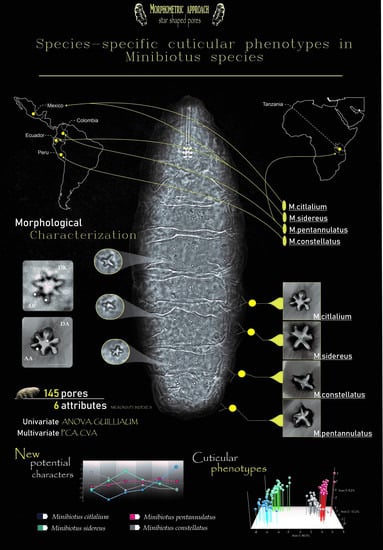
Species-Specific Cuticular Phenotypes in Eutardigrada: A Morphometric Approach to Analyze the Variation of Star-Shaped Pores in Minibiotus Species
Abstract
:1. Introduction
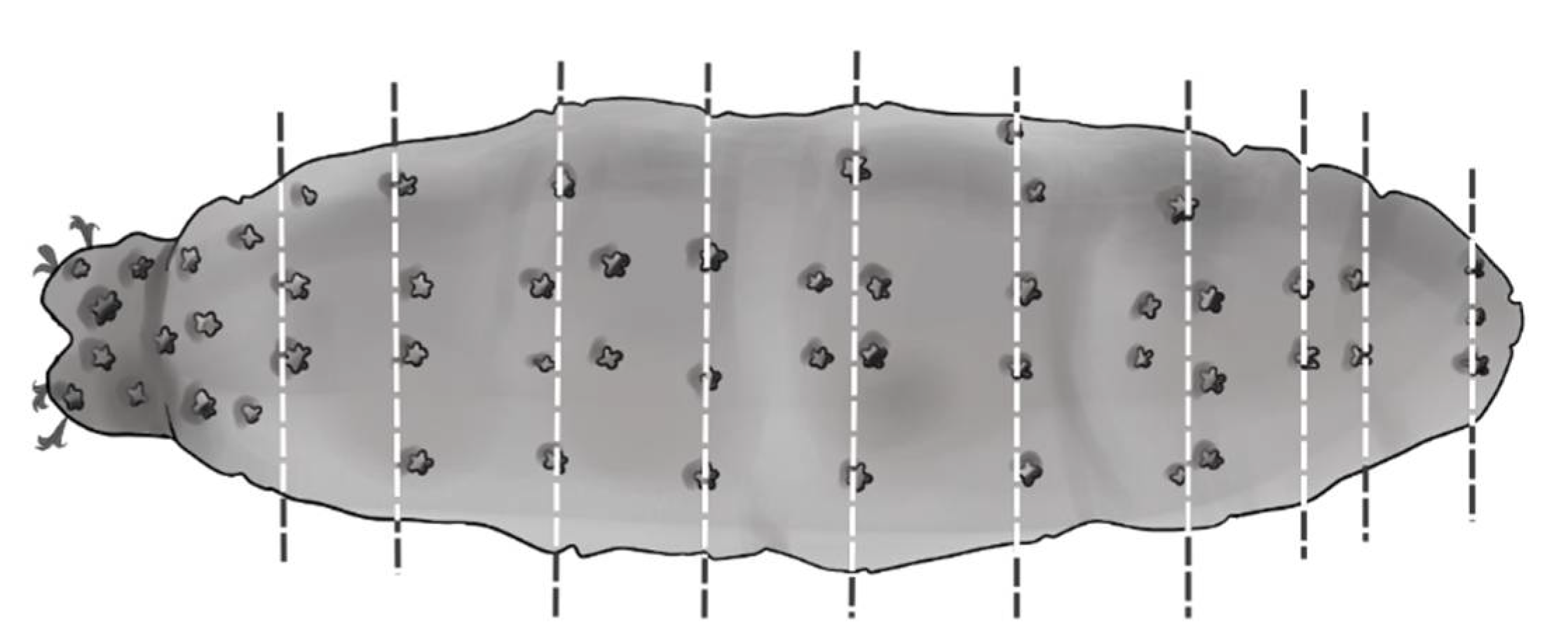
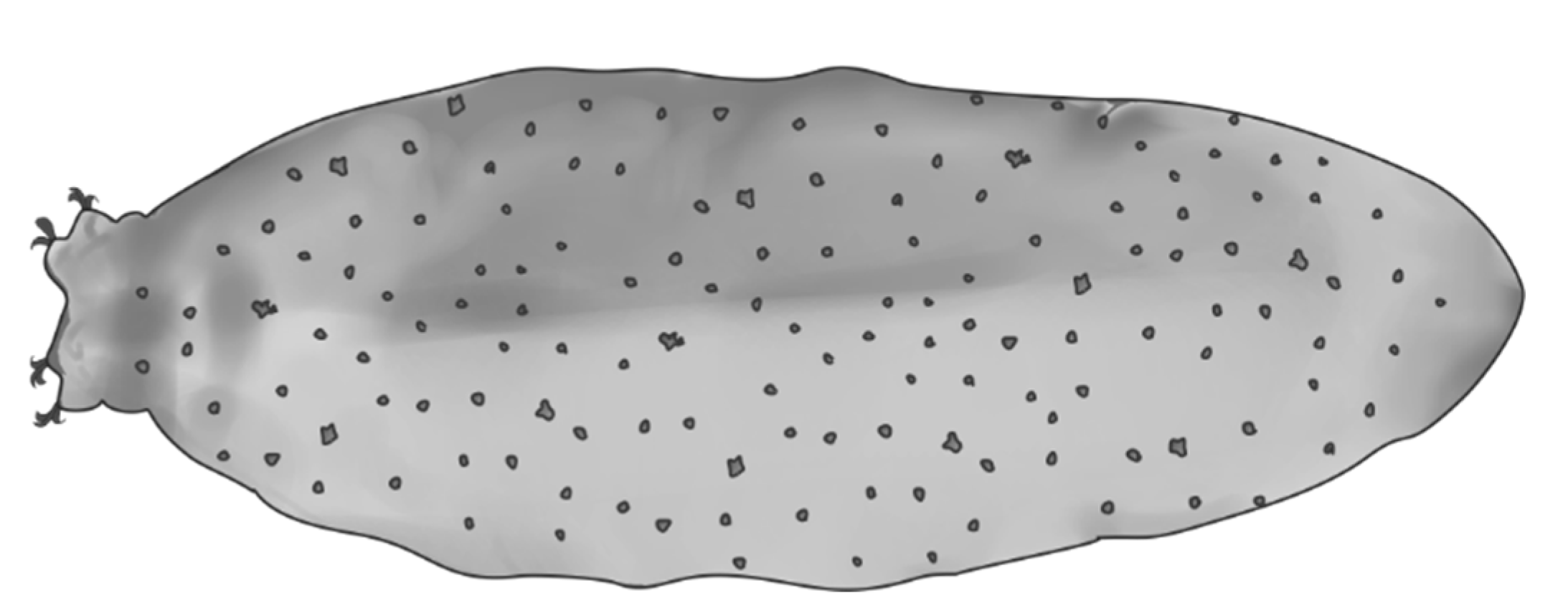
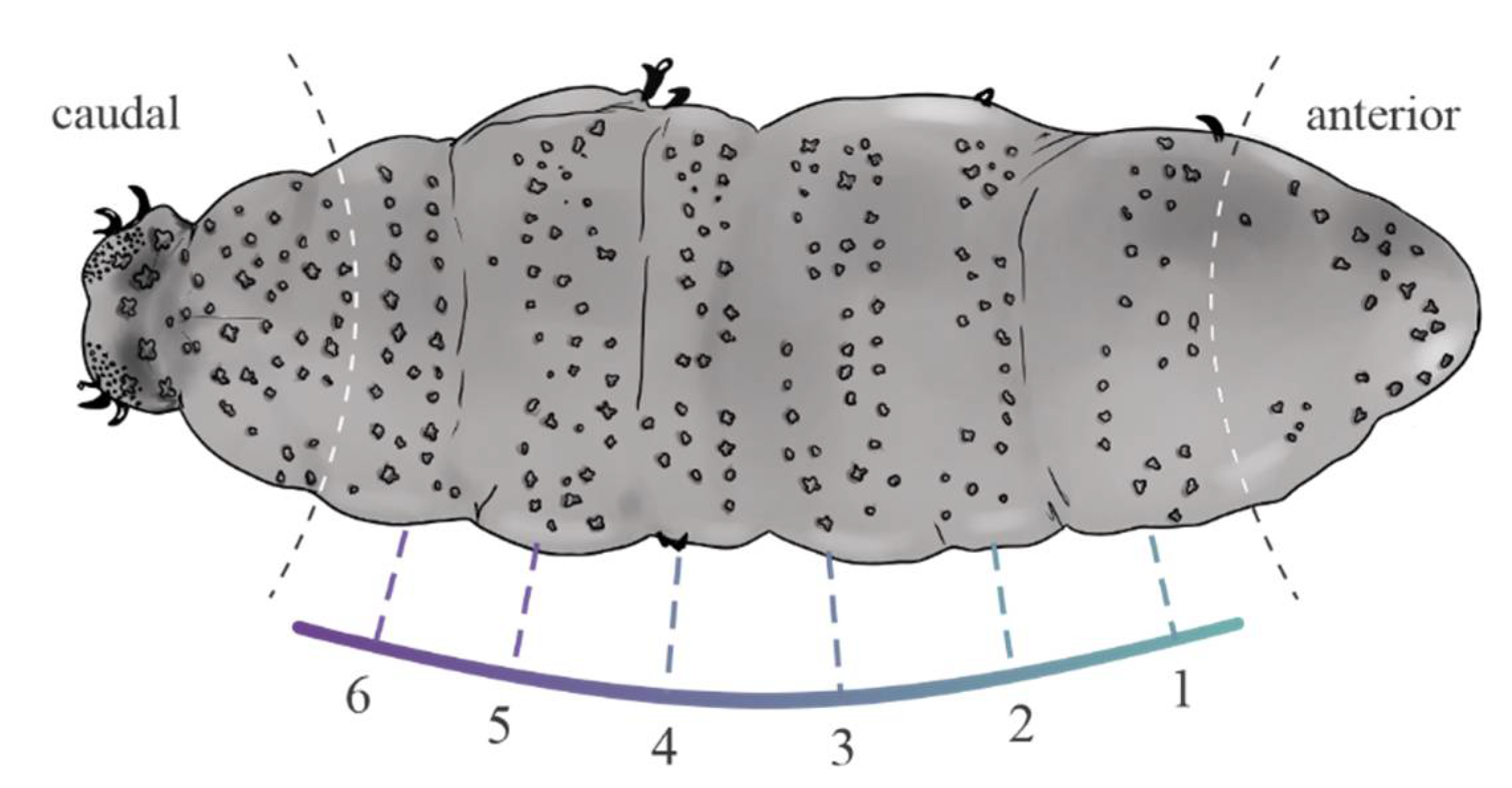
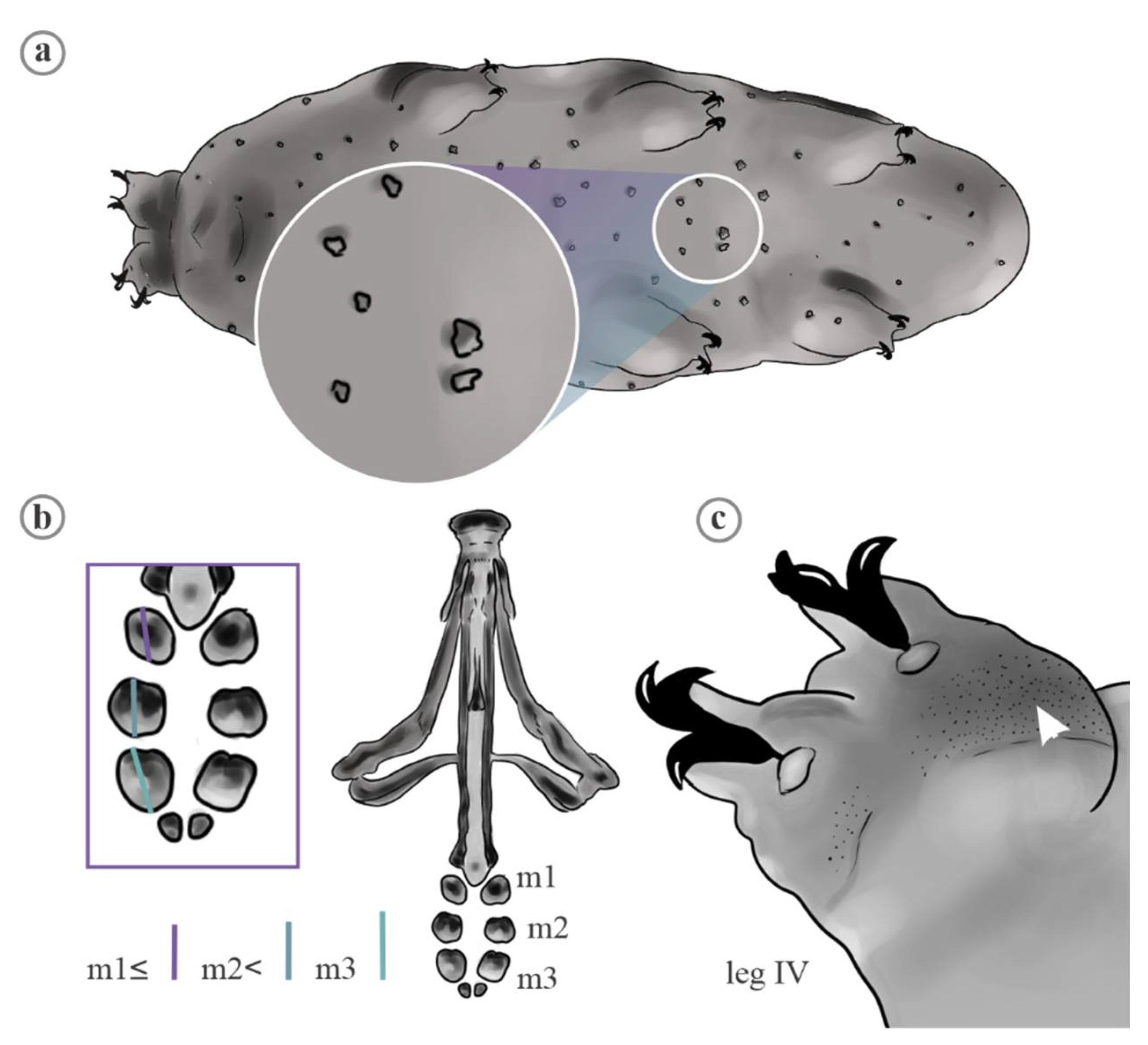
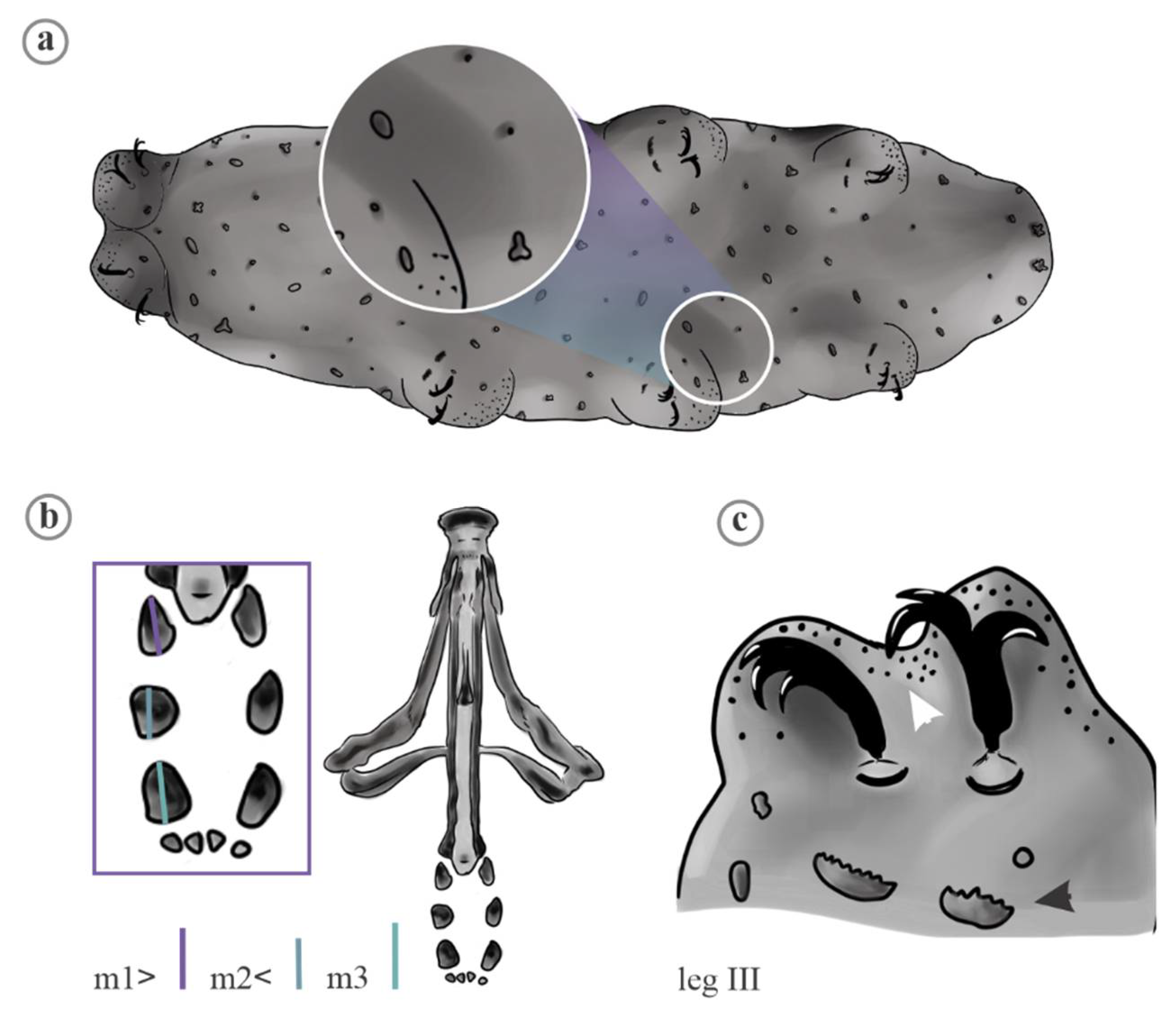
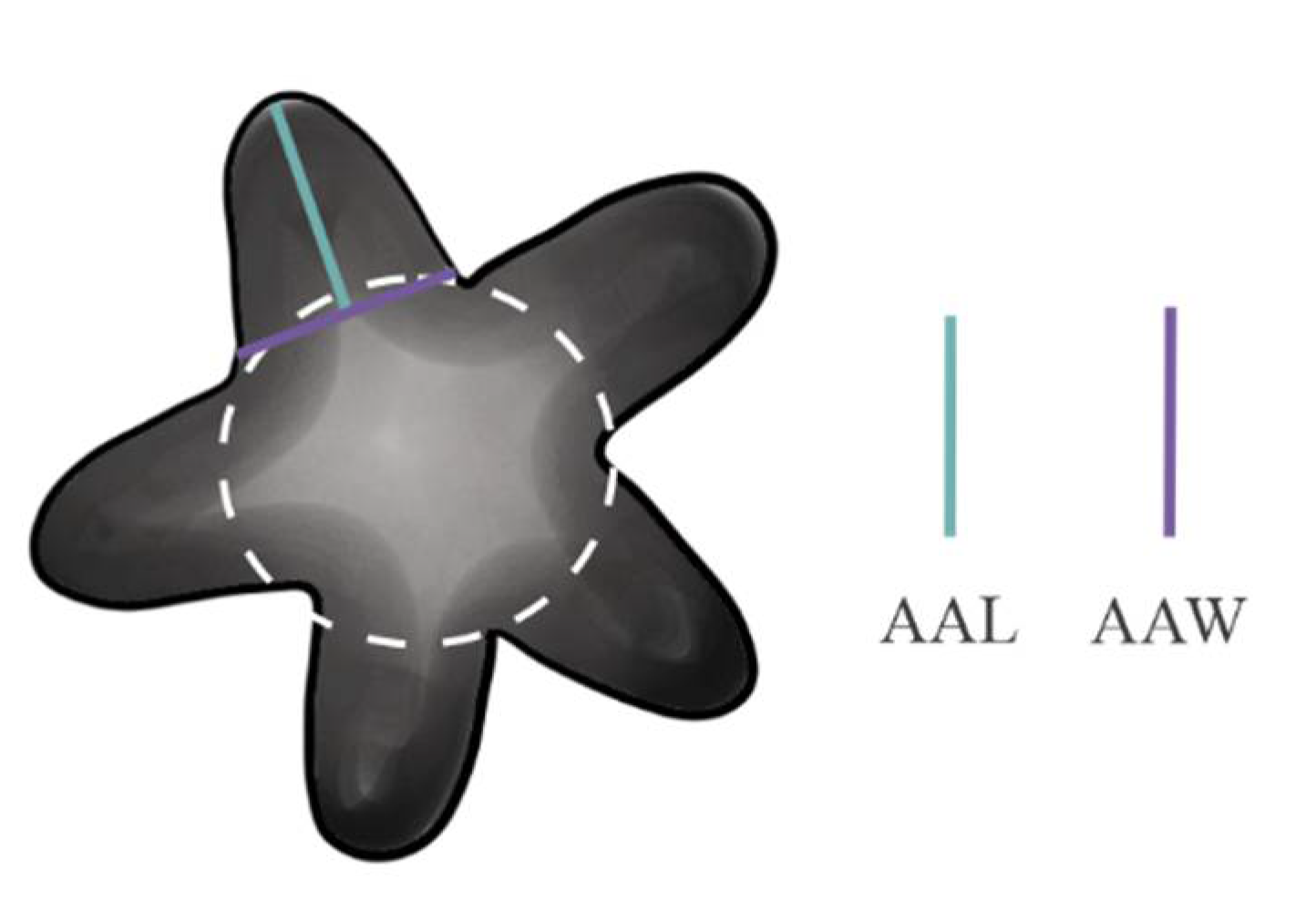
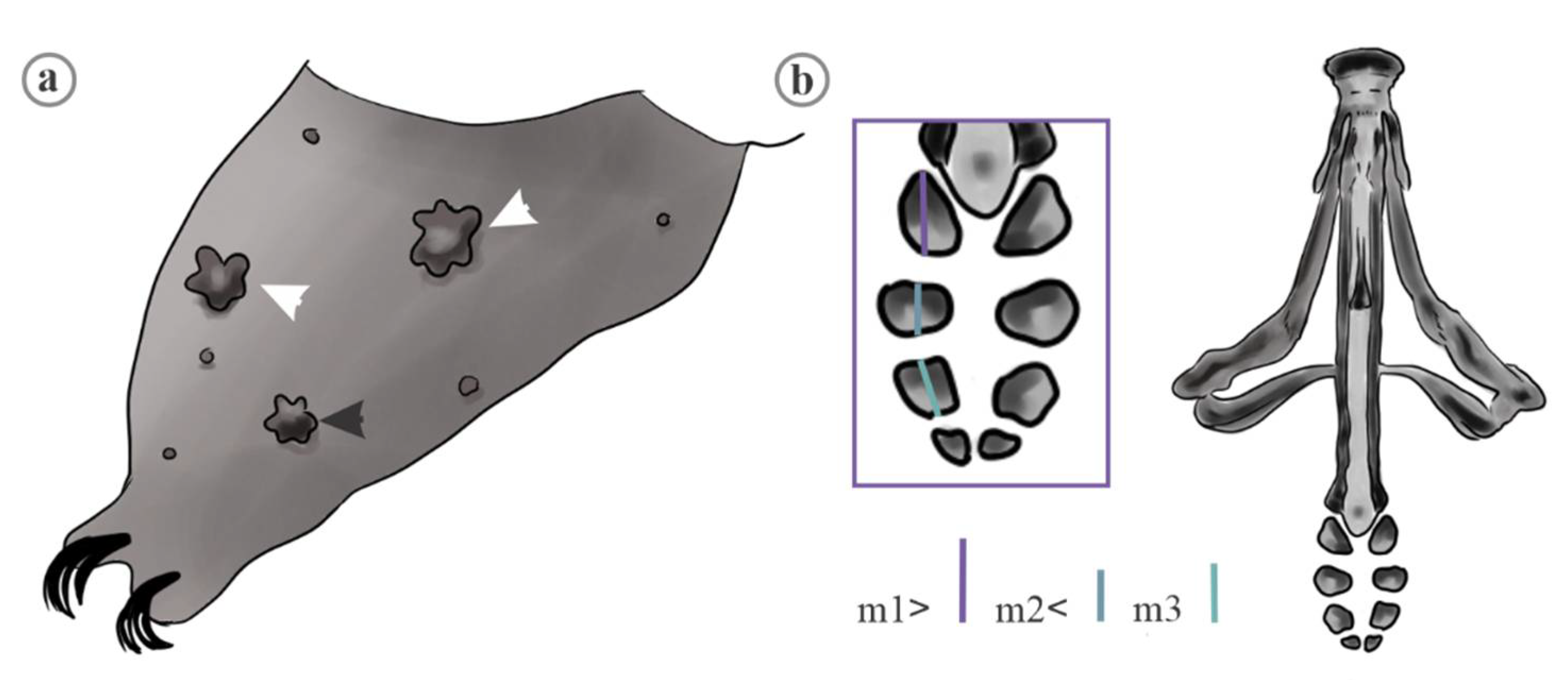
2. Material and Methods
2.1. Data Analyses
2.2. Effect of Size on the Morphometric Characters
2.3. Multivariate Analyses
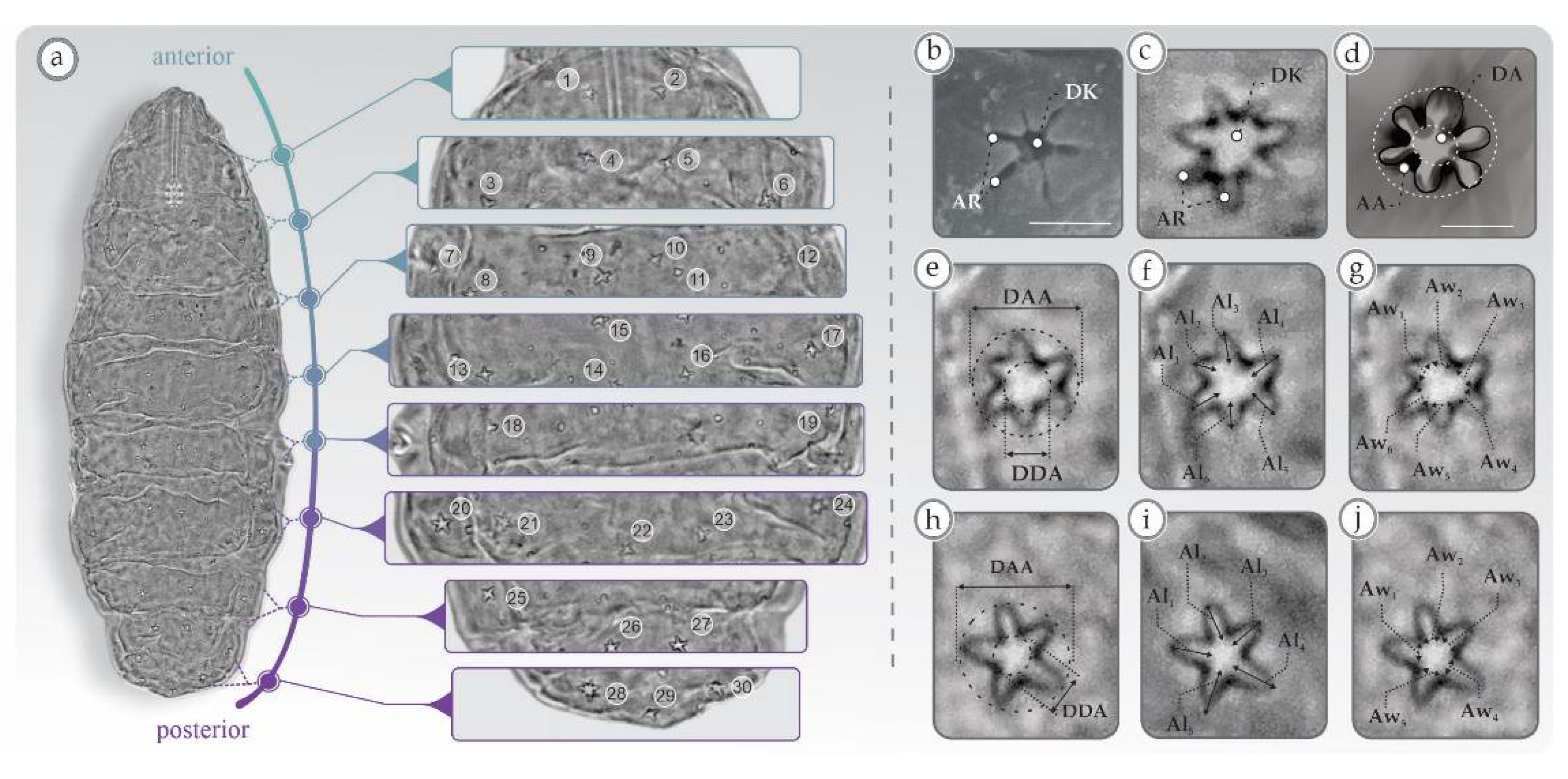
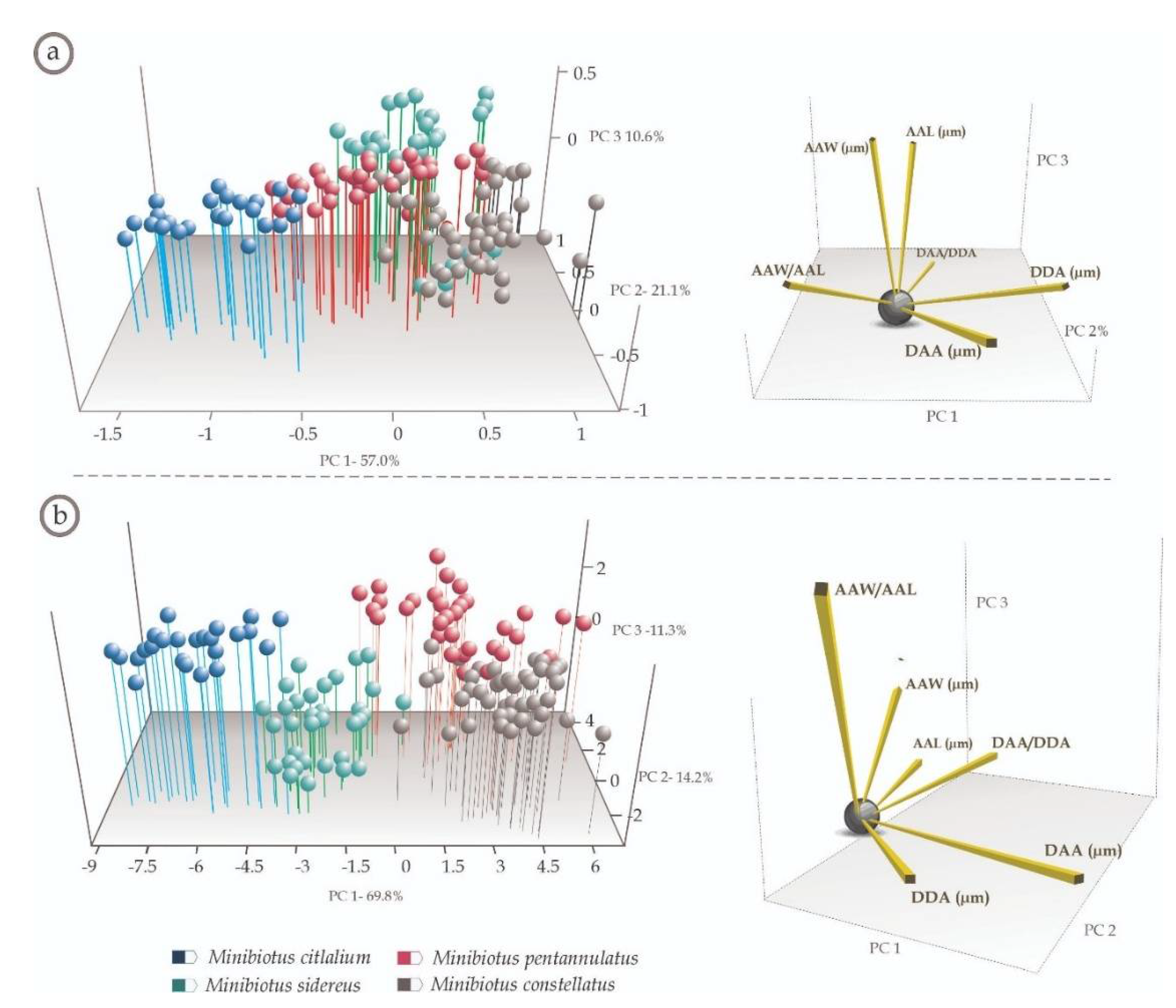
3. Results
3.1. Univariate Analyses
Size Effect on the Characteristics
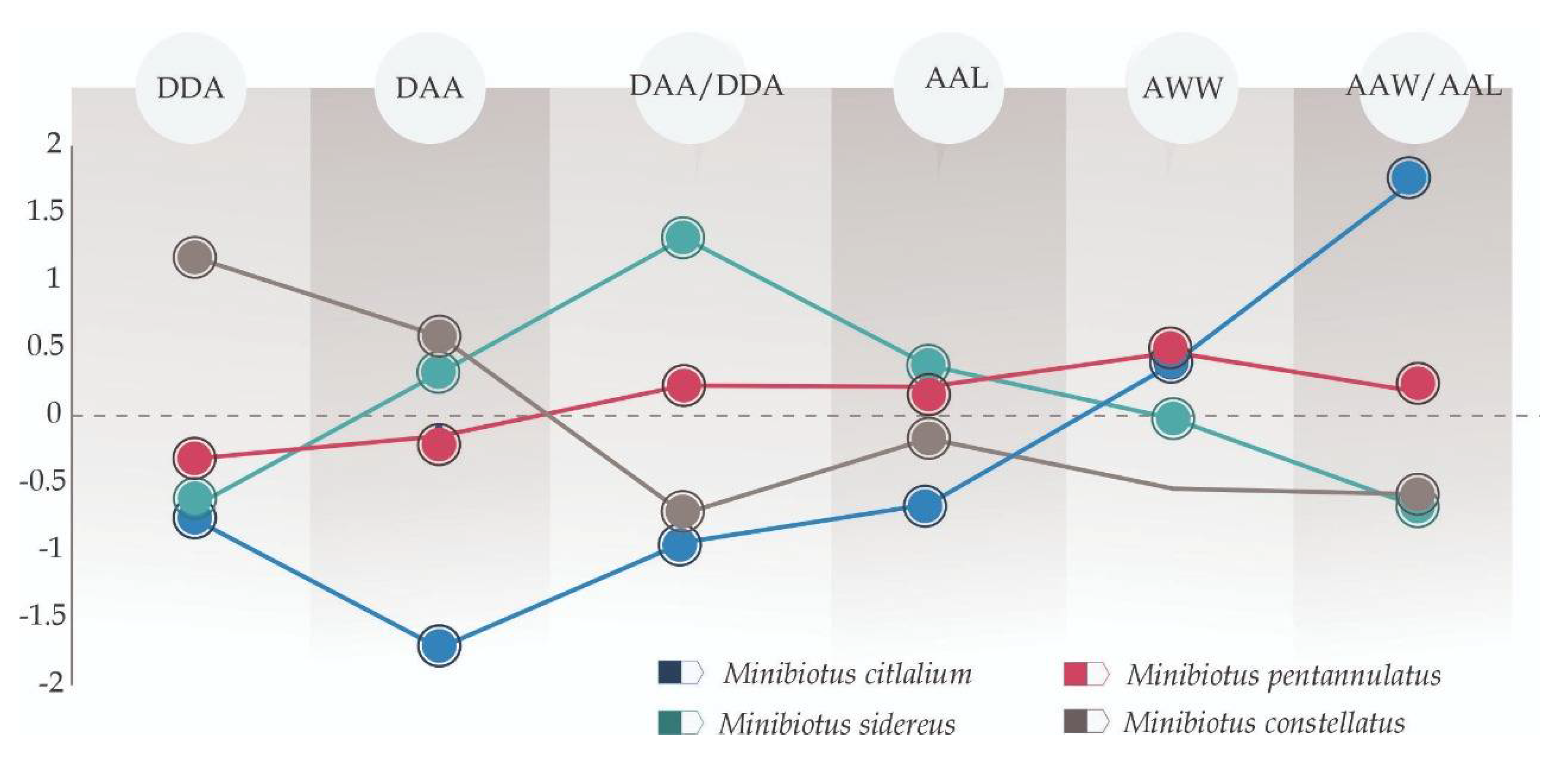
3.2. Multivariate Analyses
3.3. Dichotomous Key to the Species of Minibiotus sidereus Group
4. Discussion
4.1. Characters Analyzed
4.2. Multivariate Analyses (Species-Specific Cuticular Phenotypes)
4.3. Effect of Size in Traits and pt Values
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Claxton, S.K. A revision of the genus Minibiotus Tardigrada: Macrobiotidae with descriptions of new species from Australia. Rec. Aust. Muc. 1998, 50, 125–160. [Google Scholar] [CrossRef] [Green Version]
- Pilato, G.; Binda, M.G. A comparison of Diphascon (D.) alpinum Murray, 1906, D. (D.) chilenense Plate, 1889 and D. (D.) pingue Marcus, 1936 Tardigrada, and description of a new species. Zool. Anz. 1997, 236, 181–185. [Google Scholar]
- Kaczmarek, Ł.; Michalczyk, Ł. The Macrobiotus hufelandi group (Tardigrada) revisited. Zootaxa 2017, 4363, 101–123. [Google Scholar] [CrossRef]
- Morek, W.; Stec, D.; Gąsiorek, P.; Surmacz, B.; Michalczyk, Ł. Milnesium tardigradum Doyère, 1840: The first integrative study of interpopulation variability in a tardigrade species. J. Zool. Syst. Evol. Res. 2019, 57, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Gąsiorek, P.; Morek, W.; Stec, D.; Blagden, B.; Michalczyk, Ł. Revisiting Calohypsibiidae and Microhypsibiidae: Fractonotus Pilato, 1998 and its phylogenetic position within Isohypsibiidae (Eutardigrada: Parachela). Zoosystema 2019, 41, 71–89. [Google Scholar] [CrossRef] [Green Version]
- Binda, M.; Pilato, G.; Lisi, O. Remarks on Macrobiotus furciger Murray, 1906 and description of three new species of the furciger group (Eutardigrada, Macrobiotidae). Zootaxa 2005, 1075, 55–68. [Google Scholar] [CrossRef]
- Fontoura, P.; Pilato, G. Diphascon (Diphascon) faialense sp. nov. a new species of Tardigrada Eutardigrada, Hypsibiidae from the Azores and a key to the species of the D. pingue group. Zootaxa 2007, 1589, 47–55. [Google Scholar] [CrossRef]
- Kaczmarek, Ł.; Zawierucha, K.; Buda, J.; Stec, D.; Gawlak, M.; Michalczyk, Ł. An integrative redescription of the nominal taxon for the Mesobiotus harmsworthi group (Tardigrada: Macrobiotidae) leads to descriptions of two new Mesobiotus species from Arctic. PLoS ONE 2018, 13, e0204756. [Google Scholar] [CrossRef]
- Pilato, G. Analisi di nuovi caratterinello studio degli Eutardigradi. Animalia 1981, 8, 51–57. [Google Scholar]
- Bertolani, R.; Rebecchi, L. A revision of the Macrobiotus hufelandi group (Tardigrada, Macrobiotidaea), with some observations on the taxonomic characters of eutardigrades. Zool. Scr. 1993, 22, 127–152. [Google Scholar] [CrossRef]
- Guidetti, R.; Bertolani, R. Tardigrade taxonomy: An updated check list of the taxa and a list of characters for their identification. Zootaxa 2005, 845, 1–46. [Google Scholar] [CrossRef]
- Pilato, G.; Binda, G. Definition of families, subfamilies, genera and subgenera of the Eutardigrada, and keys to their identification. Zootaxa 2010, 2404, 1–54. [Google Scholar] [CrossRef] [Green Version]
- Michalczyk, Ł.; Kaczmarek, Ł. The Tardigrada Register: A comprehensive online data repository for tardigrade taxonomy. J. Limnol. 2013, 72, 175–181. [Google Scholar] [CrossRef]
- Pérez-Pech, W.A.; Anguas-Escalante, A.; Cutz-Pool, L.Q.; Guidetti, R. Doryphoribius chetumalensis sp. nov. (Eutardigrada: Isohypsibiidae) a new tardigrade species discovered in an unusual habitat of urban areas of Mexico. Zootaxa 2017, 4344, 347–352. [Google Scholar] [CrossRef]
- Dastych, H. The Tardigrada from Antarctic with descriptions of several new species. Acta Zool. Cracov. 1984, 27, 377–436. [Google Scholar]
- Dastych, H. Macrobiotus ramoli sp. nov., a new tardigrade species from the nival zone of the Ötztal Alps, Austria (Tardigrada). Mitt. Hambg. Zool. Mus. Inst. 2005, 102, 21–35. [Google Scholar]
- Dastych, H. A new tardigrade species of the genus Ramazzottius Binda & Pilato, 1986 (Tardigrada) from nival zone of the Mont Blanc (the Western Alps), with some morphometric remarks. Mitt. Hambg. Zool. Mus. Inst. 2006, 103, 33–45. [Google Scholar]
- Pilato, G.; Binda, M.G.; Claxton, S. Itaquason unguiculum and Itaquascon cambewarrense: Two new species of eutardigrades from Australia. N. Z. J. Zool. 2002, 29, 87–93. [Google Scholar] [CrossRef]
- Roszkowska, M.; Stec, D.; Ciobanu, D.A.; Kaczmarek, Ł. Tardigrades from Nahuel Huapi National Park (Argentina South America) with descriptions of two new Macrobiotidae species. Zootaxa 2016, 4105, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Blaxter, M.; Elsworth, B.; Daub, J. DNA taxonomy of a neglected animal phylum: An unexpected diversity of tardigrades. Proc. R. Soc. Lond. B 2004, 271, S189–S192. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, A.; Møbjerg, N.; Kristensen, R.M. A molecular study of the tardigrade Echiniscus testudo (Echiniscidae) reveals low DNA sequence diversity over a large geographical area. J. Limnol. 2007, 66, 77–83. [Google Scholar] [CrossRef]
- Cesari, M.; Bertolani, R.; Rebecchi, L.; Guidetti, R. DNA barcoding in Tardigrada: The first case study on Macrobiotus macrocalix Bertolani & Rebecchi 1993 (Eutardigrada, Macrobiotidae). Mol. Ecol. Resour. 2009, 9, 699–706. [Google Scholar] [PubMed]
- Guidetti, R.; Schill, R.O.; Bertolani, R.; Dandekar, T.; Wolf, M. New molecular data for tardigrade phylogeny, with the erection of Paramacrobiotus gen. nov. J. Zool. Syst. Evol. Res. 2009, 47, 315–321. [Google Scholar] [CrossRef]
- Guil, N.; Giribet, G. Fine scale population structure in the Echiniscus blumi-canadensis series (Heterotardigrada, Tardigrada) in an Iberian mountain range—When morphology fails to explain genetic structure. Mol. Phylogenet. Evol. 2009, 51, 606–613. [Google Scholar] [CrossRef]
- Coughlan, K.; Stec, D. Two new species of the Macrobiotus hufelandi complex (Eutardigrada: Macrobiotidae) from Australia and India, with notes on their phylogenetic position. EJT 2019, 573, 1–38. [Google Scholar] [CrossRef]
- Nelson, D.R.; Adkins, R.; Guidetti, R.; Roszkowska, M.; Grobys, D.; Kaczmarek, Ł. Two new species of Tardigrada from moss cushions (Grimmia sp.) in a xerothermic habitat in northeast Tennessee (USA, North America), with the first identification of males in the genus Viridiscus. PeerJ 2020, 8, e10251. [Google Scholar] [CrossRef] [PubMed]
- Stec, D.; Kristensen, R.M.; Michalczyk, Ł. An integrative description of Minibiotus ioculator sp. nov. from the Republic of South Africa with notes on Minibiotus pentannulatus Londoño et al., 2017 (Tardigrada: Macrobiotidae). Zool. Anz. 2020, 286, 117–134. [Google Scholar] [CrossRef]
- Kayastha, P.; Berdi, D.; Mioduchowska, M.; Gawlak, M.; Łukasiewicz, A.; Gołdyn, B.; Kaczmarek, Ł. Some tardigrades from Nepal (Asia) with integrative description of Macrobiotus wandae sp. nov (Macrobiotidae: Hufelandi group). Annal. Zool. 2020, 70, 121–142. [Google Scholar] [CrossRef]
- Kihm, J.H.; Kim, S.; McInnes, S.; Zawierucha, K.; Rho, H.S.; Kang, P.; Park, T.S. Integrative description of a new Dactylobiotus (Eutardigrada: Parachela) from Antarctica that reveals an intraspecific variation in tardigrade egg morphology. Sci. Rep. 2020, 10, 9122. [Google Scholar] [CrossRef]
- Tumanov, D.V. Integrative redescription of Hypsibius pallidoides Pilato et al., 2011 (Eutardigrada: Hypsibioidea) with the erection of a new genus and discussion on the phylogeny of Hypsibiidae. EJT 2020, 681, 1–37. [Google Scholar]
- Pilato, G. Revision of the genus Diphascon Plate, 1889, with remarks on the subfamily Itaquasconinae (Eutardigrada, Hypsibiidae). In Biology of Tardigrades. Selected Symposia and Monographs; Bertolani, R., Ed.; U.Z.I. Mucchi Editore: Modena, Italy, 1987; Volume 1, pp. 337–357. [Google Scholar]
- Dastych, G.; Thaler, K. The tardigrade Hebesuncus conjumgens (Thulin, 1911) in the Alps, with notes on morphology and distribution (Tardigrada). Entomol. Miit. Zool. Hambg. 2002, 14, 83–94. [Google Scholar]
- Kaczmarek, Ł.; Cytan, J.; Zawierucha, K.; Diduszko, D.; Michalczyk, Ł. Tardigrades from Peru (South America), with descriptions of three new species of Parachela. Zootaxa 2014, 3790, 357–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beasley, C.W.; Kaczmarek, Ł.; Michalczyk, Ł. Doryphoribius mexicanus, a new species of Tardigrada Eutardigrada: Hypsibiidae from Mexico North America. Biol. Soc. Wash. 2008, 121, 34–40. [Google Scholar] [CrossRef]
- Fontoura, P.; Morais, P. Assessment of traditional and geometric morphometrics for discriminating cryptic species of the Pseudechiniscus suillus complex (Tardigrada, Echiniscidae). J. Zool. Syst. Evol. Res. 2011, 49, 26–33. [Google Scholar] [CrossRef]
- Gąsiorek, P.; Stec, D.; Morek, W.; Michalcyk, Ł. An integrative redescription of Echiniscus testudo (Doyere, 1840), the nominal taxon for the class Heterotardigrada (Ecdysozoa: Panarthropoda: Tardigrada). Zool. Anz. 2017, 270, 107–122. [Google Scholar] [CrossRef]
- Moreno-Talamantes, A.; Roszkowska, M.; García-Aranda, M.A.; Flores-Maldonado, J.J.; Kaczmarek, Ł. Current knowledge on Mexican tardigrades with a description of Milnesium cassandrae sp. nov. (Eutardigrada: Milnesiidae) and discussion on the taxonomic value of dorsal pseudoplates in the genus Milnesium Doyère, 1840. Zootaxa 2019, 4691, 501–524. [Google Scholar] [CrossRef] [PubMed]
- Gąsiorek, P.; Stec, D.; Morek, W.; Michalczyk, Ł. An integrative redescription of Hypsibius dujardini Doyère, 1840, the nominal taxon for Hypsibioidea Tardigrada: Eutardigrada. Zootaxa 2018, 44151, 45–75. [Google Scholar] [CrossRef] [Green Version]
- Pilato, G.; Costa, G.; Conti, E.; Binda, M.G.; Lisi, O. Morphometric analysis of some metric characters of two Macrobiotus species (Eutardigrada, Macrobiotidae). J. Limnol. 2007, 66, 26–32. [Google Scholar] [CrossRef] [Green Version]
- Zawierucha, K.; Stec, D.; Lachowska-Cierlik, D.; Takeuchi, N.; Michalczyk, Ł. High mitochondrial diversity in a new water bear species (Tardigrada: Eutardigrada) from mountain glaciers in Central Asia, with the erection of a new genus Cryoconius. Ann. Zool. 2018, 68, 179–201. [Google Scholar] [CrossRef]
- Stec, D.; Gąsiorek, P.; Morek, W.; Kosztyla, P.; Zawierucha, K.; Michno, K.; Kaczmarek, Ł.; Prokop, Z.M.; Michalczyk, Ł. Estimating optimal sample size for tardigrade morphometry. Zool. J. Linn. Soc. 2016, 178, 776–784. [Google Scholar] [CrossRef] [Green Version]
- Stec, D.; Smolak, R.; Kaczmarek, Ł.; Michalczyk, Ł. An integrative description of Macrobiotus paulinae sp. nov. (Tardigrada: Eutardigrada: Macrobiotidae: Hufelandi group) from Kenya. Zootaxa 2015, 4052, 501–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roszkowska, M.; Gawlak, M.; Draga, M.; Kaczmarek, Ł. Two new species of Tardigrada from Ecuador (South America). Zootaxa 2019, 4545, 511–530. [Google Scholar] [CrossRef] [PubMed]
- Ramazzotti, G.; Maucci, W. Il Phylum Tardigrada. Terza edizione riveduta e corretta. Mem. Isituto Ital. Idrobiol. Dott. Marco Marchi 1983, 41, 1–1012. [Google Scholar]
- Stec, D.; Morek, W.; Gąsiorek, P.; Michalczyk, Ł. Unmasking hidden species diversity within the Ramazzottius oberhaeuseri complex, with an integrative redescription of the nominal species for the family Ramazzottiidae (Tardigrada: Eutardigrada: Parachela). Syst. Biodivers. 2018. [Google Scholar] [CrossRef]
- Bartylak, T.; Kulpa, A.; Grobys, D.; Kepel, M.; Kepel, A.; Kmita, H.; Gawlak, M.; Grabinski, W.; Roszkowska, M.; Kaczmarek, Ł. Variability of Echiniscus tristis Gąsiorek & Kristensen, 2018‒is morphology sufficient for taxonomic differentiation of Echiniscidae? Zootaxa 2019, 407, 1–24. [Google Scholar]
- Pilato, G.; Binda, M.G.; Lisi, O. Three new species of eutardigrades from the Seychelles. New Zealand J. Zool. 2006, 33, 39–48. [Google Scholar] [CrossRef]
- Michalczyk, Ł.; Kaczmarek, Ł. Minibiotus constellatus, new species of Tardigrada from Peru (Eutardigrada: Macrobiotidae). Genus 2003, 14, 295–305. [Google Scholar]
- Pilato, G.; Binda, M.G.; Lisi, O. Remarks on some species of tardigrades from South America with description of Minibiotus sidereus n. sp. Zootaxa 2003, 195, 1–8. [Google Scholar] [CrossRef]
- Dueñas-Cedillo, A.; Martínez-Méndez, E.; García-Román, J.; Armendáriz-Toledano, F.; Ruiz, E.A. Tardigrades from Iztaccíhuatl Volcano (Trans-Mexican Volcanic Belt), with the Description of Minibiotus citlalium sp. nov. (Eutardigrada: Macrobiotidae). Diversity 2020, 12, 271. [Google Scholar] [CrossRef]
- Londoño, R.; Daza, A.; Lisi, O.; Quiroga, S. New species of waterbear Minibiotus pentannulatus (Tardigrada: Macrobiotidae) from Colombia. Rev. Mex. Biodivers. 2017, 88, 807–814. [Google Scholar] [CrossRef]
- Michalczyk, Ł.; Kaczmarek, Ł. Minibiotus eichhorni sp. nov., a new species of eutardigrade (Eutardigrada: Macrobiotidae) from Peru. Ann. Zool. 2004, 54, 673–676. [Google Scholar]
- Chim, C.K.; Tan, K.S. Recognition of individual knobby sea stars Protoreaster nodosus (L., 1758) using aboral surface charcateristics. J. Exp. Mar. Biol. Ecol. 2012, 430, 48–55. [Google Scholar] [CrossRef]
- Rohlf, F.J. TPS Dig v 2.31; Department of Ecology and Evolution, State University of New York: Stony Brook, NY, USA, 2017; Available online: http://life.bio.sunysb.edu/morph/ (accessed on 1 May 2021).
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Guillaumin, M. Etude biometrique des populations naturelles de P. Carlivae Rbr et P. Ciasii Rbr (Lep. Hesperiidae). I Estimation du taux de chevauchement des distributions statistiques de deux populations en relation avec la notion de distance taxonomique. Arch. Zool. Exp. Gen. 1972, 115, 505–548. [Google Scholar]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Prentice Hall: Englewood Cliffs, NJ, USA, 2010; pp. 1–929. [Google Scholar]
- Bartels, P.J.; Nelson, D.R.; Exline, R.P. Allometry and the removal of body size effects in the morphometric analysis of tardigrades. J. Zool. Syst. Evol. Res. 2011, 49, 17–25. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Legendre, P.; Legendre, L. Numerical Ecology: Developments in Environmental Modeling 20, 2nd ed.; Elsevier B.V.: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Kristensen, R.M. Generic revision of the Echiniscidae (Heterotardigrada), with a discussion of the origin of the family. In Biology of Tardigrades; Bertolani, R., Ed.; Selected Symposia and Monographs; U.Z.I. Mucchi Editore: Modena, Italy, 1987; pp. 261–335. [Google Scholar]
- Fontoura, P.; Bartels, P.J.; Jørgensen, A.; Kristensen, R.M.; Hansen, J.G. A dichotomous key to the genera of the Marine Heterotardigrades (Tardigrada). Zootaxa 2017, 1–45. [Google Scholar] [CrossRef]
- Hansen, J.G.; Gallo D’Addabbo, M.; De Zio Grimaldi, S. A comparison of morphological characters within the genus Rhomboarctus (Tardigrada: Heterotardigrada) with the description of two new species. Zool. Anz. 2003, 242, 83–96. [Google Scholar] [CrossRef]
- Tumanov, D.V. Analysis of non-morphometric morphological characters used in the taxonomy of the genus Pseudechiniscus (Tardigrada:Echiniscidae). Zool. J. Linn. Soc. 2020, 188, 753–775. [Google Scholar]
- Vecchi, M.; Cesari, M.; Bertolani, R.; Jonsson, K.I.; Rebecchi, L.; Guidetti, R. Integrative systematic studies on tardigrades from Antarctica identify new genera and new species within Macrobiotoidea and Echiniscoidea. Invertebr. Syst 2016, 303–322. [Google Scholar] [CrossRef]
- Cesari, M.; Montanari, M.; Reinhardt, M.K.; Bertolani, R.; Guidetti, R.; Rebecchi, L. An integrated study of the biodiversity within the Pseudechiniscus suillus–facettalis group (Heterotardigrada: Echiniscidae). Zool. J. Linn. Soc. 2020, 188, 717–732. [Google Scholar] [CrossRef] [Green Version]
- Gąsiorek, P.; Voncina, K.; Zajac, K.; Michalczk, L. Phylogeography and morphological evolution of Pseudechiniscus (Heterotardigrada: Echiniscidae). Sci. Rep. 2021, 11, 1–16. [Google Scholar] [CrossRef]
- Nelson, D.R.; Marley, N.J. The biology and ecology of lotic Tardigrada. Freshw. Biol. 2000, 44, 93–109. [Google Scholar] [CrossRef]
- Gasiorek, P.; Stec, D.; Morek, W.; Michalczyk, L. Deceptive conservatism of claws: Distinct phyletic lineages concealed within Isohypsibioidea (Eutardigrada) revealed by molecular and morphological evidence. Contrib. Zool. 2019, 88, 78–132. [Google Scholar] [CrossRef] [Green Version]
- Guidetti, R.; Rebecchi, L.; Bertolani, R. Cuticle structure and systematics of the Macrobiotidae (Tardigrada, Eutardigrada). Acta Zool. 2000, 81, 27–36. [Google Scholar] [CrossRef]
- Bertolani, R.; Guidetti, R.; Marchioro, T.; Altiero, T.; Rebecchi, L.; Cesari, M. Phylogeny of Eutardigrada: New molecular data and their morphological support lead to the identification of new evolutionary lineages. Mol. Phylogenet. Evol. 2014, 76, 110–126. [Google Scholar] [CrossRef] [PubMed]
- Stec, D.; Krzywanski, L.; Zawierucha, K.; Michalcczyk, L. Untangling systematics of the Paramacrobiotus areolatus species complex by an integrative redescription of the nominal species for the group, with multilocus phylogeny and species delineation in the genus Paramacrobiotus. Zool. J. Linn. Soc. 2020, 188, 694–716. [Google Scholar] [CrossRef]
- Muster, C.; Michalik, P. Cryptic diversity in ant-mimic Micaria spiders (Araneae, Gnaphosidae) and a tribute to early naturalists. Zool. Scr. 2020, 49, 197–209. [Google Scholar] [CrossRef] [Green Version]
- Cai, R.; Prior, T.; Lawson, B.; Cantalapiedra-Navarrete, C.; Palomares-Rius, J.E.; Castillo, P.; Archidona-Yuste, A. An integrative taxonomic study of the needle nematode complex Longidorus goodeyi Hooper, 1961 (Nematoda: Longidoridae) with description of a new species. Eur. J. Plant. Pathol. 2020, 158, 59–81. [Google Scholar] [CrossRef]
- Cid-Muñoz, R.; Cibrián-Tovar, D.; Valadez-Moctezuma, E.; Estrada-Martinez, E.; Armendariz-Toledano, F. Biology and Life Stages of Pine Spittle Bug Ocoaxo assimilis Walker (Hemiptera: Cercopidae). Insects 2020, 11, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moly de Peluffo, M.C.; Paluffo, J.R.; Rocha, A.M.; Doma, I.L. Tardigrade distribution in a medium-sized city of central Argentina. Hydrobiologia 2006, 558, 141–150. [Google Scholar] [CrossRef]



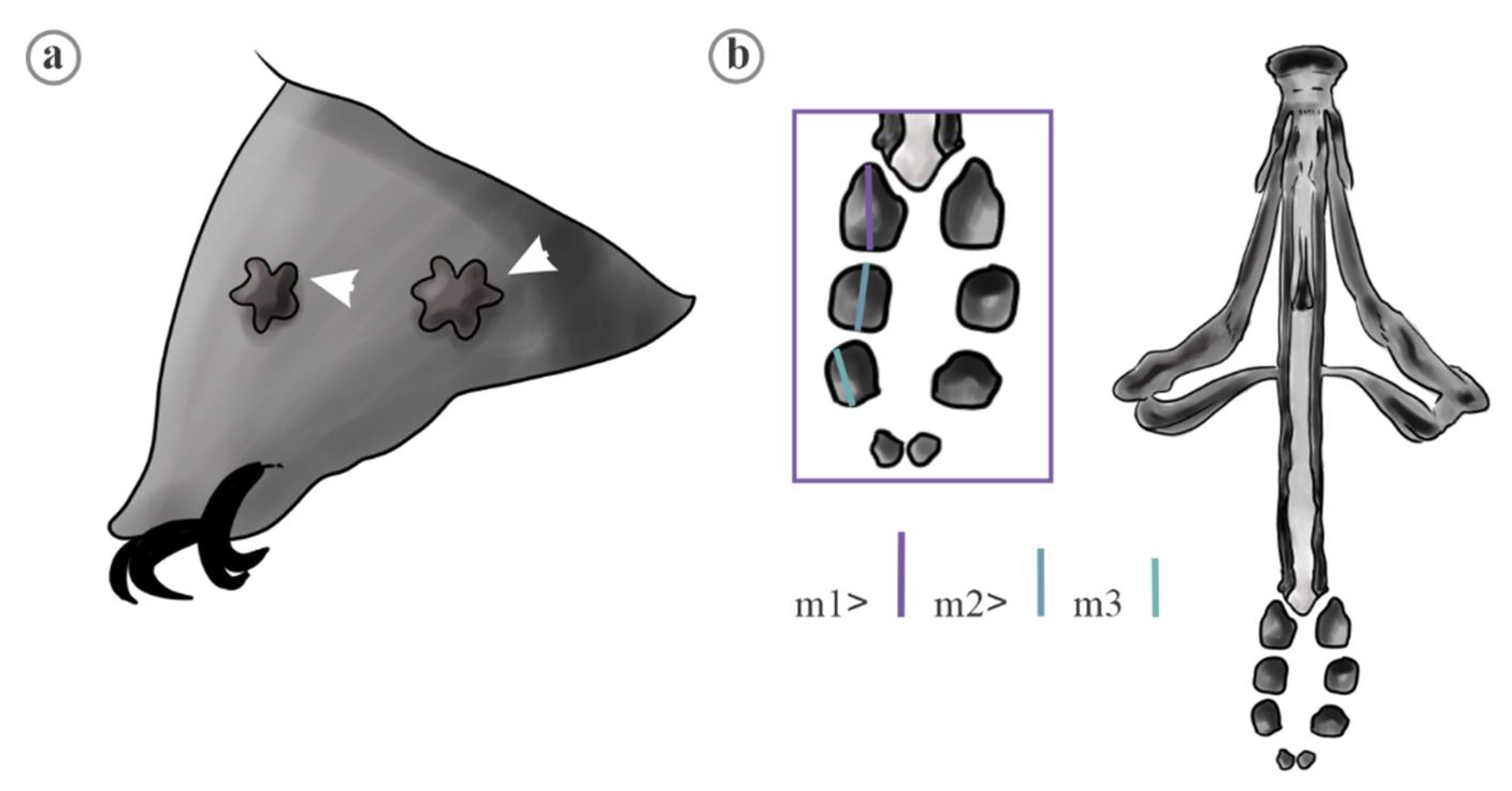

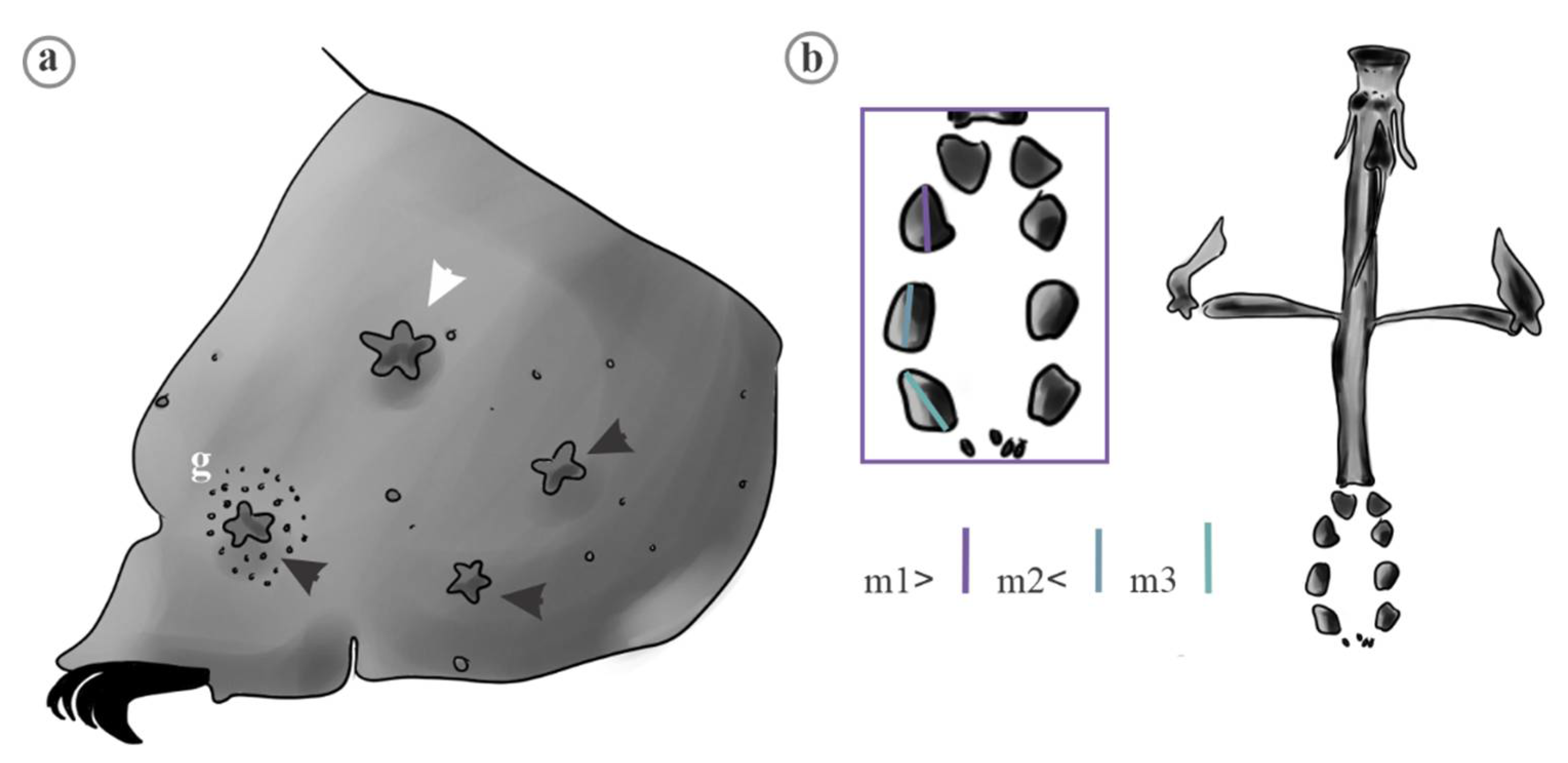
| Trait | Species | Mean | FANOVA |
|---|---|---|---|
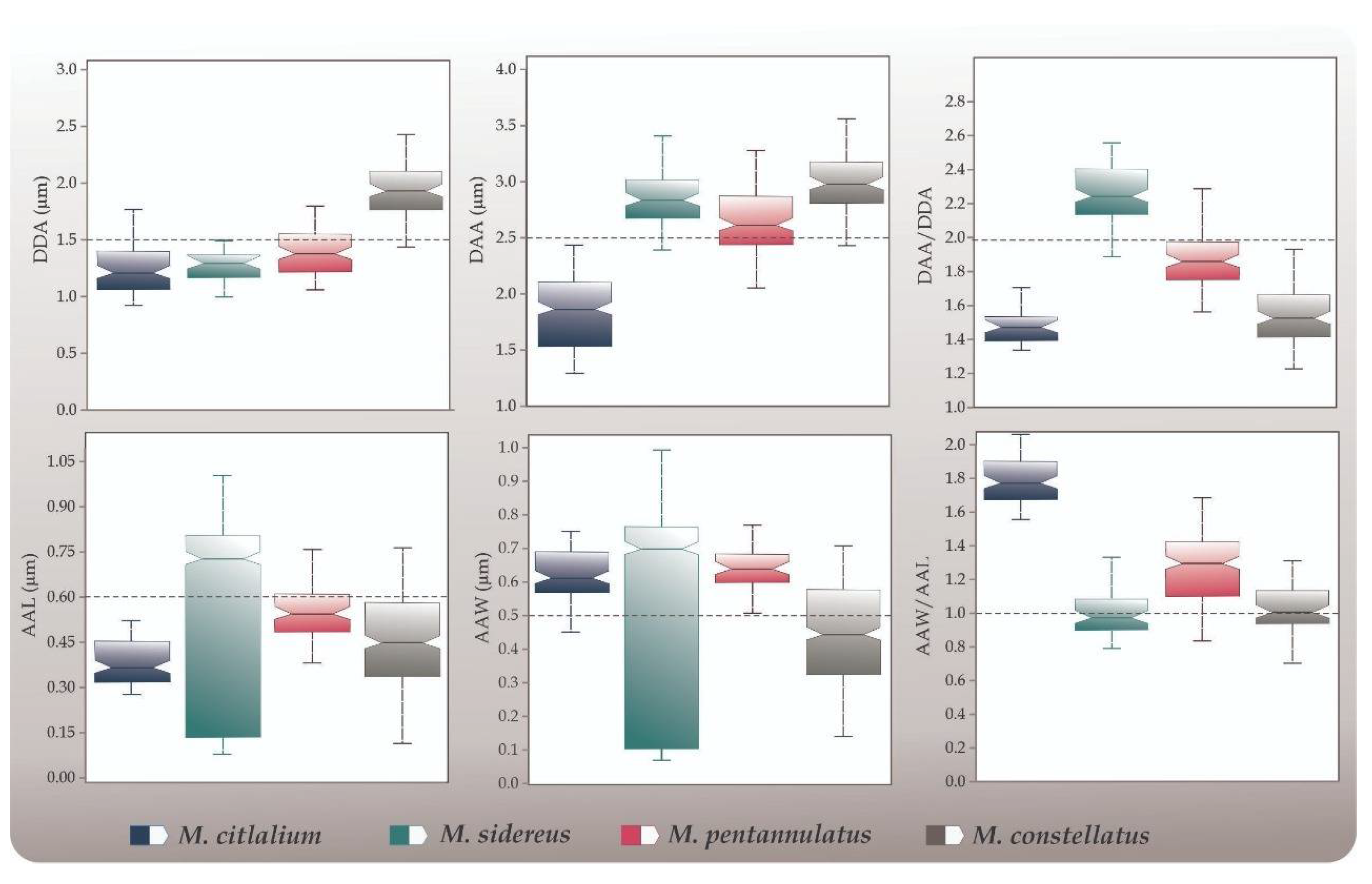
| Diameter of disc area (DDA) | M. citlalium | 1.24 ± 0.04; (0.9, 1.7) a | 193 ** |
| M. sidereus | 1.28 ± 0.02; (1.0, 1.5) a | ||
| M. pentannulatus | 1.40 ± 0.03; (1.0, 1.7) b | ||
| M. constellatus | 1.94 ± 0.03; (1.4, 2.4) c | ||
| Diameter of arm area (DAA) | M. citlalium | 1.83 ± 0.06; (1.3, 2.4) a | 90.21 ** |
| M. sidereus | 2.85 ± 0.04; (2.3, 3.4) b | ||
| M. pentannulatus | 2.62 ± 0.05; (2.0, 3.2) c | ||
| M. constellatus | 3.00 ± 0.03; (2.4, 3.6) b, d | ||
| DAA/DDA | M. citlalium | 1.48 ± 0.02; (1.3, 1.7) a | 157.3 ** |
| M. sidereus | 2.24 ± 0.03; (1.8, 2.5) b | ||
| M. pentannulatus | 1.87 ± 0.02; (1.5, 2.2) c | ||
| M. constellatus | 1.55 ± 0.02; (1.2, 1.9) a | ||
| Average arm length (AAL) | M. citlalium | 0.36 ± 0.008; (0.2, 0.4) a | 6.62 ** |
| M. sidereus | 0.57 ± 0.05; (0.07, 1.0) b | ||
| M. pentannulatus | 0.54 ± 0.01; (0.3, 0.7) c, b | ||
| M. constellatus | 0.46 ± 0.02; (0.1, 0.76) a, b | ||
| Average arm width (AAW) | M. citlalium | 0.62 ± 0.01; (0.4, 0.7) a | 8.56 ** |
| M. sidereus | 0.54 ± 0.05; (0.07, 0.9) a | ||
| M. pentannulatus | 0.64 ± 0.01; (0.5, 0.7) a | ||
| M. constellatus | 0.43 ± 0.02; (0.4, 0.7) a, b | ||
| AAW/AAL | M. citlalium | 1.70 ± 0.02; (1.5, 2.0) a | 166.9 ** |
| M. sidereus | 0.92 ± 0.02; (0.7, 1.2) b | ||
| M. pentannulatus | 1.20 ± 0.03; (0.7, 1.6) c | ||
| M. constellatus | 0.95 ± 0.01; (0.7, 1.2) b, d |
| Trait | Species | Mean | FANOVA |
|---|---|---|---|
| Diameter of disc area (DDA) | M. citlalium | 4.60 ± 0.17; (3.4, 6.6) a | 259 ** |
| M. sidereus | 4.70 ± 0.07; (3.9, 5.3) a | ||
| M. pentannulatus | 7.49 ± 0.17; (5.6, 9.6) b | ||
| M. constellatus | 9.57 ± 0.17; (7.0, 11.9) c | ||
| Diameter of arm area (DAA) | M. citlalium | 6.79 ± 0.25; (4.8, 9.08) a | 227 ** |
| M. sidereus | 10.51 ± 0.17; (8.6, 12.97) b | ||
| M. pentannulatus | 13.98 ± 0.29; (10.94, 17.46) c, e | ||
| M. constellatus | 14.74 ± 0.18; (11.97, 17.95) d, e | ||
| DAA/DDA | M. citlalium | 5.4 ± 0.06; (4.7, 6.3) a | 170 ** |
| M. sidereus | 8.24 ± 0.13; (6.7, 10.05) b | ||
| M. pentannulatus | 10 ± 0.15; (8.3, 12.20) c | ||
| M. constellatus | 7.65 ± 0.11; (6.04, 9.51) d | ||
| Average arm length (AAL) | M. citlalium | 1.33 ± 0.03; (0.97, 1.62) a | 16.95 ** |
| M. sidereus | 2.16 ± 0.21; (0.27, 3.9) b | ||
| M. pentannulatus | 2.90 ± 0.08; (2.02, 4.03) c | ||
| M. constellatus | 2.28 ± 0.12; (0.56, 3.75) d, b | ||
| Average arm width (AAW) | M. citlalium | 2.31 ± 0.06; (1.60, 2.74) a | 21.72 ** |
| M. sidereus | 2.03 ± 0.21; (0.24, 3.9) a | ||
| M. pentannulatus | 3.42 ± 0.05; (2.70, 4.10) b | ||
| M. constellatus | 2.16 ± 0.11; (0.69, 3.48) a | ||
| AAW/AAL | M. citlalium | 6.39 ± 0.11; (5.27, 7.46) a | 135 ** |
| M. sidereus | 3.25 ± 0.07; (2.55, 4.22) b | ||
| M. pentannulatus | 6.42 ± 0.18; (4.06, 8.59) a | ||
| M. constellatus | 4.69 ± 0.09; (3.49, 6.09) c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dueñas-Cedillo, A.; García-Román, J.; Ruiz, E.A.; Armendáriz-Toledano, F. Species-Specific Cuticular Phenotypes in Eutardigrada: A Morphometric Approach to Analyze the Variation of Star-Shaped Pores in Minibiotus Species. Diversity 2021, 13, 307. https://doi.org/10.3390/d13070307
Dueñas-Cedillo A, García-Román J, Ruiz EA, Armendáriz-Toledano F. Species-Specific Cuticular Phenotypes in Eutardigrada: A Morphometric Approach to Analyze the Variation of Star-Shaped Pores in Minibiotus Species. Diversity. 2021; 13(7):307. https://doi.org/10.3390/d13070307
Chicago/Turabian StyleDueñas-Cedillo, Alba, Jazmín García-Román, Enrico Alejandro Ruiz, and Francisco Armendáriz-Toledano. 2021. "Species-Specific Cuticular Phenotypes in Eutardigrada: A Morphometric Approach to Analyze the Variation of Star-Shaped Pores in Minibiotus Species" Diversity 13, no. 7: 307. https://doi.org/10.3390/d13070307
APA StyleDueñas-Cedillo, A., García-Román, J., Ruiz, E. A., & Armendáriz-Toledano, F. (2021). Species-Specific Cuticular Phenotypes in Eutardigrada: A Morphometric Approach to Analyze the Variation of Star-Shaped Pores in Minibiotus Species. Diversity, 13(7), 307. https://doi.org/10.3390/d13070307