Good Things Come in Larger Packages: Size Matters for Adult Fruit-Feeding Butterfly Dispersal and Larval Diet Breadth
Abstract
1. Introduction
2. Materials and Methods
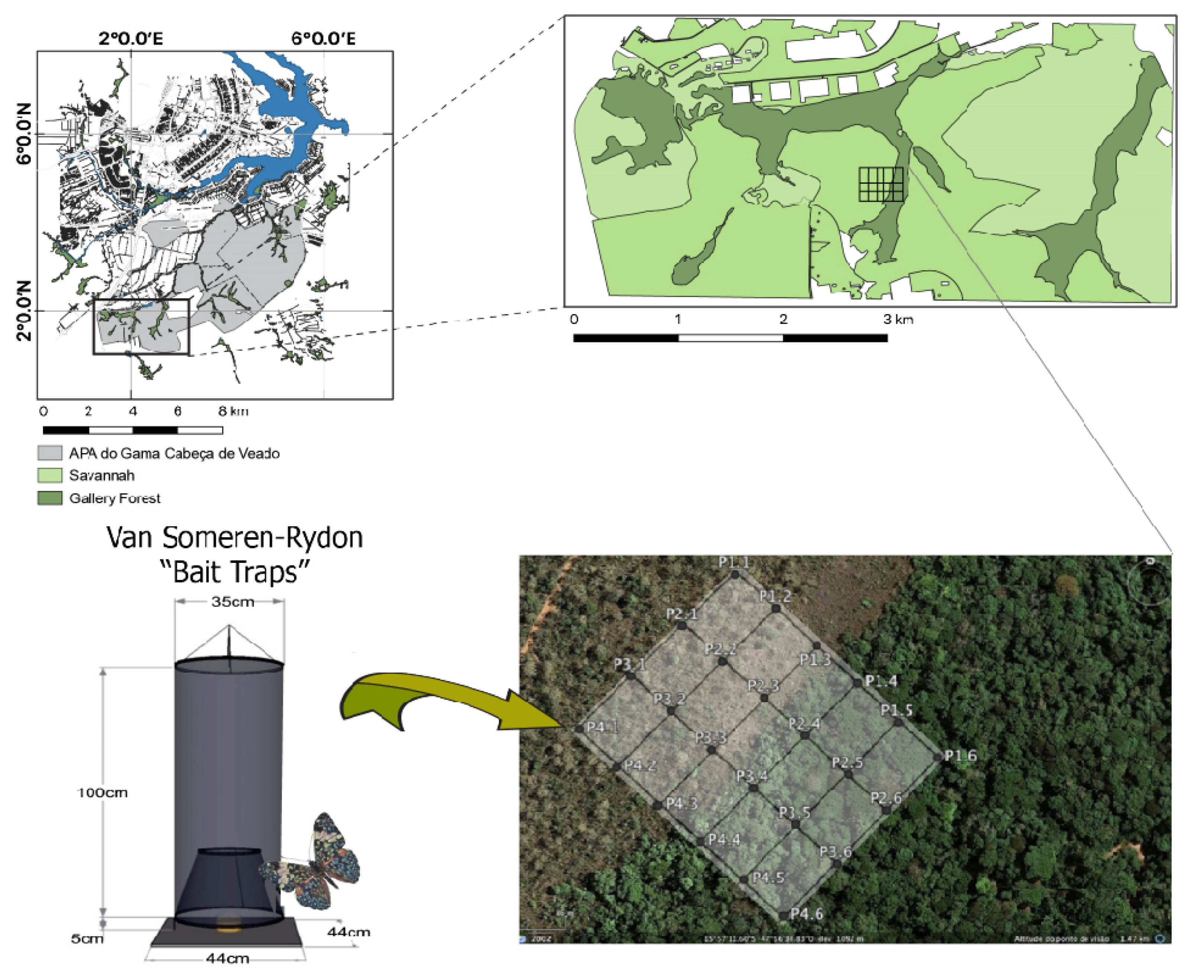
2.1. Study Site and Sampling Design
2.2. Fruit Feeding Butterflies
2.3. Phylogenetic, Morphological and Ecological Traits
2.4. Statistical Analysis
3. Results
4. Discussion
Trait-Dispersal Relationships
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Subfamily-Tribe/Species | FWL—mm | N | Disp. Level (%) | Distance—m Max (Mean ± Sd) | Perm.—Days Max (Mean ± Sd) | Diet Breadth Fam/Gen/Spp-Phy |
|---|---|---|---|---|---|---|
| Biblidinae | 30.5 ± 4.4 | 179 | 30 | 356.1 (50.7 ± 72.5) | 26 (5.8 ± 7.1) | - |
| Callicore sorana (Godart, [1824]) | 26.9 ± 1.6 | 16 | 30 | 123.1 (24.6 ± 48.3) | 6 (3.5 ± 3.6) | 1/3/3-121 |
| Catagramma pygas (Godart, [1824]) | 26.01 ± 1.4 | 4 | - | - | - | 1/2/4-21 |
| Catonephele acontius (Hübner, [1823]) | 32.9 ± 2.6 | 24 | 30 | 356.1 (140.5 ± 56.7) | 26 (6.3 ± 6.3) | 1/1/4-416 |
| Hamadryas amphinome (Linnaeus, 1767) | 39.01 ± 2.1 | 1 | - | - | 1/1/13-718 | |
| Hamadryas februa (Hübner, [1823]) | 33.4 ± 1.2 | 38 | 20 | 341.1 (55.8 ± 54.8) | 22 (5.5 ± 5.9) | 1/2/8-335 |
| Hamadryas feronia (Linnaeus, 1758) | 34.7 ± 2.1 | 70 | 40 | 266.2 (69.0 ± 88.7) | 11 (5.5 ± 6.3) | 1/1/6-618 |
| Temenis laothoe (Cramer, 1777) | 24.7 ± 1.6 | 25 | 30 | 157.1 (54.7 ± 49.1) | 20 (3.2 ± 4.3) | 1/6/21-616 |
| Temenis huebneri (Fruhstorfer, 1907) | 24.7 ± 1.3 | 1 | - | - | - | 1/6/21-na |
| Charaxinae | 28.1 ± 1.6 | 14 | 55 | 105.1 (47.8 ± 38.3) | 17(5.1 ± 5.9) | - |
| Archaeoprepona demophon (Hübner, [1814]) | 46.3 ± 1.4 | 2 | - | - | - | 11/16/46-1480 |
| Memphis acidalia (Hübner, [1819]) | 27.7 ± 1.4 | 6 | 50 | 105.1 (50.6 ± 47.3) | 17 (4.2 ± 4.5) | 2/2/3-na |
| Prepona claudina (Godart, [1824]) | 41.01 ± 1.4 | 1 | - | - | - | 6//6/7-471 |
| Zaretis strigosus (Gmelin, 1790) | 28.6 ± 1.6 | 5 | 60 | 69.1 (43.5 ± 33.5) | 11 (2.3 ± 2.9) | 1/1/2-231 |
| Nymphalinae | 30.5 ± 9.04 | 14 | 30 | 183.2 (75.8 ± 76.3) | 10 (3.5 ± 3.5) | - |
| Colobura dirce (Linnaeus, 1758) | 29.5 ± 2.3 | 12 | 30 | 183.2 (75.8 ± 76.3) | 10 (3.5 ± 3.5) | 1/1/7-699 |
| Smyrna blomfildia (Fabricius, 1781) | 40.01 ± 2.3 | 1 | - | - | - | 1/5/11-391 |
| Tigridia acesta (Linnaeus, 1758) | 22.01 ± 2.3 | 1 | - | - | - | 3/5/8-370 |
| Satyrinae-Brassolini | 52.6 ± 11.8 | 23 | 36 | 422 (124.7 ± 142.1) | 17 (3.8 ± 4.9) | - |
| Caligo illioneus (Cramer, 1775) | 65.9 ± 4.0 | 10 | 20 | 75.6 (60.4 ± 21.4) | 5 (3.2 ± 3.7) | 8//12/16-583 |
| Catoblepia berecynthia (Cramer, 1777) | 41.01 ± 2.2 | 3 | - | - | - | 1/2/2-na |
| Eryphanis automedon (C. Felder & R. Felder, 1867) | 53.9 ± 2.2 | 5 | 60 | 422.8 (237.3 ± 160.8) | 17 (4.8 ± 4.6) | 4/9/12-351 |
| Opsiphanes invirae (Hübner, [1808]) | 38.1 ± 2.0 | 5 | 30 | 40.2 (30.1 ± 12.5) | 12 (3.2 ± 3.5) | 3/17/29-566 |
| Satyrinae-Morphini | 59.1 ± 4.1 | 27 | 30 | 142.8 (86.5 ± 44.6) | 10 (3.1 ± 3.4) | - |
| Morpho helenor (C. Felder & R. Felder, 1867) | 59.1 ± 4.1 | 27 | 30 | 142.8 (86.5 ± 44.6) | 10 (3.1 ± 3.4) | 11/30/58-2094 |
| Satyrinae-Satyrini | 22.3 ± 7.1 | 282 | 15 | 272.1 (49.2 ± 53.3) | 21 (2.3 ± 3.1) | - |
| Fosterinaria quantius (Godart, [1824]) | 23.1 ± 0.8 | 20 | - | - | - | 1/1/1-na |
| Pareuptychia ocirrhoe (Fabricius, 1776) | 19.9 ± 1.4 | 174 | 10 | 151.9 (41.7 ± 38.5) | 18 (3.4 ± 4.3) | 1/8/11-359 |
| Cissia phronius (Godart, [1824]) | 19.7 ± 1.1 | 47 | 10 | 272.1 (100.9 ± 78.7) | 21 (3.3 ± 4.6) | 1/1/1-na |
| Paryphthimoides poltys (Prittwitz, 1865) | 20.2 ± 0.8 | 17 | 10 | 111.2 (45.1 ± 29.5) | 13 (2.5 ± 3.2) | 1/1/1-na |
| Taygetis virgilia (Cramer, 1776) | 36.01 ± 1.6 | 10 | - | - | - | 2/2//2-216 |
| Taygetis laches (Fabricius, 1793) | 35.7 ± 1.4 | 10 | 30 | 171.1 (66.9 ± 52.1) | 8 (2.1 ± 2.6) | 2/2/2-216 |
| Taygetis mermeria (Cramer, 1776) | 30.01 ± 1.4 | 4 | - | - | - | 1/2/2-200 |
| Total | - | 539 | 30 | 422.8 (237.3 ± 160.8) | 26 (6.3 ± 6.3) | - |
References
- Phillips, B.L.; Brown, G.P.; Shine, R. Evolutionarily accelerated invasions: The rate of dispersal evolves upwards during the range advance of cane toads. J. Evol. Biol. 2010, 23, 2595–2601. [Google Scholar] [CrossRef]
- Stevens, V.M.; Trochet, A.; Dyck, H.V.; Colbert, J.; Baguete, M. How is dispersal integrated in life histories: A quantitative analysis using butterflies. Ecol. Lett. 2012, 15, 74–86. [Google Scholar] [CrossRef]
- Fountain-Jones, N.M.; Baker, S.C.; Jordan, G.J. Moving beyond the guild concept: Developing a practical functional trait framework for terrestrial beetles. Ecol. Entomol. 2015, 40, 1–13. [Google Scholar] [CrossRef]
- Spaniol, R.L.; Duarte, L.S.; Mendonça, M.S., Jr.; Iserhard, C.A. Combining functional traits and phylogeny to disentangling Amazonian butterfly assemblages on anthropogenic gradients. Ecosphere 2019, 10, e02837. [Google Scholar] [CrossRef]
- Faleiro, F.; Machado, R.B.; Loyola, R.D. Defining spatial conservation priorities in the face of land-use and climate change. Biol Conserv. 2013, 158, 248–257. [Google Scholar] [CrossRef]
- Brown, J.H.; Gillooly, J.F.; Allen, A.P.; Savage, V.M.; West, G.B. Toward a metabolic theory of ecology. Ecology 2004, 85, 1771–1789. [Google Scholar] [CrossRef]
- Marini–Filho, O.J.; Martins, R.P. Nymphalid butterfly dispersal among forest fragments at Serra da Canastra National Park, Brazil. J. Insect Conserv. 2010, 14, 401–411. [Google Scholar] [CrossRef]
- Gillespie, M.A.K.; Birkemoe, T.; Sverdrup-Thygeson, A. Interactions between body size, abundance, seasonality and phenology in forest beetles. Ecol. Evol. 2017, 7, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Molleman, F.; Zwaan, B.J.; Brakefield, P.M.; Carey, J.R. Extraordinary long life spans in fruit-feeding butterflies can provide window on evolution of life span and aging. Exp. Gerontol. 2007, 42, 472–482. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Beck, J. Phylogenetic and ecological correlates with male adult life span of rainforest butterflies. Evol. Ecol. 2008, 22, 507–517. [Google Scholar] [CrossRef]
- Ribeiro, D.B.; Freitas, A.V.L. Large-sized insects show stronger seasonality than small-sized ones: A case study of fruit-feeding butterflies. Biol. J. Linn. Soc. 2011, 104, 820–827. [Google Scholar] [CrossRef]
- Uehara–Prado, M.; Brown, K.S., Jr.; Freitas, A.V.L. Species richness, composition and abundance of fruit-feeding butterflies in the Brazilian Atlantic Forest: Comparison between a fragmented and a continuous landscape. Glob. Ecol. Biogeogr. 2007, 16, 43–54. [Google Scholar] [CrossRef]
- Shahabuddin, G.; Ponte, C.A. Frugivorous butterfly species in tropical forest fragments: Correlates of vulnerability to extinction. Biodivers. Conserv. 2005, 14, 1137–1152. [Google Scholar] [CrossRef]
- Skórka, P.; Kudlek, J.; Pepkowska, A.; Sliwinska, E.B.; Settele, J.; Woyciechowski, M. Movements and flight morphology in the endangered large blue butterflies. Cent. Eur. J. Biol. 2013, 7, 662–669. [Google Scholar] [CrossRef]
- Ehrlich, P.R.; Raven, P.H. Butterflies and plants: A study in coevolution. Evolution 1964, 18, 586–608. [Google Scholar] [CrossRef]
- Forister, M.L.; Dyer, L.A.; Singer, M.S.; Stireman, J.O.; Lill, J.T. Revisiting the evolution of ecological specialization, with emphasis on insect–plant interactions. Ecology 2012, 93, 981–991. [Google Scholar] [CrossRef]
- MacArthur, R.H. Geographical Ecology: Patterns in the Distribution of Species; Harper & Row: New York, NY, USA, 1972. [Google Scholar]
- Davis, R.B.; Õunap, E.; Javois, J.; Gerhold, P.; Tammaru, T. Degree of specialization is related to body size in herbivorous insects: A phylogenetic confirmation. Evolution 2012, 67, 583–589. [Google Scholar] [CrossRef]
- Hjalmarsson, A.; Bergsten, J.; Monaghan, M.T. Dispersal is linked to habitat use in 59 species of water beetles (Coleoptera: Adephaga) on Madagascar. Ecography 2015, 38, 732–739. [Google Scholar] [CrossRef]
- Sudta, C.; Salcido, D.M.; Forister, M.L.; Walla, T.; Villarín-Cortez, S.; Dyer, L.A. Jack-of-all trades paradigm meets long-term data: Generalist herbivores are more widespread and locally less abundant. bioRxiv 2020. [Google Scholar] [CrossRef]
- Gaston, K.J. Patterns in local and regional dynamics of moth populations. Oikos 1988, 53, 49–57. [Google Scholar] [CrossRef]
- Reavey, D. Egg size, first instar behavior and the ecology of Lepidoptera. J. Zool. 1992, 227, 277–297. [Google Scholar] [CrossRef]
- Pavoine, S.; Baguette, M.; Stevens, V.M.; Leibold, M.A.; Turlure, C.; Bonsall, M.B. Life history traits, but not phylogeny, drive compositional patterns in a butterfly metacommunity. Ecology 2014, 95, 3304–3313. [Google Scholar] [CrossRef]
- Duarte, L.D.S.; Debastiani, V.J.; Carlucci, M.B.; Diniz-Filho, J.A.F. Analyzing community-weighted trait means across environmental gradients: Should phylogeny stay or should it go? Ecology 2014, 99, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Iserhard, C.A.; Duarte, L.; Seraphim, N.; Freitas, A.V.L. How urbanization affects multiple dimensions of biodiversity in tropical butterfly assemblages. Biodivers. Conserv. 2019, 28, 621–638. [Google Scholar] [CrossRef]
- Graça, M.B.; Pequeno, P.A.C.L.; Frankling, E.; Morais, J.W. Coevolution between flight morphology, vertical stratification and sexual dimorphism: What can we learn from tropical butterflies? J. Evol. Biol. 2017, 30, 1862–1871. [Google Scholar] [CrossRef]
- Santos, A.C.; Sales, P.C.L.; Ribeiro, D.B. Habitat conversion affects beta diversity in frugivorous butterfly assemblages. Stud. Neotrop. Fauna Environ. 2020, 55, 180–192. [Google Scholar] [CrossRef]
- Lourenço, G.M.; Soares, G.R.; Santos, T.P.; Dáttilo, W.; Freitas, A.V.L.; Ribeiro, S.P. Equal but different: Natural ecotones are dissimilar to anthropic edges. PLoS ONE 2019, 14, e0213008. [Google Scholar] [CrossRef] [PubMed]
- Freire, G.B.F., Jr.; Diniz, I.R. Temporal dynamics of fruit-feeding butterflies (Lepidoptera: Nymphalidae) in two habitats in a Brazilian environment. Fla. Entomol. 2015, 98, 1207–1216. [Google Scholar]
- De Vries, P.J. Stratification of fruit-feeding nymphalid butterflies in a Costa Rican rainforest. J. Res. Lepid. 1988, 26, 98–108. [Google Scholar]
- Freitas, A.V.L.; Iserhard, C.A.; Santos, J.P.; Carreira, J.Y.O.; Ribeiro, D.B.; Melo, D.H.A.; Rosa, A.H.B.; Marini-Filho, O.J.; Accacio, G.M.; Uehara-Prado, M. Studies with butterfly bait traps: An overview. Rev. Colomb. Entomol. 2014, 40, 209–218. [Google Scholar]
- Espírito-Santo, M.M.; Leite, M.E.; Silva, J.O.; Barbosa, R.S.; Rocha, A.M.; Anaya, F.C.; Dupin, M.G.V. Understanding patterns of land-cover change in the Brazilian Cerrado from 2000 to 2015. Philos. Trans. R. Soc. 2016, 371, 20150435. [Google Scholar] [CrossRef]
- Revell, L.J. Phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 2012, 3, 217–223. [Google Scholar] [CrossRef]
- Wahlberg, N.; Leneveu, J.; Kodandaramaiah, U.; Pena, C.; Nylin, S.; Freitas, A.V.L.; Brower, A.V.Z. Nymphalid butterflies diversify following near demise at the Cretaceous/Tertiary boundary. Proc. Biol. Sci. 2009, 276, 4295–4302. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH image to ImageJ: 25 years of image analysis. Focus Bioim. Inf. 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Beccaloni, G.W.; Hall, S.K.; Viloria, A.L.; Robinson, G.S. Catalogue of the hostplants of the Neotropical Butterflies/Catálogo de las plantas huésped de las mariposas Neotropicales. In RIBES-CYTED; Monografias, T.M., Zaragoza, S.E.A., Eds.; The Natural History Museum, Instituto Venezolano de Investigaciones Científicas: Zaragosa, Spain, 2008; Volume 8, 536p. [Google Scholar]
- Zanne, A.E.; Tank, D.C.; Cornwell, W.K.; Eastman, J.M.; Smith, A.S.; FitzJohn, R.G.; McGlinn, D.J.; O’Meara, B.C.; Moles, A.T.; Reich, P.B.; et al. Three keys to the radiation of angiosperms into freezing environments. Nature 2014, 506, 89–92. [Google Scholar] [CrossRef]
- Blomberg, S.P.; Garland, T.; Ives, A.R. Testing for phylogenetic signal in comparative data: Behavioral traits are more labile. Intern. J. Org. Evol. 2013, 57, 717–745. [Google Scholar] [CrossRef] [PubMed]
- Paradis, E.; Schliep, K. ape 5.0: An environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 2019, 35, 526–528. [Google Scholar] [CrossRef]
- Kembel, S.; Cowan, P.D.; Helmus, M.R.; Cornwell, W.K.; Morlon, H.; Ackerly, D.D.; Blomberg, S.P.; Webb, C.O. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 2010, 26, 1463–1464. [Google Scholar] [CrossRef]
- Freire, G.D.B., Jr.; Ribeiro, D.B.; Santos, A.C.; Silva, T.; Dias, J.P.; Rodrigues, H.P.; Diniz, I.R. Horizontal and vertical variation in the structure of fruit-feeding butterfly (Nymphalidae) assemblages in the Brazilian Cerrado. Insect Conserv. 2021, 14, 1–10. [Google Scholar] [CrossRef]

| Traits | Blomberg’s K | p-Value | |
|---|---|---|---|
| Body size | 1.1 | 0.001 | *** |
| Abundance | 0.39 | 0.27 | ns |
| Dispersal level | 0.76 | 0.07 | ns |
| Dispersal distance | 0.53 | 0.53 | ns |
| Visited traps | 0.39 | 0.66 | ns |
| Permanence (days) | 0.39 | 0.78 | ns |
| Diet breadth (Family of plants) | 1.08 | 0.001 | *** |
| Diet breadth (Genera of plants) | 0.84 | 0.002 | ** |
| Diet breadth (Species of plants) | 0.79 | 0.01 | ** |
| Phylogenetic Diet breadth | 0.48 | 0.06 | ns |
| Traits | F | Beta | p-Value | |
|---|---|---|---|---|
| Abundance | 7.2 | −0.09 | 0.01 | ** |
| Dispersal level | 9.69 | 1.17 | 0.01 | ** |
| Dispersal distance | 9.09 | 0.22 | 0.01 | ** |
| Visited traps | 9.69 | 0.13 | 0.01 | ** |
| Permanence (days) | 4.64 | 0.13 | 0.04 | * |
| Diet Breadth (Family of plants) | 16.23 | 3.8 | 0.001 | *** |
| Diet Breadth (Genera of plants) | 4.64 | 0.79 | 0.04 | * |
| Diet Breadth (Species of plants) | 1.83 | −0.03 | 0.81 | ns |
| Phylogenetic Diet Breadth | 4.83 | 0.11 | 0.001 | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freire, G.B., Jr.; Silva, T.; Oliveira, H.; Collier, C.; Rodrigues, H.P.; Dias, J.P.; Santos, J.P.; Marini-Filho, O.J.; Freitas, A.V.L.; Smilanich, A.M.; et al. Good Things Come in Larger Packages: Size Matters for Adult Fruit-Feeding Butterfly Dispersal and Larval Diet Breadth. Diversity 2021, 13, 664. https://doi.org/10.3390/d13120664
Freire GB Jr., Silva T, Oliveira H, Collier C, Rodrigues HP, Dias JP, Santos JP, Marini-Filho OJ, Freitas AVL, Smilanich AM, et al. Good Things Come in Larger Packages: Size Matters for Adult Fruit-Feeding Butterfly Dispersal and Larval Diet Breadth. Diversity. 2021; 13(12):664. https://doi.org/10.3390/d13120664
Chicago/Turabian StyleFreire, Geraldo B., Jr., Thayane Silva, Hernani Oliveira, Chloe Collier, Hanna P. Rodrigues, Joao P. Dias, Jessie P. Santos, Onildo J. Marini-Filho, André V. L. Freitas, Angela M. Smilanich, and et al. 2021. "Good Things Come in Larger Packages: Size Matters for Adult Fruit-Feeding Butterfly Dispersal and Larval Diet Breadth" Diversity 13, no. 12: 664. https://doi.org/10.3390/d13120664
APA StyleFreire, G. B., Jr., Silva, T., Oliveira, H., Collier, C., Rodrigues, H. P., Dias, J. P., Santos, J. P., Marini-Filho, O. J., Freitas, A. V. L., Smilanich, A. M., Dyer, L. A., & Diniz, I. R. (2021). Good Things Come in Larger Packages: Size Matters for Adult Fruit-Feeding Butterfly Dispersal and Larval Diet Breadth. Diversity, 13(12), 664. https://doi.org/10.3390/d13120664







