Record of Caromiobenella (Copepoda, Monstrilloida) in Brazil and Discovery of the Male of C. brasiliensis: Morphological and Molecular Evidence
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Morphologic Analysis
2.3. Molecular Analysis
3. Results
3.1. Taxonomy
- Subclass Copepoda Milne-Edwards, 1840
- Order Monstrilloida Thorell, 1859
- Family Monstrillidae Dana, 1849
- Genus Caromiobenella Jeon, Lee and Soh, 2018
- Caromiobenella brasiliensis (Dias and Suárez-Morales, 2000) comb. nov.
3.1.1. Material Examined
3.1.2. Type Locality
3.1.3. Diagnosis (Female and Male)
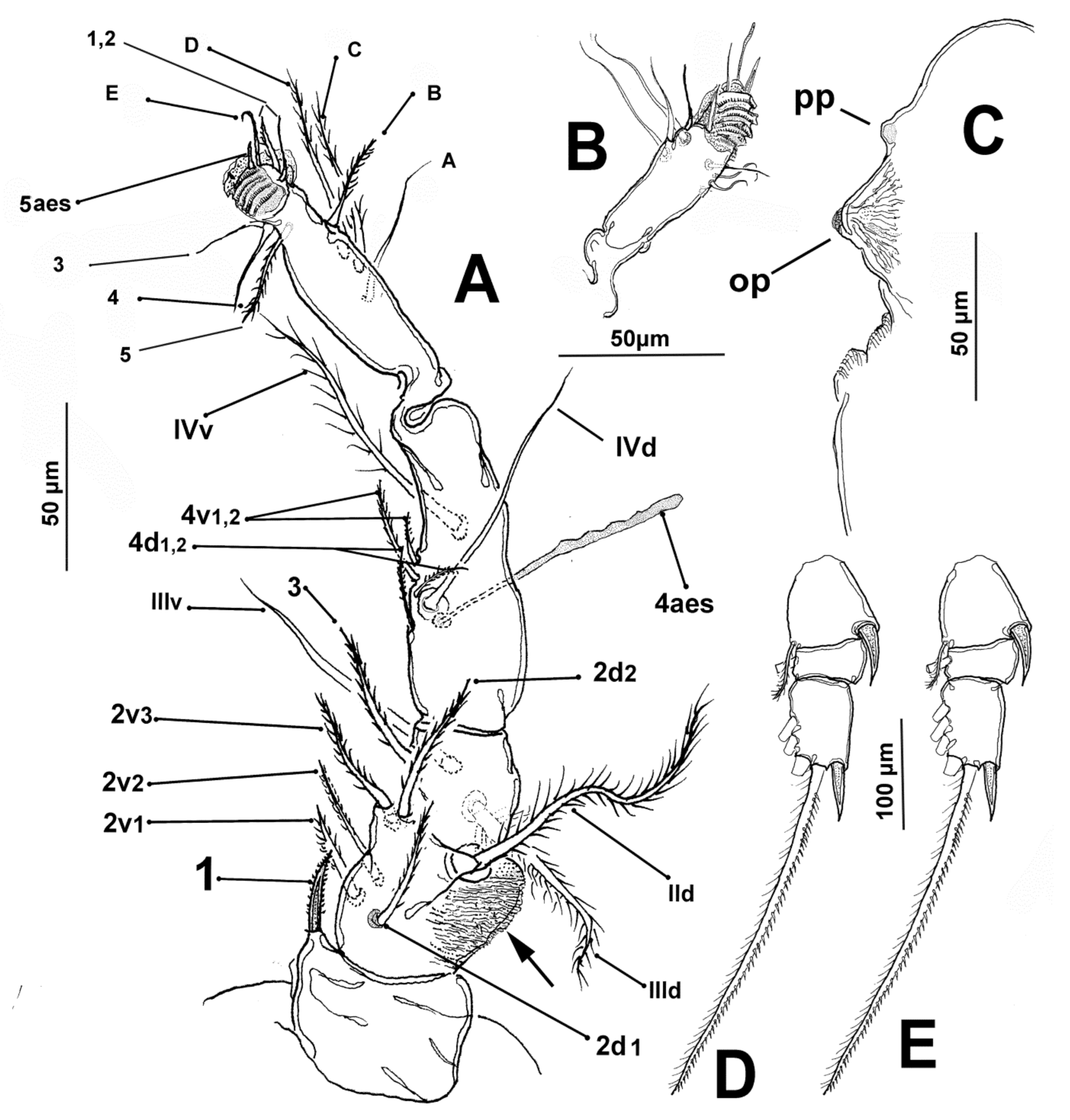
3.1.4. Description of Adult Male
3.1.5. Additional Male Specimens Measured
3.1.6. Female Specimens Measured
3.2. Molecular Analysis
4. Discussion
4.1. Ecology
4.2. Molecular Remarks
4.3. Key to Known Species of Caromiobenella (Males)
- 1.
- Antennule with wrinkled protuberance on second antennulary segment............................................................. C. brasiliensis (Dias and Suárez-Morales, 2000)
- 1A.
- Antennule lacking special processes on second segment ............................................. 2
- 2.
- Element 1 (sensu Grygier and Ohtsuka, 1995) long, reaching well beyond midlength of succeeding second segment.................................... C. polluxea Jeon, Lee and Soh, 2018
- 2A.
- Element 1(sensu Grygier and Ohtsuka, 1995) not as long, not reaching halfway of second segment .......................................................... C. ohtsukai Jeon, Lee and Soh, 2019
- 3.
- Element 2d2 (sensu Grygier and Ohtsuka, 1995) remarkably long, reaching halfway of fourth segment...................................................... C. castorea Jeon, Lee and Soh, 2018
- 3A.
- Element 2d2 (sensu Grygier and Ohtsuka, 1995) not as long, barely reaching distal margin of third segment or shorter.................................................................................................................................... 4
- 4.
- Body length (excluding caudal rami) less than 0.5 mm, 5 caudal setae.......................... ........................................................................................ C. pygmaea (Suárez-Morales, 2000)
- 4A.
- Body length (excluding caudal rami) more than 1.0 mm, 6 caudal setae.................... 5
- 5.
- Elements 2v1–3 (sensu Grygier and Ohtsuka, 1995) relatively long, elements 2v2,3 reaching beyond halfway of succeeding third segment ........................................................... 6
- 5A.
- Elements 2v1–3(sensu Grygier and Ohtsuka, 1995) short, barely reaching halfway of third segment................................................................................. C. serricornis (Sars, 1921)
- 6.
- Antennulary segment 4 with proximal bulging process.............................................. ............................................. C. patagonica (Suárez-Morales, Ramírez and Derisio, 2008)
- 6A.
- Antennulary segment 4 lacking proximal bulging process............................................7
- 7.
- Fifth leg present.................................................................................................................... 8
- 7A.
- Fifth leg absent...................................................................................................................... 9
- 8.
- Fifth leg 1- lobed, inner margin straight............................. C. helgolandica (Claus, 1863)
- 8A.
- Fifth leg 1-lobed, with two setae, inner margin produced............................................... ........................................................................ C. hamatapex (Grygier and Ohtsuka, 1995)
- 9.
- Antennulary setae A–D (sensu Huys et al., 2007) branched...................................... ........................................................................................ C. arctica (Davis and Green, 1974)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huys, R.; Llewellyn-Hughes, J.; Conroy-Dalton, S.; Olson, P.D.; Spinks, J.N.; Johnston, D.A. Extraordinary host switching in siphonostomatoid copepods and the demise of the Monstrilloida: Integrating molecular data, ontogeny and antennulary morphology. Mol. Phylogenet. Evol. 2007, 43, 368–378. [Google Scholar] [CrossRef]
- Suárez-Morales, E. Diversity of the Monstrilloida (Crustacea: Copepoda). PLoS ONE 2011, 6, e22915. [Google Scholar] [CrossRef] [Green Version]
- Jeon, D.; Lee, W.; Soh, H.Y. New genus and two new species of monstrilloid copepods (Copepoda: Monstrillidae): Inte-grating morphological, molecular phylogenetic, and ecological evidence. J. Crust. Biol. 2018, 38, 45–65. [Google Scholar] [CrossRef] [Green Version]
- Suárez-Morales, E.; Scardua, M.P.; da Silva, P.M. Occurrence and histopathological effects of Monstrilla sp. (Cope-poda: Monstrilloida) and other parasites in the brown mussel Perna perna from Brazil. J. Mar. Biol. Assoc. U. K. 2010, 90, 953–958. [Google Scholar] [CrossRef]
- Grygier, M.J. Identity of Thaumatoessa (= Thaumaleus) typica Krøyer, the first described monstrilloid copepod. Sarsia 1993, 78, 235–242. [Google Scholar] [CrossRef]
- Grygier, M.J.; Suárez-Morales, E. Recognition and partial solution of nomenclatural issues involving copepods of the family Monstrillidae (Crustacea: Copepoda: Monstrilloida). Zootaxa 2018, 4486, 497–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suárez-Morales, E.; Grygier, M.J. Mediterranean and Black Sea Monstrilloid copepods (Copepoda: Monstrilloida): Rediscovering the diversity of transient zooplankters. Water 2021, 13, 1–11. [Google Scholar]
- Suárez-Morales, E.; Dias, C. Two new species of Monstrilla (Copepoda: Monstrilloida) from Brazil. J. Mar. Biol. Assoc. U. K. 2000, 80, 1031–1039. [Google Scholar] [CrossRef]
- Suárez-Morales, E.; Dias, C. A new species of Monstrilla (Crustacea: Copepoda: Monstrilloida) from Brazil with notes on M. brevicornis Isaac. Proc. Biol. Soc. Wash. 2001, 114, 219–228. [Google Scholar]
- Suárez-Morales, E.; Dias, C. Taxonomic report of some monstrilloids (Copepoda: Monstrilloida) from Brazil with description of four new species. Bull. Inst. Royal Sci. Nat. Belg. Biologie. 2001, 71, 65–81. [Google Scholar]
- Leite, N.R.; Pereira, L.C.; Abrunhosa, F.; Pires, M.A.; Da Costa, R.M. Occurrence of Cymbasoma longispinosum Bourne, 1890 (Copepoda: Monstrilloida) in the Curuçá River estuary (Amazon Littoral). An. Acad. Bras. Ciências 2010, 82, 577–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suárez-Morales, E.; Dias, C.O.; Bonecker, S.L. Discovery of the female of Cymbasoma rochai Suárez-Morales & Dias, 2001 (Copepoda, Monstrilloida, Monstrillidae), the first Brazilian member of the C. longispinosum species-group. Crustaceana 2020, 93, 1091–1101. [Google Scholar] [CrossRef]
- Grygier, M.J.; Ohtsuka, S. A new genus of monstrilloid copepods (Crustacea) with anteriorly pointing ovigerous spines and related adaptations for subthoracic brooding. Zool. J. Linn. Soc. 2008, 152, 459–506. [Google Scholar] [CrossRef]
- Grygier, M.J.; Ohtsuka, S. Sem Observation of the Nauplius of Monstrilla hamatapex, new species, from Japan and an example of Upgraded descriptive standards for monstrilloid copepods. J. Crust. Biol. 1995, 15, 703. [Google Scholar] [CrossRef]
- Jeon, D.; Lee, W.; Soh, H.Y. New species of Caromiobenella Jeon, Lee & Soh, 2018 (Crustacea, Copepoda, Monstrilloida) from Chuja Island, Korea. ZooKeys 2019, 814, 33–51. [Google Scholar] [CrossRef]
- Huys, R.; Boxshall, G.A. Copepod Evolution; The Ray Society: London, UK, 1991; pp. 1–468. [Google Scholar]
- Harris, S.A.; Hoelzel, A.R. Molecular Genetic Analysis of Populations. A Practical Approach. J. Appl. Ecol. 1993, 30, 197. [Google Scholar] [CrossRef]
- Casquet, J.; Thebaud, C.; Gillespie, R.G. Chelex without boiling, a rapid and easy technique to obtain stable amplifiable DNA from small amounts of ethanol-stored spiders. Mol. Ecol. Resour. 2012, 12, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Tamura, S.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [Green Version]
- Raupach, M.J.; Barco, A.; Steinke, D.; Beermann, J.; Laakmann, S.; Mohrbeck, I.; Neumann, H.; Kihara, T.C.; Pointner, K.; Raulovici, A.; et al. The Application of DNA Barcodes for the Identification of Marine Crustaceans from the North Sea and Adjacent Regions. PLoS ONE 2015, 10, e0139421. [Google Scholar] [CrossRef] [Green Version]
- Unal, E.; Frost, B.W.; Armbrust, V.; Kideys, A.E. Phylogeography of Calanus helgolandicus and the Black Sea copepod Calanus euxinus, with notes on Pseudocalanus elongatus (Copepoda, Calanoida). Deep. Sea Res. Part II Top. Stud. Oceanogr. 2006, 53, 1961–1975. [Google Scholar] [CrossRef]
- Suárez-Morales, E. On the male of Monstrilla mariaeugeniae Suárez-Morales & Islas-Landeros (Copepoda: Monstrilloida) from the Mexican Caribbean Sea. Crustaceana 1998, 71, 360–362. [Google Scholar]
- Jeon, D.; Lim, D.; Lee, W.; Soh, H.Y. First use of molecular evidence to match sexes in the Monstrilloida (Crustacea: Copepoda), and taxonomic implications of the newly recognized and described, partly Maemonstrilla-like females of Monstrillopsis longilobata Lee, Kim & Chang, 2016. PeerJ 2018, 6, e4938. [Google Scholar] [CrossRef] [PubMed]
- Sale, P.F.; McWilliam, P.S.; Anderson, D.T. Composition of the near-reef zooplankton at Heron Reef, Great Barrier Reef. Mar. Biol. 1976, 34, 59–66. [Google Scholar] [CrossRef]
- Suárez-Morales, E. An aggregation of monstrilloid copepods in a western Caribbean reef area: Ecological and conceptual implications. Crustaceana 2001, 74, 689–696. [Google Scholar] [CrossRef]
- Suárez-Morales, E. Taxonomic report on a collection of monstrilloids (Copepoda: Monstrilloida) from Banco Chinchorro, Mexico with description of a new species. An. Inst. Biol. Univ. Nac. Autón. Mex. Ser. Zool. 2001, 72, 9–28. [Google Scholar]
- Dias, C.O.; Bonecker, S.L.C. Study of Monstrilloida distribution (Crustacea: Copepoda) in the Southwest Atlantic. Panamjas 2007, 2, 270–278. [Google Scholar]





| Date | Site | Collection Type | C. brasiliensis | Zoopl. Density (ind. m3) | Tidepool Volume (L) | Drained Volume (L) | Catalog Number | Temp | Sal | |
|---|---|---|---|---|---|---|---|---|---|---|
| N°, Sex | Density (ind. m3) | |||||||||
| 08.08.17 | A.Negras | TD | 3M | 15.0 | 155 | 345 | 200 | NPM00020 (1M *), (2M * lost%) | 24.2 | 30.0 |
| 21.09.17 | Remanso | TD | 2F | 5.0 | 206 | 1900 | 400 | NPM000021 (1F), (1F lost%) | 20.4 | 34.3 |
| 16.10.17 | Remanso | TD | 1 M | 5.0 | 991 | 1900 | 200 | 1M (dissected) | 21.5 | 33.2 |
| 16.10.17 | Remanso | TD | 2M | 10.0 | 335 | 600 | 200 | NPM00022 (1M *), MNRJ30136 (1M *) | 21.2 | 33.6 |
| 31.10.17 | Rasa | TD | 1F | 2.5 | 77 | 1440 | 400 | NPM00023 (1F) | 22.6 | 28.1 |
| 03.02.18 | Remanso | TD | 1M | 2.5 | 870 | 1900 | 400 | 1M * (dissected) | 28.8 | 32.0 |
| 19.09.18 | Cemiterio | PN | 1F/2M | NA | NA | NA | NA | MNRJ28990 (1M), 1M #, 1F $ * | - | - |
| 20.10.18 | Cemiterio | PN | 1F/1M | NA | NA | NA | NA | 1M #, 2F $ | - | - |
| 21.10.18 | Cemiterio | PN | 2F/1F | NA | NA | NA | NA | 2F #, 1F $ * | - | - |
| 28.10.18 | Cemiterio | PN | 1F | NA | NA | NA | NA | 1F # * | - | - |
| 24.11.18 | Cemiterio | PN | 2F | NA | NA | NA | NA | MNRJ28991 (1F), 1F # | - | - |
| 25.11.18 | Cemiterio | PN | 1F | NA | NA | NA | NA | NPM00024 (1F) | - | - |
| 02.12.18 | Cemiterio | PN | 1F | NA | NA | NA | NA | NPM00025 (1F) | - | - |
| Measure | Min (mm) | Max (mm) | Mean (mm) |
|---|---|---|---|
| Total length | 0.816 | 1.170 | 1.004 |
| Antennule length | 0.187 | 0.373 | 0.323 |
| Cephalothorax height | 0.186 | 0.269 | 0.240 |
| Cephalothorax length | 0.339 | 0.475 | 0.413 |
| Cephalothorax width | 0.283 | 0.456 | 0.381 |
| Metasome length | 0.172 | 0.239 | 0.211 |
| Urosome length | 0.816 | 1.170 | 1.004 |
| Incorporated pediger | 0.180 | 0.256 | 0.225 |
| Free pediger 1 | 0.211 | 0.294 | 0.266 |
| Free pediger 2 | 0.164 | 0.220 | 0.200 |
| Free pediger 3 | 0.131 | 0.203 | 0.178 |
| Genital somite | 0.082 | 0.810 | 0.273 |
| Preanal somite | 0.065 | 0.660 | 0.216 |
| Caudal ramus length | 0.053 | 0.066 | 0.059 |
| Caudal ramus width | 0.036 | 0.058 | 0.046 |
| Leg | Basis | Endopod | Exopod |
|---|---|---|---|
| Leg 1 | 1–0 | I-1; 0–1; 2, 2, 1 | I-1; 0–1; 2, 2, 1, I |
| Legs 2–4 | 1–0 | 0–1; 0–1; 2, 2, 1 | I-1; 0–1; 2, 2, 1, I |
| Female | Female | Female | Female | Male | Male | |
|---|---|---|---|---|---|---|
| GenBank Accesion | MZ223430 | MZ223431 | MZ223432 | MZ223433 | MZ223434 | MZ223435 |
| MZ223430 | 0.018 | 0.019 | 0.023 | 0.015 | 0.006 | |
| MZ223431 | 0.018 | 0.010 | 0.010 | 0.009 | 0.018 | |
| MZ223432 | 0.020 | 0.010 | 0.006 | 0.010 | 0.019 | |
| MZ223433 | 0.022 | 0.010 | 0.006 | 0.013 | 0.019 | |
| MZ223434 | 0.015 | 0.009 | 0.010 | 0.013 | 0.015 | |
| MZ223435 | 0.006 | 0.018 | 0.019 | 0.019 | 0.015 |
| Branches/Spp | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| (1) C. brasiliensis | 0.305 ± 0.026 | 0.308 ± 0.023 | 0.470 ± 0.038 | 0.366 ± 0.031 | 0.464 ± 0.030 | 0.505 ± 0.041 | 0.535 ± 0.036 | |
| (2) M. ilhoii | 0. 299± 0.025 | 0.401 ± 0.028 | 0.459 ± 0.039 | 0.388 ± 0.033 | 0.446 ± 0.030 | 0.595 ± 0.054 | 0.576 ± 0.038 | |
| (3) Caromiobenella | 0.302 ± 0.022 | 0.390 ± 0.027 | 0.443 ± 0.031 | 0.417 ± 0.031 | 0.503 ± 0.028 | 0.540 ± 0.041 | 0.545 ± 0.035 | |
| (4) C. helgolandica | 0.452 ± 0.035 | 0. 435± 0.035 | 0.428 ± 0.029 | 0. 398± 0.033 | 0.542 ± 0.037 | 0.573 ± 0.054 | 0.620 ± 0.043 | |
| (5) Maemonstrilla | 0.353 ± 0.028 | 0.374 ± 0.029 | 0.406 ± 0.029 | 0.385 ± 0.031 | 0.489 ± 0.032 | 0.550 ± 0.052 | 0.579 ± 0.041 | |
| (6) Cymbasoma | 0.451 ± 0.028 | 0.434 ± 0.028 | 0.488 ± 0.027 | 0.517 ± 0.033 | 0.471 ± 0.030 | 0.596 ± 0.042 | 0.593 ± 0.034 | |
| (7) M. longilobata | 0.481 ± 0.036 | 0.543 ± 0.041 | 0.515 ± 0.026 | 0.519 ± 0.040 | 0.500 ± 0.039 | 0. 561± 0.034 | 0.397 ± 0.027 | |
| (8) External group | 0.522 ± 0.034 | 0.554 ± 0.034 | 0.532 ± 0.033 | 0.588 ± 0.037 | 0.553 ± 0.035 | 0.573 ± 0.032 | 0.388 ± 0.025 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Cruz Lopes da Rosa, J.; Dias, C.d.O.; Suárez-Morales, E.; Weber, L.I.; Fischer, L.G. Record of Caromiobenella (Copepoda, Monstrilloida) in Brazil and Discovery of the Male of C. brasiliensis: Morphological and Molecular Evidence. Diversity 2021, 13, 241. https://doi.org/10.3390/d13060241
da Cruz Lopes da Rosa J, Dias CdO, Suárez-Morales E, Weber LI, Fischer LG. Record of Caromiobenella (Copepoda, Monstrilloida) in Brazil and Discovery of the Male of C. brasiliensis: Morphological and Molecular Evidence. Diversity. 2021; 13(6):241. https://doi.org/10.3390/d13060241
Chicago/Turabian Styleda Cruz Lopes da Rosa, Judson, Cristina de Oliveira Dias, Eduardo Suárez-Morales, Laura Isabel Weber, and Luciano Gomes Fischer. 2021. "Record of Caromiobenella (Copepoda, Monstrilloida) in Brazil and Discovery of the Male of C. brasiliensis: Morphological and Molecular Evidence" Diversity 13, no. 6: 241. https://doi.org/10.3390/d13060241
APA Styleda Cruz Lopes da Rosa, J., Dias, C. d. O., Suárez-Morales, E., Weber, L. I., & Fischer, L. G. (2021). Record of Caromiobenella (Copepoda, Monstrilloida) in Brazil and Discovery of the Male of C. brasiliensis: Morphological and Molecular Evidence. Diversity, 13(6), 241. https://doi.org/10.3390/d13060241






