Long Term Interactions of Native and Invasive Species in a Marine Protected Area Suggest Complex Cascading Effects Challenging Conservation Outcomes
Abstract
:1. Introduction
2. Materials and Methods
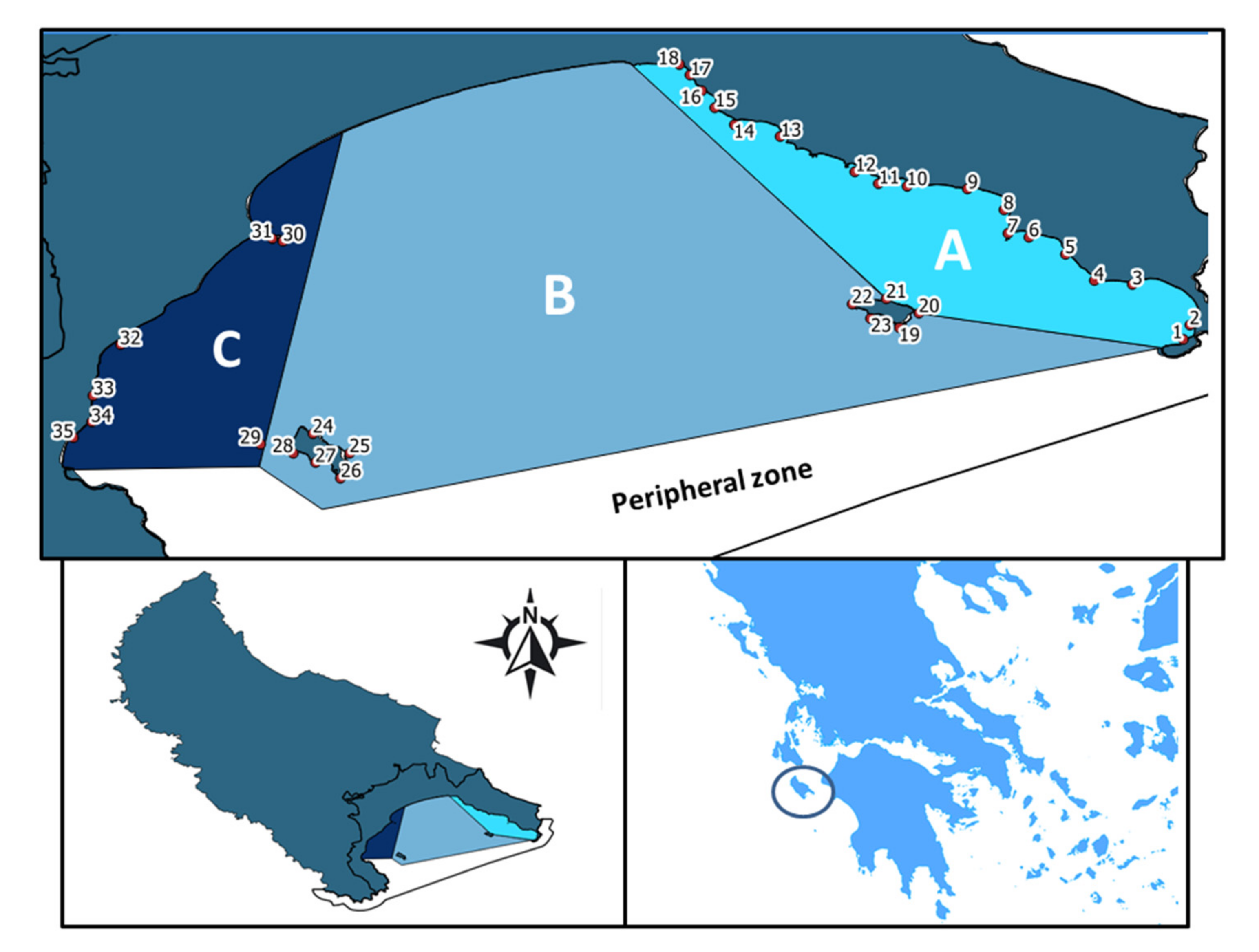
2.1. Study Area
2.2. Sampling Design
2.3. Data Analysis
3. Results
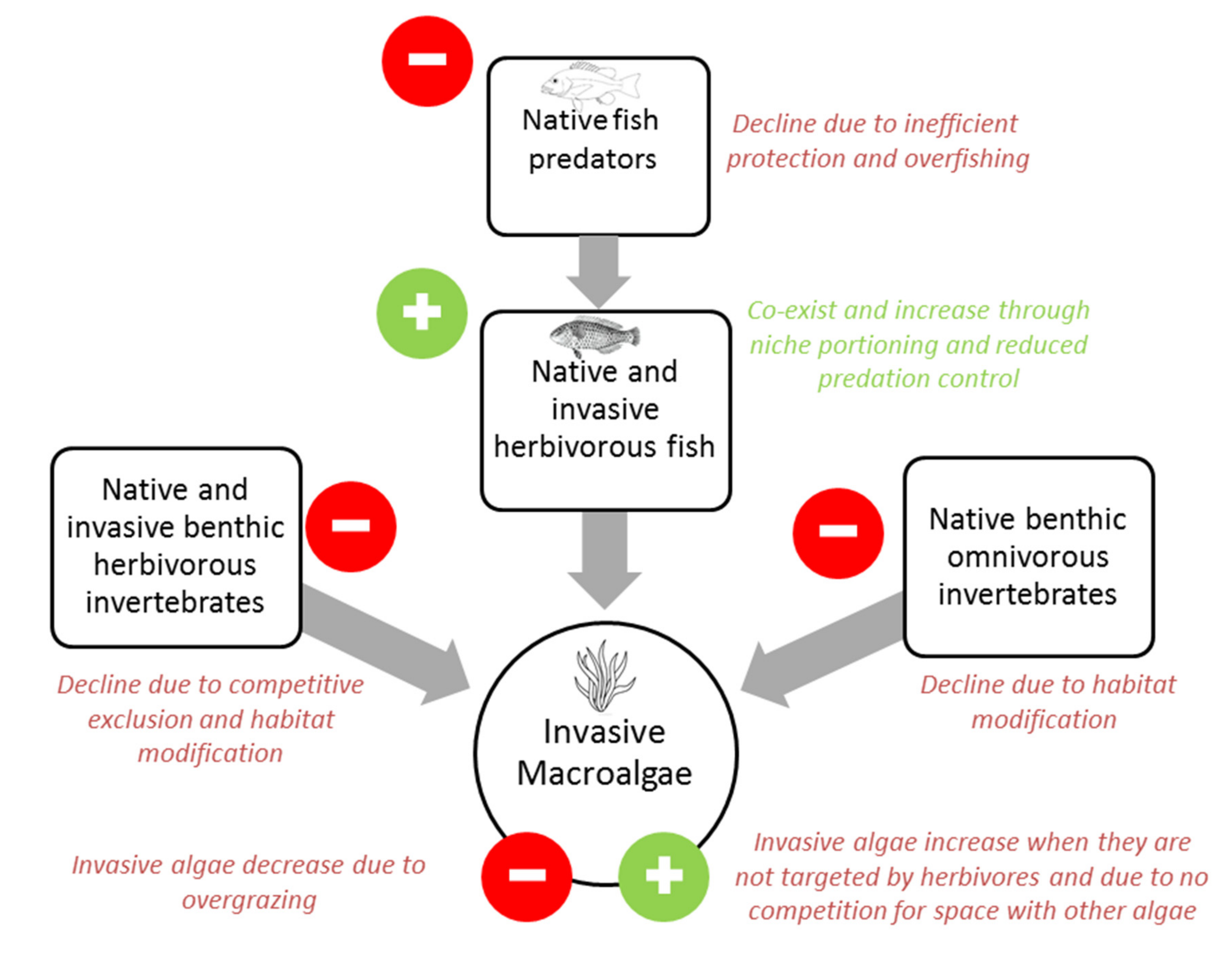
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Katsanevakis, S.; Wallentinus, I.; Zenetos, A.; Leppäkoski, E.; Çinar, M.E.; Oztürk, B.; Grabowski, M.; Golani, D.; Cardoso, A.C. Impacts of marine invasive alien species on ecosystem services and biodiversity: A pan-European review. Aquat. Invasions 2014, 9, 391–423. [Google Scholar] [CrossRef]
- Vergés, A.; Doropoulos, C.; Malcolm, H.A.; Skye, M.; Garcia-Pizá, M.; Marzinelli, E.M.; Campbell, A.H.; Ballesteros, E.; Hoey, A.S.; Vila-Concejo, A.; et al. Long-term empirical evidence of ocean warming leading to tropicalization of fish communities, increased herbivory, and loss of kelp. Proc. Natl. Acad. Sci. USA 2016, 113, 13791–13796. [Google Scholar] [CrossRef] [Green Version]
- Gallardo, B.; Clavero, M.; Sánchez, M.I.; Vilà, M. Global ecological impacts of invasive species in aquatic ecosystems. Glob. Chang. Biol. 2016, 22, 151–163. [Google Scholar] [CrossRef]
- Simberloff, D.; Martin, J.L.; Genovesi, P.; Maris, V.; Wardle, D.A.; Aronson, J.; Courchamp, F.; Galil, B.; Garcia-Berthou, E.; Pascal, M.; et al. Impacts of biological invasions: What’s what and the way forward. Trends Ecol. Evol. 2013, 28, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Giakoumi, S.; Guilhaumon, F.; Kark, S.; Terlizzi, A.; Claudet, J.; Felline, S.; Cerrano, C.; Coll, M.; Danovaro, R.; Fraschetti, S.; et al. Space invaders; biological invasions in marine conservation planning. Divers. Distrib. 2016, 22, 1220–1231. [Google Scholar] [CrossRef] [Green Version]
- Mačić, V.; Albano, P.G.; Almpanidou, V.; Claudet, J.; Corrales, X.; Essl, F.; Evagelopoulos, A.; Giovos, I.; Jimenez, C.; Kark, S.; et al. Biological invasions in conservation planning: A global systematic review. Front. Mar. Sci. 2018, 5, 178. [Google Scholar] [CrossRef] [Green Version]
- Mazaris, A.D.; Katsanevakis, S. The threat of biological invasions is under-represented in the marine protected areas of the European Natura 2000 network. Biol. Conserv. 2018, 225, 208–212. [Google Scholar] [CrossRef]
- Lubchenco, J.; Grorud-Colvert, K. Making waves: The science and politics of ocean protection. Science 2015, 15, 382–383. [Google Scholar] [CrossRef]
- Halpern, B.S.; Frazier, M.; Afflerbach, J.; Lowndes, J.S.; Micheli, F.; O’Hara, C.; Scarborough, C.; Selkoe, K.A. Recent pace of change in human impact on the world’s ocean. Sci. Rep. 2019, 9, 11609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rilov, G.; Mazaris, A.; Stelzenmüller, V.; Helmuth, B.; Wahl, M.; Guy-Haim, T.; Mieszkowska, N.; Ledoux, J.B.; Katsanevakis, S. Adaptive marine conservation planning in the face of climate change: What can we learn from physiological, genetic and ecological studies? Glob. Ecol. Conserv. 2019, 17, e00566. [Google Scholar] [CrossRef]
- Giakoumi, S.; Pey, A. Assessing the effects of marine protected areas on biological invasions: A global review. Front. Mar. Sci. 2016, 4, 49. [Google Scholar] [CrossRef]
- Blackburn, T.M.; Pyšek, P.; Bacher, S.; Carlton, J.T.; Duncan, R.P.; Jarošík, V.; Wilson, J.R.U.; Richardson, D.M. A proposed unified framework for biological invasions. Trends Ecol. Evol. 2011, 26, 333–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurevitch, J.; Fox, G.A.; Wardle, G.M.; Inderjit, D.; Taub, D. Emergent insights from the synthesis of conceptual frameworks for biological invasions. Ecol. Lett. 2011, 14, 407–418. [Google Scholar] [CrossRef]
- Levine, J.M.; D’Antonio, C.M. Elton revisited: A review of evidence linking diversity and invasibility. Oikos 1999, 87, 15–26. [Google Scholar] [CrossRef] [Green Version]
- Albins, M.A.; Hixon, M.A. Worst case scenario: Potential long-term effects of invasive predatory lionfish (Pterois volitans) on Atlantic and Caribbean coral-reef communities. Environ. Biol. Fishes 2013, 96, 1151–1157. [Google Scholar] [CrossRef]
- Stohlgren, T.J.; Barnett, D.; Flather, C.; Fuller, P.; Peterjohn, B.; Kartesz, J.; Master, L.L. Species richness and patterns of invasion in plants, birds, and fishes in the United States. Biol. Invasions 2006, 8, 427–447. [Google Scholar] [CrossRef] [Green Version]
- Fridley, J.D.; Stachowicz, J.J.; Naeem, S.; Sax, D.F.; Seabloom, E.W.; Smith, M.D.; Stohlgren, T.J.; Tilman, D.; Von Holle, B. The invasion paradox: Reconciling pattern and process in species invasions. Ecology 2007, 88, 3–17. [Google Scholar] [CrossRef]
- Keane, R.M.; Crawley, M.J. Exotic plant invasions and the enemy release hypothesis. Trends Ecol. Evol. 2002, 17, 164–170. [Google Scholar] [CrossRef]
- Daleo, P.; Alberti, J.; Iribarne, O. Biological invasions and the neutral theory. Divers. Distrib. 2009, 15, 547–553. [Google Scholar] [CrossRef]
- Ardura, A.; Juanes, F.; Planes, S.; Garcia-Vazquez, E. Rate of biological invasions is lower in coastal marine protected areas. Sci. Rep. 2016, 6, 33013. [Google Scholar] [CrossRef]
- Gestoso, I.; Ramalhosa, P.; Oliveira, P.; Canning-Clode, J. Marine protected communities against biological invasions: A case study from an offshore island. Mar. Pollut. Bull. 2017, 119, 72–80. [Google Scholar] [CrossRef]
- Giakoumi, S.; Pey, A.; Di Franco, A.; Francour, P.; Kizilkaya, Z.; Arda, Y.; Raybaud, V.; Guidetti, P. Exploring the relationships between marine protected areas and invasive fish in the world’s most invaded sea. Ecol. Appl. 2019, 29, e01809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kindinger, T.L.; Albins, M.A. Consumptive and non-consumptive effects of an invasive marine predator on native coral-reef herbivores. Biol. Invasions 2017, 19, 131–146. [Google Scholar] [CrossRef]
- Godoy, O. Coexistence theory as a tool to understand biological invasions in species interaction networks: Implications for the study of novel ecosystems. Funct. Ecol. 2017, 33, 1190–1201. [Google Scholar] [CrossRef]
- Epstein, G.; Hawkins, S.J.; Smale, D.A. Identifying niche and fitness dissimilarities in invaded marine macroalgal canopies within the context of contemporary coexistence theory. Sci. Rep. 2019, 9, 8816. [Google Scholar] [CrossRef] [Green Version]
- Strayer, D.L.; Eviner, V.T.; Jeschke, J.M.; Pace, M.L. Understanding the long-term effects of species invasions. Trends Ecol. Evol. 2006, 21, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Margaritoulis, D. Nesting activity and reproductive output of loggerhead sea turtles, Caretta caretta, over 19 seasons (1984–2002) at Laganas Bay, Zakynthos, Greece: The largest rookery in the Mediterranean. Chelonian Conserv. Biol. 2005, 4, 916–929. [Google Scholar]
- Dimitriadis, C.; Sini, M.; Gerovasileiou, V.; Sourbes, L.; Koutsoubas, D. Assessment of fish communities in a Mediterranean MPA: Can a seasonal no-take zone provide effective protection? Estuar. Coast. Shelf Sci. 2018, 207, 223–231. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Weber, A.; Pipitone, C.; Leopold, M.; Cronin, M.; Scheidat, M.; Doyle, T.K.; Buhl-Mortensen, L.; Buhl-Mortensen, P.; D’Anna, G.; et al. Monitoring marine populations and communities: Review of methods and tools dealing with imperfect detectability. Aquat. Biol. 2012, 16, 31–52. [Google Scholar] [CrossRef] [Green Version]
- Issaris, Y.; Katsanevakis, S.; Salomidi, M.; Tsiamis, K.; Katsiaras, N.; Verriopoulos, G. Occupancy estimation of marine demersal species: Dealing with imperfect detectability. Mar. Ecol. Prog. Ser. 2012, 453, 95–106. [Google Scholar] [CrossRef]
- Hanspach, J.; Kuehn, I.; Pyšek, P.; Boos, E.; Klotz, S. Correlates of naturalization and occupancy of introduced ornamentals in Germany. Perspect. Plant Ecol. Evol. Syst. 2008, 10, 241–250. [Google Scholar] [CrossRef]
- MacKenzie, D.I.; Nichols, J.D.; Royle, J.A.; Pollock, K.H.; Bailey, L.L.; Hines, J.E. Occupancy Estimation and Modeling: Inferring Patterns and Dynamics of Species Occurrence; Academic Press: Burlington, MA, USA, 2006; p. 324. [Google Scholar]
- Thessalou-Legaki, M.; Nikolaidou, A.; Katsanevakis, S.; Tsiamis, K.; Poursanidis, D.; Issaris, Y.; Louizidou, P.; Delimitsou, A.; Kytinou, E.; Maroulakis, M. Part 1: Recording Benthic Fauna in the National Marine Park of Zakynthos, Final Report; National and Kapodistrian University of Athens: Athens, Greece, 2009; p. 65. [Google Scholar]
- Puccio, V.; Relini, M.; Azzurro, E.; Relini, O. Feeding habits of Percnon gibbesi (H. Milne Edwards, 1853) in the Sicily Strait. Hydrobiologia 2006, 557, 79–84. [Google Scholar] [CrossRef]
- Bariche, M. Diet of the Lessepsian fishes, Siganus rivulatus and S. luridus (Siganidae) in the eastern Mediterranean: A bibliographic analysis. Cybium 2006, 30, 41–49. [Google Scholar]
- Harmelin, J.G.; Harmelin-Vivien, M. A review on habitat, diet and growth of the dusky grouper Epinephelus marginatus (Lowe, 1834). Mar. Life 1999, 9, 11–20. [Google Scholar]
- Karachle, P.; Stergiou, K. An update on the feeding habits of fish in the Mediterranean Sea (2002–2015). Mediterr. Mar. Sci. 2017, 18, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, J. Feeding activity in Echinaster and its induction with dissolved nutrients. Biol. Bull. 1969, 136, 374–384. [Google Scholar] [CrossRef]
- Wangensteen, O.S.; Turon, X.; García-Cisneros, A.; Recasens, M.; Romero, J.; Palacín, C. A wolf in sheep’s clothing: Carnivory in dominant sea urchins in the Mediterranean. Mar. Ecol. Prog. Ser. 2011, 441, 117–128. [Google Scholar] [CrossRef] [Green Version]
- Hines, J.E. PRESENCE2-Software to Estimate Patch Occupancy and Related Parameters. 2006. Available online: www.mbr-pwrc.usgs.gov/software/presence.html (accessed on 15 September 2019).
- Burnham, K.P.; Anderson, D. Model Selection and Multi-Model Inference; Springer: Berlin, Germany, 2002; p. 488. [Google Scholar]
- Burfeind, D.D.; Pitt, K.A.; Connolly, R.M.; Byers, J.E. Performance of non-native species within marine reserves. Biol. Invasions 2013, 15, 17–28. [Google Scholar] [CrossRef]
- Rilov, G.; Peleg, O.; Yeruham, E.; Garval, T.; Vichik, A.; Raveh, O. Alien turf: Overfishing, overgrazing and invader domination in southeastern Levant reef ecosystems. Aquat. Conserv. 2017, 28, 351–369. [Google Scholar] [CrossRef]
- Mumby, P.J.; Harborne, A.R.; Brumbaugh, D.R. Grouper as a natural biocontrol of invasive lionfish. PLoS ONE 2011, 6, e21510. [Google Scholar] [CrossRef]
- Dimitriadis, C.; Sourbes, L.; Koutsoubas, D. Nodavirus Have Reached the Marine Protected Area of National Marine Park of Zakynthos. Scientific Watch Section of the MedPAN Network Website. 2013. Available online: http://www.medpan.org/en/ennews/-/blogs/is-the-nodavirus-present-in-your-mpa- (accessed on 4 April 2015).
- Condinin, M.V.; García-Charton, J.A.; Garcia, A.M. A review of the biology, ecology, behaviour and conservation status of the dusky grouper, Epinephelus marginatus (Lowe 1834). Rev. Fish Biol. Fish. 2018, 28, 301–330. [Google Scholar] [CrossRef]
- Giakoumi, S.; Pey, A.; Thiriet, P.; Francour, P.; Guidetti, P. Patterns of predation on native and invasive alien fish in Mediterranean protected and unprotected areas. Mar. Environ. Res. 2019, 150, 104792. [Google Scholar] [CrossRef] [PubMed]
- Parravicini, V.; Azzurro, E.; Kulbicki, M.; Belmake, J. Niche shift can impair the ability to predict invasion risk in the marine realm: An illustration using Mediterranean fish invaders. Ecol. Lett. 2015, 18, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Sala, E.; Kizilkaya, Z.; Yildirim, D.; Ballesteros, E. Alien marine fishes deplete algal biomass in the eastern Mediterranean. PLoS ONE 2011, 6, e17356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vergès, A.; Tomas, F.; Cebrian, E.; Ballesteros, E.; Kizilkaya, Z.; Dendrinos, P.; Karamanlidis, A.A.; Spiegel, D.; Sala, E. Tropical rabbitfish and the deforestation of a warming temperate sea. J. Ecol. 2014, 102, 1518–1527. [Google Scholar] [CrossRef]
- Giakoumi, S. Distribution patterns of the invasive herbivore Siganus luridus (Rüppell, 1829) and its relation to native benthic communities in the central Aegean Sea, Northeastern Mediterranean. Mar. Ecol. 2014, 35, 96–105. [Google Scholar] [CrossRef]
- Azzurro, E.; Fanelli, E.; Mostarda, E.; Catra, M.; Andaloro, F. Resource partitioning among early colonizing Siganus luridus and native herbivorous fish in the Mediterranean: An integrated study based on gut-content analysis and stable isotope signatures. J. Mar. Biolog. Assoc. UK 2007, 87, 991–998. [Google Scholar] [CrossRef] [Green Version]
- Bonaldo, R.M.; Pires, M.M.; Guimarães, P.R.J.; Hoey, A.S.; Hay, M.E. Small marine protected areas in Fiji provide refuge for reef fish assemblages, feeding groups, and corals. PLoS ONE 2017, 12, e0170638. [Google Scholar] [CrossRef]
- Tsirika, A.; Haritonidis, S. A survey of the benthic flora in the National Marine Park of Zakynthos (Greece). Bot. Mar. 2005, 48, 38–45. [Google Scholar] [CrossRef]
- Cheminée, A.; Sala, E.; Pastor, J.; Bodilis, P.; Thiriet, P.; Mangialajo, L.; Cottalorda, J.M.; Francour, P. Nursery value of Cystoseira forests for Mediterranean rocky reef fishes. J. Exp. Mar. Biol. Ecol. 2013, 442, 70–79. [Google Scholar] [CrossRef]
- Bianchelli, S.; Buschi, E.; Danovaro, R.; Pusceddu, A. Biodiversity loss and turnover in alternative states in the Mediterranean Sea: A case study on meiofauna. Sci. Rep. 2016, 6, 34544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsanevakis, S.; Issaris, Y.; Poursanidis, D.; Thessalou-Legaki, M. Vulnerability of marine habitats to the invasive green alga Caulerpa racemosa var. cylindracea within a marine protected area. Mar. Environ. Res. 2010, 70, 210–218. [Google Scholar]
- Thessalou-Legaki, M.; Aydogan, Ö.; Bekas, P.; Bilge, G.; Boyaci, Y.O.; Brunelli, E.; Circosta, V.; Crocetta, F.; Durucan, F.; Erdem, M.; et al. New Mediterranean Biodiversity Records (December 2012). Mediterr. Mar. Sci. 2012, 13, 312–327. [Google Scholar] [CrossRef]
- Gerovasileiou, V.; Akel, E.H.K.; Akyol, O.; Alongi, G.; Azevedo, F.; Babali, N.; Bakiu, R.; Bariche, M.; Bennoui, A.; Castriota, L.; et al. New Mediterranean Biodiversity Records (July 2017). Mediterr. Mar. Sci. 2017, 18, 355–384. [Google Scholar]
- Villamor, A.M.; Becerro, A. Matching spatial distributions of the sea star Echinaster sepositus and crustose coralline algae in shallow rocky Mediterranean communities. Mar. Biol. 2010, 157, 2241–2251. [Google Scholar] [CrossRef]
- Trapani, D.; Bonaviri, C.; Costa, V.; Badalamenti, F.; Gianguzza, P. Feeding behaviour of Ophidiaster ophidianus (Lmk.) (Asteroidea) in Mediterranean rocky reefs. In Proceedings of the Libro degli Abstract La Ricerca Ecologica in un Mondo che Cambia 28, Congresso Società Italiana di Ecologia, Napoli, Italy, 12–15 September 2017. [Google Scholar]
- Dimitriadis, C.; Sini, M.; Gerovasileiou, V.; Poursanidis, D.; Evagelopoulos, A.; FournariKonstantinidou, I.; Badouvas, N.; Lekkas, V.; Giourgis, E.; Akritopoulou, E.; et al. Management Measures for Fisheries in the Marine Protected Area of the National Marine Park of Zakynthos; US Environmental Protection Agency (EPA), Ed.; University of the Aegean: Lesbos, Greece, 2013; p. 121.
- Bates, A.E.; Barrett, N.S.; Stuart-Smith, R.D.; Holbrook, N.J.; Thompson, P.A.; Edgar, G.J. Resilience and signatures of tropicalization in protected reef fish communities. Nat. Clim. Chang. 2014, 4, 62–67. [Google Scholar] [CrossRef]
- Cacabelos, E.; Martins, G.M.; Faria, J.; Prestes, A.C.L.; Costa, T.; Moreu, I.; Neto, A.I. Limited effects of marine protected areas on the distribution of invasive species, despite positive effects on diversity in shallow-water marine communities. Biol. Invasions 2020, 22, 1169–1179. [Google Scholar] [CrossRef]
- Letten, A.D.; Ke, P.J.; Fukami, T. Linking modern coexistence theory and contemporary niche theory. Ecol. Monogr. 2017, 87, 161–177. [Google Scholar] [CrossRef]
- Kraft, N.J.B.; Godoy, O.; Levine, J.M. Plant functional traits and the multidimensional nature of species coexistence. Proc. Natl. Acad. Sci. USA 2017, 12, 797–802. [Google Scholar] [CrossRef] [Green Version]
- Pearson, D.E.; Ortega, Y.K.; Eren, Ö.; Hierro, J.L. Community assembly theory as a framework for biological invasions. Trends Ecol. Evol. 2018, 33, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Giakoumi, S.; Katsanevakis, S.; Albano, P.G.; Azzurro, E.; Cardoso, A.C.; Cebrian, E.; Deidun, A.; Edelist, D.; Francour, P.; Jimenez, C.; et al. Management priorities for marine invasive species. Sci. Total Environ. 2019, 688, 976–982. [Google Scholar] [CrossRef] [PubMed]
| Alien | Native | |
|---|---|---|
| Arthropoda | ||
| Percnon gibbesi (H. Milne Edwards, 1853) | + | |
| Chordata | ||
| Siganus rivulatus Forsskål & Niebuhr, 1775 | + | |
| Siganus luridus (Rüppell, 1829) | + | |
| Epinephelus marginatus (Lowe, 1834) | + | |
| Epinephelus costae (Steindachner, 1878) | + | |
| Sparisoma cretense (Linnaeus, 1758) | + | |
| Rhodophyta | ||
| Lophocladia lallemandii (Montagne) F.Schmitz, 1893 | + | |
| Ganonema farinosum (J.V.Lamouroux) K.C.Fan & Yung C.Wang, 1974 | + | |
| Chlorophyta | ||
| Caulerpa cylindracea Sonder, 1845 | + | |
| Ochrophyta | ||
| Stypopodium schimperi (Kützing) M.Verlaque & Boudouresque, 1991 | + | |
| Echinodermata | ||
| Echinaster sepositus (Retzius, 1783) | + | |
| Ophidiaster ophidianus (Lamarck, 1816) | + | |
| Hacelia attenuata Gray, 1840 Paracentotus lividus (Lamarck, 1816) | + + |
| Species | Trophic Group | Reference |
|---|---|---|
| Percnon gibbesi | benthic herbivorous invertebrate | [34] |
| Siganus rivulatus | herbivorous fish | [35] |
| Siganus luridus | herbivorous fish | [35] |
| Epinephelus marginatus | predatory fish | [36] |
| Epinephelus costae | predatory fish | [37] |
| Sparisoma cretense | herbivorous fish | [37] |
| Echinaster sepositus | benthic omnivorous invertebrate | [38] |
| Paracentrotus lividus | benthic herbivorous invertebrate | [39] |
| Ophidiaster ophidianus | benthic omnivorous invertebrate | [38] |
| Hacelia attenuata | benthic omnivorous invertebrate | [38] |
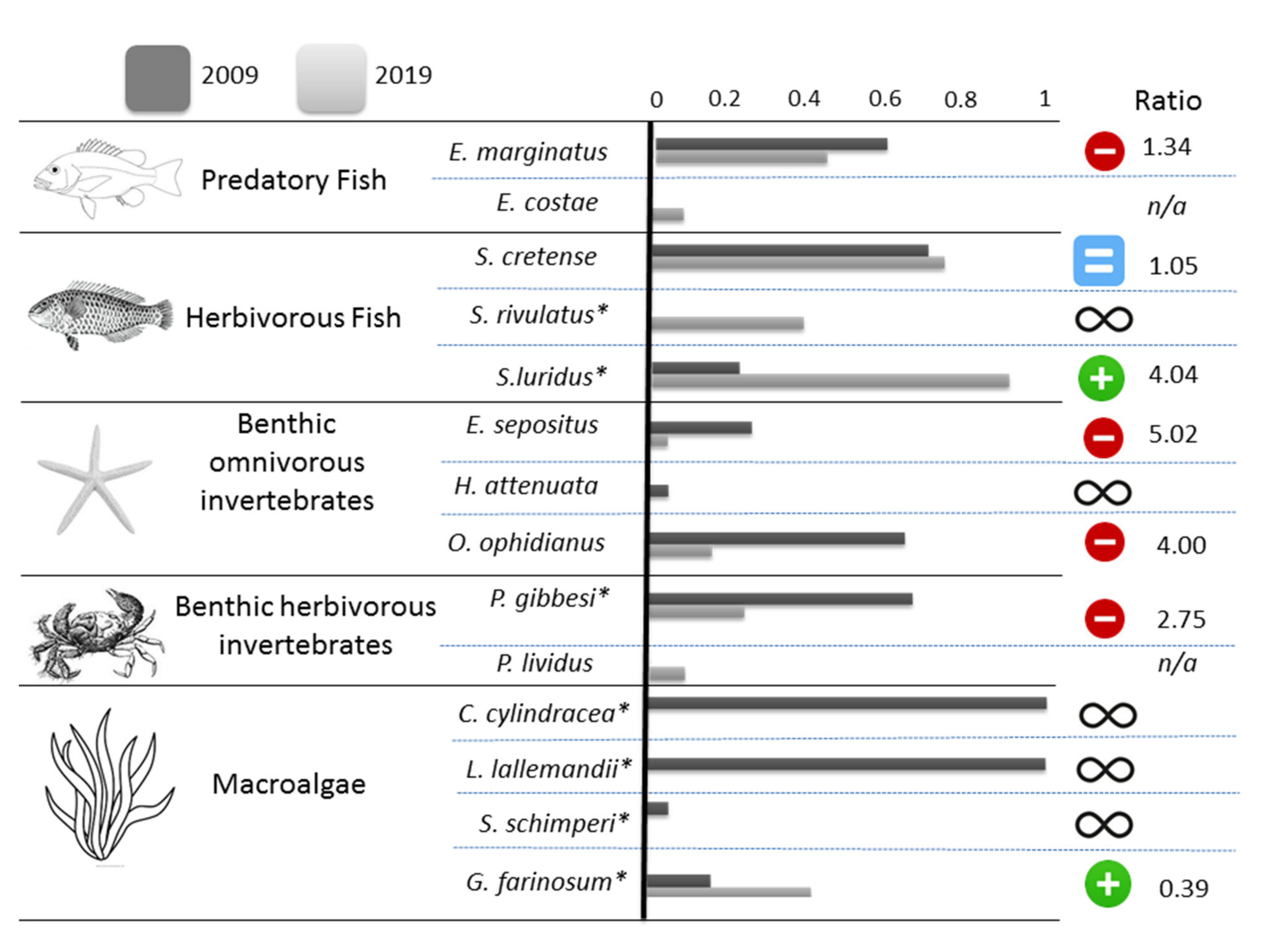
| Best Model | Support of the Assumption of Differing Occupancy between 2009 and 2019 | Occupancy | ||
|---|---|---|---|---|
| 2009 | 2019 | |||
| Predatory Fish | ||||
| Epinephelus marginatus | ψ(period) p(obs) | 59.1% | 0.58 | 0.43 |
| Epinephelus costae1 | ψ(.) p(.) | n.a. | n.a. | 0.09 |
| Herbivorous Fish | ||||
| Sparisoma cretense | ψ(.) p(obs) | 28.7% | 0.69 | 0.73 |
| Siganus rivulatus *,2 | ψ(.) p(.) | n.a. | 0.00 | 0.39 |
| Siganus luridus * | ψ(period) p(.) | 100.0% | 0.22 | 0.89 |
| Benthic omnivorous invertebrates | ||||
| Echinaster sepositus | ψ(period) p(.) | 92.7% | 0.26 | 0.05 |
| Hacelia attenuate3 | n.a. | n.a. | 0.06 | 0.00 |
| Ophidiaster ophidianus | ψ(period) p(.) | 92.4% | 0.64 | 0.16 |
| Benthic herbivorous invertebrates | ||||
| Percnon gibbesi * | ψ(period) p(.) | 99.4% | 0.66 | 0.24 |
| Paracentrotus lividus1 | ψ(.) p(.) | n.a. | n.a. | 0.09 |
| Macroalgae | ||||
| Caulerpa cylindracea * | n.a. | n.a. | 1.00 | 0.00 |
| Lophocladia lallemandii * | n.a. | n.a. | 1.00 | 0.00 |
| Stypopodium schimperi *,3 | n.a. | n.a. | 0.06 | 0.00 |
| Ganonema farinosum * | ψ(period) p(.) | 93.1% | 0.16 | 0.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimitriadis, C.; Fournari-Konstantinidou, I.; Sourbès, L.; Koutsoubas, D.; Katsanevakis, S. Long Term Interactions of Native and Invasive Species in a Marine Protected Area Suggest Complex Cascading Effects Challenging Conservation Outcomes. Diversity 2021, 13, 71. https://doi.org/10.3390/d13020071
Dimitriadis C, Fournari-Konstantinidou I, Sourbès L, Koutsoubas D, Katsanevakis S. Long Term Interactions of Native and Invasive Species in a Marine Protected Area Suggest Complex Cascading Effects Challenging Conservation Outcomes. Diversity. 2021; 13(2):71. https://doi.org/10.3390/d13020071
Chicago/Turabian StyleDimitriadis, Charalampos, Ivoni Fournari-Konstantinidou, Laurent Sourbès, Drosos Koutsoubas, and Stelios Katsanevakis. 2021. "Long Term Interactions of Native and Invasive Species in a Marine Protected Area Suggest Complex Cascading Effects Challenging Conservation Outcomes" Diversity 13, no. 2: 71. https://doi.org/10.3390/d13020071
APA StyleDimitriadis, C., Fournari-Konstantinidou, I., Sourbès, L., Koutsoubas, D., & Katsanevakis, S. (2021). Long Term Interactions of Native and Invasive Species in a Marine Protected Area Suggest Complex Cascading Effects Challenging Conservation Outcomes. Diversity, 13(2), 71. https://doi.org/10.3390/d13020071









