Abstract
Numerous amphibian species are declining because of habitat loss and fragmentation due to urbanization of landscapes and the construction of roads. This is a mounting threat to species restricted to habitats close to urban areas, such as agricultural wetlands in North East Asia. The Suweon treefrog (Dryophytes suweonensis) falls into the list of species threatened with habitat loss and most populations are under threat of extirpation. Over the last decades, sub-populations have become increasingly disconnected and specifically the density of paved roads has increased around the only site connecting northern and southern Seoul populations. We surveyed this locality in Hojobeol, Siheung, Republic of Korea in 2012, 2015 and 2019 to first confirm the decline in the number of sites where D. suweonensis was present. The second objective was to analyze the habitat characteristics and determine the remaining suitable habitat for D. suweonensis through a species distribution model following the maximum entropy method. Our results show that rice paddy cover and distance from the paved road are the most important factor defining suitable habitat for D. suweonensis. At this locality, uninterrupted rice paddies are a suitable habitat for the species when reaching at least 0.19 km2, with an average distance of 138 ± 93 m2 from the roads. We link the decrease in the number of sites where D. suweonensis is present with the decrease in rice paddy cover, generally replaced by localized infrastructures, greenhouses and habitat fragmentation. Rice paddies should remain connected over a large area for the protection of the remaining populations. In addition, habitat requirements should be integrated in the requisites to designate protected areas.
1. Introduction
Amphibian decline is a global issue [1,2,3,4] and the destruction of habitats, along with fragmentation and disturbance, is one of the primary factors resulting in biodiversity decline [1,5,6,7]. Habitat connectivity plays a primordial role in the subsistence of species [8,9], with population isolation, inbreeding and edge effect caused by habitat loss and fragmentation having a significant impact on species declines [10,11,12]. Specifically, while destroying patches of habitat, urbanization also leaves the remaining fragments disconnected, further accentuating its negative impact. As a result, population sizes decrease because of the loss of genetic diversity [13] and eventually populations are caught in an extinction vortex [14].
A variety of landscape features can result in habitat disconnection, including natural barriers such as rivers and mountains [15,16,17]. Artificial structures are a more recent cause of habitat fragmentation and roads are the main driver resulting in the loss of habitat connectivity and the increase in fragmentation [18]. In addition, most man-made structures impair connectivity and even light structures such as the transformation of agricultural fields into greenhouse will negatively affect the species within the habitat [19]. Therefore, habitat fragmentation and disturbance must be prevented when aiming at preserving species diversity [20].
Despite their artificial origins, rice paddies are adequate substitute habitats for organisms when natural wetland are not available [21]. Even if agriculture has been listed as one of the principal cause for decrease of wildlife and destruction of the natural habitat [22], the biodiversity conservation function of rice paddies is becoming increasingly prominent [23]. For instance, rice paddies were developed to enhance biodiversity conservation [24,25] and in some areas they are the only habitat left. In the Republic of Korea, 10 of the 13 anuran species use rice paddies during their life cycle [26]. However, urbanization and land development are also damaging the rice paddy environment, critically affecting biodiversity [24,27].
Analyzing the relation between species specific environmental factors and habitat type is therefore needed to understand species requirements and prevent population declines [3]. Our research area in the Republic of Korea focuses on the only remaining locality connecting populations of Dryophytes suweonensis north and south of Seoul’s urban area, a species now present exclusively in rice paddies [28]. The area is composed of rice paddies that used to cover more than 5 km2 a few decades ago, followed by a markedly decrease in rice paddy area due to urbanization and resulting in the landscape with the remaining rice paddies being now fragmented by roads and urban areas. We conducted this study to examine the habitat requirements in relation with habitat connectivity for D. suweonensis through species distribution models. Lastly, we evaluated how each of the determined environmental variables affects the presence of D. suweonensis in the habitat and determined the variables importance for the survival of the species.
2. Materials and Methods
2.1. Species Introduction
The Suweon treefrog (Dryophytes suweonensis) is a Hylidae species distributed on the western lowlands of the Korean Peninsula, relying on lentic shallow water bodies to breed. The species is declining, even when controlling for natural fluctuations in amphibian population size [29], mostly because its natural habitat has been highly modified by human activities [30] and the species is now found breeding in rice paddies only [28]. Agricultural wetlands are adequate substitute breeding habitat as the species takes advantage of the controlled hydroperiod [31] and about 40% of the sites where the species occurs are rice paddies on land reclaimed from tidal flats since the 1960’s [30]. According to habitat analyses, D. suweonensis is present far from forest edges and close to rivers [32] and also generally avoids rice paddy margins when calling, especially when paved with concrete [19].
2.2. Study Area
This study is focused on Siheung, in Gyeonggi Province, Republic of Korea. The mean annual temperature of the area between 2010 and 2019 was 12.5 °C, ranging from −15.1 °C to 36.2 °C and the mean value of annual precipitations was 1150 mm (Climate Korean Meteorological Administration; https://data.kma.go.kr/ (accessed on 21 January 2021)). To collect data to build our ecological models, we first surveyed the area in Hojobeol (37.407° N, 126.805° E; Figure 1), the focal locality of this project. The area is about 5 km2 and it is now covered with agricultural fields and localized infrastructures (Figure 1). The area was originally a tidal flat that was reclaimed in 1721.
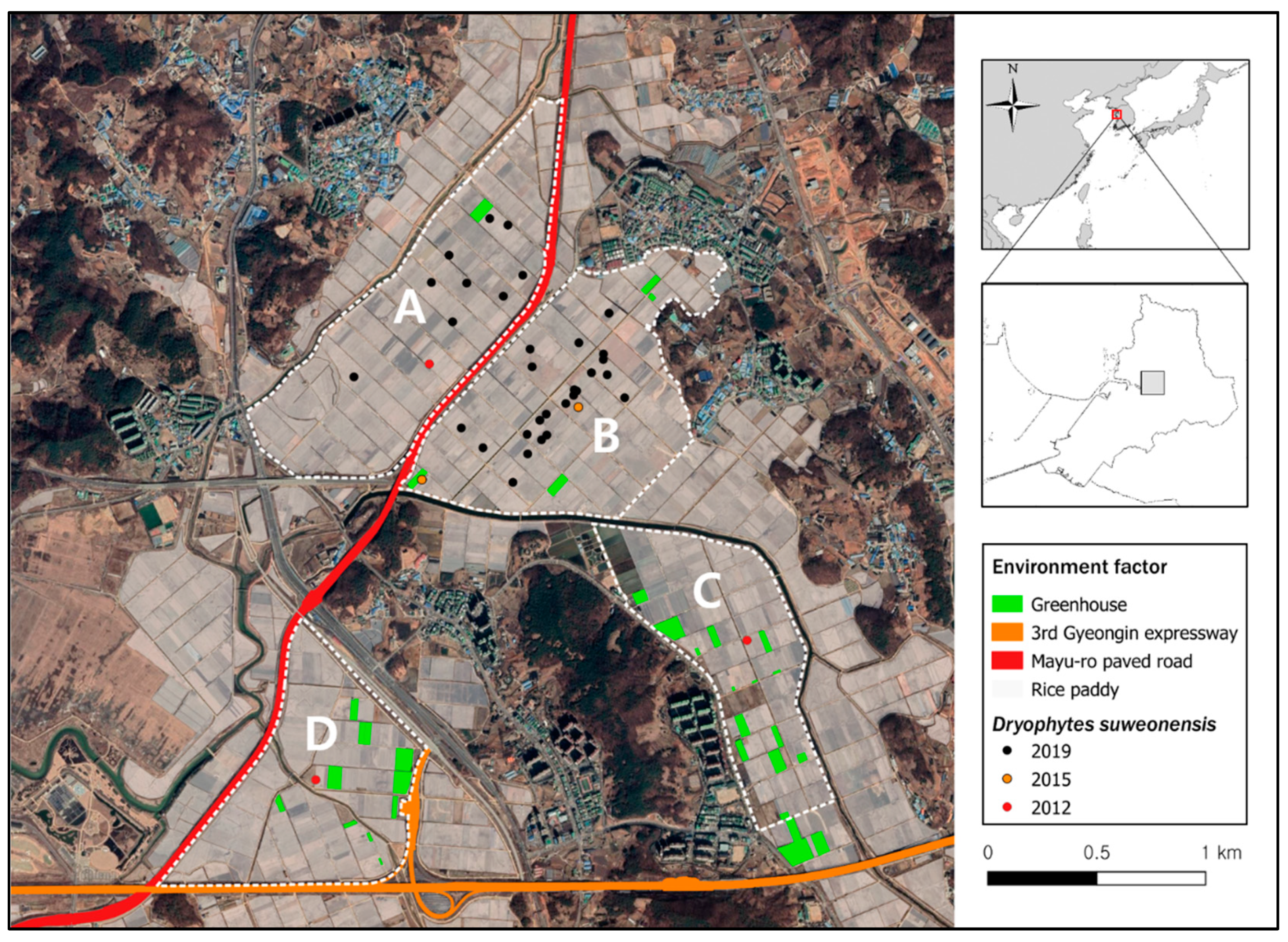
Figure 1.
Spatial location of the research sites in this study. Sites (A–D) are large rice paddies areas, in Hojobeol, Siheung, Republic of Korea, where Dryophytes suweonensis was observed between 2012 and 2019. Habitat disturbance due to paved road and green house is highlighted in green, orange and red. The sites (A) and (B) were separated by the Mayu-ro paved road (red) constructed in 2016. The site (D) and (C) were fragmented in 2010 when the 3rd Gyeongin expressway was opened.
Since 1721 and until recent times, the area around Hojobeol was covered by contiguous rice paddies but it is now fragmented due to urbanization and the construction of roads such as the 3rd Gyeongin expressway, an eight-lane expressway completed in 2010; and the Mayu-ro, a six-lane paved road completed in 2016 (Figure 1). The locality studied here is now divided into four independent areas by a ≥25 m-wide paved road and a ≥40 m-wide river. These landscape structures are generally impermeable to amphibian dispersal [33], including to D. suweonensis [34]. We designated four study sites such as site A (1.05 km2), site B (1.05 km2), site C (0.67 km2) and site D (0.78 km2; Figure 1).
We conducted survey at each of these sites to determine the presence of D. suweonensis individuals in 2012, 2015 and 2019. The surveys were conducted in the form of aural point- and transect-surveys, a method demonstrated to be adequate to accurately determine the presence of the species [35]. The surveys were supplemented by traditional spotlight surveys following the edges of rice paddy at night. We conducted surveys during the breeding season and the period when metamorphs and adults are easily visible on the vegetation, between June and August of each year.
2.3. Modeling Method
We used species distribution models (SDMs) to analyze habitat preferences and predict habitat usage by D. suweonensis in the focal area. SDMs have recently demonstrated to be highly reliable for biodiversity conservation [36,37,38,39]. The SDMs were performed with the maximum entropy (MaxEnt) modeling software MaxEnt (v. 3.4.1). In addition, we have conducted other machine learning SDMs based on presence-absence data for comparison. Here we used the: random forest (RF) and boosted regression trees (BRT) methods as they generally provide robust predictions in comparison to MaxEnt [40]. However, here we excluded RF and BRT from the main results (Figure A1) as MaxEnt best explained the distribution of D. suweonensis. We modeled the probability of presence of D. suweonensis in the four areas studied utilizing the known presence datapoints originating from the surveys and using 10,000 random datapoints as a proxy for absence, together with the environmental factors of the research locality. The parameters were set such as 25% random test, 15 bootstrap replicates, 5000 iterations and regularization multiplier = 1 [41,42]. A smaller regularization multiplier concentrates the habitat suitability closer to the presence data and it increases the possibility of overfitting, while a higher regularization multiplier can result in rougher and underfitted models which have low discrimination [43]. However, the results of both alternative models (Appendix A Figure A2) were not biologically meaningful as they went against the known ecology of the species. For instance, D. suweonensis does not prefer the edges of rice paddy adjacent to roads but prefers the center of the rice paddy [44]. As a result, we used a regularization multiplier value of 1 for all analyses.
We used a 10 m × 10 m grid resolution of environmental variables, computed from 1:5000 map data that can be expressed at 1 m resolution. To determine which habitat is suitable for D. suweonensis, we determined a threshold following the average suitability approach. The value for average suitability is calculated as the mean predicted suitability value for the areas with known presence datapoints. The areas with values over the threshold were designated as suitable habitat for the species while areas below the threshold were designated as unsuitable. The average suitability approach is simple and easily applicable but robust because it relies on flexible criteria according to the data used to build the model and does not rely on a fixed standard [45]. The reliability of the modeling result was evaluated through the area under the receiver operating characteristic (ROC) curve (AUC) value, built in the MaxEnt software and the true skill statistic (TSS). We used the AUC to statistically assess the test results, following the literature where the AUC is the most common quantitative index describing the ROC curve [46,47]. TSS is a method to determine the accuracy and reliability of a model, based on sensitivity and specificity [48].
The model was based on the environmental variables important to D. suweonensis. We selected the distance to forest, freshwater bodies such as streams and lakes and paved road as variables to be included in the model. In addition, as the vegetation index is an important variable for habitat analysis because it is highly correlated with climate change and landscape modification, we included the normalized difference vegetation index (NDVI) in the model, one of the most widely used vegetation indices [49,50]. Altitude and slope were also included as basic terrain.
We used seven environmental variables, including rice paddy cover, distance to forest, distance to freshwater, distance to paved road, NDVI, altitude and slope to build our models. Correlation analysis were performed with SPSS v 24.0 (IBM Corp. 2016) to check for multicollinearity (i.e., correlation between a pair of variable over |0.7| [51,52]. No variable pair crossed the threshold (r < 0.61). Distance to forest, distance to freshwater and rice paddy cover were extracted from the 2019 land cover layer downloaded from the Environmental Geographic Information Service (EGIS; https://egis.me.go.kr/main.do (accessed on 21 January 2021)). Distance to paved road was extracted from the 2019 road data downloaded from the National Spatial Data Infrastructure Portal (NSDI; http://www.nsdi.go.kr/lxportal/?menuno=2679 (accessed on 21 January 2021)). NDVI was built from satellite images taken in July 2019 and downloaded from the United States Geological Survey (USGS; https://www.usgs.gov/ (accessed on 21 January 2021)). Altitude and slope data were extracted from the 2014 digital elevation model from the National Geographic Information Institute (http://map.ngii.go.kr/mn/mainPage.do (accessed on 21 January 2021)). Lastly, we compared our modeling results with the actual environment values at the four sites surveyed for the two variables the most important to explain habitat suitability for D. suweonensis, namely rice paddy cover and distance from paved road.
3. Results
3.1. Sampling and Habitat Analysis
We found Dryophytes suweonensis in the rice paddies of all four sites during the surveys in 2012. We observed D. suweonensis at the site B only in 2015 but we found D. suweonensis at sites A and B in 2019. In total, we obtained 36 datapoints to analyze the habitat used by D. suweonensis and we used these points to build the models. To prepare the data, we analyzed the seven environmental values selected for the 36 points where we observed D. suweonensis (Table 1) and later compared these values with the modeling result.

Table 1.
Environmental values at the locality where Dryophytes suweonensis was observed (sites A, B, C and D; Figure 1) in Siheung in 2012, 2015 and 2019. This table highlights the relationship between habitat suitability values, ranges of model results and contribution of each environmental variables.
3.2. Modeling Results
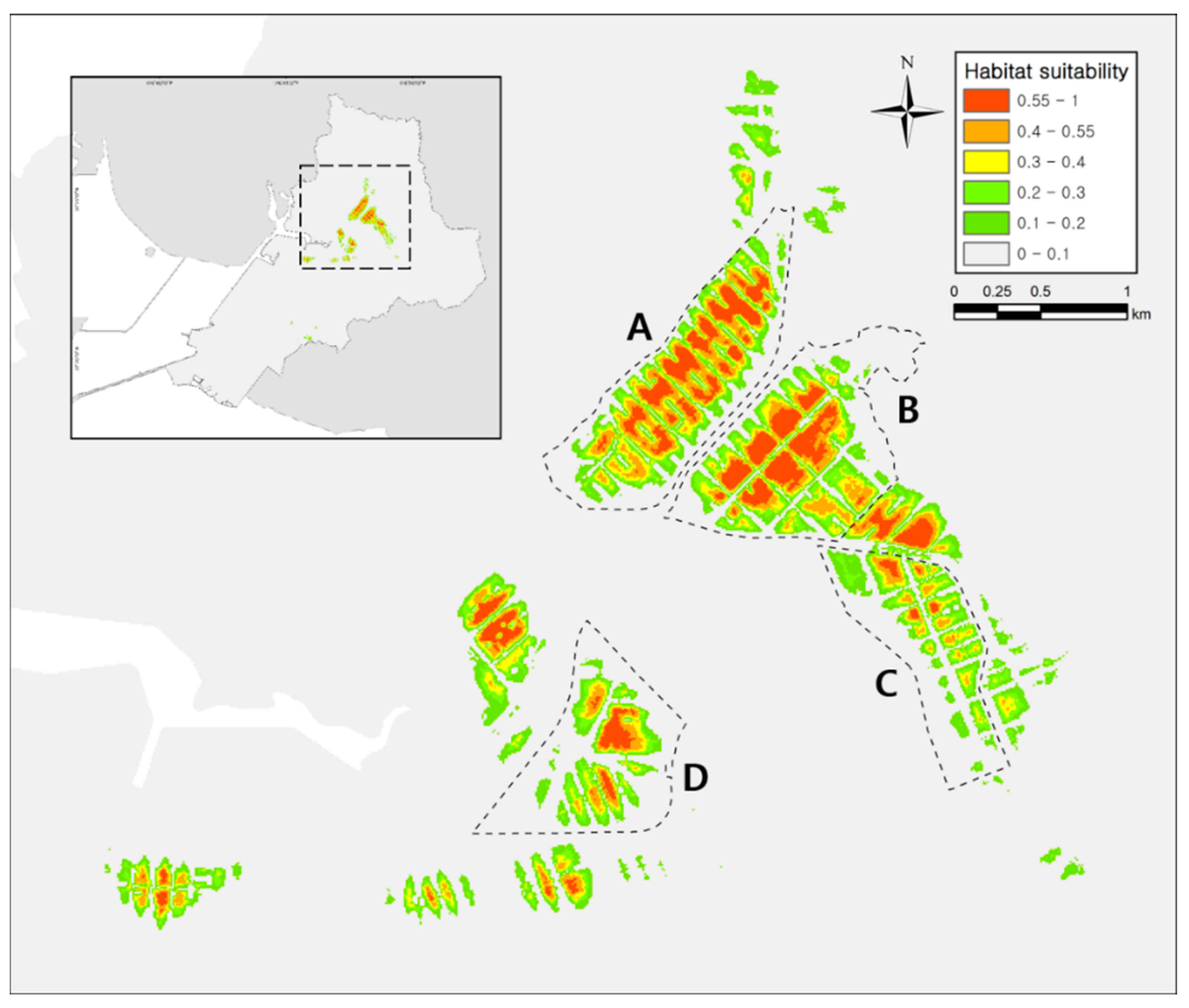
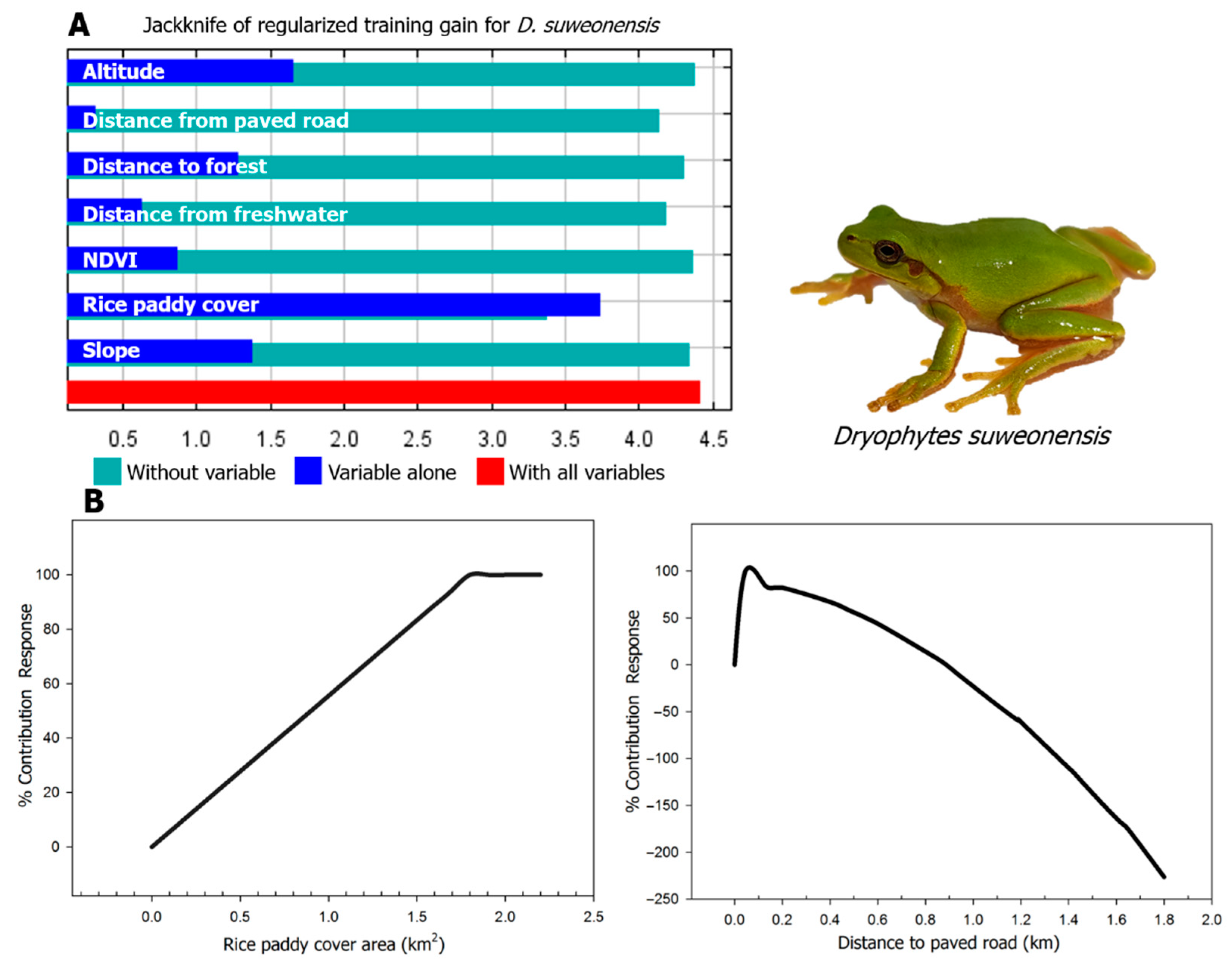
The AUC (area under curve) and TSS (true skill statistic) values indicate that the reliability of the model was excellent (test AUC value = 0.994; training AUC value = 0.996; test TSS value = 0.977; training TSS value = 0.981). The modeling results converged within a mean iteration value of 716 and provided a habitat suitability index ranging from 0 to 1, from most unsuitable to most suitable habitat [53]. The average suitability approach resulted in a threshold of 0.55 and we therefore considered the habitat with a value between 0.55 and 1 to be suitable for the species. The suitable habitat for the species covered 0.43 km2 or 4.35% of the total area of rice paddies in Siheung (10.69 km2; Figure 2). According to the Jackknife test result, rice paddy cover (81.9%), distance to paved road (5.1%), NDVI (normalized difference vegetation index; 4.5%), distance to freshwater (4.1%), distance to forest (2.5%), slope (1.4%) and altitude (0.5%) had the highest contribution to the model.
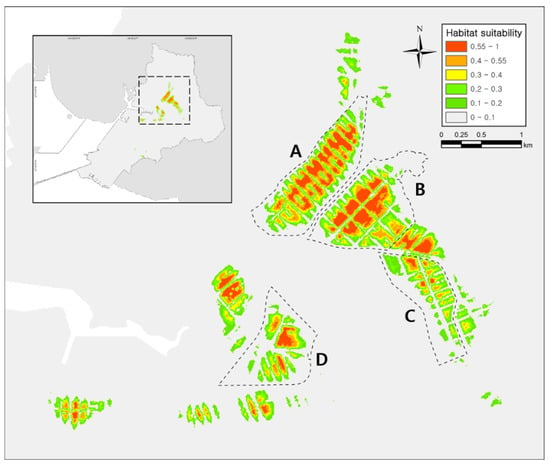
Figure 2.
Result of Dryophytes suweonensis distribution model for Hojobeol, Siheung, in the Republic of Korea. Our analysis highlights 0.55 as the threshold above which an area was suitable for D. suweonensis. Sites (A) and (B) contain the largest suitable while sites (C) and (D) are less suitable for the species.
For the variable rice paddy cover, habitat suitability increased with the size of the area of connected rice paddy, until reaching a plateau phase at 0.19 km2 (Figure 3). The species was found in areas with a rice paddy cover of at least 0.12 km2. The closer to a paved road, the less suitable the habitat was for D. suweonensis and paved roads had an effect over 1 km. NDVI had a positive relationship with habitat suitability of D. suweonensis, until reaching a plateau phase at about 0.65 and the species was estimated to be able to exploit habitats with values ranging from 0 to 0.78, although individuals were observed at sites with values between 0.31 to 0.73. The habitat was determined to be suitable for the species up to 500 m away from freshwater but habitat suitability also decreased with distance from freshwater. Habitat suitability was also higher when close to forests, with a threshold in impact until 600 m. Regarding slope and altitude, the lowest values were associated with higher habitat suitability for D. suweonensis (Table 1).
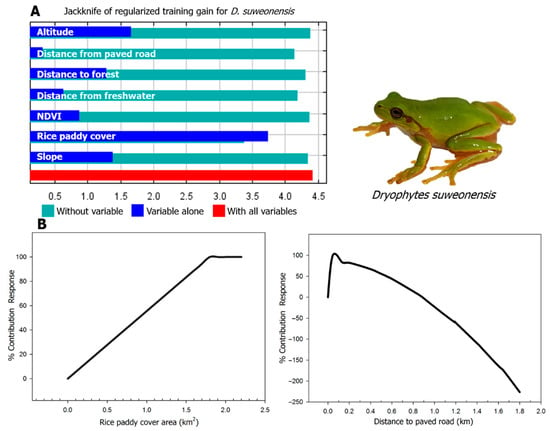
Figure 3.
Jackknife test result and response curves of Dryophytes suweonensis to the two most important environmental variables in Hojobeol, Siheung, Republic of Korea. (A) Jackknife test for seven environmental variables. The horizontal blue bar shows that rice paddy cover has the highest gain and therefore it is most useful environmental variable to the model. The green horizontal bars also show that the omission of the variable rice paddy cover results in the largest decreases in gain, indicating a stronger impact than the other variables. (B) Response of D. suweonensis to rice paddy cover and distance to paved road. According to the response curve of rice paddy cover, habitat suitability increases with the size of the area of connected rice paddy, until reaching a plateau phase at about 0.19 km2. The distance to paved road to 45 m increased steeply up the suitability of D. suweonensis and gradually decreased thereafter.
The in-depth analyses at the survey sites showed that based on rice paddy cover requirement only, the habitable was suitable on 0.66 km2 (62.9%) out of a total area of 1.05 km2 at site A, with a mean (± standard deviation) rice paddy cover of 0.14 ± 0.04 km2. At site B (1.05 km2), the suitable habitat covered 0.66 km2 (62.8%), with a mean rice paddy cover of 0.13 ± 0.03 km2. At site C (0.67 km2), the suitable habitat was 0.32 km2 (47.9%), with a mean rice paddy cover of 0.11 ± 0.02 km2. At site D (0.78 km2), the suitable habitat was 0.25 km2 (31.3%), with a mean rice paddy cover of 0.10 ± 0.02 km2. The mean value and standard error for the distance from paved road was 138 ± 93 m2 at the site A, 49 ± 28 m2 at the site B, 37 ± 28 m2 at the site C and 39 ± 28 m2 at the site D. According to the response curve, an increase in distance to paved road up to 45 m matched with the steep increase in habitat suitability for D. suweonensis and gradually decreased thereafter (Figure 3). Mean values of distance to paved road were 138 ± 93 m at the site A, 49 ± 28 m at the site B, 37 ± 24 m at the site C and 39 ± 28 m at the site D (Table 2). The mean distance to paved roads at sites A and B was relatively higher than at the sites C and D and the sites A and B had a higher habitat suitability.

Table 2.
Descriptive statistics for the two environmental variables having the largest effect on habitat suitability for Dryophytes suweonensis in Siheung, Republic of Korea. These two variables are rice paddy cover and distance from paved road, extracted from 36 points where D. suweonensis were observed in 2012, 2015 and 2019. The data is provided broken down by sites A, B, C and D (details on Figure 1).
4. Discussion
This study determined that rice paddy cover and distance to paved roads were the principal characteristics explaining habitat suitability for Dryophytes suweonensis in Siheung, Republic of Korea, a reasonable proxy for the species in general. Our results showed that the remaining suitable habitats for D. suweonensis in the area are the large rice paddies near Hojobeol (Figure 1). Specifically, we found that rice paddy cover was critical and had the highest contribution (81.9%) to habitat suitability, with all other variables combined contributing to only about 5%. We conclude that, the highest the rice paddy cover, the more suitable the habitat is for D. suweonensis. While a new quantitative result, this finding is reflected by the fact that rice paddies are the only habitat of D. suweonensis [28] and the size of the rice paddy complexes is known to be significant factor to the presence of the species [30,54].
For the habitat to be suitable for D. suweonensis, the rice paddy cover should be at least 0.12 km2, although the species was only found in areas with a rice paddy cover higher than 0.19 km2 and a larger area may be needed to host stable populations as the average size of rice paddy complexes where the species is present in the Republic of Korea is 4.78 ± 4.36 km2 [30]. At the locality studied in this project, the site C was isolated from the other sub-populations by the Botong stream, villages and high hills, while the site D was isolated from the other rice paddies by a wide eight-lane road. Thus, the habitat suitability at the sites C and D is below the threshold required for D. suweonensis to be present and it likely explains why the species was not detected at these sites recently. Also, while the species was detected at the site A, a localities where the species is functionally extirpated can still be characterized by the presence of a few remaining males.
The distance from paved road was also a significant variable determining the presence of D. suweonensis. Being at least 45 m from a paved road drastically increased the suitability of the habitat for D. suweonensis, with paved road having an impact as far as 1006 m away. The impact of roads on amphibians is widespread and sometimes reaching even further. For instance, populations of the Leopard frog (Lithobates pipiens) are negatively affected by traffic density within a radius of 1.5 km [55]. Similarly, populations of Spotted salamanders (Ambystoma maculatum) drop when the breeding pond is near a paved road [56]. In addition, our results show that not only large paved roads but also small dirt road around rice paddies negatively affect habitat suitability for D. suweonensis. Thus, it is important to consider the removal of paved roads and dirt roads when considering the conservation of the species.
Landscape urbanization and habitat fragmentation have a negative impact on D. suweonensis [34]. In 2012, individuals were observed at all four sites but since 2015 individuals have not been observed at the sites C and D. The site C was composed of rice paddies only until 2010, when the number of greenhouse began to increase. This transformation resulted in a decrease in rice paddy cover, while at the same time increasing landscape fragmentation. The transformation of rice paddies into greenhouse is known to have an adverse effect on the anuran species breeding in rice paddies [19] and D. suweonensis at the site C is likely have become extirpated for these reasons. Regarding the site D, the original area was about 2 km2, before being divided into three sections with the opening of the 3rd Gyeongin expressway in 2010 (Figure 1). As a result, the surface of rice paddy was greatly reduced, down to 0.78 km2. Greenhouses also cropped up at the sites and it is highly likely that D. suweonensis became extirpated at the site D for the same reasons. Hojobeol has become divided into sites A and B following the construction of the Mayu highway, a six-lane paved road completed in 2016 (Figure 1). The number of individuals at the site A was found to be fluctuating, a potential sign of future extirpation and further habitat fragmentation and decrease in rice paddy cover, such as resulting from the construction of the new motorway going through site B under discussion, would therefore likely lead the extirpation of D. suweonensis in Siheung.
While this study is focused on a single amphibian species, habitat fragmentation and degradation caused by urbanization and greenhouses development can result in the extirpation of other species present at the site [7]. Preserving or restoring undisturbed habitats and preventing further degradation and fragmentation is a highly efficient method to protect species and populations [20] and damages to the ecosystem when constructing roads and buildings must be minimized. Furthermore, rice paddies play a significant role in replacing natural wetland [21] and preserving rice paddies is a primary requirement to conserve the ecosystem at the study locality. This is also likely to be the only method to prevent the extirpation of D. suweonensis and as Siheung is the connecting point for the populations south and north of Seoul the loss of the population would results in a gap in the range of D. suweonensis, the interruption of gene exchange and a step toward the extinction vortex.
While this study follows all statistical requirements, a potential spatial autocorrelation of the data cannot be excluded. The potential autocorrelation here arises from the small number of datapoints, inherent to the work on Endangered species and the small scale of the study, as the purpose of this study is to determine the variables important to the species at this locality only.
Currently, the only guidelines to evaluate environmental protection areas for wildlife habitat in the Republic of Korea are the Article 12* (documentation criterion for establishing first-class catchment area in ecology and natural map) and Article 13* (documentation criterion for establishing second-class catchment area in ecology and natural map) of the Documentation Guideline for Ecological and Nature Maps [57]. However, in this documentation, regulations are provided irrespectively of species characteristics and requirements, only stipulating that protected areas should be established within a 1-km radius and 500-m radius from the spot where Endangered species are found, for class I and class II respectively. Some additional details are provided for birds and fish but not for amphibians despite their need for both terrestrial and aquatic habitat to complete their life cycles. In addition, several species are known to disperse and migrate and have special conservation needs—for example, Dryophytes sp. [58], Onychodactylus koreanus [59], Bufo gargarizans [60] and Rana sp. [61]. Therefore, specific criteria and regulations must be developed based on habitat requirements, such as determined here for D. suweonensis, to determine protected areas for amphibians. The use of habitat analysis and modeling applied in this study provide such tools.
5. Conclusions
In the last ten years, the development of paved roads near Hojobeol in Siheung has resulted in a decrease in the density of rice paddies and in the fragmentation of the main habitat of the Dryophytes Suweonensis. As a result, the species became extirpated from some sites and the population declined at others. Therefore, in order to preserve the remaining D. suweonensis population in Siheung, development should be limited to activities that do not result in the decrease of rice paddy cover and decrease distance to roads. In addition the current method to determine wildlife reserve should be updated to take into account the ecological characteristics of each species. We suggest adopting a more suitable and detailed conservation methods considering the habitat environment of each species to preserve biodiversity.
Author Contributions
Conceptualization, I.-K.P. and A.B.; methodology, I.-K.P.; software, I.-K.P.; validation, I.-K.P., D.P. and A.B.; formal analysis, I.-K.P.; investigation, I.-K.P., A.B.; resources, I.-K.P. and A.B.; data curation, I.-K.P. and A.B.; writing—original draft preparation, I.-K.P.; writing—review and editing, I.-K.P., D.P. and A.B.; visualization, I.-K.P.; supervision, D.P. and A.B.; project administration, A.B.; funding acquisition, I.-K.P. and A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in article.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
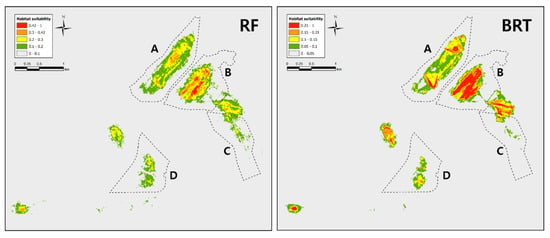
Figure A1.
Dryophytes suweonensis SDM results using RF (random forest) and BRT (boosted regression trees). These results follow an applied average suitability approaches to designate a threshold for D. suweonensis habitability suitability. RF and BRT show that site B mainly is suitable to D. suweonensis.
Figure A1.
Dryophytes suweonensis SDM results using RF (random forest) and BRT (boosted regression trees). These results follow an applied average suitability approaches to designate a threshold for D. suweonensis habitability suitability. RF and BRT show that site B mainly is suitable to D. suweonensis.
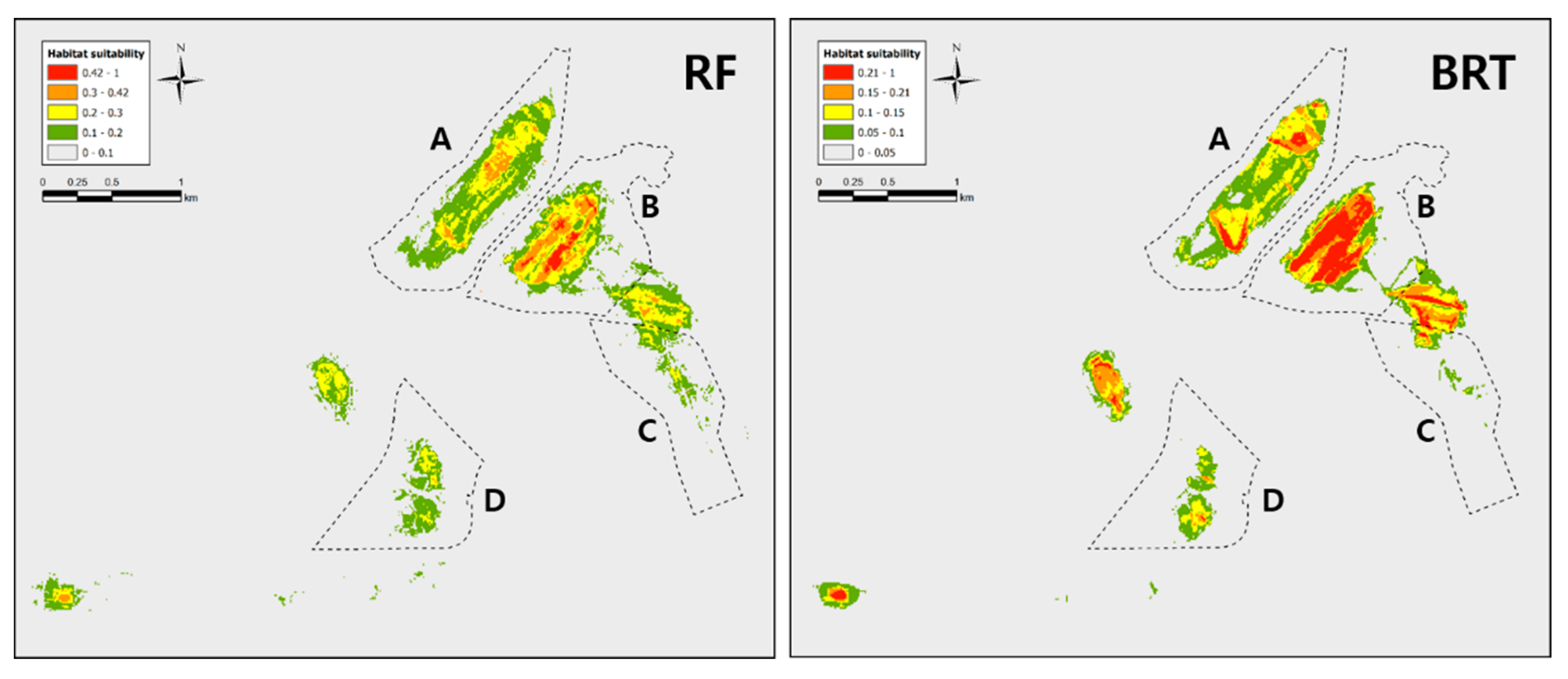
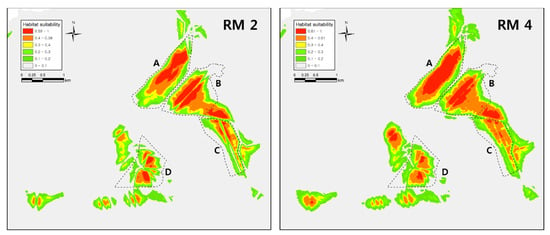
Figure A2.
SDM results for Dryophytes suweonensis conducted with different regularization multiplier values. The model in (RM 2) used a regularization multiplier = 2 and the model in (RM 4) used a regularization multiplier = 4. We did not use these models in analyses as they clearly do no follow the ecological requirements of the species.
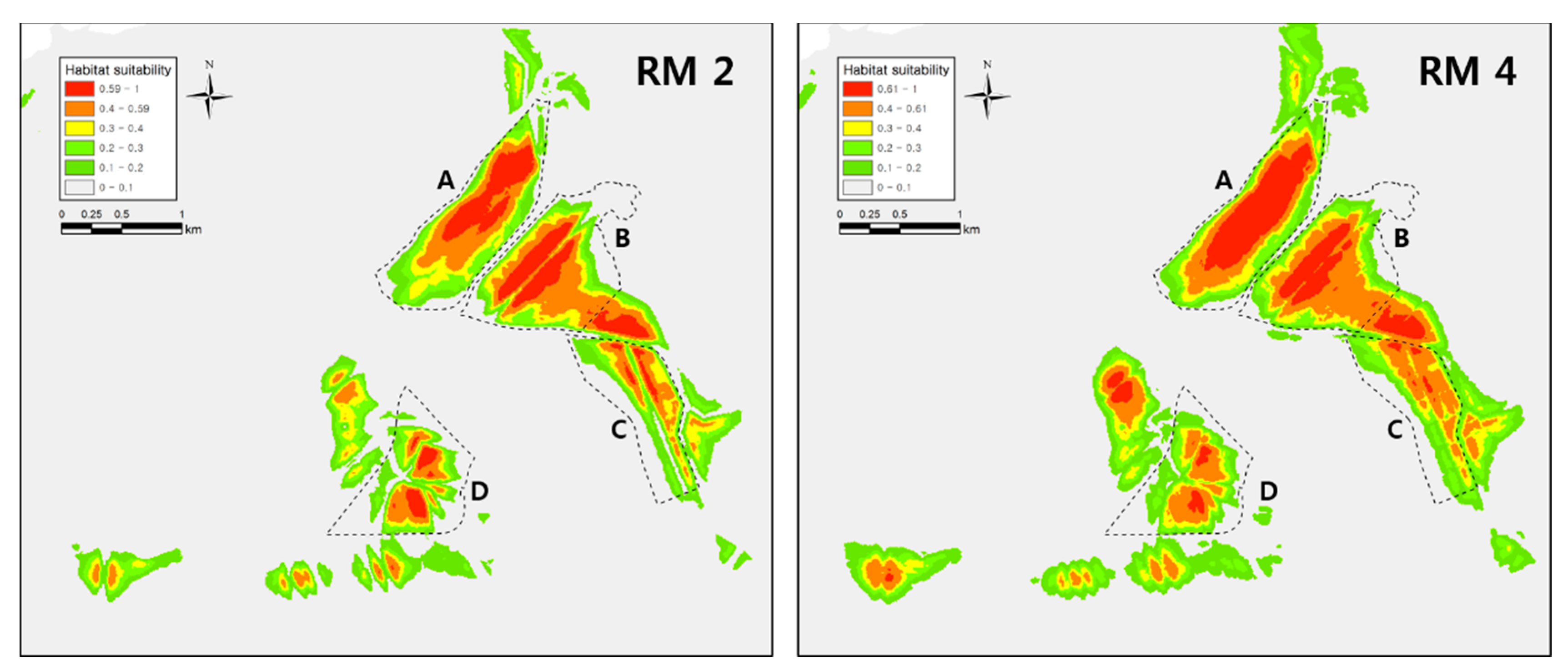
Figure A2.
SDM results for Dryophytes suweonensis conducted with different regularization multiplier values. The model in (RM 2) used a regularization multiplier = 2 and the model in (RM 4) used a regularization multiplier = 4. We did not use these models in analyses as they clearly do no follow the ecological requirements of the species.
References
- Fisher, R.N.; Shaffer, H.B. The Decline of amphibians in California’s Great Central Valley. Conserv. Biol. 1996, 10, 1387–1397. [Google Scholar] [CrossRef]
- Alford, R.A.; Richards, S.J. Global amphibian declines: A problem in applied ecology. Annu. Rev. Ecol. Syst. 1999, 30, 133–165. [Google Scholar] [CrossRef]
- Gardner, T. Declining amphibian populations: A global phenomenon in conservation of biology. Anim. Biodivers. Conserv. 2001, 24, 25–44. [Google Scholar]
- Collins, J.P. Amphibian decline and extinction: What we know and what we need to learn. Dis. Aquat. Org. 2010, 92, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; May, R.M.; Lehman, C.L.; Nowak, M.A. Habitat destruction and the extinction debt. Nature 1994, 371, 65–66. [Google Scholar] [CrossRef]
- Marsh, D.M.; Trenham, P.C. Metapopulation dynamics and amphibian conservation. Conserv. Biol. 2001, 15, 40–49. [Google Scholar] [CrossRef]
- Fahrig, L. Effects of habitat fragmentation on biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 487–515. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Hobbs, R.J.; Montague-Drake, R.; Alexandra, J.; Bennett, A.; Burgman, M.; Cale, P.; Calhoun, A.; Cramer, V.; Cullen, P.; et al. A checklist for ecological management of landscapes for conservation. Ecol. Lett. 2007, 11, 78–91. [Google Scholar] [CrossRef]
- Olds, A.D.; Connolly, R.M.; Pitt, K.A.; Maxwell, P.S. Habitat connectivity improves reserve performance. Conserv. Lett. 2011, 5, 56–63. [Google Scholar] [CrossRef]
- Hanski, I. Metapopulation dynamics: Does it help to have more of the same? Trends Ecol. Evol. 1989, 4, 113–114. [Google Scholar] [CrossRef]
- Cushman, S.A. Effects of habitat loss and fragmentation on amphibians: A review and prospectus. Biol. Conserv. 2006, 128, 231–240. [Google Scholar] [CrossRef]
- Hamer, A.J.; McDonnell, M.J. Amphibian ecology and conservation in the urbanising world: A review. Biol. Conserv. 2008, 141, 2432–2449. [Google Scholar] [CrossRef]
- Radeloff, V.C.; Hammer, R.B.; Stewart, S.I. Rural and suburban sprawl in the U.S. Midwest from 1940 to 2000 and its relation to forest fragmentation. Conserv. Biol. 2005, 19, 793–805. [Google Scholar] [CrossRef]
- Burkey, T.V. Extinction in Nature Reserves: The effect of fragmentation and the importance of migration between reserve fragments. Oikos 1989, 55, 75–81. [Google Scholar] [CrossRef]
- Feldman, C.R.; Spicer, G.S. Comparative phylogeography of woodland reptiles in California: Repeated patterns of cladogenesis and population expansion. Mol. Ecol. 2006, 15, 2201–2222. [Google Scholar] [CrossRef] [PubMed]
- Castoe, T.A.; Spencer, C.L.; Parkinson, C.L. Phylogeographic structure and historical demography of the western diamondback rattlesnake (Crotalus atrox): A perspective on North American desert biogeography. Mol. Phylogenet. Evol. 2007, 42, 193–212. [Google Scholar] [CrossRef]
- Lemmon, E.M.; Lemmon, A.R.; Collins, J.T.; Lee-Yaw, J.A.; Cannatella, D.C. Phylogeny-based delimitation of species boundaries and contact zones in the trilling chorus frogs (Pseudacris). Mol. Phylogenet. Evol. 2007, 44, 1068–1082. [Google Scholar] [CrossRef] [PubMed]
- Forman, R.T.T. Connecting wildlife populations in fractured landscapes. In Safe Passages: Highways, Wildlife, and Habitat Connectivity; Beckmann, J.P., Clevenger, A.P., Huijser, M.P., Hillty, J.A., Eds.; Island Press: Washington, DC, USA, 2012; pp. 3–16. [Google Scholar]
- Groffen, J.; Borzée, A.; Jang, Y. Preference for natural borders in rice paddies by two treefrog species. Anim. Cells Syst. 2018, 22, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Marvier, M.; Kareiva, P.; Neubert, M.G. Habitat destruction, fragmentation, and disturbance promote invasion by habitat generalists in a multispecies metapopulation. Risk Anal. 2004, 24, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Kurechi, M. Restoring rice paddy wetland environments and the local sustainable society-project for achieving co-existence of rice paddy agriculture with waterbirds at Kabukuri-Numa, Miyagi Prefecture, Japan. Glob. Environ. Res. 2007, 11, 141–152. (In English) [Google Scholar]
- Green, R.E.; Cornell, S.J.; Scharlemann, J.P.W.; Balmford, A. Farming and the fate of wild nature. Science 2005, 307, 550–555. [Google Scholar] [CrossRef]
- Ramsar, COP10. Enhancing biodiversity in rice paddies as wetland systems. In Proceedings of the 10th Meeting of the Conference of the Parties to the Convention on Wetlands (Ramsar, Iran, 1971), Changwon, Korea, 28 October–4 November 2008. [Google Scholar]
- Fuller, R.J.; Norton, L.; Feber, R.; Johnson, P.; Chamberlain, D.; Joys, A.; Mathews, F.; Stuart, R.; Townsend, M.; Manley, W.; et al. Benefits of organic farming to biodiversity vary among taxa. Biol. Lett. 2005, 1, 431–434. [Google Scholar] [CrossRef]
- Butler, S.J.; Brooks, D.; Feber, R.E.; Storkey, J.; Vickery, J.A.; Norris, K. A Cross-Taxonomic index for quantifying the health of farmland biodiversity. J. Appl. Ecol. 2009, 46, 1154–1162. [Google Scholar] [CrossRef]
- Jang, H.J.; Suh, J.H. Distribution of amphibian species in South Korea. Korean J. Herpetol. 2010, 2, 45–51. [Google Scholar]
- Hazell, D.; Hero, J.-M.; Lindenmayer, D.; Cunningham, R. A comparison of constructed and natural habitat for frog conservation in an Australian agricultural landscape. Biol. Conserv. 2004, 119, 61–71. [Google Scholar] [CrossRef]
- Borzée, A.; Jang, Y. Description of a seminatural habitat of the endangered Suweon treefrog Hyla suweonensis. Anim. Cells Syst. 2015, 19, 216–220. [Google Scholar] [CrossRef]
- Borzée, A.; Andersen, D.; Jang, Y. Population trend inferred from aural surveys for calling anurans in Korea. PeerJ 2018, 6, e5568. [Google Scholar] [CrossRef]
- Borzée, A.; Heo, K.; Jang, Y. Relationship between agro-environmental variables and breeding Hylids in rice paddies. Sci. Rep. 2018, 8, 8049. [Google Scholar] [CrossRef]
- Borzée, A.; Kim, K.; Heo, K.; Jablonski, P.G.; Jang, Y. Impact of land reclamation and agricultural water regime on the distribution and conservation status of the endangered Dryophytes suweonensis. PeerJ 2017, 5, e3872. [Google Scholar] [CrossRef]
- Kim, I.H.; Son, S.H.; Kang, S.W.; Kim, J.B. Distribution and habitat characteristics of the endangered Suweon-Tree Frog (Hyla suweonensis). Korean J. Herpetol. 2012, 4, 15–22. [Google Scholar]
- Smith, M.A.; Green, D.M. Dispersal and the metapopulation paradigm in amphibian ecology and conservation: Are all amphibian populations metapopulations? Ecography 2005, 28, 110–128. [Google Scholar] [CrossRef]
- Borzée, A. Why Are Anurans Threatened? The case of Dryophytes suweonensis. Ph.D. Thesis, Seoul National University, Seoul, Korea, February 2018. [Google Scholar]
- Borzée, A.; Kosch, T.A.; Kim, M.; Jang, Y. Introduced bullfrogs are associated with increased Batrachochytrium dendrobatidis prevalence and reduced occurrence of Korean treefrogs. PLoS ONE 2017, 12, e0177860. [Google Scholar]
- Rodríguez, J.P.; Brotons, L.; Bustamante, J.; Seoane, J. The application of predictive modelling of species distribution to biodiversity conservation. Divers. Distrib. 2007, 13, 243–251. [Google Scholar] [CrossRef]
- Franklin, J. Species distribution models in conservation biogeography: Developments and challenges. Divers. Distrib. 2013, 19, 1217–1223. [Google Scholar] [CrossRef]
- Guisan, A.; Tingley, R.; Baumgartner, J.B.; Naujokaitis-Lewis, I.; Sutcliffe, P.R.; Tulloch, A.I.T.; Regan, T.J.; Brotons, L.; McDonald-Madden, E.; Mantyka-Pringle, C.; et al. Predicting species distributions for conservation decisions. Ecol. Lett. 2013, 16, 1424–1435. [Google Scholar] [CrossRef]
- Guillera-Arroita, G.; Lahoz-Monfort, J.J.; Elith, J.; Gordon, A.; Kujala, H.; Lentini, P.E.; McCarthy, M.A.; Tingley, R.; Wintle, B.A. Is my species distribution model fit for purpose? Matching data and models to applications. Glob. Ecol. Biogeogr. 2015, 24, 276–292. [Google Scholar] [CrossRef]
- Heikkinen, R.K.; Marmion, M.; Luoto, M. Does the interpolation accuracy of species distribution models come at the expense of transferability? Ecography 2012, 35, 276–288. [Google Scholar] [CrossRef]
- Phillips, S.J. A brief tutorial on Maxent. ATT Res. 2005, 190, 231–259. [Google Scholar]
- Young, N.; Carter, L.; Evangelista, P. A MaxEnt Model v3. 3.3 e Tutorial (ArcGIS v10); Colorado State University: Fort Collins, CO, USA, 2011. [Google Scholar]
- Radosavljevic, A.; Anderson, R.P. Making better Maxent models of species distributions: Complexity, overfitting and evaluation. J. Biogeogr. 2014, 41, 629–643. [Google Scholar] [CrossRef]
- Borzée, A.; Kim, J.Y.; Jang, Y. Asymmetric competition over calling sites in two closely related treefrog species. Sci. Rep. 2016, 6, 32569. [Google Scholar] [CrossRef]
- Liu, C.; Berry, P.M.; Dawson, T.P.; Pearson, R.G. Selecting thresholds of occurrence in the prediction of species distributions. Ecography 2005, 28, 385–393. [Google Scholar] [CrossRef]
- Hanley, J.A.; McNeil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Bradley, A.P. The use of the area under the ROC curve in the evaluation of machine learning algorithms. Pattern Recognit. 1997, 30, 1145–1159. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Beck, P.S.A.; Atzberger, C.; Høgda, K.A.; Johansen, B.; Skidmore, A.K. Improved monitoring of vegetation dynamics at very high latitudes: A new method using MODIS NDVI. Remote Sens. Environ. 2006, 100, 321–334. [Google Scholar] [CrossRef]
- Lee, B.; Kim, E.; Lee, J.; Chung, J.M.; Lim, J.H. Detecting Phenology Using MODIS vegetation indices and Forest Type Msp in South Korea. Korean J. Remote Sens. 2018, 34, 267–282. [Google Scholar]
- Ziegel, E.R.; Neter, J.; Kutner, M.; Nachtsheim, C.; Wasserman, W. Applied Linear Statistical Models. Technometrics 1997, 39, 342. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Roh, G.; Borzée, A.; Jang, Y. Spatiotemporal distributions and habitat characteristics of the endangered treefrog, Hyla suweonensis, in relation to sympatric H. japonica. Ecol. Inform. 2014, 24, 78–84. [Google Scholar] [CrossRef]
- Carr, L.W.; Fahrig, L. Effect of road traffic on two amphibian species of differing vagility. Conserv. Biol. 2001, 15, 1071–1078. [Google Scholar] [CrossRef]
- Karraker, N.E.; Gibbs, J.P. Contrasting road effect signals in reproduction of long-versus short-lived amphibians. Hydrobiologia 2011, 664, 213–218. [Google Scholar] [CrossRef]
- Ministry of Environment. Documentation Guideline for Ecological Nature Map; Technical report No. 645; Ministry of Environment Regulations: Sejong, Korea, 2018. [Google Scholar]
- Borzée, A.; Choi, Y.; Kim, Y.E.; Jablonski, P.G.; Jang, Y. Interspecific variation in seasonal migration and brumation behavior in two closely related species of treefrogs. Front. Ecol. Evol. 2019, 7, 55. [Google Scholar] [CrossRef]
- Shin, Y.; Jeong, D.; Borzée, A. Mass displacement of Korean clawed salamanders (Onychodactylus koreanus) and the threat of Road-Kill. Herpetol. Bull. 2020, 151, 28–31. [Google Scholar] [CrossRef][Green Version]
- Lee, J.H.; Lee, C.W.; Yang, H.S.; Kim, T.S.; Lee, J.H.; Park, S.J.; Yang, B.G. Post-Breeding Dispersal and Movement patterns of Male Asian Toads (Bufo gargarizans). Korean J. Herpetol. 2013, 5, 1–8. [Google Scholar]
- Do, M.S.; Jang, H.J.; Kim, D.I.; Koo, K.S.; Lee, S.C.; Nam, H.K. The study on habitat analysis and ecological niche of Korean Brown Frogs (Rana dybowskii, R. coreana and R. huanrensis) using the species distribution model. Korean J. Herpetol. 2018, 9, 1–11. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).