Component Endoparasite Communities Mirror Life-History Specialization in Syntopic Reed Frogs (Hyperolius spp.)
Abstract
:1. Introduction
2. Materials and Methods
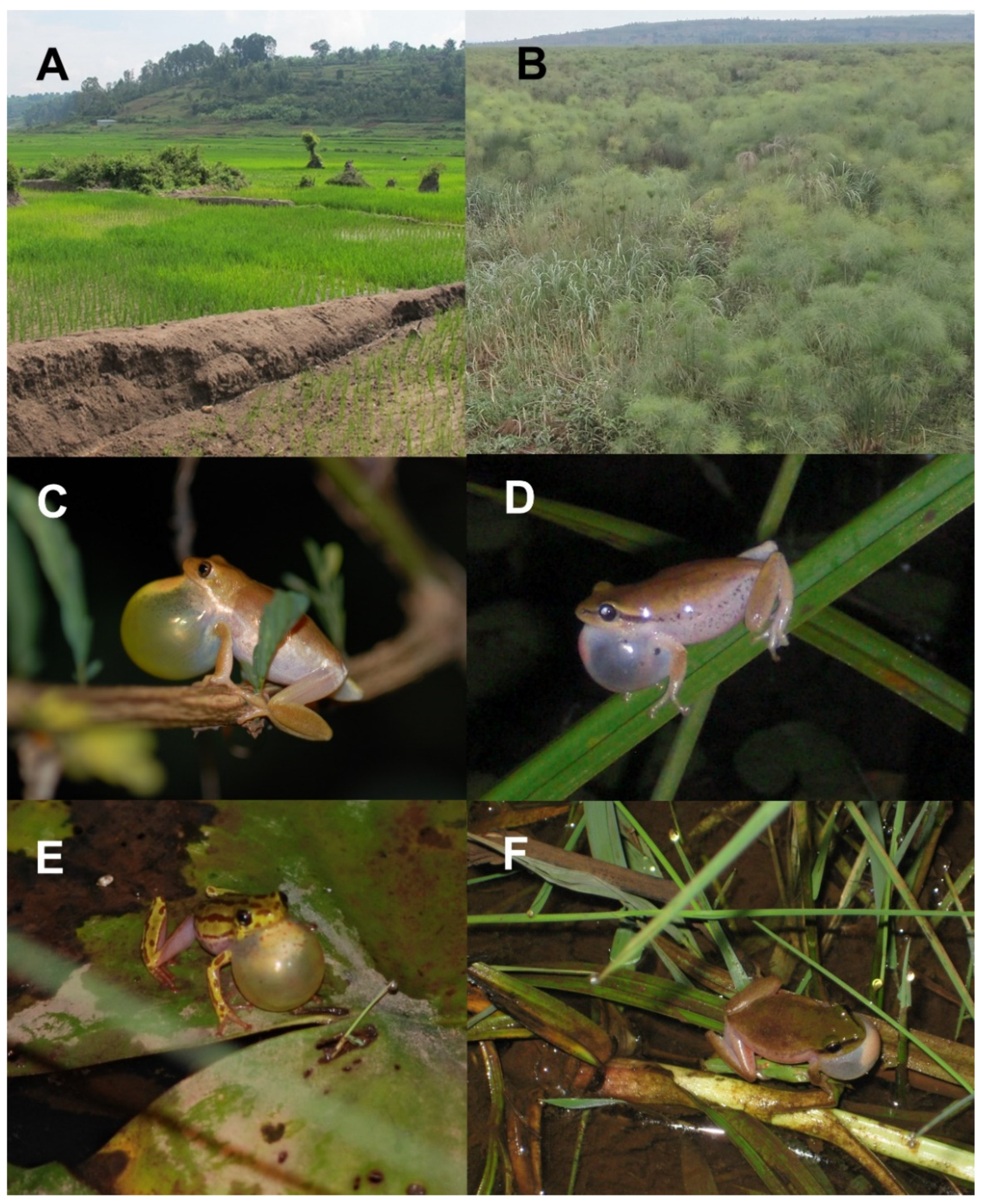
2.1. Sampling
2.2. Morphological Identification of Parasites and Endocommensals
2.3. Molecular Characterisation of Parasites
| Gene | Primer | Sequence | Reference |
|---|---|---|---|
| 18S-ITS1–5.8S | S1 | 5′-ATTCCGATAACGAACGAGACT-3′ | [38] |
| IR8 | 5′-GCTAGCTGCGTTCTTCATCGA-3′ | [39] | |
| 18S | F18 | 5′-ACCTGGTTGATCCTGCCAGTAG-3′ | [40] |
| 18RG | 5′-CTCTCTTAACCATTACTTCGG-3′ | [40] | |
| 18F3 | 5′-GGACGGCATGTTTACTTTGA-3′ | [40] | |
| IR5 | 5′-TACGGAAACCTTGTTACGAC-3′ | [40] | |
| 28S | LSU5′ | 5′-TAGGTCGACCCGCTGAAYTTAAGCA-3′ | [41] |
| IR14 | 5′-CATGTTAAACTCCTTGGTCCG-3′ | [40] | |
| IF15 | 5′-GTCTGTGGCGTAGTGGTAGAC-3′ | [40] | |
| LSU3′ | 5′-TAGAAGCTTCCTGAGGGAAACTTCGG-3′ | [41] | |
| COI | MplatCOX1dF | 5′-TGTAAAACGACGGCCAGTTTWCITTRGATCATAAG-3′ | [42] |
| MplatCOX1dR | 5′-CAGGAAACAGCTATGACTGAAAYAAYAIIGGATCICCACC-3′ | [42] |
2.4. Host Life-History Traits: Age and Size
3. Results
3.1. Local Host Biology: H. kivuensis and H. viridiflavus
3.2. Parasite-Community Structure: Species Richness, Abundance and Organ Systems Infested
3.3. Host-Parasite Interactions
3.3.1. Susceptibility of Hosts to Parasite Infestation
3.3.2. Seasonal Variation of Infestation
3.3.3. Impact on Host Survival
4. Discussion
4.1. Prediction 1: The Component Parasite Community of the More Aquatic Host Species Is Trematode-Dominated and More Diverse Than That of the More Terrestrial Host
4.2. Prediction 2: Prevalence of Infested Hosts Increases with the Species Richness of the Component Parasite Community
4.3. Prediction 3: Syntopic Host Populations Share More Parasite Species Than Allopatric Ones
4.4. Prediction 4: Prevalence of Shared Parasite Species, Infestation Intensity and Impact on Host Life-History Are Similar in the Two Host Species
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, P.T.J.; Buller, I.D. Parasite competition hidden by correlated coinfection: Using surveys and experiments to understand parasite interactions. Ecology 2011, 92, 535–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poulin, R.; Forbes, M.R. Meta-analysis and research on host-parasite interactions: Past and future. Evol. Ecol. 2012, 26, 1169–1185. [Google Scholar] [CrossRef]
- Koprivnikar, J.; Marcogliese, D.J.; Rohr, J.R.; Orlofske, S.A.; Raffel, T.R.; Johnson, P.T.J. Macroparasite Infections of Amphibians: What Can They Tell Us? Ecohealth 2012, 9, 342–360. [Google Scholar] [CrossRef] [PubMed]
- Thomas, F.; Renaud, F.; Rousset, F.; Cezilly, F.; Meeuûs, T.D. Differential mortality of two closely related host species induced by one parasite. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1995, 260, 349–352. [Google Scholar] [CrossRef]
- Rauque, C.; Paterson, R.; Poulin, R.; Tompkins, D. Do different parasite species interact in their effects on host fitness? A case study on parasites of the amphipod Paracalliope fluviatilis. Parasitology 2011, 138, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Wuerthner, V.P.; Hua, J.; Hoverman, J.T. The benefits of coinfection: Trematodes alter disease outcomes associated with virus infection. J. Anim. Ecol. 2017, 86, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Brooks, D.R.; Leon-Regagnon, V.; McLennan, D.A.; Zelmer, D. Ecological fitting as a determinant of the community structure of platyhelminth parasites of anurans. Ecology 2006, 87, S76–S85. [Google Scholar] [CrossRef]
- Aho, J.M. Helminth communities of amphibians and reptiles: Comparative approaches to understanding patterns and processes. In Parasite Communities: Patterns and Processes; Esch, G.W., Bush, A.O., Aho, J.M., Eds.; Springer: Dordrecht, The Netherlands, 1990; pp. 157–195. [Google Scholar] [CrossRef]
- Martins, P.M.; Poulin, R.; Gonçalves-Souza, T. Drivers of parasite β-diversity among anuran hosts depend on scale, realm and parasite group. Philos. Trans. R. Soc. B Biol. Sci. 2021, 376, 20200367. [Google Scholar] [CrossRef]
- Moss, W.E.; McDevitt-Galles, T.; Calhoun, D.M.; Johnson, P.T.J. Tracking the assembly of nested parasite communities: Using beta-diversity to understand variation in parasite richness and composition over time and scale. J. Anim. Ecol. 2020, 89, 1532–1542. [Google Scholar] [CrossRef] [PubMed]
- Bush, A.; Lafferty, K.; Lotz, J.; Shostak, A. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef]
- Sinsch, U.; Lümkemann, K.; Rosar, K.; Schwarz, C.; Dehling, J.M. Acoustic niche partitioning in an anuran community inhabiting an Afromontane wetland (Butare, Rwanda). Afr. Zool. 2012, 47, 60–73. [Google Scholar] [CrossRef]
- Dehling, J.M.; Sinsch, U. Partitioning of morphospace in larval and adult reed frogs (Anura: Hyperoliidae: Hyperolius) of the Central African Albertine Rift. Zool. Anz. 2019, 280, 65–77. [Google Scholar] [CrossRef]
- Portik, D.M.; Bell, R.C.; Blackburn, D.C.; Bauer, A.M.; Barratt, C.D.; Branch, W.R.; Burger, M.; Channing, A.; Colston, T.J.; Conradie, W.; et al. Sexual Dichromatism Drives Diversification Within a Major Radiation of African Amphibians. Syst. Biol. 2019, 68, 859–875. [Google Scholar] [CrossRef]
- Mindje, M.; Tumushimire, L.; Sinsch, U. Diversity assessment of anurans in the Mugesera wetland (eastern Rwanda): Impact of habitat disturbance and partial recovery. Salamandra 2020, 56, 27–38. [Google Scholar]
- Tumushimire, L.; Mindje, M.; Sinsch, U.; Dehling, J.M. Anuran diversity of cultivated wetlands in Rwanda: Melting pot of generalists? Salamandra 2020, 56, 99–112. [Google Scholar]
- Dehling, D.M.; Dehling, J.M. Elevated alpha diversity in disturbed sites obscures regional decline and homogenization of amphibian diversity. bioRxiv 2021. [Google Scholar] [CrossRef]
- Sinsch, U.; Dehling, J.M.; Scheid, P.; Balczun, C. A new African species of parasitic Dero (Annelida, Clitellata, Naididae) in the urinary tract of reed frogs. Parasitol. Res. 2019, 118, 3359–3370. [Google Scholar] [CrossRef] [PubMed]
- Sinsch, U.; Dehling, J.M.; Scheid, P.; Balczun, C. Molecular Diversity of Nematode Parasites in Afrotropical Reed Frogs (Hyperolius spp.). Divers.-Basel 2020, 12, 265. [Google Scholar] [CrossRef]
- Sinsch, U.; Dehling, J.M.; Scheid, P.; Balczun, C. Alternative Development Strategies of Clinostomum chabaudi (Digenea) Metacercariae in Frog Hosts (Hyperolius spp.). Diversity 2021, 13, 93. [Google Scholar] [CrossRef]
- Anderson, R.C.; Chabaud, A.G.; Willmott, S. Keys to the Nematode Parasites of Vertebrates: Archival Volume; CABI: Wallingford, UK, 2009. [Google Scholar]
- Gibbons, L.M. Keys to the Nematode Parasites of Vertebrates: Supplementary Volume; CABI: Wallingford, UK, 2010; Volume 10. [Google Scholar]
- Gibson, D.I.; Jones, A.; Bray, R. (Eds.) Keys to Trematoda; CABI: Wallingford, UK, 2002; Volume 1, p. 544. [Google Scholar]
- Jones, A.; Bray, R.A.; Gibson, D.I. (Eds.) Keys to the Trematoda; CABI: Wallingford, UK, 2005; Volume 2, p. 768. [Google Scholar]
- Bray, R.A.; Gibson, D.I.; Jones, A. (Eds.) Keys to the Trematoda; CABI: Wallingford, UK, 2008; Volume 3, p. 805. [Google Scholar]
- Harman, W.J. A Review of the Subgenus Allodero (Oligochaeta: Naididae: Dero) with a Description of D. (A.) floridana n. sp. from Bufo terrestris. Trans. Am. Microsc. Soc. 1971, 90, 225–228. [Google Scholar] [CrossRef]
- Andrews, J.M.; Childress, J.N.; Iakovidis, T.J.; Langford, G.J. Elucidating the Life History and Ecological Aspects of Allodero hylae (Annelida: Clitellata: Naididae), A Parasitic Oligochaete of Invasive Cuban Tree Frogs in Florida. J. Parasitol. 2015, 101, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Oda, F.H.; Petsch, D.K.; Ragonha, F.H.; Batista, V.G.; Takeda, A.M.; Takemoto, R.M. Dero (Allodero) lutzi Michaelsen, 1926 (Oligochaeta: Naididae) associated with Scinax fuscovarius (Lutz, 1925) (Anura: Hylidae) from Semi-deciduous Atlantic Rain Forest, southern Brazil. Braz. J. Biol. 2015, 75, 86–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douglas, L.T. The Taxonomy of Nematotaeniid Cestodes. J. Parasitol. 1958, 44, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Mariaux, J. Cestode systematics: Any progress? Int. J. Parasitol. 1996, 26, 231–243. [Google Scholar] [CrossRef]
- Scholz, T.; Besprozvannykh, V.V.; Boutorina, T.E.; Choudhury, A.; Cribb, T.H.; Ermolenko, A.V.; Faltynkova, A.; Shedko, M.B.; Shimazu, T.; Smit, N.J. Trematode diversity in freshwater fishes of the Globe I: ‘Old World’. Syst. Parasitol. 2016, 93, 257–269. [Google Scholar] [CrossRef]
- Wilbert, N.; Schmeier, U. Survey of the Intestinal Opalines and Ciliates in Central European Amphibians. Arch. Protistenkd. 1982, 125, 271–285. [Google Scholar] [CrossRef]
- Delvinquier, B.L.J.; Markus, M.B.; Passmore, N.I. Opalinidae in African Anura. IV. Genus Protoopalina. Syst. Parasitol. 1995, 30, 81–120. [Google Scholar] [CrossRef]
- Li, M.; Hu, G.; Li, C.; Zhao, W.S.; Zou, H.; Li, W.X.; Wu, S.G.; Wang, G.T.; Ponce-Gordo, F. Morphological and molecular characterization of a new ciliate Nyctotheroides grimi n. sp. (Armophorea, Clevelandellida) from Chinese frogs. Acta Trop. 2020, 208, 105531. [Google Scholar] [CrossRef] [PubMed]
- Colwell, R.K.; Elsensohn, J.E. EstimateS turns 20: Statistical estimation of species richness and shared species from samples, with non-parametric extrapolation. Ecography 2014, 37, 609–613. [Google Scholar] [CrossRef]
- Sørensen, T.A. A method of establishing groups of equal amplitude in plant sociology based on similarity of species content and its application to analyses of the vegetation on Danish commons. K. Dan. Vidensk. Selskab. 1948, 5, 1–34. [Google Scholar]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Sinnappah, N.D.; Lim, L.-H.S.; Rohde, K.; Tinsley, R.; Combes, C.; Verneau, O. A paedomorphic parasite associated with a neotenic amphibian host: Phylogenetic evidence suggests a revised systematic position for Sphyranuridae within anuran and turtle Polystomatoineans. Mol. Phyl. Evol. 2001, 18, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Kaci-Chaouch, T.; Verneau, O.; Desdevises, Y. Host specificity is linked to intraspecific variability in the genus Lamellodiscus (Monogenea). Parasitology 2008, 135, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Badets, M.; Verneau, O. Origin and evolution of alternative developmental strategies in amphibious sarcopterygian parasites (Platyhelminthes, Monogenea, Polystomatidae). Org. Divers. Evol. 2009, 9, 155–164. [Google Scholar] [CrossRef]
- Snyder, S.D.; Loker, E.S. Evolutionary relationships among the Schistosomatidae (Platyhelminthes: Digenea) and an Asian origin for Schistosoma. J. Parasitol. 2000, 86, 283–288. [Google Scholar] [CrossRef]
- Moszczynska, A.; Locke, S.A.; McLaughlin, J.D.; Marcogliese, D.J.; Crease, T.J. Development of primers for the mitochondrial cytochrome c oxidase I gene in digenetic trematodes (Platyhelminthes) illustrates the challenge of barcoding parasitic helminths. Mol. Ecol. Resour. 2009, 9, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Sinsch, U. Review: Skeletochronological assessment of demographic life-history traits in amphibians. Herpetol. J. 2015, 25, 5–13. [Google Scholar]
- Dollfus, R.P. Trematodes récoltés au Congo Belge par le Professeur Paul Brien (mai-août 1937). Ann. Mus. Congo Belge. C.-Zool. 1950, 1, 1–135. [Google Scholar]
- Meskal, F.H. Trematodes of anurans from Ethiopia. Arb. Univ. Bergen Mat.-Naturv. 1970, 1, 1–73. [Google Scholar]
- Aisien, S.O.; Ugbo, A.D.; Ilavbare, A.N.; Ogunbor, O. Endoparasites of amphibians from South-Western Nigeria. Acta Parasitol. 2001, 46, 299–305. [Google Scholar]
- Aisien, M.; Sampson, S.A.; Amuzie, C. Anuran parasites from three biotopes in Rivers State, Nigeria. Niger. J. Parasitol. 2017, 38, 129–135. [Google Scholar] [CrossRef]
- Okere, S.; Joseph, F.; Amuzie, C. Endo-parasitic helminths of amphibians, Ptychadena mascareniensis and Ptychadena pumilio at Rumuji-Emohua, Rivers state, Nigeria. Afr. J. Environ. Nat. Sci. Res. 2019, 2, 71–76. [Google Scholar]
- Edo-Taiwo, O.; Aisien, M. Helminth parasitic infections of leaf litter frogs (Arthroleptis and Phrynobatrachus spp.) from cocoa plantations in southern Nigeria. Niger. J. Parasitol. 2020, 41, 93–100. [Google Scholar] [CrossRef]
- Imasuen, A.; Aisien, M. Helminth parasites of Silurana tropicalis from the Okomu National Park, Edo State, Nigeria. Niger. J. Parasitol. 2015, 36, 61–66. [Google Scholar]
- Imasuen, A.; Aisien, M. Helminth parasitofauna of Ptychadena species from altered rainforest. Niger. J. Parasitol. 2019, 40, 193–197. [Google Scholar] [CrossRef]
- Imasuen, A.A.; Ojo, O.O.; Adesina, O.O.; Enabuele, E.E.; Aisien, M. Parasitic endohelminths of tree frogs from two rainforest habitats in Edo State, Nigeria. Zoologist 2020, 17, 13–17. [Google Scholar] [CrossRef]
- Tinsley, R.C. Parasites of Xenopus. In The Biology of Xenopus; Tinsley, R.C., Kobel, H.R., Eds.; Clarendon Press: London, UK, 1996; pp. 233–262. [Google Scholar]
- Paul, A.A. Life History Studies of North American Fresh-Water Polystomes. J. Parasitol. 1938, 24, 489–510. [Google Scholar] [CrossRef]
- Badets, M.; Morrison, C.; Verneau, O. Alternative parasite development in transmission strategies: How time flies! J. Evol. Biol. 2010, 23, 2151–2162. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, R.C. Correlation between life-cycle of Eupolystoma alluaudi (Monogenea) and ecology of its host Bufo regularis. Parasitology 1975, 71, R16–R17. [Google Scholar]
- Hamann, M.I. Seasonal maturation of Glypthelmins vitellinophilum (Trematoda: Digenea) in Lysapsus limellus (Anura: Pseudidae) from an Argentinian subtropical permanent pond. Rev. Bras. Biol. 2006, 66, 85–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolek, M.G.; Snyder, S.D.; Janovy, J., Jr. Alternative Life Cycle Strategies and Colonization of Young Anurans by Gorgoderina attenuata in Nebraska. J. Parasitol. 2009, 95, 604–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nollen, P.M.; Alberico, R.A. Absence of testes in Gorgoderina attenuata. J. Parasitol. 1972, 58, 1011–1012. [Google Scholar] [CrossRef] [PubMed]
- Hoole, D.; Mitchell, J.B. Gorgoderina vitelliloba (Digenea: Gorgoderidae) in Hyla arborea. Z. Parasitenkd.-Parasitol. Res. 1984, 70, 829–831. [Google Scholar] [CrossRef]
- Johnson, P.T.J.; Hoverman, J.T. Parasite diversity and coinfection determine pathogen infection success and host fitness. Proc. Natl. Acad. Sci. USA 2012, 109, 9006–9011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoverman, J.T.; Hoye, B.J.; Johnson, P.T.J. Does timing matter? How priority effects influence the outcome of parasite interactions within hosts. Oecologia 2013, 173, 1471–1480. [Google Scholar] [CrossRef]
- Johnson, P.T.; McKenzie, V.J. Effects of environmental change on helminth infections in amphibians: Exploring the emergence of Ribeiroia and Echinostoma infections in North America. In The Biology of Echinostomes; Toledo, R., Fried, B., Eds.; Springer: New York, NY, USA, 2009; pp. 249–280. [Google Scholar] [CrossRef]
- Orlofske, S.A.; Belden, L.K.; Hopkins, W.A. Effects of Echinostoma trivolvis metacercariae infection during development and metamorphosis of the wood frog (Lithobates sylvaticus). Comp. Biochem. Physiol. Mol. Integr. Physiol. 2017, 203, 40–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LaFonte, B.E.; Johnson, P.T.J. Experimental infection dynamics: Using immunosuppression and in vivo parasite tracking to understand host resistance in an amphibian-trematode system. J. Exp. Biol. 2013, 216, 3700–3708. [Google Scholar] [CrossRef] [Green Version]


| Hyperolius kivuensis (Prevalence) | Hyperolius viridiflavus (Prevalence) | GenBank Accession Numbers | |||
|---|---|---|---|---|---|
| Huye n = 89 | Musanze n = 26 | Huye n = 100 | Muhanga n = 29 | ||
| Annelida: | |||||
| Dero rwandae (nHK = 53) | 6 (5.2%) | - | - | MN215405, MN555814, MN555815, MN215422, MN555439, MN555440, MN215423, MN555442, MN555443 | |
| Nematoda: | |||||
| Orneoascaris chrysanthemoides (nHK = 24, nHV = 3) | 4 (4.5%) | - | 2 (2.0%) | - | MN912507, MN912509 |
| Orneoascaris schoutedeni (nHK = 3) | - | 1 (0.9%) | - | MN912510 | |
| Aplectana chamaeleonis (nHK = 23) | 5 (5.6%) | 5 (19.2%) | - | MN907375, MN907376, MN907377, MN907378 | |
| Rhabdias collaris (nHV = 1) | - | 1 (1.0%) | - | MN792646, MN927222 | |
| Trematoda, Digenea: | |||||
| Clinostomum chabaudi (nHK = 81, nHV = 80) | 17 (19.1%) | 4 (15.4%) | 20 (20.0%) | 3 (10.3%) | MW528862, MW528861, MW528859, MW528855, MW528854, MW528856, MW528857, MW528858, MW528860, MW528863, MW525123, MW525125, MW525129, MW525124, MW525122, MW525126, MW525128, MW525127, MW525121, MW525130 |
| Undet. echinostomatides (nHK = 27, nHV = 939) | 4 (4.5%) | 2 (7.6%) | 19 (19.0%) | 28 (96.6%) | OL598857, OL598858, OL598859, OL598860, OL598861, OL598862, OL598863, OL598864 |
| Glypthelmins africana (nHV = 39) | - | 13 (13.0%) | - | OL413039, OL413040, OL413041, OL413042, OL413043, OL413046 | |
| Gorgoderinaafricana (nHV = 6) | - | 5 (5.0%) | - | OL413045, OL413037, OL413038 | |
| Trematoda, Monogenea: | |||||
| Polystoma sp. (nHV = 20) | - | 5 (5.0%) | 1 (3.4%) | OL413034, OL413047 | |
| Cestoda, Cyclophyllidea: | |||||
| Nematotaenia sp. (nHV = 28) | - | 4 (4.0%) | 2 (6.8%) | OL396583, OL396584, OL396586, OL396587 | |
| Ciliata, Armophorea: | |||||
| Nyctotheroides sp. | 19 (21.1%) | 3 (11.5%) | 3 (3.0%) | 1 (3.4%) | - |
| Heterokonta, Opalinidae: | |||||
| Protoopalina sp. | 25 (28.0%) | 6 (23.1%) | 16 (16.0%) | 2 (6.8%) | - |
| Endoparasite Load N Species | Hyperolius kivuensis Prevalence [n Parasites Per Host] | Parasite Associations (Number of Infested Frogs) | Hyperolius viridiflavus Prevalence [n Parasites Per Host] | Parasite Associations (Number of Infested Frogs) |
|---|---|---|---|---|
| 0 | 65.2% [-] | - | 38.0% [-] | - |
| 1 | 32.2% [1–22] | Clinostomum (n = 18) Aplectana (n = 9) E. metacercaria (n = 5) O. chrysanthemoides (n = 4) Dero (n = 3) | 46.5% [1–100] | E. metacercaria (n = 32) Clinostomum (n = 10) Glypthelmins (n = 8) Polystoma (n = 4) Gorgoderina (n = 3) O. chrysanthemoides (n = 2) Nematotaenia (n = 1) |
| 2 | 1.7% [9–12] | Dero, Aplectana (n = 1) Dero, Clinostomum (n = 1) | 14.0% [2–35] | Clinostomum, E. metacercaria (n = 10) Clinostomum, Nematotaenia (n = 1) Clinostomum, Glypthelmins (n = 1) Glypthelmins, Gorgoderina (n = 1) Glypthelmins, Rhabdias (n = 1) Nematotaenia, E. metacercaria (n = 1) Polystoma, E. metacercaria (n = 1) Polystoma, Nematotaenia (n = 1) |
| 3 | 0.9% [12] | Clinostomum, O. schoute-deni, E. metacercaria (n = 1) | 1.6% [7–12} | Nematotaenia, Clinostomum, E. metacercaria (n = 1) Nematotaenia, Glypthelmins, Gorgoderina (n = 1) |
| Hyperolius kivuensis | Hyperolius viridiflavus | |||||
|---|---|---|---|---|---|---|
| October | January | March | October | January | March | |
| Dero rwandae | - | - | - | - | - | |
| Prevalence [%] | 23.1 | |||||
| Intensity of infestation | 8.8 ± 2.8 | |||||
| Range | 1–20 | |||||
| Orneoascaris chrysanthemoides | - | - | ||||
| Prevalence [%] | 2.2 | 8.6 | 1 | 4 | ||
| Intensity of infestation | 1 | 7.7 ± 4.4 | 2 | 1 | ||
| Range | 1 | 1–16 | 2 | 1 | ||
| Aplectana chamaeleonis | - | - | - | |||
| Prevalence [%] | 13.6 | 8.6 | 3.8 | |||
| Intensity of infestation | 1.7 ± 0.4 | 1.7 ± 1.0 | 7 | |||
| Range | 1–3 | 1–4 | 7 | |||
| Clinostomum chabaudi | ||||||
| Prevalence [%] | 20.5 | 14.3 | 26.9 | 16.7 | 8 | 27.8 |
| Intensity of infestation | 4.7 ± 1.5 | 1.8 ± 0.4 | 4.3 ± 3.0 | 3.8 ± 1.2 | 2.5 ± 0.5 | 3.0 ± 1.3 |
| Range | 1–15 | 1–3 | 1–22 | 1–15 | 2–3 | 1–7 |
| Undet. echinostomatid | - | |||||
| Prevalence [%] | 4.4 | 15.4 | 42.7 | 8 | 22.2 | |
| Intensity of infestation | 1.5 ± 0.5 | 6.0 ± 1.7 | 19.3 ± 3.2 | 13.0 ± 12.0 | 30.3 ± 23.3 | |
| Range | 1–2 | 1–8 | 1–100 | 1–25 | 4–100 | |
| Gorgoderina africana | - | - | - | - | ||
| Prevalence [%] | 1 | 16 | ||||
| Intensity of infestation | 1 | 1.3 ± 0.3 | ||||
| Range | 1 | 1–2 | ||||
| Glypthelmins africana | - | - | - | |||
| Prevalence [%] | 10 | 44 | 11.1 | |||
| Intensity of infestation | 1.8 ± 0.6 | 3.1 ± 0.9 | 2.5 ± 1.5 | |||
| Range | 1–3 | 1–10 | 1–4 | |||
| Polystoma sp. | - | - | - | - | - | |
| Prevalence [%] | 6.3 | |||||
| Intensity of infestation | 3.3 ± 1.4 | |||||
| Range | 1–10 | |||||
| Nematotaenia sp. | - | - | - | - | ||
| Prevalence [%] | 5.2 | 4 | ||||
| Intensity of infestation | 5.4 ± 1.9 | 1 | ||||
| Range | 2–11 | 1 | ||||
| Host Infestation | Hyperolius kivuensis Survival [d] | Hyperolius viridiflavus Survival [d] |
|---|---|---|
| Parasite-free controls | 52.4 ± 4.3 n = 16 | 51.9 ± 3.3 n = 12 |
| Single-species infested frogs | ||
| Orneoascaris chrysanthemoides | - | 31 n = 1 |
| Aplectana chamaeleonis | 60 ± 0.0 n = 6 | - |
| Clinostomum chabaudi | 45.8 ± 7.4 n = 8 | 53.7 ± 6.3 n = 3 |
| Undet. echinostomatid | 60 n = 1 | 60 ± 0.0 n = 2 |
| Gorgoderina africana | - | 60 n=1 |
| Polystoma sp. | - | 40.3 ± 9.9 n = 3 |
| Nematotaenia sp. | - | 34 n = 1 |
| Multi-species infested frogs | 60 n = 1 | 39.3 ± 10.3 n = 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinsch, U.; Balczun, C.; Scheid, P.; Dehling, J.M. Component Endoparasite Communities Mirror Life-History Specialization in Syntopic Reed Frogs (Hyperolius spp.). Diversity 2021, 13, 669. https://doi.org/10.3390/d13120669
Sinsch U, Balczun C, Scheid P, Dehling JM. Component Endoparasite Communities Mirror Life-History Specialization in Syntopic Reed Frogs (Hyperolius spp.). Diversity. 2021; 13(12):669. https://doi.org/10.3390/d13120669
Chicago/Turabian StyleSinsch, Ulrich, Carsten Balczun, Patrick Scheid, and Jonas Maximilian Dehling. 2021. "Component Endoparasite Communities Mirror Life-History Specialization in Syntopic Reed Frogs (Hyperolius spp.)" Diversity 13, no. 12: 669. https://doi.org/10.3390/d13120669
APA StyleSinsch, U., Balczun, C., Scheid, P., & Dehling, J. M. (2021). Component Endoparasite Communities Mirror Life-History Specialization in Syntopic Reed Frogs (Hyperolius spp.). Diversity, 13(12), 669. https://doi.org/10.3390/d13120669








