Common Vole as a Focal Small Mammal Species in Orchards of the Northern Zone
Abstract
:1. Introduction
2. Material and methods
2.1. Study Sites
2.2. Small Mammal Trapping
2.3. Data Analysis
3. Results
3.1. Share of Common Vole in Small Mammal Communities
3.2. Abundance of Common Vole
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balčiauskas, L.; Trakimas, G.; Juškaitis, R.; Ulevičius, A.; Balčiauskienė, L. Atlas of Lithuanian Mammals, Amphibians and Reptiles, 2nd ed.; Akstis: Vilnius, Lithuania, 1999; p. 112. [Google Scholar]
- Burgin, C.J.; Colella, J.P.; Kahn, P.L.; Upham, N.S. How many species of mammals are there? J. Mammal. 2018, 99, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Gasperini, S.; Bonacchi, A.; Bartolommei, P.; Manzo, E.; Cozzolino, R. Seasonal cravings: Plant food preferences of syntopic small mammals. Ethol. Ecol. Evol. 2017, 30, 12–25. [Google Scholar] [CrossRef]
- Arregoitia, L.D.V.; D’Elía, G. Classifying rodent diets for comparative research. Mammal. Rev. 2021, 51, 51–65. [Google Scholar] [CrossRef]
- Cramer, M.J.; Willig, M.R. Habitat heterogeneity, species diversity and null models. Oikos 2005, 108, 209–218. [Google Scholar] [CrossRef]
- Riojas-López, M. Response of rodent assemblages to change in habitat heterogeneity in fruit-oriented nopal orchards in the Central High Plateau of Mexico. J. Arid. Environ. 2012, 85, 27–32. [Google Scholar] [CrossRef]
- Balestrieri, A.; Remonti, L.; Morotti, L.; Saino, N.; Prigioni, C.; Guidali, F. Multilevel habitat preferences of Apodemus sylvaticus and Clethrionomys glareolus in an intensively cultivated agricultural landscape. Ethol. Ecol. Evol. 2017, 29, 38–53. [Google Scholar] [CrossRef]
- Moreno-García, P.; Baiser, B. Assessing functional redundancy in Eurasian small mammal assemblages across multiple traits and biogeographic extents. Ecography 2020. [Google Scholar] [CrossRef]
- Rivera, M.; Guarín, A.; Pinto-Correia, T.; Almaas, H.; Mur, L.A.; Burns, V.; Czekaj, M.; Ellis, R.; Galli, F.; Grivins, M.; et al. Assessing the role of small farms in regional food systems in Europe: Evidence from a comparative study. Glob. Food Secur. 2020, 26, 100417. [Google Scholar] [CrossRef]
- Butet, A.; Paillat, G.; Delettre, Y. Factors Driving Small Rodents Assemblages from Field Boundaries in Agricultural Landscapes of Western France. Landscape Ecol. 2006, 21, 49–461. [Google Scholar]
- Fischer, C.; Schröder, B. Predicting spatial and temporal habitat use of rodents in a highly intensive agricultural area. Agric. Ecosyst. Environ. 2014, 189, 145–153. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Balčiauskienė, L.; Stirkė, V. Mow the Grass at the Mouse’s Peril: Diversity of Small Mammals in Commercial Fruit Farms. Animals 2019, 9, 334. [Google Scholar] [CrossRef] [Green Version]
- Fischer, C.; Gayer, C.; Kurucz, K.; Riesch, F.; Tscharntke, T.; Batáry, P. Ecosystem services and disservices provided by small rodents in arable fields: Effects of local and landscape management. J. Appl. Ecol. 2017, 55, 548–558. [Google Scholar] [CrossRef] [Green Version]
- Schlötelburg, A.; Plekat, A.; Bellingrath-Kimura, S.; Jacob, J. Self-service traps inspected by avian and terrestrial predators as a management option for rodents. Pest. Manag. Sci. 2020, 76, 103–110. [Google Scholar] [CrossRef]
- Horváth, A.; Bank, L.; Horváth, G.F. Variation in the diet and breeding biology of the Common Barn-owl (Tyto alba) in a demographic cycle of Common Vole (Microtus arvalis) between two outbreaks. Ornis Hung. 2020, 28, 37–65. [Google Scholar] [CrossRef]
- Ims, R.A.; Henden, J.A.; Thingnes, A.V.; Killengreen, S.T. Indirect food web interactions mediated by predator-rodent dy-namics: Relative roles of lemmings and voles. Biol. Lett. 2013, 9, 20130802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zárybnická, M.; Riegert, J.; Kouba, M. Indirect food web interactions affect predation of Tengmalm’s Owls Aegolius funreus nests by Pine Martens Martes martes according to the alternative prey hypothesis. Ibis 2015, 157, 459–467. [Google Scholar] [CrossRef]
- Broughton, R.K.; Shore, R.F.; Heard, M.S.; Amy, S.R.; Meek, W.R.; Redhead, J.W.; Turk, A.; Pywell, R.F. Agri-environment scheme enhances small mammal diversity and abundance at the farm-scale. Agric. Ecosyst. Environ. 2014, 192, 122–129. [Google Scholar] [CrossRef] [Green Version]
- Kleijn, D.; Rundlöf, M.; Scheper, J.; Smith, H.G.; Tscharntke, T. Does conservation on farmland contribute to halting the biodiversity decline? Trends Ecol. Evol. 2011, 26, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.K.; Morris, A.J.; Cristinacce, A.; Dadam, D.; Grice, P.V.; Peach, W.J. Effects of higher-tier agri-environment scheme on the abundance of priority farmland birds. Anim. Conserv. 2018, 21, 183–192. [Google Scholar] [CrossRef]
- Singleton, G.R.; Brown, P.R.; Jacob, J.; Aplin, K.P. Sudarmaji Unwanted and unintended effects of culling: A case for ecologically-based rodent management. Integr. Zool. 2007, 2, 247–259. [Google Scholar] [CrossRef]
- Jacob, J.; Manson, P.; Barfknecht, R.; Fredricks, T. Common vole (Microtus arvalis) ecology and management: Implications for risk assessment of plant protection products. Pest. Manag. Sci. 2014, 70, 869–878. [Google Scholar] [CrossRef]
- Roos, D.; Caminero Saldaña, C.; Arroyo, B.; Mougeot, F.; Luque-Larena, J.J.; Lambin, X. Unintentional effects of environ-mentally-friendly farming practices: Arising conflicts between zero-tillage and a crop pest, the common vole (Microtus arvalis). Agric. Ecosyst. Environ. 2019, 272, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Krojerová-Prokešová, J.; Homolka, M.; Heroldová, M.; Barančeková, M.; Baňař, P.; Kamler, J.; Modlinger, R.; Purchart, L.; Zejda, J.; Suchomel, J. Patterns of vole gnawing on saplings in managed clearings in Central European forests. For. Ecol. Manag. 2018, 408, 137–147. [Google Scholar] [CrossRef]
- Sánchez-Barbudo, I.S.; Camarero, P.R.; Mateo, R. Primary and secondary poisoning by anticoagulant rodenticides of non-target animals in Spain. Sci. Total. Environ. 2012, 420, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Elmeros, M.; Christensen, T.K.; Lassen, P. Concentrations of anticoagulant rodenticides in stoats Mustela erminea and weasels Mustela nivalis from Denmark. Sci. Total. Environ. 2011, 409, 2373–2378. [Google Scholar] [CrossRef]
- Brooks, A.C.; Fryer, M.; Lawrence, A.; Pascual, J.; Sharp, R. Reflections on bird and mammal risk assessment for plant pro-tection products in the European Union: Past, present, and future. Environ. Toxicol. Chem. 2017, 36, 565–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walther, B.; Geduhn, A.; Schenke, D.; Jacob, J. Exposure of passerine birds to brodifacoum during management of Norway rats on farms. Sci. Total. Environ. 2021, 762, 144160. [Google Scholar] [CrossRef]
- Risk Assessment for Birds and Mammals. EFSA J. 2009, 7. [CrossRef]
- Northern Zone Pesticide Risk Assessment for Birds and Mammals. Selection of Relevant Species and Development of Standard Scenarios for Higher Tier Risk Assessment in the Northern Zone in Accordance with Regulation EC 1107/2009. Version 2.1. December 2021. Available online: https://eng.mst.dk/media/211955/birds-and-mammals-higher-tier-risk-assesment-northern-zone-april-2020-ver-2-0.docx (accessed on 15 February 2021).
- Balčiauskas, L.; Juškaitis, R. Diversity of small mammal communities in Lithuania (1. a review). ACTA Zool. Litu. 1997, 7, 29–45. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Čepukienė, A.; Balčiauskienė, L. Small mammal community response to early meadow–forest succession. For. Ecosyst. 2017, 4, 11. [Google Scholar] [CrossRef]
- Balčiauskas, L. Results of the Long-Term Monitoring of Small Mammal Communities in the Ignalina Nuclear Power Plant Region (Drūkšiai LTER Site). ACTA Zool. Litu. 2005, 15, 79–84. [Google Scholar] [CrossRef]
- Šinkūnas, R.; Balčiauskas, L. Small Mammal Communities in the Fragmented Landscape in Lithuania. ACTA Zool. Litu. 2006, 16, 130–136. [Google Scholar] [CrossRef]
- Official Statistics Portal. Available online: https://osp.stat.gov.lt/statistiniu-rodikliu-analize?indicator=S9R017#/ (accessed on 2 February 2021).
- Balčiauskas, L. Methods of Investigation of Terrestrial Ecosystems. Part I. Animal Surveys; VU leidykla: Vilnius, Lithuania, 2004; p. 183. [Google Scholar]
- Prūsaitė, J. (Comp.). Fauna of Lithuania. Mammals; Mokslas: Vilnius, Lithuania, 1988; p. 295. [Google Scholar]
- Brown, L.D.; Cat, T.T.; DasGupta, A. Interval Estimation for a proportion. Stat. Sci. 2001, 16, 101–133. [Google Scholar]
- Dean, A.G.; Sullivan, K.M.; Soe, M.M. OpenEpi: Open Source Epidemiologic Statistics for Public Health. Available online: http://OpenEpi.com (accessed on 19 January 2021).
- G-Test Calculator. Available online: https://elem.com/~btilly/effective-ab-testing/g-test-calculator.html (accessed on 16 March 2021).
- Thomas, J.R.; Salazar, W.; Landers, D.M. What is missing in p less than 0.05? Effect size. Res. Q. Exerc. Sport 1991, 62. [Google Scholar]
- Balčiauskienė, L.; Balčiauskas, L.; Timm, U. Changes in size of Baltic field voles over the last 50 years: Are they really shrinking? Biologia 2018, 73, 247–257. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Balčiauskienė, L.; Janonytė, A. The influence of spring floods on small mammal communities in the Nemunas River Delta, Lithuania. Biologia 2012, 67, 1220–1229. [Google Scholar] [CrossRef] [Green Version]
- Wegge, P.; Rolstad, J. Cyclic small rodents in boreal forests and the effects of even-aged forest management: Patterns and predictions from a long-term study in southeastern Norway. For. Ecol. Manag. 2018, 422, 79–86. [Google Scholar] [CrossRef]
- Korpimaki, E.; Norrdahl, K. Numerical and Functional Responses of Kestrels, Short-Eared Owls, and Long-Eared Owls to Vole Densities. Ecology 1991, 72, 814–826. [Google Scholar] [CrossRef]
- Tome, D. Changes in the diet of long-eared owl Asio otus: Seasonal patterns of dependence on vole abundance. Ardeola 2009, 56, 49–56. [Google Scholar]
- Zárybnická, M.; Riegert, J.; Št’Astný, K. The role of Apodemus mice and Microtus voles in the diet of the Tengmalm’s owl in Central Europe. Popul. Ecol. 2013, 55, 353–361. [Google Scholar] [CrossRef]
- Fay, R.; Michler, S.; Laesser, J.; Jeanmonod, J.; Schaub, M. Large-Scale Vole Population Synchrony in Central Europe Revealed by Kestrel Breeding Performance. Front. Ecol. Evol. 2020, 7, 512. [Google Scholar] [CrossRef] [Green Version]
- Balčiauskienė, L.; Naruševičius, V. Coincidence of small mammal trapping data with their share in the Tawny Owl diet. ACTA Zoologica Lituanica 2006, 16, 93–101. [Google Scholar] [CrossRef]
- Tulis, F.; Baláž, M.; Obuch, J.; Šotnár, K. Responses of the long-eared owl Asio otus diet and the numbers of wintering indi-viduals to changing abundance of the common vole Microtus arvalis. Biologia 2015, 70, 667–673. [Google Scholar] [CrossRef]
- Gryz, J.; Krauze-Gryz, D.; Jakub, G.; Dagny, K.-G. Seasonal variability in the diet of the long-eared owl Asio otus in a mosaic of field and forest habitats in central Poland. ACTA Zool. Cracoviensia 2015, 58, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Kitowski, I. Winter diet of the barn owl (Tyto alba) and the long-eared owl (Asio otus) in Eastern Poland. North-West J. Zool. 2013, 9, 16–22. [Google Scholar]
- Romanowski, J.; Żmihorski, M. Effect of season, weather and habitat on diet variation of a feeding-specialist: A case study of the long-eared owl, Asio otus in Central Poland. Folia Zool. 2008, 57, 411–419. [Google Scholar]
- Santamaría, A.E.; Olea, P.P.; Viñuela, J.; García, J.T. Spatial and seasonal variation in occupation and abundance of common vole burrows in highly disturbed agricultural ecosystems. Eur. J. Wildl. Res. 2019, 65, 52. [Google Scholar] [CrossRef]
- Jacob, J.; Imholt, C.; Caminero-Saldaña, C.; Couval, G.; Giraudoux, P.; Herrero-Cofreces, S.; Horváth, G.; Luque-Larena, J.J.; Tkadlec, E.; Wymenga, E. Europe-wide outbreaks of common voles in 2019. J. Pest Sci. 2020, 93, 703–709. [Google Scholar] [CrossRef] [Green Version]
- Sîtnic, V.; Nistreanu, V.; Larion, A.; Savin, A. The demographic structure of Microtus arvalis and Microtus rossiaemeridionalis (Mammalia, Rodentia, Cricetidae) populations in agrocenoses from the Republic of Moldova. Oltenia-studii si comunicari stiintele naturii 2020, 36, 129–136. [Google Scholar]
- Heroldová, M.; Šipoš, J.; Suchomel, J.; Zejda, J. Interactions between common vole and winter rape. Pest. Manag. Sci. 2021, 77, 599–603. [Google Scholar] [CrossRef]
- Somogyi, B.A.; Horváth, G.F. Seasonal activity of common vole (Microtus arvalis) in alfalfa fields in southern Hungary. Biol. 2019, 74, 91–96. [Google Scholar] [CrossRef]
- Wang, M.; Ebeling, M.; Hahne, J. Relevance of body weight effects for the population development of common voles and its significance in regulatory risk assessment of pesticides in the European Union. Environ. Sci. Eur. 2019, 31, 1–9. [Google Scholar] [CrossRef]
- Nilsson, I.N. Seasonal Changes in Food of the Long-Eared Owl in Southern Sweden. Ornis Scand. 1981, 12, 216. [Google Scholar] [CrossRef]
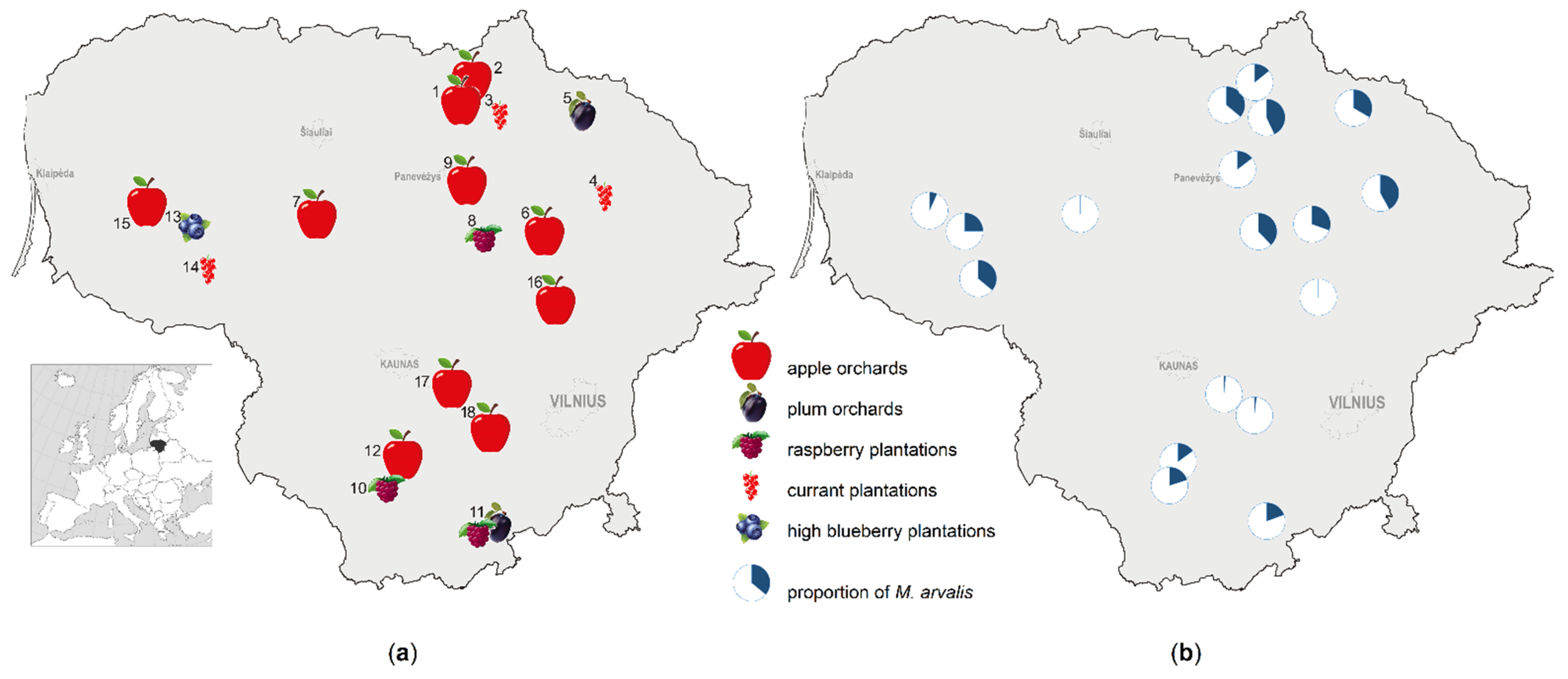


| Parameter | Values | Sites | Trapping Effort |
|---|---|---|---|
| Crop age | old | 1,2,6,7,9,12,16–18 | 9768 |
| medium | 3,4,8,11,13–15 | 5050 | |
| young | 1,5,10,12 | 1900 | |
| Intensity of agriculture | high | 2,6,10–13,15,17 | 8218 |
| medium | 1,5,9,14 | 4450 | |
| low | 3,4,7,8,11,16,18 | 4050 | |
| Control | forest | 11,17 | 525 |
| mowed meadow | 1,2,4,6,8–10,12,13–16 | 5560 | |
| non-mowed meadow | 1,3,5,7–9,11,18 | 2700 |
| Season | Species | 2018 | 2019 | 2020 |
|---|---|---|---|---|
| Summer | Dominant | M. arvalis (27.2%) | M. arvalis (38.3%) | A. flavicollis (39.7%) |
| Sub-dominant | A. flavicollis (20.7%) | A. flavicollis (27.1%) | M. arvalis (26.9%) | |
| Autumn | Dominant | A. agrarius (37.1%) | M. arvalis (36.2%) | A. agrarius (32.4%) |
| Sub-dominant | M. arvalis (25.7%) | A. flavicollis (29.9%) | A. flavicollis (30.3%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stirkė, V.; Balčiauskas, L.; Balčiauskienė, L. Common Vole as a Focal Small Mammal Species in Orchards of the Northern Zone. Diversity 2021, 13, 134. https://doi.org/10.3390/d13030134
Stirkė V, Balčiauskas L, Balčiauskienė L. Common Vole as a Focal Small Mammal Species in Orchards of the Northern Zone. Diversity. 2021; 13(3):134. https://doi.org/10.3390/d13030134
Chicago/Turabian StyleStirkė, Vitalijus, Linas Balčiauskas, and Laima Balčiauskienė. 2021. "Common Vole as a Focal Small Mammal Species in Orchards of the Northern Zone" Diversity 13, no. 3: 134. https://doi.org/10.3390/d13030134
APA StyleStirkė, V., Balčiauskas, L., & Balčiauskienė, L. (2021). Common Vole as a Focal Small Mammal Species in Orchards of the Northern Zone. Diversity, 13(3), 134. https://doi.org/10.3390/d13030134








