Bigger Is Better, Sometimes: The Interaction between Body Size and Carcass Size Determines Fitness, Reproductive Strategies, and Senescence in Two Species of Burying Beetles
Abstract
1. Introduction
2. Materials and Methods
2.1. Burying Beetle Natural History
2.2. Experimental Design
2.3. Statistical Analyses
2.3.1. Analysis of Optimal Carcass Size
2.3.2. Analysis of Patterns of Reproductive Allocation
2.3.3. Analysis of Patterns of Reproductive Senescence
3. Results
3.1. Optimal Carcass Size
3.2. Patterns of Reproductive Allocation
3.3. Patterns of Reproductive Senescence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, G.C. Natural selection, the costs of reproduction, and a refinement of Lack’s principle. Am. Nat. 1966, 100, 687–690. [Google Scholar] [CrossRef]
- Carlisle, T.R. Brood success in variable environments: Implications for parental care allocation. Anim. Behav. 1982, 30, 824–836. [Google Scholar] [CrossRef]
- Heimpel, G.E.; Rosenheim, J.A. Dynamic host feeding by the parasitoid Aphytis melinus: The balance between current and future reproduction. J. Anim. Ecol. 1995, 64, 153–167. [Google Scholar] [CrossRef]
- Rosenheim, J.A. Characterizing the cost of oviposition in insects: A dynamic model. Evol. Ecol. 1999, 13, 141. [Google Scholar] [CrossRef]
- Pianka, E.R.; Parker, W.S. Age–Specific Reproductive Tactics. Am. Nat. 1975, 109, 453–464. [Google Scholar] [CrossRef]
- Clutton–Brock, T.H. Reproductive effort and terminal investment in iteroparous animals. Am. Nat. 1984, 123, 212–229. [Google Scholar] [CrossRef]
- McNamara, J.M.; Houston, A.I.; Barta, Z.; Scheuerlein, A.; Fromhage, L. Deterioration, death and the evolution of reproductive restraint in late life. Proc. Royal Soc. B Biol. Sci. 2009, 276, 4061–4066. [Google Scholar] [CrossRef]
- Duffield, K.R.; Bowers, E.K.; Sakaluk, S.K.; Sadd, B.M. A dynamic threshold model for terminal investment. Behav. Ecol. Sociobiol. 2017, 71, 185. [Google Scholar] [CrossRef] [PubMed]
- van Noordwijk, A.J.; de Jong, G. Acquisition and allocation of resources: Their influence on variation in life history tactics. Am. Nat. 1986, 128, 137–142. [Google Scholar] [CrossRef]
- Trumbo, S.T. Monogamy to communal breeding: Exploitation of a broad resource base by burying beetles (Nicrophorus). Ecol. Entomol. 1992, 17, 289–298. [Google Scholar] [CrossRef]
- Billman, E.J.; Belk, M.C. Effect of age–based and environment–based cues on reproductive investment in Gambusia affinis. Behav. Ecol. 2014, 4, 1611–1622. [Google Scholar] [CrossRef] [PubMed]
- Billman, E.J.; Creighton, J.C.; Belk, M.C. Prior experience affects allocation to current reproduction in a burying beetle. Behav. Ecol. 2014, 25, 813–818. [Google Scholar] [CrossRef]
- Brannelly, L.A.; Webb, R.; Skerratt, L.F.; Berger, L. Amphibians with infectious disease increase their reproductive effort: Evidence for the terminal investment hypothesis. Open Biol. 2016, 6, 150251. [Google Scholar] [CrossRef] [PubMed]
- Cotter, S.C.; Littlefair, J.E.; Grantham, P.J.; Kilner, R.M. A direct physiological trade–off between personal and social immunity. J. Anim. Ecol. 2013, 82 4, 846–853. [Google Scholar] [CrossRef]
- Creighton, J.C.; Heflin, N.D.; Belk, M.C. Cost of reproduction, resource quality, and terminal investment in a burying beetle. Am. Nat. 2009, 174, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Duffield, K.R.; Hunt, J.; Rapkin, J.; Sadd, B.M.; Sakaluk, S.K. Terminal investment in the gustatory appeal of nuptial food gifts in crickets. J. Evol. Biol. 2015, 28, 1872–1881. [Google Scholar] [CrossRef] [PubMed]
- Farchmin, P.; Eggert, A.; Duffield, K.; Sakaluk, S. Dynamic terminal investment in male burying beetles. Anim. Behav. 2020, 163, 1–7. [Google Scholar] [CrossRef]
- Heinze, J.; Schrempf, A. Terminal investment: Individual reproduction of ant queens increases with age. PLoS ONE 2012, 7, e35201. [Google Scholar] [CrossRef]
- Krams, I.A.; Krama, T.; Moore, F.R.; Rantala, M.J.; Mänd, R.; Mierauskas, P.; Mänd, M. Resource availability as a proxy for terminal investment in a beetle. Oecologia 2015, 178, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Mason, T.H.E.; Chirichella, R.; Richards, S.A.; Stephens, P.A.; Willis, S.G.; Apollonio, M. Contrasting life histories in neighbouring populations of a large mammal. PLoS ONE 2011, 6, e28002. [Google Scholar] [CrossRef] [PubMed]
- Reavey, C.E.; Silva, F.W.S.; Cotter, S.C. Bacterial infection increases reproductive investment in burying beetles. Insects 2015, 6, 926–942. [Google Scholar] [CrossRef] [PubMed]
- Velando, A.; Drummond, H.; Torres, R. Senescent birds redouble reproductive effort when ill: Confirmation of the terminal investment hypothesis. Proc. Biol. Sci. 2006, 273, 1443–1448. [Google Scholar] [CrossRef] [PubMed]
- Balbontín, J.; Møller, A.P.; Hermosell, I.G.; Marzal, A.; Reviriego, M.; de Lope, F. Geographical variation in reproductive ageing patterns and life–history strategy of a short–lived passerine bird. J. Evol. Biol. 2012, 25, 2298–2309. [Google Scholar] [CrossRef] [PubMed]
- Elliott, K.H.; O’Reilly, K.M.; Hatch, S.A.; Gaston, A.J.; Hare, J.F.; Anderson, W.G. The prudent parent meets old age: A high stress response in very old seabirds supports the terminal restraint hypothesis. Horm. Behav. 2014, 66, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Lecomte, V.J.; Sorci, G.; Cornet, S.; Jaeger, A.; Faivre, B.; Arnoux, E.; Gaillard, M.; Trouvé, C.; Besson, D.; Chastel, O.; et al. Patterns of aging in the long–lived wandering albatross. Proc. Natl. Acad. Sci. USA 2010, 107, 6370–6375. [Google Scholar] [CrossRef] [PubMed]
- McCallum, M.L.; Trauth, S.E. Physiological trade–offs between immunity and reproduction in the northern cricket frog (Acris crepitans). Herpetologica 2007, 63, 269–274. [Google Scholar] [CrossRef]
- Morin, A.; Rughetti, M.; Rioux–Paquette, S.; Festa–Bianchet, M. Older conservatives: Reproduction in female Alpine chamois (Rupicapra rupicapra) is increasingly risk–averse with age. Canad. J. Zool. 2016, 94, 311–321. [Google Scholar] [CrossRef]
- Smith, A.N.; Belk, M.C.; Creighton, J.C. Residency time as an indicator of reproductive restraint in male burying beetles. PLoS ONE 2014, 9, e109165. [Google Scholar] [CrossRef]
- Davison, R.; Boggs, C.L.; Baudisch, A. Resource allocation as a driver of senescence: Life history tradeoffs produce age patterns of mortality. J. Theor. Biol. 2014, 360, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Le Lann, C.; Visser, B.; Van Baaren, J.; Van Alphen, J.J.; Ellers, J. Comparing resource exploitation and allocation of two closely related aphid parasitoids sharing the same host. Evol. Ecol. 2012, 26, 79–94. [Google Scholar] [CrossRef]
- Scott, M.P. The ecology and behavior of burying beetles. Annu. Rev. Entomol. 1998, 43, 595–618. [Google Scholar] [CrossRef]
- Trumbo, S.T.; Fiore, A.J. Interspecific competition and the evolution of communal breeding in burying beetles. Am. Mid. Nat. 1994, 131, 169–174. [Google Scholar] [CrossRef]
- Smith, R.J.; Heese, B. Carcass selection in a high altitude population of the burying beetle, Nicrophorus investigator (Silphidae). Southwest. Nat. 1995, 40, 50–55. [Google Scholar]
- Sikes, D.S.; Venables, C. Molecular phylogeny of the burying beetles (Coleoptera: Silphidae: Nicrophorinae). Mol. Phylogenet. Evol. 2013, 69, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Peck, S.B.; Kaulbars, M.M. A synopsis of the distribution and bionomics of the carrion beetles (Coleoptera, Silphidae) of the conterminous United States. Proc. Entomol. Soc. Ont. 1987, 118, 47–81. [Google Scholar]
- Ratcliffe, B.C. The Carrion Beetles (Coleoptera: Silphidae) of Nebraska. Bull. Univ. Neb. State Mus. 2011, 13, 100. [Google Scholar]
- Bartlett, J.; Ashworth, C.M. Brood size and fitness in Nicrophorus vespilloides (Coleoptera: Silphidae). Behav. Ecol. Sociobiol. 1988, 22, 429–434. [Google Scholar] [CrossRef]
- Müller, J.K.; Eggert, A.-K.; Dressel, J. Intraspecific brood parasitism in the burying beetle, Necrophorus vespilloides (Coleoptera: Silphidae). Anim. Behav. 1990, 40, 491–499. [Google Scholar] [CrossRef]
- Creighton, J.C.; Smith, A.N.; Komendat, A.; Belk, M.C. Dynamics of biparental care in a burying beetle: Experimental handicapping results in partner compensation. Behav. Ecol. Sociobiol. 2015, 69, 265–271. [Google Scholar] [CrossRef]
- Anderson, R.S. Resource partitioning in the carrion beetle (Coleoptera:Silphidae) fauna of southern Ontario: Ecological and evolutionary considerations. Canad. J. Zool. 1982, 60, 1314–1325. [Google Scholar] [CrossRef]
- Trumbo, S.T. Interference competition among burying beetles (Silphidae, Nicrophorus). Ecol. Entomol. 1990, 15, 347–355. [Google Scholar] [CrossRef]
- Lomolino, M.; Creighton, C.; Schnell, G.; Certain, D. Ecology and conservation of the endangered American burying beetle (Nicrophorus americanus). Conserv. Biol. 1995, 9, 605–614. [Google Scholar] [CrossRef]
- Hocking, M.; Darimont, C.; Christie, K.; Reimchen, T. Niche variation in burying beetles (Nicrophorus spp.) associated with marine and terrestrial carrion. Canad. J. Zool. 2007, 85, 437–442. [Google Scholar] [CrossRef]
- Smith, G.; Trumbo, S.T.; Sikes, D.S.; Scott, M.P.; Smith, R.L. Host shift by the burying beetle, Nicrophorus pustulatus, a parasitoid of snake eggs. J. Evol. Biol. 2007, 20, 2389–2399. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.L.; Howard, D.R.; Hall, C.L. Spatiotemporal niche partitioning in a specious silphid community (Coleoptera: Silphidae Nicrophorus). Sci. Nat. 2019, 106, 57. [Google Scholar] [CrossRef]
- Hopwood, P.; Moore, A.; Tregenza, T.; Royle, N. Niche variation and the maintenance of variation in body size in a burying beetle. Ecol. Entomol. 2015, 41. [Google Scholar] [CrossRef]
- Smith, C.B.; Urness, P.J. Small mammal abundance on native and improved foothill ranges, Utah. J. Range Manag. 1984, 37, 353–357. [Google Scholar] [CrossRef]
- Smith, A.N.; Belk, M.C. Evidence for interspecific brood parasite detection and removal in burying beetles. Psyche 2018. [Google Scholar] [CrossRef] [PubMed]
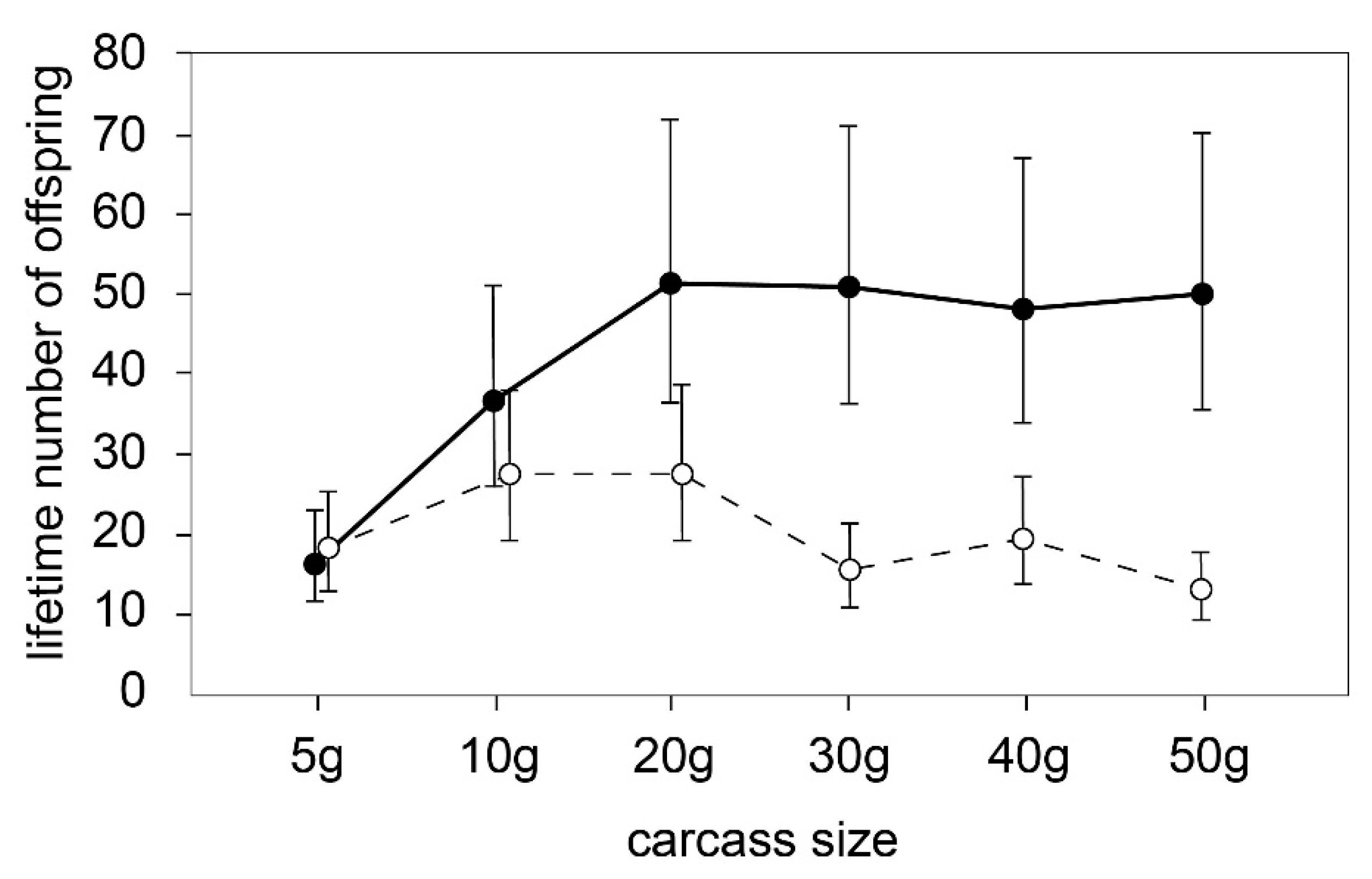
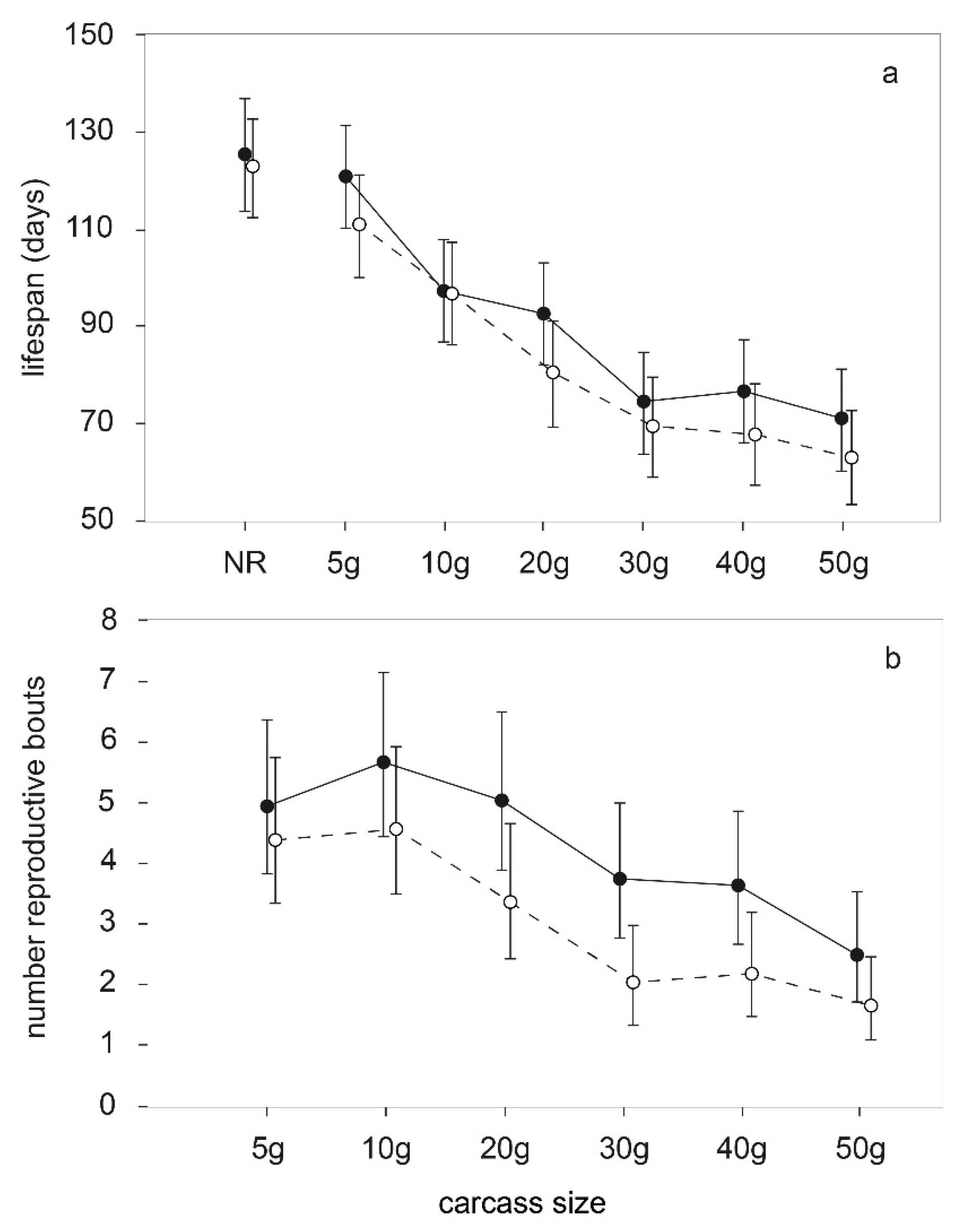

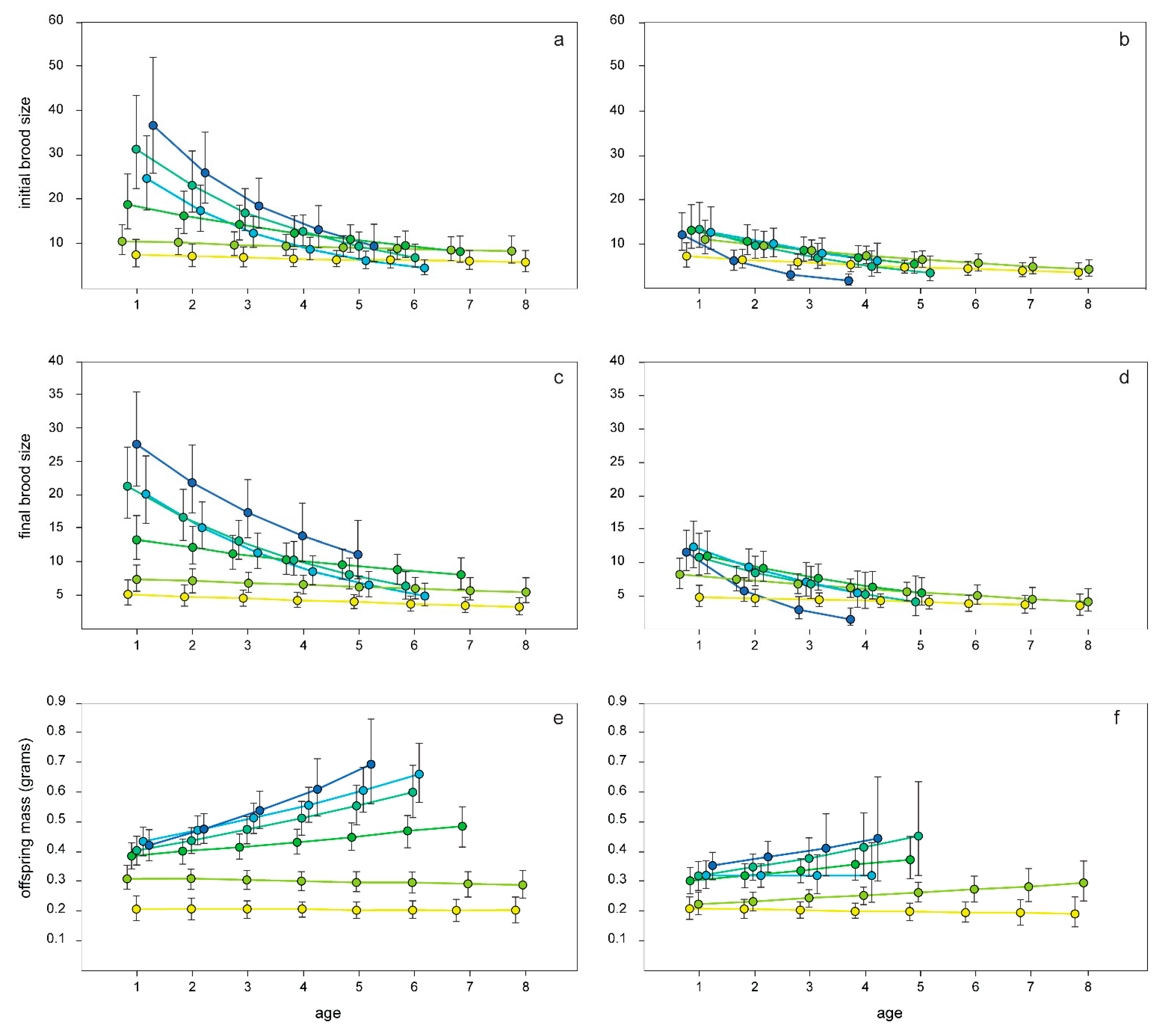
| Effect. | DF (num/den) | F | p |
|---|---|---|---|
| species | 1/132 | 54.07 | <0.0001 |
| carcass | 5/132 | 4.78 | 0.0005 |
| carcass × species | 5/132 | 5.36 | 0.0002 |
| standardized size | 1/132 | 0.16 | 0.6891 |
| Effect | DF (num/den) | F | p |
|---|---|---|---|
| species | 1/153 | 5.60 | 0.0192 |
| carcass | 6/153 | 35.78 | <0.0001 |
| carcass × species | 6/153 | 0.32 | 0.9244 |
| standardized size | 1/153 | 16.75 | <0.0001 |
| Effect | DF | χ2 | p |
|---|---|---|---|
| species | 1 | 16.79 | <0.0001 |
| carcass | 5 | 51.88 | <0.0001 |
| carcass × species | 5 | 3.80 | 0.5784 |
| standardized size | 1 | 1.50 | 0.2202 |
| Effect | DF (num/den) | F | p |
|---|---|---|---|
| Mass Change | |||
| species | 1/462 | 0.04 | 0.8387 |
| carcass | 5/462 | 0.79 | 0.5565 |
| age | 1/462 | 0.05 | 0.824 |
| standardized size | 1/462 | 0.73 | 0.3923 |
| species × carcass | 5/462 | 0.73 | 0.602 |
| age × species | 1/462 | 1.12 | 0.2915 |
| age × carcass | 5/462 | 0.86 | 0.5065 |
| age × species × carcass | 5/462 | 0.58 | 0.719 |
| Proportion Brood Culled | |||
| species | 1/212.8 | 5.65 | 0.0184 |
| carcass | 5/167.2 | 2.88 | 0.0072 |
| age | 1/202 | 8.71 | 0.0035 |
| standardized size | 1/135.8 | 8.45 | 0.0043 |
| species × carcass | 5/186.4 | 2.58 | 0.0275 |
| age × species | 1/265.6 | 0.29 | 0.5892 |
| age × carcass | 5/117.1 | 0.75 | 0.6305 |
| age × species × carcass | 5/129.2 | 2.03 | 0.0788 |
| Effect | DF (num/den) | F | p |
|---|---|---|---|
| Initial Brood Size | |||
| species | 1/118.7 | 9.87 | 0.0021 |
| carcass | 5/117.4 | 12.54 | <0.0001 |
| age | 1/337 | 135.6 | <0.0001 |
| standardized size | 1/100.6 | 0.03 | 0.8525 |
| species × carcass | 5/117.5 | 1.97 | 0.0885 |
| age × species | 1/341.6 | 4.35 | 0.0378 |
| age × carcass | 5/236.5 | 10.07 | <0.0001 |
| age × species × carcass | 5/236.9 | 1.25 | 0.2845 |
| Final Brood Size | |||
| species | 1/139 | 6.86 | 0.0098 |
| carcass | 5/131.8 | 22.28 | <0.0001 |
| age | 1/489 | 116.9 | <0.0001 |
| standardized size | 1/94.6 | 0.15 | 0.6982 |
| species × carcass | 5/132 | 1.64 | 0.153 |
| age × species | 1/489 | 6.81 | 0.0093 |
| age × carcass | 5/395.3 | 9.35 | <0.0001 |
| age × species × carcass | 5/396.1 | 2.01 | 0.0764 |
| Mean Offspring Mass | |||
| species | 1/212 | 12.43 | 0.0005 |
| carcass | 5/191.6 | 7.53 | <0.0001 |
| age | 1/447.2 | 18.84 | <0.0001 |
| standardized size | 1/100.5 | 0.61 | 0.4382 |
| species × carcass | 5/191.9 | 0.75 | 0.5834 |
| age × species | 1/449.6 | 0.27 | 0.6038 |
| age × carcass | 5/414.9 | 2.8 | 0.0169 |
| age × species × carcass | 5/415.1 | 1.02 | 0.4041 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belk, M.C.; Meyers, P.J.; Creighton, J.C. Bigger Is Better, Sometimes: The Interaction between Body Size and Carcass Size Determines Fitness, Reproductive Strategies, and Senescence in Two Species of Burying Beetles. Diversity 2021, 13, 662. https://doi.org/10.3390/d13120662
Belk MC, Meyers PJ, Creighton JC. Bigger Is Better, Sometimes: The Interaction between Body Size and Carcass Size Determines Fitness, Reproductive Strategies, and Senescence in Two Species of Burying Beetles. Diversity. 2021; 13(12):662. https://doi.org/10.3390/d13120662
Chicago/Turabian StyleBelk, Mark C., Peter J. Meyers, and J. Curtis Creighton. 2021. "Bigger Is Better, Sometimes: The Interaction between Body Size and Carcass Size Determines Fitness, Reproductive Strategies, and Senescence in Two Species of Burying Beetles" Diversity 13, no. 12: 662. https://doi.org/10.3390/d13120662
APA StyleBelk, M. C., Meyers, P. J., & Creighton, J. C. (2021). Bigger Is Better, Sometimes: The Interaction between Body Size and Carcass Size Determines Fitness, Reproductive Strategies, and Senescence in Two Species of Burying Beetles. Diversity, 13(12), 662. https://doi.org/10.3390/d13120662







