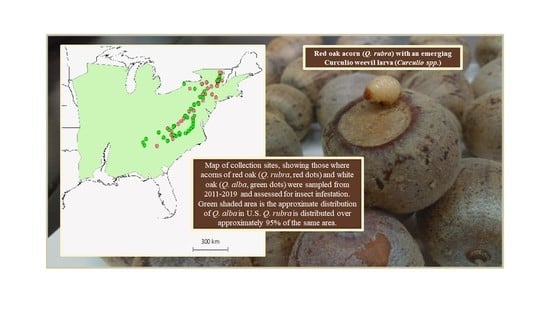
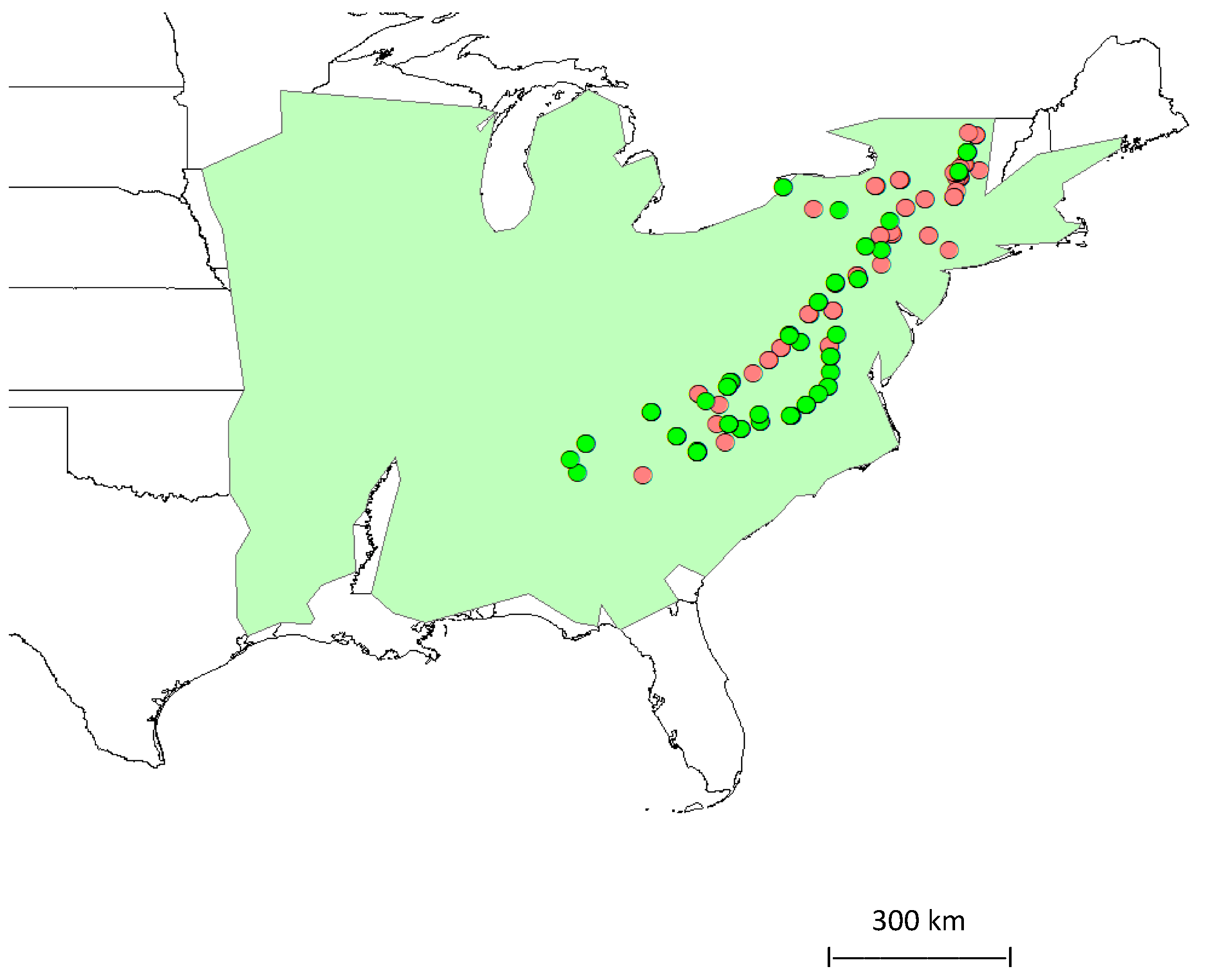
Oak (Acorn)–Weevil Interactions across an Extensive Latitudinal Gradient in Eastern North America
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Weevil Prevalence across Years and Latitudinal Gradient
3.2. Seed Size across a Latitudinal Gradient
3.3. Relationship between Cotyledon Damage and Latitude
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nixon, K. The genus Quercus in Mexico. In Biological Diversity of Mexico: Origins and Distribution; Ramamoorthy, T., Bye, R., Lot, A., Fa, J., Eds.; Oxford University Press: Oxford, UK, 1993; pp. 447–458. [Google Scholar]
- Nixon, K. Fagaceae. In Flora of North America, North of Mexico; Morin, N.R., Ed.; Oxford University Press: New York, NY, USA, 1997; pp. 436–437. [Google Scholar]
- Nixon, K. Global and neotropical distribution and diversity of oak (genus Quercus) and oak forests. In Ecology and Conservation of Neotropical Montane Oak Forests; Caldwell, M., Heldmaier, G., Jackson, R., Lange, O., Mooney, H., Schulze, E., Sommer, U., Eds.; Springer: Berlin, Germany, 2006; Volume 185, pp. 3–13. [Google Scholar]
- Steele, M. Oak Seed Dispersal: A Study in Plant-Animal Interaction; Johns Hopkins University Press: Baltimore, MD, USA, 2021; 431p. [Google Scholar]
- Fukumoto, H.; Kajimura, H. Effects of asynchronous acorn production by co-occurring Quercus trees on resource utilization by acorn-feeding insects. J. For. Res. 2011, 16, 62–67. [Google Scholar] [CrossRef]
- Bogdziewicz, M.; Espelta, J.M.; Bonal, R. Tolerance to seed predation mediated by seed size increases at lower latitudes in a Mediterranean oak. Ann. Bot. 2019, 123, 707–714. [Google Scholar] [CrossRef]
- Boucher, D.H.; Sork, V.L. Early drop of nuts in response to insect infestation. Oikos 1979, 33, 440–443. [Google Scholar] [CrossRef]
- Bonal, R.; Munoz, A. Seed growth suppression constrains the growth of seed parasites: Premature acorn abscission reduces Curculio elephas larval size. Ecol. Entomol. 2008, 33, 31–36. [Google Scholar] [CrossRef]
- Bogdziewicz, M.; Marino, S.A.; Bonal, R.; Zwolak, R.; Steele, M.A. Rapid aggregative and reproductive responses of weevils to masting of North American oaks counteract predator satiation. Ecology 2018, 99, 2575–2582. [Google Scholar] [CrossRef]
- Riccardi, C.L.; McCarthy, B.C.; Long, R.P. Oak seed production, weevil (Coleoptera: Curculionidae) populations and predation rates in mixed oak forests of southeast Ohio. In Forest Ecology and Management, General Technical Reports, NE-316, Proceedings of the 14th Central Hardwood Forest Conference, Wooster, OH, USA, 16–19 March 2004; Gen. Tech. Rep. NE-316; Yaussy, D.A., Hix, D.M., Long, R.P., Goebel, P.C., Eds.; U.S. Department of Agriculture, Forest Service, Northeastern Research Station: Newtown Square, PA, USA, 2004; pp. 10–20. [Google Scholar]
- Bonal, R.; Espelta, J.M.; Vogler, A.P. Complex selection on life-history traits and the maintenance of variation in exaggerated rostrum length in acorn weevils. Oecologia 2011, 167, 1053–1061. [Google Scholar] [CrossRef]
- Bonal, R.; Munoz, A.; Diaz, M. Satiation of predispersal seed predators: The importance of considering both plant and seed levels. Evol. Ecol. 2007, 21, 367–380. [Google Scholar] [CrossRef]
- Yi, X.; Yang, Y. Apical thickening of epicarp is responsible for embryo protection in acorns of Quercus variabilis. Isr. J. Ecol. Evol. 2010, 56, 153–164. [Google Scholar] [CrossRef]
- Bartlow, A.W.; Agosta, S.J.; Curtis, R.; Yi, X.; Steele, M.A. Acorn size and tolerance to seed predators: The multiple roles of acorns as food for seed predators, fruit for dispersal and fuel for growth. Integr. Zool. 2018, 13, 251–266. [Google Scholar] [CrossRef]
- Bogdziewicz, M.; Crone, E.E.; Steele, M.A.; Zwolak, R. Effects of nitrogen deposition on reproduction in a masting tree: Benefits of higher seed production are trumped by negative biotic interactions. J. Ecol. 2017, 105, 310–320. [Google Scholar] [CrossRef]
- Steele, M.; Gavel, K.; Bachman, W. Dispersal of half-eaten acorns by gray squirrels: Effects of physical and chemical seed characteristics. In Ecology and Evolutionary Biology of Tree Squirrels, Proceedings of the International Colloquium on the Ecology of Tree Squirrels, Powdermill Biological Station, Carnegie Museum of Natural History, Pittsburgh, PA, USA, 22–28 April 1994; Steele, M., Merritt, J., Zegers, D., Eds.; Virginia Museum of Natural History: Matinsville, VA, USA, 1998; pp. 223–231. [Google Scholar]
- Steele, M.A.; Knowles, T.; Bridle, K.; Simms, E.L. Tannins and partial consumption of acorns: Implications for dispersal of oaks by seed predators. Am. Midl. Nat. 1993, 130, 229–238. [Google Scholar] [CrossRef]
- Hadj-Chikh, L.Z.; Steele, M.A.; Smallwood, P.D. Caching decisions by grey squirrels: A test of the handling time and perishability hypotheses. Anim. Behav. 1996, 52, 941–948. [Google Scholar] [CrossRef] [Green Version]
- Bonal, R.; Espelta, J.M.; Munoz, A.; Ortega, J.; Aparicio, J.M.; Gaddis, K.; Sork, V. Diversity in insect seed parasite guilds at large geographical scale: The roles of host specificity and spatial distance. J. Biogeogr. 2016, 43, 1620–1630. [Google Scholar] [CrossRef] [Green Version]
- Xia, K.; Harrower, W.L.; Turkington, R.; Tan, H.-Y.; Zhou, Z.-K. Pre-dispersal strategies by Quercus schottkyana to mitigate the effects of weevil infestation of acorns. Sci. Rep. 2016, 6, 37520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogdziewicz, M.; Espelta, J.M.; Muñoz, A.; Aparicio, J.M.; Bonal, R. Effectiveness of predator satiation in masting oaks is negatively affected by conspecific density. Oecologia 2018, 186, 983–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steele, M.A.; Smallwood, P.D.; Spunar, A.; Nelsen, E. The proximate basis of the oak dispersal syndrome: Detection of seed dormancy by rodents. Am. Zool. 2001, 41, 852–864. [Google Scholar] [CrossRef]
- Bates, D.; Machler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using Ime4. J. Stat. Softw. 2015, 67, 127003. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: http://www.R-project.org/ (accessed on 12 December 2020).
- Ricca, M.A.; Weckerly, F.D.; Semlitsch, R.D. Effects of Moisture and Temperature on Overwintering Survival of Curculio Larvae (Coleoptera: Curculionidae). Am. Midl. Nat. 1996, 136, 203–206. [Google Scholar] [CrossRef]
- Weckerly, F.W.; Sugg, W.D.; Semlitsch, R.D. Germination success of acorns (Quercus): Insect predation and tannins. Can. J. For. Res. 1989, 19, 811–815. [Google Scholar] [CrossRef]
- Schultz, J.C.; Baldwin, I.T. Oak leaf quality declines in response to defoliation by gypsy-moth larvae. Science 1982, 217, 203–206. [Google Scholar] [CrossRef]
- Govindan, B.N. The Role of Resource Predictability in the Metapopulation Dynamics of Insects. Ph.D. Thesis, Purdue University, West Lafayette, IN, USA, 2013. [Google Scholar]
- Govindan, B.N.; Kery, M.; Swihart, R.K. Host selection and responses to forest fragmentation in acorn weevils: Inferences rom dynamic occupancy models. Oikos 2012, 121, 623–633. [Google Scholar] [CrossRef]
- Myczko, Ł.; Dylewski, Ł.; Chrzanowski, A.; Sparks, T.H. Acorns of invasive Northern Red Oak (Quercus rubra) in Europe are larval hosts for moths and beetles. Biol. Invasions 2017, 19, 2419–2425. [Google Scholar] [CrossRef]
- Bogdziewicz, M.; Bonal, R.; Espelta, J.M.; Kalemba, E.; Steele, M.A.; Zwolak, R. Invasive oaks escape pre-dispersal insect seed predation and trap enemies in their seeds. Integr. Zool. 2018, 13, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Ostfeld, R.S.; Jones, C.G.; Wolff, J.O. Of mice and mast. BioScience 1996, 46, 323–330. [Google Scholar] [CrossRef]
- Ostfeld, R.S.; Keesing, F. Pulsed resources and community dynamics of consumers in terrestrial ecosystems. Trends Ecol. Evol. 2000, 15, 232–237. [Google Scholar] [CrossRef]
- Menu, F.; Roebuck, J.-P.; Viala, M. Bet hedging diapause strategies in stochastic environments. Am. Nat. 2000, 155, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Rajon, E.; Desouhant, E.; Cheavalier, M.; Debias, F.; Menu, F. The evolution of bet hedging in response to local ecological conditions. Am. Nat. 2014, 183, E1–E15. [Google Scholar] [CrossRef] [PubMed]
- Weeks, H.P.; Kirkpatrick, C.M. Salt preferences and sodium drive phenology in fox squirrels and woodchucks. J. Mammal. 1978, 59, 531–542. [Google Scholar] [CrossRef]
- Aizen, M.A.; Patterson, W.A., III. Acorn size and geographical range in the North American oaks (Quercus L.). J. Biogeogr. 1990, 17, 327–332. [Google Scholar] [CrossRef] [Green Version]
- Aizen, M.A.; Woodcock, H. Latitudinal trends in acorn size in eastern North American species of Quercus. Can. J. Bot. 1992, 70, 1218–1222. [Google Scholar] [CrossRef]
- Koenig, W.D.; Knops, J.M.; Dickinson, J.L.; Zuckerberg, B. Latitudinal decrease in acorn size in bur oak (Quercus macrocarpa) is due to environmental constraints, not avian dispersal. Botany 2009, 87, 349–356. [Google Scholar] [CrossRef] [Green Version]





| Year | No. Q. rubra Trees | Mean Q. rubra Acorns per Tree | SE (+/−) | No. Q. alba Trees | Mean Q. alba Acorns per Tree | SE (+/−) | Northern Most | Southern Most | ||
|---|---|---|---|---|---|---|---|---|---|---|
| City | Latitude | City | Latitude | |||||||
| 2011 | 34 | 15.53 | 2.69 | 17 | 9.47 | 1.6 | Syracuse, NY | 43°1′55″ | Williamsburg, NC | 35°58′35″ |
| 2012 | 18 | 54 | 4.25 | 22 | 49.36 | 2.74 | Clarkstown, NY | 41°7′51″ | Kings Mountain, NC | 35°10′0.8″ |
| 2013 | 18 | 64.89 | 6.87 | 9 | 57.56 | 10.9 | Syracuse, NY | 43°1′53″ | Redland, NC | 35°59′13″ |
| 2014 | 31 | 55.61 | 2.13 | 35 | 51.14 | 2.28 | Queensbury, NY | 43°16′1″ | Calhoun, GA | 34°33′0.7″ |
| 2015 | 23 | 47.13 | 3.72 | 16 | 41.06 | 4.29 | Lake Champlain, NY | 44°35′2″ | West Marion, NC | 35°38′20″ |
| 2016 | 18 | 37.17 | 1.66 | 21 | 36.9 | 2.41 | Mountain Top, PA | 41°7′49″ | West Marion, NC | 35°38′17″ |
| 2017 | 46 | 25.43 | 1.31 | 23 | 23.22 | 2.66 | Colchester, VT | 44°30′8″ | Fairplay, SC | 34°29′12″ |
| 2019 | 25 | 32.2 | 1.22 | 22 | 29.64 | 1.04 | Hershey, PA | 40°17′7″ | Athens, TN | 35°25′0.8″ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steele, M.A.; Dalgleish, H.J.; Marino, S.; Bartlow, A.W.; Curtis, R.; Stratford, J.A. Oak (Acorn)–Weevil Interactions across an Extensive Latitudinal Gradient in Eastern North America. Diversity 2021, 13, 303. https://doi.org/10.3390/d13070303
Steele MA, Dalgleish HJ, Marino S, Bartlow AW, Curtis R, Stratford JA. Oak (Acorn)–Weevil Interactions across an Extensive Latitudinal Gradient in Eastern North America. Diversity. 2021; 13(7):303. https://doi.org/10.3390/d13070303
Chicago/Turabian StyleSteele, Michael A., Harmony J. Dalgleish, Shealyn Marino, Andrew W. Bartlow, Rachel Curtis, and Jeffrey A. Stratford. 2021. "Oak (Acorn)–Weevil Interactions across an Extensive Latitudinal Gradient in Eastern North America" Diversity 13, no. 7: 303. https://doi.org/10.3390/d13070303
APA StyleSteele, M. A., Dalgleish, H. J., Marino, S., Bartlow, A. W., Curtis, R., & Stratford, J. A. (2021). Oak (Acorn)–Weevil Interactions across an Extensive Latitudinal Gradient in Eastern North America. Diversity, 13(7), 303. https://doi.org/10.3390/d13070303