Genetic Diversity of Ancient Camellia sinensis (L.) O.Kuntze in Sandu County of Guizhou Province in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Phenotypic Data Analysis
2.3. DNA Extraction, SSR Marker Development and PCR Analysis
2.4. Genetic Diversity Analysis Using SSR Markers
3. Results
3.1. Diversity Analysis of Phenotypic Characters
3.2. Principal Component Analysis of Phenotypic Characters
3.3. Cluster Analysis of Phenotypic Characters
3.4. SSR Markers Development
3.5. Genetic Diversity Analysis of Ancient Tea Plant Germplasm Using SSR Markers
3.6. Analysis of Molecular Variance (AMOVA)
3.7. Population Structure Analysis
3.8. UPGMA Cluster Analysis Using SSR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statements
Acknowledgments
Conflicts of Interest
References
- Ji, P.Z.; Li, H.; Gao, L.Z.; Zhang, J.; Cheng, Z.Q.; Huang, X.Q. ISSR diversity and genetic differentiation of ancient tea (camellia sinensis var. assamica) plantations from china: Implications for precious tea germplasm conservation. Pak. J. Bot. 2011, 43, 281–291. [Google Scholar]
- Beris, F.Ş.; Pehlivan, N.; Kac, M.; Haznedar, A.; Coşkun, F.; Sandallı, C. Evaluation of genetic diversity of cultivated tea clones (Camellia sinensis (L.) KUNTZE) in the eastern black sea coast by inter-simple sequence repeats (ISSRS). Genetika 2016, 48, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Wang, P.; Tan, Y.; Song, W.; Yi, B.; Chen, M. Studied on the distribution and diversity about ancient tea trees ceno species in Yunan, China. Southwest China J. Agric. Sci. 2006, 19, 123–126. [Google Scholar]
- Chen, Z.; Liu, H.; Cao, Y. Protection and utilization of variation type of wild tea resources and local tea varieties in Guizhou. Guizhou Agric. Sci. 2009, 21, 296–299. [Google Scholar]
- Karthigeyan, S.; Rajkumar, S.; Sharma, R.K.; Gulati, A.; Sud, R.K.; Ahuja, P.S. High level of genetic diversity among the selected accessions of tea (Camellia sinensis) from abandoned tea gardens in Western Himalaya. Biochem. Genet. 2008, 46, 810. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Mi, X.; Zhao, S.; Guo, R.; Xia, X.; Liu, S.; Wei, C. Revealing distinctions in genetic diversity and adaptive evolution between two varieties of Camellia sinensis by whole-genomer esequencing. Front. Plant Sci. 2020, 11, 603819. [Google Scholar] [CrossRef]
- Rao, V.R.; Hodgkin, T. Genetic diversity and conservation and utilization of plant genetic resources. Plant Cell Tissue Organ Cult. 2002, 68, 1–19. [Google Scholar]
- Ding, S.T.; Cheng, X.M.; Zhang, Y.; Ren, H.J.; Jiang, C.J.; Xiao-Ming, J.I. The research on phenotypic traits genetic diversity of the ancient tea germplasm resources in Shaanxi province. Guangdong Agric. Sci. 2018, 45, 52–60, 62. (In Chinese) [Google Scholar] [CrossRef]
- Ben-Ying, L.I.U.; You-Yong, L.I.; Yi-Chun, T.A.N.G.; Li-Yuan, W.A.N.G.; Cheng, H.; Ping-Sheng, W.A.N.G. Assessment of genetic diversity and relationship of tea germplasm in Yunnan as revealed by ISSR markers. Acta Agron. Sin. 2010, 36, 391–400. [Google Scholar]
- Ebrahimi, R.; Zamani, Z.; Kashi, A. Genetic diversity evaluation of wild Persian shallot (Allium hirtifolium Boiss.) using morphological and RAPD markers. Sci. Hortic. 2009, 119, 345–351. [Google Scholar] [CrossRef]
- Fang, W.; Cheng, H.; Duan, Y.; Jiang, X.; Li, X. Genetic diversity and relationship of clonal tea (Camellia sinensis) cultivars in China as revealed by SSR markers. Plant Syst. Evol. 2012, 298, 469–483. [Google Scholar] [CrossRef]
- Chopkar, R.; Sarawgi, A.K. Study of genetic diversity for agro-morphological and quality characters in diverse aromatic rice germplasm ORYZA. Int. J. Rice 2017, 54, 342. [Google Scholar]
- Akalusi, M.E.; Bourque, C.P.A. Effect of climatic variation on the morphological characteristics of 37-year-old balsam fir provenances planted in a common garden in New Brunswick, Canada. Ecol. Evol. 2018, 8, 3208–3218. [Google Scholar] [CrossRef] [PubMed]
- Petit, J.; Salentijn, E.M.; Paulo, M.J.; Thouminot, C.; van Dinter, B.J.; Magagnini, G.; Gusovius, H.J.; Tang, K.; Amaducci, S.; Wang, S.; et al. Genetic variability of morphological, flowering, and biomass quality traits in Hemp (Cannabis sativa L.). Front. Plant Sci. 2020, 11, 102. [Google Scholar] [CrossRef] [Green Version]
- Poljak, I.; Vukelić, J.; Vidaković, A.; Vuković, M.; Idžojtić, M. Variability of the populations of Scots pine (Pinus sylvestris L.) in the northwestern part of Mala Kapela according to the morphological characteristics of the needles and cones. Šumarski List 2020, 144, 539–548. [Google Scholar] [CrossRef]
- Poljak, I.; Idžojtić, M.; Šapić, I.; Korijan, P.; Vukelić, J. Diversity and structure of croatian continental and alpine-dinaric populations of grey alder (Alnus incana/L./Moench subsp. incana): Isolation by distance and environment explains phenotypic divergence. Šumarski List 2018, 142, 19–31. [Google Scholar]
- Botha, A.M.; Venter, E. Molecular marker technology linked to pest and pathogen resistance in wheat breeding. South Afr. J. Sci. 2000, 96, 233–240. [Google Scholar]
- Prasanna, B.M.; Hoisington, D. Molecular breeding for maize improvement: An overview. Indian J. Biotechnol. 2003, 2, 85–98. [Google Scholar]
- Buhariwalla, H.K.; Jayashree, B.; Eshwar, K.; Crouch, J.H. Development of ESTs from chickpea roots and their use in diversity analysis of the Cicer genus. BMC Plant Biol. 2005, 5, 16. [Google Scholar] [CrossRef] [Green Version]
- Thungo, Z.; Shimelis, H.; Odindo, A.; Mashilo, J.; Shayanowako, A.I.T. Genetic relationship among selected heat and drought tolerant bread wheat genotypes using SSR markers, agronomic traits and grain protein content. Acta Agric. Scand. 2020, 70, 594–604. [Google Scholar] [CrossRef]
- Mujaju, C.; Werlemark, G.; Garkava-Gustavsson, L.; Nybom, H. High levels of rapd and SSR marker diversity in landraces of watermelon (Citrullus Lanatus) in Southern Africa. Acta Hortic. 2011, 918, 291–295. [Google Scholar] [CrossRef]
- Lu, F.H.; Yoon, M.Y.; Cho, Y.I.; Chung, J.W.; Kim, K.T.; Cho, M.C.; Cheong, S.R.; Park, Y.J. Transcriptome analysis and SNP/SSR marker information of red pepper variety YCM334 and Taean. Sci. Hortic. 2011, 129, 38–45. [Google Scholar] [CrossRef]
- Yu, P.; Sun, X.; Li, C.; Zhang, X.; Zheng, W.; Liu, C. Chromosome karyotype analysis and development of SSR molecular markers in Toona sinensis. Acta Hortic. Sin. 2019, 46, 1172–1182. [Google Scholar]
- Raza, Q.; Riaz, A.; Saher, H.; Bibi, A.; Sabar, M. Grain Fe and Zn contents linked SSR markers based genetic diversity in rice. PLoS ONE 2020, 15, e0239739. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Hayes, D.J.; Fraver, S.; Chen, G. Global pyrogenic carbon production during recent decades has created the potential for a large, long-term sink of atmospheric CO2. J. Geophys. Res. Biogeosci. 2018, 123, 3682–3696. [Google Scholar] [CrossRef]
- Seidl, R.; Thom, D.; Kautz, M.; Martin-Benito, D.; Peltoniemi, M.; Vacchiano, G.; Wild, J.; Ascoli, D.; Petr, M.; Honkaniemi, J. Forest disturbances under climate change. Nat. Clim. Chang. 2017, 7, 395–402. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Larsen, C.P. Methods to detect edge affected reductions in fire frequency in simulated forest landscapes. ISPRS Int. J. Geo-Inf. 2019, 8, 277. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Zhang, Y.; Zhu, Z.; Zhao, L.; Chen, T.; Zhou, L.; Wang, C. Genetic diversity of parental lines in japonica hybrid rice based on cluster analysis of SSR markers and phenotypic characters. Hybrid Rice 2010, 25, 68–74. [Google Scholar]
- Kim, Y.D.; Jeong, M.J.; Song, H.J.; Kim, J.C.; Choi, M.S. Phenotypic characters and genetic relationship between catechins-rich and-poor tea tree (Camellia sinensis L.) lines. For. Sci. Technol. 2012, 8, 28–33. [Google Scholar]
- Rajanna, L.; Ramakrishnan, M.; Simon, L. Evaluation of morphological diversity in south Indian tea clones using statistical methods. Maejo Int. J. Sci. Technol. 2011, 5, 1–12. [Google Scholar]
- Sensoy, S.; Büyükalaca, S.; Abak, K. Evaluation of genetic diversity in Turkish melons (Cucumis melo L.) based on phenotypic characters and RAPD markers. Genet. Resour. Crop Evol. 2007, 54, 1351–1365. [Google Scholar] [CrossRef]
- Benedetti, S.; Menditto, I.; Degano, M.; Rodolico, C.; Merlini, L.; D’Amico, A.; Palmucci, L.; Berardinelli, A.; Pegoraro, E.; Trevisan, C.P.; et al. Phenotypic clustering of lamin A/C mutations in neuromuscular patients. Neurology 2007, 69, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2008. [Google Scholar]
- Tamari, F.; Hinkley, C.S.; Ramprashad, N. A comparison of DNA extraction methods using petunia hybrida tissues. J. Biomol. Tech. 2013, 24, 113–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandra, A.; Grisham, M.P.; Pan, Y.B. Allelic divergence and cultivar-specific SSR alleles revealed by capillary electrophoresis using fluorescence-labeled SSR markers in sugarcane. Genome 2014, 57, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yeh, F.; Yang, R.C.; Boyle, T. POPGENE-for the analysis of genetic variation among and within populations using co-dominant and dominant markers. Genetics 2000, 155, 945–959. [Google Scholar]
- Belalia, N.; Lupini, A.; Djemel, A.; Morsli, A.; Mauceri, A.; Lotti, C.; Khelifi-Slaoui, M.; Khelifi, L.; Sunseri, F. Analysis of genetic diversity and population structure in Saharan maize (Zea mays L.) populations using phenotypic traits and SSR markers. Genet. Resour. Crop Evol. 2019, 66, 243–257. [Google Scholar] [CrossRef]
- Zhang, L.; Yuan, M.; Tao, A.; Xu, J.; Lin, L.; Fang, P.; Qi, J. Genetic structure and relationship analysis of an association population in Jute (Corchorus spp.) evaluated by SSR markers. PLoS ONE 2015, 10, e0128195. [Google Scholar] [CrossRef] [Green Version]
- Upadyayula, N.; Wassom, J.; Bohn, M.O.; Rocheford, T.R. Quantitative trait loci analysis of phenotypic traits and principal components of maize tassel inflorescence architecture. Theor. Appl. Genet. 2006, 113, 1395–1407. [Google Scholar] [CrossRef]
- Tehrim, S.; Pervaiz, Z.H.; Mirza, M.Y.; Rabbani, M.A.; Shahid, M. Assessment of phenotypic variability in rice (Oryza sativa L.) cultivars using multivariate analysis. Pak. J. Bot. 2012, 44, 999–1006. [Google Scholar]
- Rohini, M.R.; Sankaran, M.; Rajkumar, S.; Prakash, K.; Gaikwad, A.; Chaudhury, R.; Malik, S.K. Morphological characterization and analysis of genetic diversity and population structure in Citrus × jambhiri Lush. using SSR markers. Die Kult. 2020, 67, 1259–1275. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.J.; Donnelly, P.J. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Lee, J.R.; Sebastin, R.; Shin, M.J.; Kim, S.H.; Cho, G.T.; Hyun, D.Y. Assessment of genetic diversity of tea germplasm for its management and sustainable use in Korea genebank. Forests 2019, 10, 780. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Wenshan, Z.; Chen, Y.; Hong, Y.; Wang, H. Genetic diversity analysis of cherry tomato germplasms based on phenotypic traits. J. Trop. Crop. 2019, 40, 1095–1101. [Google Scholar]
- Poljak, I.; Vahčić, N.; Vidaković, A.; Tumpa, K.; Žarković, I.; Idžojtić, M. Traditional sweet chestnut and hybrid varieties: Chemical composition, morphometric and qualitative nut characteristics. Agronomy 2021, 11, 516. [Google Scholar] [CrossRef]
- Meegahakumbura, M.K.; Wambulwa, M.C.; Li, M.-M.; Thapa, K.K.; Sun, Y.-S.; Möller, M.; Xu, J.-C.; Yang, J.-B.; Liu, J.; Liu, B.-Y.; et al. Domestication origin and breeding history of the tea plant (Camellia sinensis) in China and India based on nuclear microsatellites and cpDNA sequence data. Front. Plant Sci. 2018, 8, 2270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patil, R.; Viswanatha, K.P.; Upadhyaya, H.D.; Lokesha, R.; Khan, H.; Gururaj, S. Genetic diversity, association and principle component analyses for agronomical and quality traits in genomic selection training population of groundnut (Arachis hypogaea L.). Indian J. Genet. Plant Breed. 2020, 80, 282–290. [Google Scholar]
- Niu, S.Z.; Song, Q.F.; An, H.W.; Huang, Z.; Liu, X.; Zhao, D.G.; Chen, Z.W. Diversity of ancient tea germplasm resources based on morphological characteristics in Guizhou. Acta Agric. Zhejiangensis 2019, 31, 1689–1699. (In Chinese) [Google Scholar]
- Huang, H.T.; Zheng, X.X.; Mao, Y.X.; Li, H.L.; Yu, J.Z. Analysis of phenotypic traits and diversity of West Lake Longjing Tea plant resources. Zhejiang Agric. Sci. 2019, 60, 1793–1797. (In Chinese) [Google Scholar]
- Xie, W.G.; Li, X.S.; Li, W.; Tang, X. Phenotypic genetic diversity of small leaf ancient tea germplasm in Sichuan province. Acta Agric. Zhejiangensis 2019, 31, 1405–1415. (In Chinese) [Google Scholar]
- Rossetto, M.; Slade, R.W.; Baverstock, P.R.; Henry, R.J.; Lee, L.S. Microsatellite variation and analysis of genetic structure in tea tree (Melaleuca alternifolia Myrtaceae). Mol. Ecol. 1999, 8, 633–643. [Google Scholar] [CrossRef]
- Wu, W.F.; Chen, F.X.; Yeh, K.; Chen, J.J. ISSR Analysis of genetic diversity and structure of plum varieties cultivated in Southern China. Biology 2019, 8, 2. [Google Scholar] [CrossRef] [Green Version]
- Diethard, T.; Manfred, R. Simple sequences are ubiquitous repetitive components of eukaryotic genomes. Nucleic Acids Res. 1984, 12, 4127–4138. [Google Scholar]
- Liu, S.; Liu, H.; Wu, A.; Hou, Y.; An, Y.; Wei, C. Construction of fingerprinting for tea plant (Camellia sinensis) accessions using new genomic SSR markers. Mol. Breed. 2017, 37, 93. [Google Scholar] [CrossRef]
- Singh, N.; Vasudev, S.; Kumar Yadava, D.; Kumar, S.; Naresh, S.; Ramachandra Bhat, S.; Vinod Prabhu, K. Assessment of genetic diversity in Brassica juncea (Brassicaceae) genotypes using phenotypic differences and SSR markers. Rev. De Biol. Trop. 2013, 61, 1919–1934. [Google Scholar]
- Zhou, Q.; Li, H.; Hoang, T.X.; Ruan, X.; Zhang, Y.; Arkorful, E.; Chen, X.; Sun, K.; Li, X. Genetic diversity and relationship of dongting Biluochun tea Germplasm in Suzhou revealed by SSR markers. Pak. J. Bot. 2019, 51, 895–902. [Google Scholar] [CrossRef] [Green Version]
- Yao, M.Z.; Ma, C.L.; Qiao, T.T.; Jin, J.Q.; Chen, L. Diversity distribution and population structure of tea germplasms in China revealed by EST-SSR markers. Tree Genet. Genomes 2012, 8, 205–220. [Google Scholar] [CrossRef]
- Liu, S.; An, Y.; Tong, W.; Qin, X.; Samarina, L.; Guo, R.; Xia, X.; Wei, C. Characterization of genome-wide genetic variations between two varieties of tea plant (Camellia sinensis) and development of InDel markers for genetic research. BMC Genom. 2019, 20, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciccarese, L.; Piotto, B. Pre-sowing treatment of seeds of forest shrubs and trees and loss of genetic diversity. Monti E Boschi 2000, 51, 37–41. [Google Scholar]
- Liang, C.; Yamaguchi, S. Genetic diversity and phylogeny of tea plant (Camellia sinensis) and its related species and varieties in the section Thea genus Camellia determined by randomly amplified polymorphic DNA analysis. J. Pomol. Hortic. Sci. 2002, 77, 729–732. [Google Scholar]
- Chen, J.; Wang, P.; Xia, Y.; Xu, M.; Pei, S. Genetic diversity and differentiation of Camellia sinensis L. (cultivated tea) and its wild relatives in Yunnan province of China, revealed by morphology, biochemistry and allozyme studies. Genet. Resour. Crop Evol. 2005, 52, 41–52. [Google Scholar] [CrossRef]
- Niu, S.; Song, Q.; Koiwa, H.; Qiao, D.; Zhao, D.; Chen, Z.; Liu, X.; Wen, X. Genetic diversity, linkage disequilibrium, and population structure analysis of the tea plant (Camellia sinensis) from an origin center, Guizhou plateau, using genome-wide SNPs developed by genotyping-by-sequencing. BMC Plant Biol. 2019, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Ma, L.; Cai, Q.; Ying, X.M.; Lu, X.; Su, H.S.; Mao, J.; Fan, Y.H. Genetic diversity analysis of phenotypic traits in Yunnan sugarcane varieties. J. Plant Genet. Resour. 2010, 11, 703–708. [Google Scholar]
- Xia, E.H.; Tong, W.; Wu, Q.; Wei, S.; Zhao, J.; Zhang, Z.Z.; Wei, C.L.; Wan, X.C. Tea plant genomics: Achievements, challenges and perspectives. Hortic. Res. 2020, 7, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
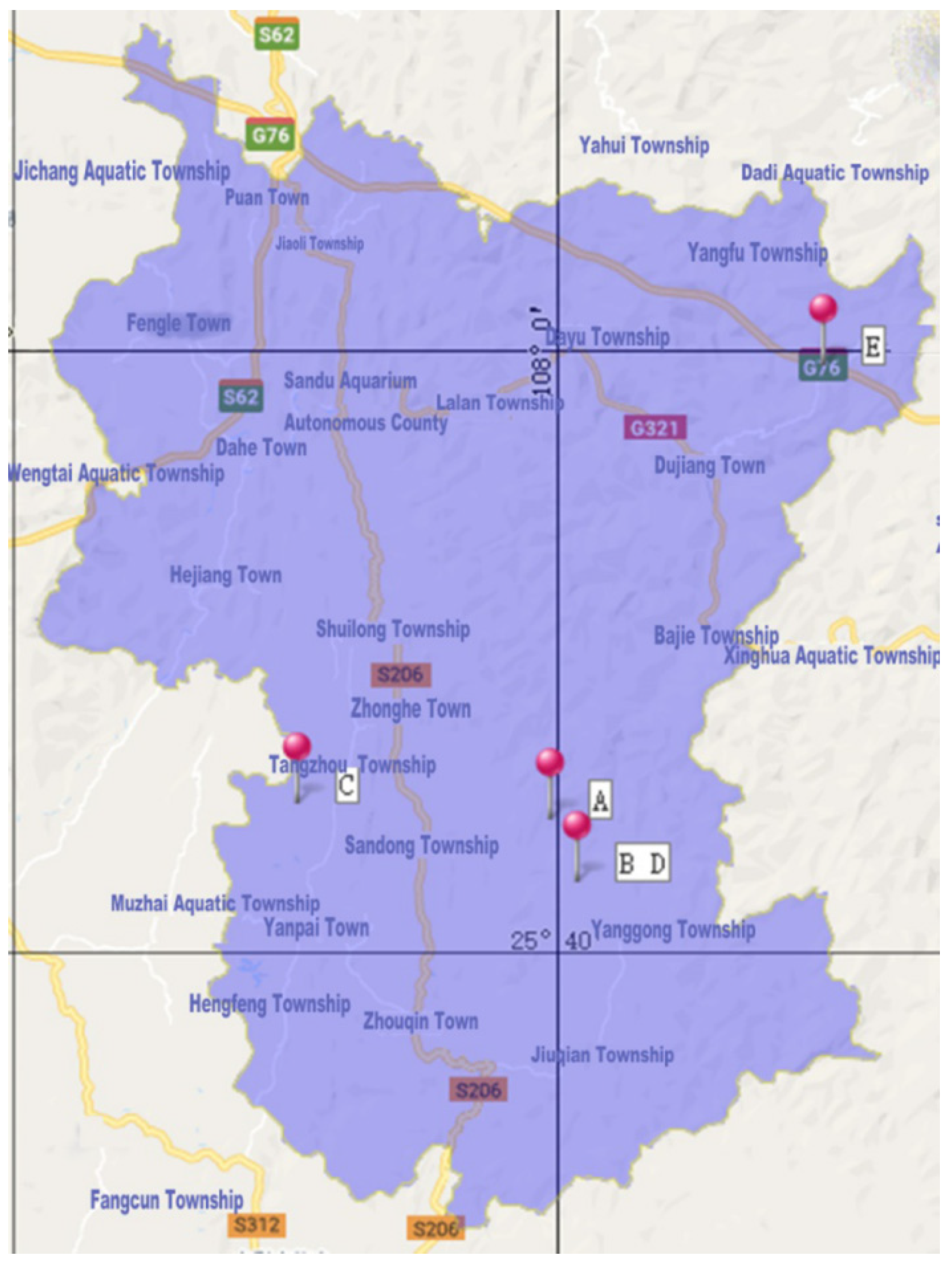


| Location | Tree Form | Longitude | Latitude | Mean Altitude (m) | Sample Size |
|---|---|---|---|---|---|
| Guqi Village | Bush | 107°59′34″ E | 25°44′34″ N | 689.1 | 22 |
| Landong Village | Bush | 108°0′20″ E | 25°42′40″ N | 988.7 | 15 |
| Yangmeng Village | Arbor/Bush | 107°50′11″ E | 25°45′8″ N | 884.0 | 10 |
| Landong Village | Arbor | 108°0′30″ E | 25°42′30″ N | 905.2 | 44 |
| Zenya Village | Arbor | 108°9′37″ E | 25°59′36″ N | 1299.6 | 54 |
| Variable | Factor Loading | |||||
|---|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | |
| Height of tree (m) | 0.555 | 0.208 | −0.495 | −0.118 | 0.222 | 0.096 |
| Tree shape | 0.457 | 0.126 | −0.686 | 0.357 | 0.057 | 0.186 |
| Leaf texture | 0.172 | −0.042 | 0.200 | 0.174 | 0.890 | −0.270 |
| Leaf apex | −0.123 | −0.356 | 0.037 | 0.591 | −0.307 | −0.450 |
| Leaf shape | 0.339 | 0.799 | 0.343 | 0.125 | −0.105 | −0.135 |
| Leaf size | 0.871 | −0.209 | 0.109 | −0.027 | −0.093 | −0.190 |
| Leaf tooth depth | −0.143 | −0.301 | 0.568 | 0.355 | 0.091 | 0.058 |
| Length of leaf (cm) | 0.960 | 0.055 | 0.179 | −0.065 | −0.096 | −0.067 |
| Width of leaf (cm) | 0.859 | −0.467 | −0.056 | −0.078 | −0.040 | −0.024 |
| Leaf shape index | 0.291 | 0.841 | 0.371 | −0.006 | −0.064 | −0.057 |
| Leaf area | 0.946 | −0.231 | 0.081 | −0.086 | −0.076 | −0.064 |
| Leaf veins logarithmic | 0.351 | 0.031 | 0.197 | 0.632 | 0.011 | 0.580 |
| Leaf tooth logarithmic | 0.087 | −0.351 | 0.584 | −0.376 | 0.032 | 0.345 |
| Number | Primer ID | Forward Primer Sequence (5′-3′) | Reverse Primer Sequence (5′-3′) | Repeat Moti | Expected Product Size (bp) | PIC Value |
|---|---|---|---|---|---|---|
| 1 | Cs1 | ATGCCCTCTACATGCCTTTG | ATGACGTAGGCGGAAACAAC | (TC)6 | 179 | 0.48 |
| 2 | Cs2 | GAGCTGAGGCAGTCCATAGG | AAAAGGGAGAAAGACGTGGG | (GT)7 | 217 | 0.74 |
| 3 | Cs3 | GCCATCATAGACTGCTCGGT | GGTTGGCTTGACAAAAAGGA | (TA)10 | 278 | 0.73 |
| 4 | Cs4 | CTCTTCCTCAGCCACCAAAG | TGAGGAGTTGTGGCAGAATG | (AAG)6 | 199 | 0.81 |
| 5 | Cs5 | GGGATCAGATATGGAGCCAA | CAGCAAATTCTTGAGGAAAC | (TTC)5 | 240 | 0.82 |
| 6 | Cs6 | GCTCATTGGCTTTGGCTTT | AATGCATTCCGTAAGCTTGG | (CTA)5 | 180 | 0.31 |
| 7 | Cs7 | GAACAGGCGAACAAGTAGGG | CGACCTCTGAGGCAATCTTC | (GAA)6 | 253 | 0.77 |
| 8 | Cs8 | GGCTTGCATGCCAGTTTATT | ACGTGGGGTTGGAAGACATA | (GT)7 | 195 | 0.87 |
| 9 | Cs9 | CCACGCTTTCTAACACCCAT | AAGGCTCCAAATGCTGAAGA | (TC)6 | 183 | 0.62 |
| 10 | Cs10 | CTGAGTCGGGACAGTTTGGT | CCCAAGAGGTGGAAATAGCA | (CT)6 | 253 | 0.63 |
| 11 | Cs11 | CGTGCAATTGAGAATGCTGT | GGGTCGCTGTCTCTACTTGG | (TC)7 | 222 | 0.62 |
| 12 | Cs12 | AACCATGCAGCAAGACACTG | CCCGTAGGAGGTGCATAAGA | (CAA)5 | 129 | 0.36 |
| 13 | Cs13 | AAAGTGGTCGGTGTCCAAAG | TAACAGGTTTCATCCCTGGC | (GAT)5 | 235 | 0.78 |
| 14 | Cs14 | CTTTTGGCCATTGTCAAGGT | CAGACCTATCGAAAACCCGA | (CAC)6 | 242 | 0.68 |
| 15 | Cs15 | CCCACTCCTAAACTCACCCA | AGCCATCACATTGTCCAACA | (CAC)5 | 254 | 0.65 |
| Parameter | Na | Ne | H | I | PPB (%) | Ho | He |
|---|---|---|---|---|---|---|---|
| Site | |||||||
| Landong (arbor) | 1.9188 | 1.2264 | 0.1595 | 0.2730 | 91.88 | 0.899 | 0.710 |
| Zenya (arbor) | 1.9612 | 1.2238 | 0.1637 | 0.2840 | 96.12 | 0.899 | 0.733 |
| Yangmeng (arbor) | 1.6659 | 1.2283 | 0.1524 | 0.2499 | 66.50 | 0.899 | 0.665 |
| Guqi (bush) | 1.8680 | 1.2329 | 0.1603 | 0.2719 | 86.80 | 1.000 | 0.795 |
| Landong (bush) | 1.7745 | 1.2325 | 0.1591 | 0.2652 | 77.45 | 1.000 | 0.793 |
| Sources | Df | SS | MS | Est.Var | % |
|---|---|---|---|---|---|
| Among Populations | 4 | 56.46 | 14.12 | 0.22 | 5.00 |
| Within Populations | 144 | 643.50 | 4.47 | 4.47 | 95.00 |
| Total | 148 | 699.96 | 4.68 | 100.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Wang, R.; Liu, Q.; Dong, X.; Zhao, D.-G. Genetic Diversity of Ancient Camellia sinensis (L.) O.Kuntze in Sandu County of Guizhou Province in China. Diversity 2021, 13, 276. https://doi.org/10.3390/d13060276
Zhao Y, Wang R, Liu Q, Dong X, Zhao D-G. Genetic Diversity of Ancient Camellia sinensis (L.) O.Kuntze in Sandu County of Guizhou Province in China. Diversity. 2021; 13(6):276. https://doi.org/10.3390/d13060276
Chicago/Turabian StyleZhao, Yichen, Runying Wang, Qing Liu, Xuan Dong, and De-Gang Zhao. 2021. "Genetic Diversity of Ancient Camellia sinensis (L.) O.Kuntze in Sandu County of Guizhou Province in China" Diversity 13, no. 6: 276. https://doi.org/10.3390/d13060276






