a minor increase in RF-PA (Figure 4a). The results of five estimators (Chao, Chao2, Jacknife1, Jacknife2, and Bootstrap) were all greater than the number of observed species (112 species), particularly the Jacknife2 result, which gave the greatest maximum value of 133.9 species. In contrast, the estimation of the number of zooplankton species in RF-PA revealed that all five estimators gave values that were close to the number of observed species (88). The Chao2 method gave the closest estimate (89.9 species, Figure 4b,c). This finding suggests that many species have yet to be discovered in RF-NPA. In RF-PA, on the other hand, few unknown species are expected to be discovered.
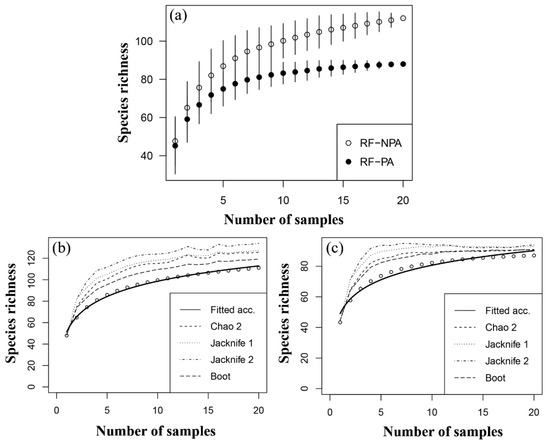
Figure 4.
Species accumulation curve (a), and the estimation curves of zooplankton in RF-NPA (b) and RF-PA (c).
3.2.3. Abundance, Density, and Dominant Species
The total abundance of all zooplankton in RF-NPA was greater than in RF-PA, with 9769 and 6649 individuals, respectively. The difference between RF-NPA and RP-PA in the total number of individuals of zooplankton was mainly due to rotifers (7355 individuals in RF-NPA versus 3952 individuals in RF-PA) and cladocerans (1605 individuals in RF-NPA versus 2136 individuals in RF-PA) (Table 2).
The mean zooplankton density in RF-NPA was significantly higher than that in RF-PA (p < 0.001) (24.4 and 16.6 ind./L, respectively). The total densities of the RF-NPA zooplankton at the first and second samplings were 250.3 and 238.1 ind./L, respectively. By comparison, the corresponding figures for RF-PA were lower (179.4 and 153.1 ind./L, respectively). The first sampling in RF-PA showed significantly higher average cladoceran and nauplii densities than the second sampling (Table 3).

Table 3.
The mean ± SD of the density and range of relative density of zooplankton in RF-NPA and RF-PA on two sample occasions. The asterisks (*) indicate statistically significant differences.
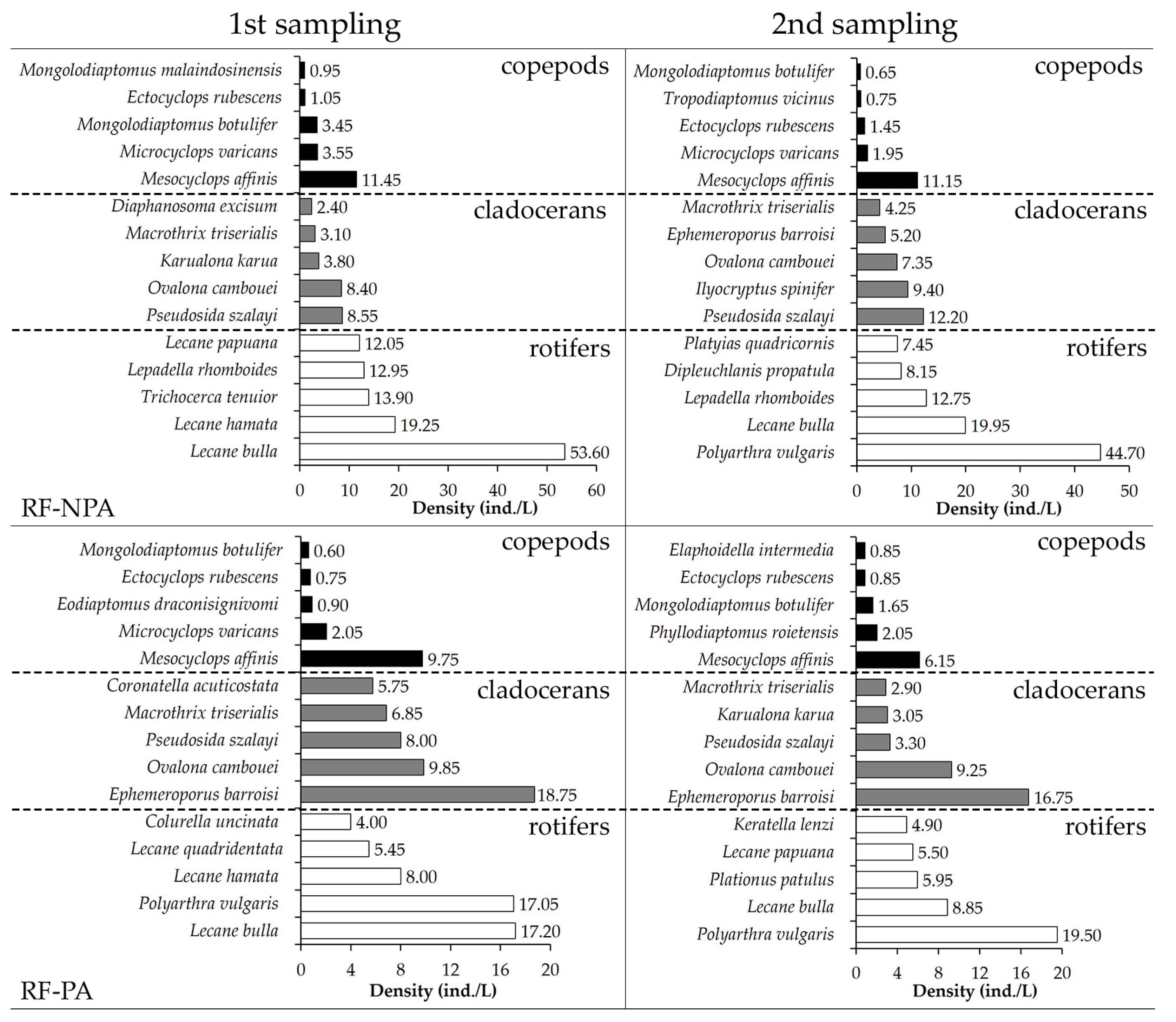
The dominating zooplankton in RF-NPA and RF-PA were comparable. In the first sample, Lecane bulla was the most common species in both rice fields (53.6 ind./L in RF-NPA versus 17.2 ind./L in RF-PA), with a DCi of 0.71 and 0.60, respectively. In the second sample, Polyarthra vulgaris had the greatest density in RF-NPA (44.7 ind./L) and RF-PA (19.5 ind./L), whereas L. bulla had reduced in both fields. Mesocyclops affinis was the most common copepod seen in each rice field on both occasions. Pseudosida szalayi had the highest density in both the first (8.6 ind./L) and second (12.2 ind./L) RF-NPA samplings. Ephemeroporus barroisi was the most common cladoceran in RF-PA in both the first and second samplings, with a DCi of 0.60 and 0.61, respectively (Figure 5).
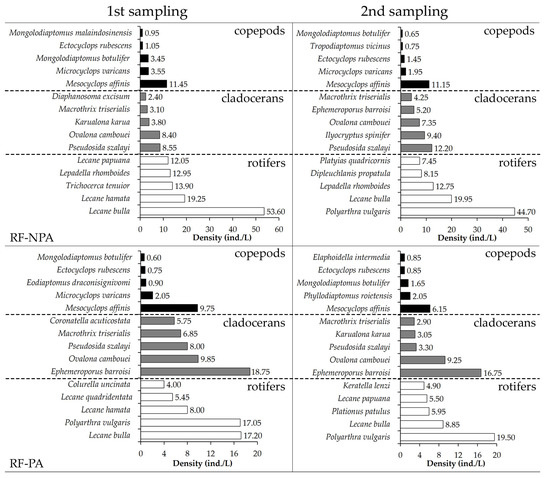
Figure 5.
Highest ranking of five dominant zooplankton in RF-NPA and RF-PA at two sampling times.
4. Discussion
4.1. Environmental Factors
One week after glyphosate treatment, the EC of RF-PA revealed significantly elevated values, while other parameters showed no significant differences. This implied that glyphosate remains in the water. Conductivity and glyphosate concentrations had a positive relationship [33]. After three weeks, the EC decreased, indicating that glyphosate might have degraded due to its short half-life with a range of 7–14 days [34]. Similarly, conductivity in rice fields had slightly higher values in herbicide treatments with imazethapyr, imazapic, bispyribac-sodium, and penoxsulam [16]. In RF-NPA, EC, TDS, DO, nitrate, and chlorophyll a content significantly differed between the first and second samples. This was due to the use of 169 kg/ha of urea fertilizer before the first sampling. The application of urea fertilizer resulted in high conductivity and low dissolved oxygen levels in RF-NPA [35]. The amount of chlorophyll a in the two rice fields exhibited a positive relationship with nitrate. A strong positive relationship between total nitrogen and the total density of key phytoplankton species was found in the rice fields, including Chlorophyta and Bacillariophyta [36]. Organophosphate pesticides were not detected in the water samples of RF-APA and RF-PA. Chlorpyrifos had been applied 90 days before the first sampling in RF-PA and might have degraded. Chlorpyrifos residue in water is non-detectable 14 days after application [37]. In addition, Fu et al. reported that the half-life of chlorpyrifos in a rice field in Zhejiang, China, was 0.9–3.8 days, with the highest residue detected one day after application [38].
4.2. Zooplankton Community Structure in RF-NPA and RF-PA
The rotifer species richness in RF-NPA was 79, which was higher than the 71 species found in Plangklang and Athibai [39]. As more samples were collected, more species were found. Even though 19 species discovered by Plangklang and Athibai were not found, there were 27 more taxa discovered. Twenty-one species of cladoceran were identified, four of which were common species, including Diaphanosoma excisum, Ilyocryptus spinifer, Moinodaphnia macleayi, and Scapholeberis kingi [40,41,42]. Six species of calanoid copepods, namely Eodiaptomus draconisignivomi, Heliodiaptomus elegans, Mongolodiaptomus botulifer, M. malaindosinensis, Neodiaptomus yangtsekiangensis, and Tropodiaptomus vicinus were found in the Mun River Basin, which is in the same RF-NPA and RF-PA areas [43]. Phyllodiaptomus praedictus has been discovered in rice fields that have been irrigated [44], and has been found to be common in Northeast Thailand [45]. These findings confirm that the diversity and occurrence of species in rice fields depend on the characteristics of the habitat, irrigation sources, and native species [44,46].
The total species richness of zooplankton and the number of species of rotifers were significantly higher in RF-NPA than in RF-PA. This concurred with the finding of Romero et al. [47] that the species richness of zooplankton in an agroecological rice field is greater than in a conventional rice field in Argentina. Pesticides have affected zooplankton communities by reducing species richness and diversity [48]. In RF-PA, the species richness of zooplankton dropped from the first sample to the second sample. The toxicity of herbicides may be dependent on exposure duration and half-life. Baker et al. [49] demonstrated that one day after applying glyphosate, zooplankton richness was significantly reduced; moreover, the decrease in species richness persisted for two weeks. The half-life of glyphosate in aquatic environments has been reported as ranging from 1−4.8 days in wetlands [49,50] to 3.5−11.2 days in forest ponds [51]. Moreover, the commercial formulation of glyphosate is more toxic than the active ingredient [52].
The density of zooplankton was considerably higher in RF-NPA than in RF-PA. Rotifer density of RF-NPA (18.38 ind./L) was comparable to that observed by Plangklang and Athibai [39], which established an average of 16.20 ind./L in the same field where pesticides had been applied. The density of rotifers in RF-NPA was higher than that in RF-PA for both samplings. This result is consistent with a reduction in rotifer abundance in subtropical rice fields after receiving the herbicides imazethapyr, imazapic, bispyribac-sodium, and penoxsulam [16]. Similarly, glyphosate and 2,4-D have also been found to affect emerged rotifers in hatching treatments by significantly decreasing their abundance relative to a control group [53].
In the present work, the glyphosate application had apparent effects on cladocerans and nauplii in RF-PA. The average density of cladocerans and nauplii in the second sampling were significantly lower than in the first sampling. Pesticides are not directly toxic to cladocerans immediately after spraying, but these chemicals could restrain cladoceran populations by a sublethal effect that disturbs their reproductive ability, habitat selection, and food habits, and creates changes in predator-prey relationships and interspecific competition [54]. The population of nauplii in RF-PA, like that of cladocerans, seemed to suffer from glyphosate application. It is likely that nauplii are more sensitive to glyphosate than copepodites and the adult stages. This is consistent with the case of the nauplii of Pseudodiaptomus annandalei, which have exhibited more sensitivity to glyphosate than copepodites and adult stages [15]. Also, glyphosate affects the development of Notodiaptomus carteri by increasing the development time of the nauplii and interrupting copepod development during the transition from the larva stage to the adult stage [55]. However, applications of glyphosate and other agrochemicals show marginal effects on the populations of nauplii and copepodites in a conventional rice field [47].
Although pesticides could not be detected in the water samples of the rice field, the zooplankton community structure in RF-NPA was different than it was in RF-PA. The presence of several species that are only found in RF-NPA indicates that avoidance of pesticide use in rice fields provides favorable conditions for the existence of some exclusive species, when compared to the presence of species in RF-PA. This led to higher density, a higher diversity index, and a higher evenness index in RF-NPA, in both samplings.
5. Conclusions
The findings provided a realistic picture of the effects of herbicide usage on zooplankton assemblages and environmental variables under field conditions. Glyphosate treatment had a greater effect on the species richness and the diversity of zooplankton in RF-PA, when compared to RF-NPA. The application of glyphosate in RF-PA resulted in decreases in the total species richness of zooplankton, in the diversity index, and in the density of cladocerans and copepod nauplii in the second sampling. Furthermore, its application led to an increase in the EC levels of rice field water. Thus, inappropriate use of pesticides might cause a reduction in zooplankton diversity and density in rice fields.
Author Contributions
Conceptualization, S.A.; methodology, N.P. and S.A.; formal analysis, N.P.; investigation, N.P. and S.A.; data curation, N.P.; writing—original draft preparation, N.P.; writing—review and editing, S.A.; supervision, S.A.; funding acquisition, S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a grant from the Government of Thailand’s Grants to Khon Kaen University (KKU) (Project code I62-00-27-20). The first author was supported by the Human Resource Development in Science Project (Science Achievement Scholarship of Thailand, SAST).
Institutional Review Board Statement
The study was reviewed and approved by the Institutional Animal Care and Use Committee of Khon Kaen University, Thailand (No. IACUC-KKU-42/61).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available from the authors upon request.
Acknowledgments
The authors thank Supatra Tiang-Nga, Rungnapa Somnark and Benjamart Suksai for assistance in the field. The authors would like to thank Bradley Rader for helping with English corrections.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Salem, H.; Olajos, E.J. Review of pesticides: Chemistry, uses and toxicology. Toxicol. Ind. Health 1988, 4, 291–321. [Google Scholar] [CrossRef]
- Praneetvatakul, S.; Schreinemachers, P.; Pananurak, P.; Tipraqsa, P. Pesticides, external costs and policy options for Thai agriculture. Environ. Sci. Pol. 2013, 27, 103–113. [Google Scholar] [CrossRef]
- Laohaudomchok, W.; Nankongnab, N.; Siriruttanapruk, S.; Klaimala, P.; Lianchamroon, W.; Ousap, P.; Jatiket, M.; Kajitvichyanukul, P.; Kitana, N.; Siriwong, W.; et al. Pesticide use in Thailand: Current situation, health risks, and gaps in research and policy. Hum. Ecol. Risk Assess. 2020, 27, 1147–1169. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.R.; Bajet, C.M.; Matin, M.A.; Nhan, D.D.; Sulaiman, A.H. Ecotoxicology of pesticides in the tropical paddy field ecosystem. Environ. Toxicol. Chem. 1997, 16, 59–70. [Google Scholar] [CrossRef]
- Aktar, M.W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Iwai, C.B.; Sujira, H.; Somparn, A.; Komarova, T.; Mueller, J.; Noller, B. Monitoring pesticides in the paddy field ecosystem of North-eastern Thailand for environmental and health risks. In Rational Environmental Management of Agrochemicals: Risk Assessment, Monitoring, and Remedial Action; Kennedy, I., Solomon, K., Gee, S., Crossan, A., Wang, S., Sánchez-Bayo, F., Eds.; American Chemical Society: Washington, DC, USA, 2007; pp. 259–273. [Google Scholar]
- Keister, J.E.; Bonnet, D.; Chiba, S.; Johnson, C.L.; Mackas, D.L.; Escribano, R. Zooplankton population connections, community dynamics, and climate variability. ICES J. Mar. Sci. 2012, 63, 347–350. [Google Scholar] [CrossRef] [Green Version]
- Lawler, S.P. Rice fields as temporary wetlands: A review. Isr. J. Zool. 2001, 47, 513–528. [Google Scholar] [CrossRef]
- Segers, H.; Sanoamuang, L. Note on a highly diverse rotifer assemblage (Rotifera: Monogononta) in a Laotian rice paddy and adjacent pond. Int. Rev. Hydrobiol. 2007, 92, 640–646. [Google Scholar] [CrossRef]
- Ferdous, Z.; Muktadir, A. A review: Potentiality of zooplankton as bioindicator. Am. J. Appl. Sci. 2009, 6, 1815–1819. [Google Scholar] [CrossRef]
- Xiong, W.; Huang, X.; Chen, Y.; Fu, R.; Du, X.; Chen, X.; Zhan, A. Zooplankton biodiversity monitoring in polluted freshwater ecosystems: A technical review. Environ. Sci. Ecotech. 2020, 1, 100008. [Google Scholar] [CrossRef]
- Snell, T.W.; Joaquim-Justo, C. Workshop on rotifers in ecotoxicology. Hydrobiologia 2007, 593, 227–232. [Google Scholar] [CrossRef]
- Vera, M.S.; Di Fiori, E.; Lagomarsino, L.; Sinistro, R.; Escaray, R.; Iummato, M.M.; Juárez, A.; de Molina, M.d.C.R.; Tell, G.; Pizarro, H. Direct and indirect effects of the glyphosate formulation Glifosato Atanor® on freshwater microbial communities. Ecotoxicology 2012, 21, 1805–1816. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.; Liu, F.; Liu, Y.; Yao, S.; Zhu, G. Effects of pesticide mixtures on zooplankton assemblages in aquatic microcosms simulating rice paddy fields. Bull. Environ. Contam. Toxicol. 2017, 99, 27–32. [Google Scholar] [CrossRef]
- Lim, X.; Koksong, L.; Koksong, L.; Liew, H.; Loh, J.; Loh, J. Acute toxicity of glyphosate on various life stages of calanoid copepod, Pseudodiaptomus annandalei. Asia Pac. J. Mol. Biol. Biotechnol. 2019, 27, 24–31. [Google Scholar] [CrossRef]
- Reimche, G.B.; Machado, S.L.; Oliveira, M.A.; Zanella, R.; Dressler, V.L.; Flores, E.M.; Gonçalves, F.F.; Donato, F.F.; Nunes, M.A. Imazethapyr and imazapic, bispyribac-sodium and penoxsulam: Zooplankton and dissipation in subtropical rice paddy water. Sci. Total Environ. 2015, 514, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Segers, H. Rotifera 2. The Lecanidae (Monogononta). In Guides to the Identification of the Microinvertebrates of the Continental Waters of the World; Dumont, H.J., Nogrady, T., Eds.; SPB Academic Publishing: The Hague, The Netherlands, 1995; Volume 6, pp. 1–226. [Google Scholar]
- Smirnov, N.N. Cladocera: The Chydorinae and Sayciinae (Chydoridae) of the world. In Guides to the Identification of the Microinvertebrates of the Continental Waters of the World; Dumont, H., Ed.; SPB Academic Publishing: Amsterdam, The Netherlands, 1996; pp. 1–197. [Google Scholar]
- Nogrady, T.; Segers, H. Rotifera 6: Asplanchnidae, Gastropodidae, Lindiidae, Microcodidae, Synchaetidae, Trochosphaeridae and Filinia. In Guides to the Identification of the Microinvertebrates of the Continental Water of the World; Dumont, H., Ed.; Backhuys Publishers: Leiden, The Netherlands, 2002; Volume 18, pp. 1–264. [Google Scholar]
- Sanoamuang, L. Freshwater Zooplankton: Calanoid Copepods in Thailand; Klungnana Vitthaya Press: Khon Kaen, Thailand, 2002; pp. 1–159. [Google Scholar]
- Hołyńska, M. Copepoda: Cyclopoida: Genera Mesocyclops and Thermocyclops. In Guides to the Identification of the Microinvertebrates of the Continental Water of the World; Dumont, H., Ed.; Backhuys Publishers: Leiden, The Netherlands, 2003; Volume 20, pp. 1–318. [Google Scholar]
- Lim, R.; Fernando, C. A review of Malaysian freshwater Copepoda with notes on new records and little known species. Hydrobiologia 1985, 128, 71–89. [Google Scholar] [CrossRef]
- Sinev, A.Y. Key for identification of Cladocera of the subfamily Aloninae (Anomopoda: Chydoridae) from South-East Asia. Zootaxa 2016, 4200, 451–486. [Google Scholar] [CrossRef]
- Winkler, L.W. Die bestimmung des im wasser gelösten sauerstoffes. Ber. Dtsch. Chem. Ges. 1888, 21, 2843–2854. [Google Scholar] [CrossRef] [Green Version]
- APHA-AWWA-WPCF. Standard Methods for Examination of Water and Waste Water; American Public Health Association, American Water Work Association and Water Pollution Control Federation: Washington, DC, USA, 1998; pp. 1–2671. [Google Scholar]
- Chao, A.; Chazdon, R.L.; Colwell, R.K.; Shen, T.J. Abundance-based similarity indices and their estimation when there are unseen species in samples. Biometrics 2006, 62, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Shannon, C.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949; pp. 1–125. [Google Scholar]
- Krebs, C. Ecological Methodology; Addison-Wesley Educational Publishers, Inc.: Menlo Park, CA, USA, 1999; pp. 1–631. [Google Scholar]
- Magurran, A.E. Measuring Biological Diversity; Blackwell Publishing: Oxford, UK, 2004; pp. 1–215. [Google Scholar]
- Avolio, M.L.; Forrestel, E.J.; Chang, C.C.; La Pierre, K.J.; Burghardt, K.T.; Smith, M.D. Demystifying dominant species. New Phytol. 2019, 223, 1106–1126. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.; O’Hara, R.; Simpson, G.; Solymos, P.; et al. Vegan: Community Ecology Package. Available online: https://CRAN.R-project.org/package=vegan (accessed on 6 April 2020).
- Colwell, R.K.; Coddington, J.A. Estimating terrestrial biodiversity through extrapolation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1994, 345, 101–118. [Google Scholar] [PubMed] [Green Version]
- Lautenschlager, R.; Schaertl, G.R. Electrical conductivity of five concentrations of two glyphosate-containing herbicides. S. J. Appl. Forest. 1991, 15, 85–88. [Google Scholar] [CrossRef]
- Giesy, J.P.; Dobson, S.; Solomon, K.R. Ecotoxicological risk assessment for Roundup® herbicide. Rev. Environ. Contam. Toxicol. 2000, 167, 35–120. [Google Scholar]
- Khrueakham, A.; Anurugsa, B.; Hungspreug, N. Influence of chemical fertilizer applications on water quality in paddy fields in Nong Harn, Sakon Nakhon Province, Thailand. Agr. Nat. Resour. 2015, 49, 868–879. [Google Scholar]
- Liu, Y.; Zou, G.; Yuan, Q.; Huang, W.; Zhou, W. Phytoplankton community characteristics in rice paddy fields under different nitrogen fertiliser applications. Acta Physiol. Plant. 2020, 42, 33. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, Y.; Yu, X.-y.; Liu, X.-j. Dissipation of chlorpyrifos and residue analysis in rice, soil and water under paddy field conditions. Ecotoxicol. Environ. Saf. 2012, 78, 276–280. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, F.; Zhao, C.; Zhao, Y.; Liu, Y.; Zhu, G. Distribution of chlorpyrifos in rice paddy environment and its potential dietary risk. J. Environ. Sci. 2015, 35, 101–107. [Google Scholar] [CrossRef]
- Plangklang, N.; Athibai, S. Species diversity and abundance of rotifer fauna in a conventional rice field in Nakhon Ratchasima Province. Khon Kaen Agr. J. 2019, 47, 651–656. [Google Scholar]
- Kotov, A.A.; Dumont, H.J. Analysis of the Ilyocryptus spinifer-species group (Anomopoda, Branchiopoda), with description of a new species. Hydrobiologia 2000, 428, 85–113. [Google Scholar] [CrossRef]
- Korovchinsky, N.M.; Sanoamuang, L. Overview of Sididae (Crustacea: Cladocera: Ctenopoda) of Northeast and East Thailand, with description of a new species of the genus Diaphanosoma. Zootaxa 2008, 1682, 45–61. [Google Scholar] [CrossRef]
- Korovchinsky, N.M. Cladocera (Crustacea: Branchiopoda) of South East Asia: History of exploration, taxon richness and notes on zoogeography. J. Limnol. 2013, 72, 109–124. [Google Scholar] [CrossRef] [Green Version]
- Sanoamuang, L.; Faitakum, S. Species diversity of cladocerans and copepods in the floodplain of the River Mun, Northeast Thailand. Asia-Pac. J. Sci. Technol. 2005, 10, 106–113. [Google Scholar]
- Chittapun, S.; Pholpunthin, P.; Sanoamuang, L. Diversity and composition of zooplankton in rice fields during a crop cycle at Pathum Thani province, Thailand. Songklanakarin J. Sci. Technol. 2009, 31, 261–267. [Google Scholar]
- Sanoamuang, L. Species composition and distribution of freshwater Calanoida and Cyclopoida (Copepoda) of north-east Thailand. In Crustaceans and Biodiversity Crisis; Schram, F., Klein, J., Eds.; Brill Academic Publishers: Leiden, The Netherlands, 1999; pp. 217–230. [Google Scholar]
- Fernando, C.; Furtado, J.; Lim, R. The Aquatic Fauna of the World’s Rice Fields; Department of Zoology, University of Malaya: Kuala Lumpur, Malaysia, 1979; pp. 1–105. [Google Scholar]
- Romero, N.; Attademo, A.M.; Reno, U.; Regaldo, L.; Repetti, M.R.; Lajmanovich, R.; Gagneten, A.M. Analysis of the zooplanktonic community in rice fields during a crop cycle in agroecological versus conventional management. Limnetica 2022, 41. [Google Scholar] [CrossRef]
- Hanazato, T. Pesticide effects on freshwater zooplankton: An ecological perspective. Environ. Pollut. 2001, 112, 1–10. [Google Scholar] [CrossRef]
- Baker, L.F.; Mudge, J.F.; Thompson, D.G.; Houlahan, J.E.; Kidd, K.A. The combined influence of two agricultural contaminants on natural communities of phytoplankton and zooplankton. Ecotoxicology 2016, 25, 1021–1032. [Google Scholar] [CrossRef]
- Degenhardt, D.; Humphries, D.; Cessna, A.J.; Messing, P.; Badiou, P.H.; Raina, R.; Farenhorst, A.; Pennock, D.J. Dissipation of glyphosate and aminomethylphosphonic acid in water and sediment of two Canadian prairie wetlands. J. Environ. Sci. Health B 2012, 47, 631–639. [Google Scholar] [CrossRef]
- Goldsborough, L.G.; Brown, D.J. Dissipation of glyphosate and aminomethylphosphonic acid in water and sediments of boreal forest ponds. Environ. Toxicol. Chem. 1993, 12, 1139–1147. [Google Scholar] [CrossRef]
- Reno, U.; Doyle, S.R.; Momo, F.R.; Regaldo, L.; Gagneten, A.M. Effects of glyphosate formulations on the population dynamics of two freshwater cladoceran species. Ecotoxicology 2018, 27, 784–793. [Google Scholar] [CrossRef]
- Portinho, J.L.; Nielsen, D.L.; Daré, L.; Henry, R.; Oliveira, R.C.; Branco, C.C. Mixture of commercial herbicides based on 2, 4-D and glyphosate mixture can suppress the emergence of zooplankton from sediments. Chemosphere 2018, 203, 151–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, R.; Abdullah, M.; Fernando, C. Ecological studies of Cladocera in the ricefields of Tanjung Karang, Malaysia, subjected to pesticide treatment. Hydrobiologia 1984, 113, 99–103. [Google Scholar] [CrossRef]
- Fantón, N.; Bacchetta, C.; Rossi, A.; Gutierrez, M.F. Effects of a glyphosate-based herbicide on the development and biochemical biomarkers of the freshwater copepod Notodiaptomus carteri (Lowndes, 1934). Ecotoxicol. Environ. Saf. 2020, 196, 110501. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).