Abstract
The lava tubes at Undara became internationally recognised in the late 1980s, when 24 species of terrestrial cave-adapted invertebrates (troglobionts) were recorded from Bayliss Cave, making it one of the 20 richest known cave communities in the world at the time. Over the last decades, several of the Undara species have been taxonomically described and a great deal of research has been undertaken in other parts of Australia, which has revealed additional subterranean hotspots. It is therefore timely to update the list of Undara cave fauna, and to evaluate the Undara cave system in relation to other subterranean hotspots in Australia. The updated species list was compiled from the published literature and museum databases. Minimally, 78 species of arthropods have been recorded from 17 lava tube caves in the Undara Basalt. Sixteen species have been taxonomically described; 30 identified to genus and/or morpho-species; and 32 remain unidentified to species or genus level. Thirty troglobionts and one stygobiont species were recorded. Seven caves harboured obligate subterranean species; Bayliss Cave harboured the most obligate subterranean species: 23 troglobionts and one stygobiont. All these caves contained deep zone environments with high humidity, of which three also contained ‘bad air’ (CO2). The unique combination of geomorphic structure and environmental parameters (high humidity) and multiple energy sources (tree roots, bats and guano, organic material wash-in) are the main factors responsible for Bayliss Cave’s extraordinary local richness. Further research is needed to investigate CO2 as a factor influencing troglobiont richness and distribution in ‘bad air’ caves. Undara remains the richest subterranean hotspot in humid tropical Australia; however, significantly richer subterranean assemblages are found in arid and semi-arid calcrete aquifers, karst and iron-ore terrains, mostly in Western Australia.
1. Introduction
The lava tubes at Undara became internationally recognised when, in the late 1980s, 24 species of terrestrial cave-adapted invertebrates (troglobionts) were recorded from Bayliss Cave [1,2], making it one of the 20 richest known cave communities in the world at the time [3]. Since then, the troglobionts at Undara have been the subject of landmark studies on reductive evolutionary trends and acoustic communication in cixiid planthoppers [4,5], adaptive shift and non-relictual tropical cave-adapted animals [1], and carbon dioxide and implications for the evolution of cave-adapted animals [2,6].
The research at Undara thrust these caves into the limelight of biospeleology in Australia on four counts. Firstly, it broke the existing paradigm that mainland Australia was poor in cave-adapted animals [7]. That view was further invalidated by additional discoveries in Western Australia [8,9]. Secondly, it upended the global paradigm that cave-adapted animals were rare in tropical caves [10]. The third important aspect was that lava tubes were also considered to be devoid of cave-adapted animals [11]; and fourth, some of these tropical cave-adapted animals were non-relictual, in the sense that some cave species had closely related congeners living in surface habitats nearby.
The latter finding held important implications for understanding the evolutionary processes leading to speciation and adaptation to subterranean habitats, i.e., the adaptive shift hypothesis [12]. The situation with Undara and some other tropical cave faunas contrasts with many temperate zone cave faunas, where the surface lineages that gave rise to cave forms had long since disappeared or migrated elsewhere due to changing conditions on the surface particularly during Pleistocene glacial periods, i.e., the climatic relict hypothesis [11].
These global paradigms were also challenged after troglobionts were discovered in tropical and subtropical limestone caves and lava tubes in the Galapagos [13], Hawaii [14], Canary Islands [15,16], Jamaica [17], Congo, Thailand, Indonesia, and Central and South America [18,19]. On the other hand, recent molecular and morphological studies appear to support the climate relict hypothesis even for tropical cave animals, e.g., Soulier-Perkins [20] study of Chillagoe and Undara cixiid planthoppers and Slaney and Blair [21] study of Chillagoe and Undara ectobiid cockroaches. However, the question of which came first, isolation or cave adaptation, cannot be answered with current methods. Nonetheless, clear examples of adaptive shifts are known [22] suggesting that cave adaptation comes first at least in some cases [23].
As demonstrated in the young lava tubes on Hawaii [6,24] the primary habitat for troglobionts and cave-adapted aquatic species (stygobionts) in basaltic terrains is within intermediate sized voids (=mesocaverns). Mesocaverns (5–500 mm diameter) are distinguished from large-sized caves generally enterable by humans (>500 mm) and fine-grained interstitial habitats in porous sediments (<5 mm) [25]. Troglobionts occupy accessible cave-sized passages when environmental conditions are suitable [26]. These animals can disperse entirely underground throughout basalt flows, and they can potentially disperse into adjoining flows if there are suitable connecting voids. Since these caves comprise an interconnected lava tube system without barriers to underground dispersal, the five tube systems in the Undara Basalt are treated as one integrated cave ecosystem. Since the pool of potential colonizers is similar throughout the basalt flow, comparisons between community composition and the physical environment can provide useful insights into cave ecology.
Since publication of the first hotspot list in 2000, knowledge of global cave biodiversity has grown substantially, and the number of hotspot caves has more than doubled. However tropical hotspot caves remain in the minority with only five tropical caves harbouring 20 or more specialized cave species recognised in the last update [27]. The small representation of tropical hotspot caves may be partly because many tropical cave regions have not been adequately investigated and because many species remain undescribed. Additionally, tropical caves often contain specialized guano fauna (guanobionts) that are only weakly troglomorphic but still not known outside caves [19,28].
With publication of this special issue featuring world hotspots of subterranean biodiversity, it is thus timely to update and review the species list for Bayliss Cave and include the fauna of other cave segments in the Undara lava flow. Several of the cavernicolous species have been described in the years since the last updates by Stone [29] and Clarke [30]. The current state of knowledge on the geology, ecology, physical environment, and natural history of the subterranean animals in the Undara caves is the focus of this review. We briefly mention other biodiversity hotspots in Australia to place Undara in context and discuss the challenge of classifying cavernicoles in the face of limited taxonomic and ecological knowledge. We hope this paper stimulates renewed field sampling and taxonomic interest in Undara’s remarkable tropical lava tube system.
2. Materials and Methods
The taxonomic impediment is a global problem exacerbated by reduced funding for systematic research, the ‘orphaning’ of taxa by retirement and passing of experts, and the increasing complexity of the discipline as new species must be compared with an increasing number of related taxa. Fontaine et al. [31] determined that the average delay between collection and description of a new species was over two decades, and that the time was further increased when the new species occurred in species-rich areas, and for poorly known taxa lacking a recent revision. These universal limitations mean that many Undara cave species have not been identified to species level and numerous putative new species await formal description.
The updated species list was compiled from the published literature and registered specimen databases of the Queensland Museum (QM) and Australian Museum (AM). In some cases, undescribed taxa reported in earlier collection lists could not be located in museum databases, probably because a substantial portion of cave collections made from Undara caves remain unsorted and unregistered. Portions of the (registered) Undara cave collections remain on loan to the BP Bishop Museum (BPBM), Hawaii, or have been loaned to taxonomists pending description. Taxa collected by Clarke [30] are deposited at various institutions including QM, AM, Australian National Insect Collection (ANIC), Tasmanian Museum and Art Gallery (TMAG) and the Northern Territory Museum and Art Gallery (NTMAG). Taxonomists holding material were contacted for information about the taxonomic and ecological status of taxa. Data on other subterranean hotspots in Australia were sourced from the published literature, unpublished consultancy reports and collection records (SE). Other collectors [32,33,34,35] list additional unidentified taxa in groups represented in our lists. Without inspecting voucher specimens, it is not possible to determine whether the taxon is already listed; therefore, the conservative option is to exclude these records from the list.
Determining whether a species is a troglobiont, exclusively restricted to caves and associated mesocaverns, rather than a soil or surface inhabitant is often problematic especially in poorly known groups and in groups that possess troglomorphic characters but are not confined to caves. To help discriminate true troglobionts from troglomorphic troglophiles and edaphophiles we also relied on field observations of behavior as well as morphology [36]. That is, in addition to reduced pigment, eyes and wings, elongated appendages, and sometimes a larger size compared to their soil or surface relatives, troglobionts generally exhibit no response to light and move comparatively slowly even when disturbed. For example, all polydesmid millipedes lack eyes, and many species occur in both caves and cryptic surface habitats. Surface dwelling polydesmids that have been studied can detect light and respond by moving into dark refuges when exposed to light [37]. Although not studied experimentally, cave-restricted polydesmids, including the pale-coloured species we observed at Undara, usually do not respond to light and move slowly.
Oxygen and carbon dioxide concentrations (as volume percentage) were measured 15 cm above the cave floor using a Draeger Multi Gas Detector with oxygen (5%/B) and carbon dioxide (0.1%/a) tubes, respectively. A few additional readings using appropriate Draeger tubes were made to test for the presence of carbon monoxide, ammonia and methane; the results were negative. Generally, two readings for carbon dioxide were taken at selected locations within each zone and corrected for barometric pressure (96.4–96.5 kPa) measured with a Thommen no. 2000 (5000 m) altimeter. Temperature and relative humidity were measured 15 cm and 2 m above the cave floor as noted using a battery-powered Bendix aspirating psychrometer. The psychrometer was accurate up to 95% RH, and the presence and thickness of fog was used to indicate higher humidity levels.
3. Geology, Geomorphology, Hydrology
‘Undara’ is an Aboriginal word meaning ‘long way’. The extensive lava tube system that developed in the Undara Basalt flow is one of several basalt flows making up the McBride Volcanic Province. The province is situated on the Atherton Tableland approximately 200 km southwest of Cairns in tropical northeast Queensland. It comprises a broad topographic dome roughly 100 km in diameter, which reaches an elevation of around 1020 m descending to its margins at approximately 400 m above sea level. The dome is believed to have been built over a period of 10 million years by multiple lava flows from more than 160 vents [38] (Figure 1). Much of the basalt dome is devoid of surface watercourses. The high porosity of basalt facilitates rapid infiltration of surface water to groundwater drainages that largely correspond to now buried drainages incised in the underlying granites prior to volcanism [35]. Swampy resurgences occur at the margins where the original watercourses have been filled with basalt. Some lava tubes contain temporary or permanent perched lakes which are fed by diffuse infiltration and local surface runoff into entrances during the wet season.
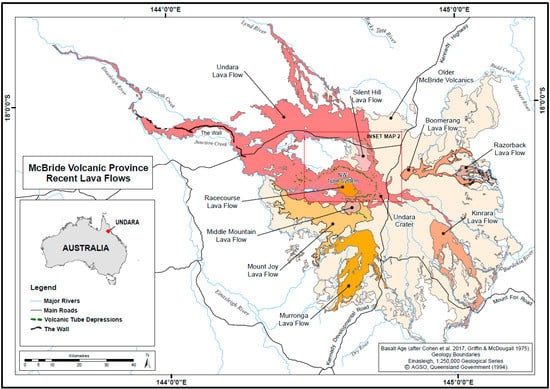
Figure 1.
McBride Volcanic Province showing recent (<1 my) lava flows. Map adapted from Pearson [35] with permission from Chillagoe Caving Club. Geology boundaries from Queensland Government 1:250,000 Geological Series.
The Undara Basalt is the third youngest flow (~190,000 years, ka) [39,40], covering 1374 km2. It is the most extensive of the recent (<1 million years, ma) basalt flows in the province. Lava flowed radially outwards from the Undara Volcano. The lava was distributed down slope by three major, and two minor, lava tube systems. The largest system is the north-western flow, which extends 176 km from the crater [35,41].
Sixty-seven arches and caves with a combined mapped length of 6.38 km are recorded, with the majority (70%) found in the north-western (NW) tube system, all of which occur within 32 km of the crater (Figure 2). The caves and arches are humanly accessible segments of the subsurface tube system, created where the roof of the tube collapsed to form an entrance. Additional caves certainly exist but are difficult to locate over the rough untracked terrain. Most of the known caves are relatively short segments of breached lava tube less than 100 m long, and the longest segment is Bayliss Cave at 1300 m. The original lava floor is rarely visible, only occurring as small patches in a few caves.

Figure 2.
Lava tube systems and caves in the Undara Basalt flow. Bayliss Cave and other major cave segments are labelled. Map adapted from Pearson [35] with permission from Chillagoe Caving Club. Geology boundaries from Queensland Government 1:250,000 Geological Series.
Other lava tube caves are developed in adjacent Murronga, Kinrara and Silent Hill Basalts (Figure 1), however only cave segments in Murronga and Silent Hill lava flows have been sampled for cave fauna (Table 1). These neighbouring lava flows and lava tube cave systems are important for understanding relationships between taxa and tube systems. Sampling in Murronga and Silent Hill systems has identified locally endemic troglobiont species, as well as troglobionts whose distribution range encompasses caves in the Undara Basalt tube system. As envisioned for Hawaiian cave animals [25], the cave-adapted fauna in Undara caves, may have colonised mesovoids and caves within the Undara flow from neighbouring older flows. Thus, the age of the Undara lava tube system may not indicate a maximum age of cave adaptation [42].

Table 1.
McBride Province basalt flows (youngest to oldest) and a synopsis of their recorded lava tube systems and caves (where known) including caves which have been sampled for fauna. Geological information from [35,38,40] and 40Ar/39Ar ages (ka/ma) from [39]; biological information compiled from [1,29,30,32,33,34].
Lava tubes at Undara and other McBride flows formed in pāhoehoe basalt [43]. Pāhoehoe lava has a relatively low viscosity and flows like a river. These lava rivers crust over insulating the interior and transporting the fluid lava many kilometres downslope. These flows respond to changes in the eruption rate, composition of lava, slope, and condition of the land surface; therefore, cave formation is dynamic [44]. Overflows, from breaks in the forming roof, release lava over the cooling lava; thus, strengthening the roof downslope. These overflows build flow on flow, layer on layer [45]. These layers poorly fuse to the cooled, older surfaces so that there are numerous gaps preserved between each flow unit. Escaping gas can inflate voids within the flow and additional voids and cave-like spaces within the lava can be created by tree moulds, earthquakes, and cooling cracks. Furthermore, sections of older lava tubes are buried as the eruption continues. In this way, basaltic lava flows can cover large areas and create extensive and abundant underground habitats throughout young pāhoehoe flows [6].
Subterranean habitats in basalt are potentially as deep as the thickness of the basalt flow. Tube-fed lava flows are thicker near the vent and thin as they flow downslope. For the Undara lava flow, the minimum thickness of the habitat is indicated by the exposed lava at the base of Undara Crater, which is 50 to 60 m deep [46]. However, the actual depth of the basalt at the crater rim is likely to be much deeper. The rim of Undara Crater is 1020 m above sea level, and, at a 40-km long lava ridge (‘The Wall’) beginning 60 km downslope from the vent, the elevation is approximately 600 m suggesting that the basalt thickness near the rim might be 400 m. Geological drilling at “The Wall” found the thickness of basalt to be about 40 m [47]. However, the thickness at the crater may be less than 400 m since uplift of the underlying granitic inlier could affect the height of the basalt dome [46,48].
4. Environment
4.1. Surface
The climate is monsoonal, characterised by hot humid summers and warm dry winters. Mean annual maximum temperature (at Mount Surprise) is 31.1 °C and mean annual minimum 16.1 °C. Mean annual rainfall is 793 mm, which falls mostly during the hotter months from November to March [49]. Rainfall sinks rapidly underground through cracks in the porous basalt. The soil layer is thin since developing soil is washed or subsides underground with rain and gravity, where it forms thick deposits on the floor of the caves. Leaflitter is rare in most Undara lava tubes, except in a few twilight zones of caves with large, exposed entrances such as Road Cave and Barkers Cave.
The surface vegetation is a dry savannah woodland, with widely spaced Eucalytpus trees and grassland understory (Figure 3). In stark contrast to the savannah are conspicuous patches of dark-green vine-thicket growing in larger depressions and collapsed caves (Figure 3). The vine-thicket is thought to be a remnant of a once more widespread vegetation type with strong Gondwana affinities. These botanical ‘islands’ generally mark the course of the major lava tubes that fed the advancing lava; some are drained lava lakes.

Figure 3.
Oblique view looking East showing Undara Crater in the upper right corner and the entrance to Taylor Cave about one kilometre downslope (arrowed). Contrasting with the surrounding dry savanna woodland, the conspicuous ribbon of dark-green ‘islands’ are remnant vine-thicket vegetation growing in depressions and collapsed caves and revealing the course of the lava tube system. Picture from Google Earth. Scale not shown in this oblique view since apparent distances will vary depending on position.
4.2. Underground
Globally, terrestrial cave habitats are zonal with three main zones defined by the amount of light: entrance zone, twilight zone, and dark zone. The dark zone can be further divided into two or three subzones: transition zone where diurnal and weather events on the surface affect the moisture, temperature, and airflow and deep zone where the environment is buffered from events on the surface and characterized by calm and water-saturated atmosphere. A few researchers recognize a stagnant air zone in which the environment is even more stable than in the deep zone [6,26]. The extent of each zone depends on the length and shape of the entrance(s) and passage configurations.
At Undara, CO2 accumulates in deeper portions of most sampled caves, usually at low (<2%) but detectable levels. However, in both Bayliss Cave and Nasty Cave, CO2 concentration approaches 6% by volume. The troglobiont species are characteristically found only in the deep and stagnant air zones. The Undara caves vary in size and shape as well as in the amount of moisture, carbon dioxide, and type and quality of nutrients present. Thus, each cave comprises a different mix of environmental variables which in turn favour differing communities of cavernicoles.
5. Results
5.1. Overview of Invertebrate Sampling in the Undara Basalt Lava Tube Systems
- Records were found for biological inventories in 17 cave segments: 14 in the northwest tube system and three in the northern and north-northeast tube systems (Table 2). Biological surveys have been conducted in a few additional caves [29,30], but the results are unpublished.
 Table 2. Named lava tube segments within the Undara Lava Flow from which cavernicoles have been reported, arranged by tube system, from upslope to downslope. * Indicates cave identification number, Australian Speleological Federation. See also Figure 2.
Table 2. Named lava tube segments within the Undara Lava Flow from which cavernicoles have been reported, arranged by tube system, from upslope to downslope. * Indicates cave identification number, Australian Speleological Federation. See also Figure 2. - Minimally 78 species of arthropods have been recorded from Undara Basalt lava tubes, along with a cavernicolous fern (Psilotum sp.) and four bat genera (Hipposideros, Miniopterus, Rhinolophus, Vespadelus).
- Of the arthropods: 16 (21%) have been taxonomically described; 30 (39%) identified to genus and/or morpho-species; and 32 (40%) remain unidentified to species or genus level.
- Seven caves harboured obligate subterranean species; all these caves contained deep zone environments with high humidity, of which three also contained bad air (CO2).
- Overall, 30 troglobionts and one stygobiont species were recorded in the Undara Basalt Flow (Table 3). Bayliss Cave harboured the most obligate subterranean species: 23 troglobionts and one stygobiont.
 Table 3. List of obligate cave species recorded from the Undara lava tube system. Troglobionts (TB), stygobionts (SB). * Indicates cave identification number, Australian Speleological Federation (ASF). Refer to Supplementary Materials for a list of troglophiles (Table S1) and trogloxenes, accidentals (Table S2).
Table 3. List of obligate cave species recorded from the Undara lava tube system. Troglobionts (TB), stygobionts (SB). * Indicates cave identification number, Australian Speleological Federation (ASF). Refer to Supplementary Materials for a list of troglophiles (Table S1) and trogloxenes, accidentals (Table S2). - Twenty-two species of arthropods and one plant are classified as troglophiles, being native cavernicoles capable of living their entire life cycle underground but populations of the same species may also be found in surface habitats (Table S1) [2,21,30,32,50,51,52,53,54]. Some of these may prove to be troglobionts once more is known of their biology.
- Twenty-five species of arthropods are classified as ‘visitors’ (Table S2) [2,30,35,55,56]. Some habitually use caves for shelter or to find food (=trogloxenes). Others occasionally enter caves for shelter, and some wander or fall into caves accidentally (=‘incidental’ or ‘accidental’ cavernicoles). In addition, unidentified mites (Arachnida: Acari) have been reported from most surveyed caves. Many are associated with guano, and a few are parasites of other cavernicoles including bats; however, because both the identity and ecological status of the mites recorded from Undara caves are unknown, they are not enumerated further in Table S2.
- Besides Bayliss, the other caves with a high diversity of troglobionts are Nasty Cave (eight species) and Barkers Cave (seven species), which are located, respectively, 3 km and 3.7 km downflow of Bayliss (Figure 4).
 Figure 4. Aerial photograph showing cave entrances and major cave outlines in the north-western tube system at 23.5 to 31.5 km from Undara Crater. This 8 km section contains the two longest mapped tube segments in the Undara Flow, Bayliss Cave and Barkers Cave, and other biologically important segments including Nasty Cave and Road Cave (refer Table 2).
Figure 4. Aerial photograph showing cave entrances and major cave outlines in the north-western tube system at 23.5 to 31.5 km from Undara Crater. This 8 km section contains the two longest mapped tube segments in the Undara Flow, Bayliss Cave and Barkers Cave, and other biologically important segments including Nasty Cave and Road Cave (refer Table 2).
5.2. Bayliss Cave Environment
Bayliss Cave is by far the most biodiverse lava tube segment in the Undara lava flow. Therefore, we describe the cave and its environment to help understand the factors contributing to its richness. With a length of 1300 m Bayliss Cave is the longest mapped lava tube in Australia. It is entered through a narrow entrance crawlway about eight metres long and less than one metre in diameter, which opens into the main lava tube at the top of a 10 m high talus slope. From the base of the talus slope the tube descends gently downslope for 950 m to a low crawlway, only accessible when the CO2 concentration is low, that extends the cave 350 m to a mud blockage. The average tube width and height is 8 × 5 m (maximum 25 × 11.5 m) [2,35]. The single small entrance limits air circulation and helps to maintain the high internal humidity which is sustained by condensation, infiltrating drip water, and small vadose seepages. Cave environment zones are well distinguished. The entrance zone is reduced owing to the small dimensions of the opening, and the twilight zone is limited to the eight-metre crawlway and top of the talus slope. The transition zone extends from the talus slope to the “Duck-Under”, a low passage one to two metres high about 350 m into the cave; the deep and stagnant air zones are beyond the Duck-Under. The transition zone experiences the tropical winter effect, which is caused by cool, dry air entering the cave at night [69], and except for scattered ceiling drips, the floor in the transition zone is usually dry clay with a thin caliche crust. Beyond the Duck-Under, the floor is covered with moist to wet clayey soil and thin accumulations of bat guano. Thick ‘curtains’ of tree roots penetrate the ceiling to the floor especially beyond the Duck-Under (Figure 5).

Figure 5.
Root ‘curtains’ of Eucalyptus penetrate from ceiling to floor in the deep zone about 100 m beyond the Duck-Under. Photograph by F.G. Howarth.
Savannah woodland overlies almost all the mapped cave passage, except the entrance depression and a 200 m section near the end, where the tube skirts the margin of a surface depression with vine thicket (Figure 4). The seasonally xeric environment and thin, poor soils force Eucalyptus woodland trees to send massive roots deep underground to obtain sufficient moisture and nutrients. The wide spacing (~9 m apart) of trees in the savannah suggests that all the root masses visible in Figure 5 connect to only one or perhaps up to three trees. Elsewhere in Australia, Eucalyptus spp. are renowned for their deeply penetrating root systems which may penetrate up to 70 m below the surface to tap groundwater in caves [70,71].
Between 14–15 June 1985, environmental parameters (temperature, relative humidity, carbon dioxide, oxygen), were measured at five stations located progressively deeper inside Bayliss Cave (Figure 6a,b). The atmosphere beyond the Duck-Under was foggy with water vapor condensing on surfaces; RH ranged from 98% to >100% (Figure 6a). The downward sloping tunnel accumulates carbon dioxide, which is denser than air. The concentration of CO2 increased from about 1% at the Duck-Under to 6% by volume below The Wall > 650 m from the entrance (Figure 6b). Concomitantly oxygen concentrations decreased, indicating a biogenic origin of CO2. Potential sources of CO2 include respiration of tree roots, bats, invertebrates, and microorganisms. Oxygen concentration increased unexpectedly at 450 m from the entrance before decreasing again at 650 m, in parallel with increasing CO2. The cause of the anomalous oxygen level is unknown, but this area had the highest concentration of root curtains.

Figure 6.
Environment and arthropod distribution in Bayliss Cave plots measured 14–15 June 1985 at progressively greater distances from the entrance: (a) temperature and relative humidity; (b) oxygen and carbon dioxide; (c) abundance of trogloxenes, troglophiles, partially troglomorphic and highly troglomorphic at the numbered positions in (d) map profile view with sampling stations numbered: 1—near entrance, 2—before the Duck-Under, 3—beyond the Duck-Under, 4—The Wall, 5—beyond The Wall. After [2], reproduced with author permission.
The amount of moisture and CO2 in Bayliss Cave and other Undara caves varies with the seasons and major climatic events as evidenced by the data in Table S3. Although the environmental zones in Bayliss Cave are well constrained by passage shape and length, severe floods and prolonged droughts occasionally destabilize the zones in the cave. For example, on 31 May 1986, high water marks were noted at 2 and 2.4 m above the floor in the transition zone, which indicated that at least part of the cave occasionally floods. Additionally, the caliche surface crusts characteristically form where the soil is alternately wetted then dried such as in desert regions.
In other less extensive caves with more open entrances, e.g., Barkers and Pinwill, the zones are more seasonally dynamic, with the boundary of the deep zone moving closer to the entrance during the warmer wet periods and retreating during cold spells. The dynamic nature of the cave environment was observed in Nasty Cave between 29 and 30 May 1986. The entrance to Nasty is a narrow vertical crevice about 0.75 m wide, 3 m long and 3–4 m deep. A horizontal crawlway less than one metre high at the bottom of the crevice leads into the cave. A large boulder lies across the top of the crevice and blocks access. On 29 May, one edge of the rock was raised and tied in place to gain access and conduct an environmental survey. On 29 May, the cave was mostly in the deep-stagnant air zone, and the arthropod fauna was relatively diverse and abundant. During the repeat survey conducted one day later, far fewer animals were observed, and the troglobiontic Nocticola and polyxenid millipedes had disappeared. The cave air had become fresher from a change in weather and increased ventilation after enlarging of the entrance. The night of 28–29 May was cloudy and relatively warm; whereas the next was clear and cold, which resulted in a significant ‘winter’ effect; CO2 levels near the inner end of the cave dropped from 5.1% to 3.4% (Table S3). The boulder was replaced across the entrance at the end of the survey.
5.3. Bayliss Cave Invertebrate Fauna Distribution
A survey of arthropod diversity was undertaken at the same time and stations as the environmental parameters in June 1985. The survey documented 46 species of arthropods and inferred their ecological status [1,2] (Figure 6c above). Combined with subsequent sampling, 50 arthropod species and one plant are currently recorded from Bayliss Cave, including 24 troglobionts and one stygobiont (Table 3); 16 inferred troglophiles (Table S1), some of which may be troglobionts when more is known of their biology; and ten cave visitors including trogloxenes, accidentals (Table S2).
In the 1985 survey, all species of terrestrial troglobionts were found beyond the Duck-Under in conditions of >98% RH and elevated CO2, and only a few individuals of eight of these species were also found in moist areas and drip holes in the transition zone (Figure 6c) [2]. The stygobiontic amphipod was found among tree root mats growing in the intermittent stream near the bottom of the entrance slope. A total of 24 arthropod species were recorded from the talus slope to the Duck-Under, of which about 33% were troglobionts; 50% were troglophiles; and 17% were trogloxenes. Thirty species were found from the Duck-Under to The Wall, of which about 67% were troglobionts; 23% were troglophiles; and 10% were trogloxenes. From The Wall to the crawlway, there were 28 species, of which about 68% were troglobionts; 18% were troglophiles; and 14% were trogloxenes (Figure 6c). Since some species were found in more than one station, the number of observations exceeded the number of species (i.e., 82 observations of 46 species).
In addition to the animals (Figure 7), a primitive plant grows in the high CO2 portion of Bayliss Cave (Figure 7). The plant is a whisk fern (Psilotales: Psilotum species) [72]. It is a saprophyte obtaining nutrients from the substrate. Whisk ferns are widespread in the tropics but rarely reported from caves. A different species of Psilotum also occurs in lava tubes on Maui and Hawaii islands.


Figure 7.
Bayliss Cave troglobionts and subterranean plant: (a) scutigeromorph centipede, photo F.G. Howarth; (b) Millipedes on bait, photo F.G. Howarth; (c) ectobiid cockroach Neotemnopteryx baylissensis Slaney, 2000, photo G. Thompson, Queensland Museum; (d) nocticolid cockroach Nocticola sp. 1, photo G. Smith; (e) cixiid planthopper Solonaima baylissa Hoch and Howarth, 1989, photo H. Reimer; (f) whisk fern, Psilotum sp. photo F.G. Howarth. (g) reduviid Peirates sp. preying on cockroach, N. baylissensis, photo F.G. Howarth; (h) atelurine silverfish, Pseudogastrotheus undarae Smith, 2016, photo G. Smith; (i) Bayliss Cave isopod, cf. Porcellionidae, photo J. Sydney; (j) Pinwill Cave isopod, photo J. Sydney; (k) pseudoscorpion, Protochelifer sp. nr. cavernarum Beier, 1967, photo A. Clarke; (l) Erebid moth, cf. Schrankia sp. adult with cocoon on tree root, photo G. Smith.
5.4. Notable Cave Species
5.4.1. Aquatic Fauna
Only one species of stygobiont is known from Undara: a blind amphipod (cf. Chillagoe sp.) from Road and Bayliss Caves. The nominate species, Chillagoe thea Barnard and Williams, 1995 was described from Chillagoe caves located 110 km north from Undara. An additional undescribed amphipod in the same genus is recorded from Camooweal caves, 600 km to the west [70].
5.4.2. Terrestrial Fauna
Spiders are well represented with at least six troglobiontic species known and several others that remain unidentified. A large eyeless ctenid, Amauropelma undara Raven and Gray 2001 and a tiny eyeless unidentified oonopid do not build webs but are a ‘sit and wait’ ambush predators. The blind zodariid spider, Nosterella cavicola, is also an ambush predator but females hunt from holes with raised turrets constructed in mud. An unidentified linyphiid builds intricate horizontal sheet webs across cracks and drip holes and hangs under the sheet and safely captures prey falling or landing on the sheet. A scaffold web spider, Nesticella species, builds tangled webs over drip holes, and prey become tangled in the loose strands of silk. Two species of long-legged spiders, Spermophora species 1 and 2, build loose webs on walls and in drip holes. Their webs act as tripwires and when alerted, the spider throws additional silk to envelope prey. Pisaurids are hunting spiders that prey on aquatic animals. Little has been reported on the status of the Dolomedes species 1 found near the lake in Barkers Cave. One potential troglobiont arachnid in Barkers Cave, a schizomid, was not detected in Bayliss.
One of the most remarkable troglobionts is the large scutigerid centipede. Troglophilic and epigean species of these long-legged centipedes escape threats and overtake prey by running extremely fast; whereas the Undara cave species (Figure 7) walks slowly (several seconds to move one body length) even when disturbed. With a body length of around seven centimetres, it is one of the largest terrestrial troglobionts known.
Two blind cockroaches live within Undara lava: the ectobiid, Neotemnopteryx baylissensis Slaney, 2000 and the nocticolid, Nocticola sp. 1 (Figure 7). Other species of Neotemnopteryx and Nocticola are recorded from other caves and tube systems in the McBride Volcanics, namely Neotemnopteryx undarensis Slaney, 2000 from the Hot Hole and Wishing Well in the northern tube system, and Nocticola sp. 2 from the Murronga flow. Two other ectobiid cockroaches are classified as troglophiles, Paratemnopteryx stonei Roth, 1990 in Bayliss and Barkers Caves, and Paratemnopteryx howarthi Roth, 1990 in Nasty Cave (Table S1).
At least four species of cave-adapted beetles are known. At time of writing the staphylinid and pselaphine have not been described; and descriptions of the two weevils, Curculionidae, are in preparation [67]. These unusual, long-legged and eyeless weevils are the first cave-adapted weevils to be described in Australia, while other undescribed subterranean Curculionidae and Pselaphinae are known from iron-ore terrains in Western Australia.
The most studied cave invertebrates at Undara (and Chillagoe) are multiple species of planthopper bugs in the family Cixiidae. Six species in the genus Solonaima exhibit varying degrees of cave adaptation, from epigean to troglophilic and fully troglobiontic species. At Chillagoe, two troglophilic species were generally found in the most open caves, the two moderately troglomorphic species were found in deeper caves, and the most highly modified—completely eyeless, colourless and nearly completely wingless—species, Solonaima baylissa Hoch and Howarth, 1989 (Figure 7) is restricted to humid cave passages with high CO2 levels in Bayliss and Nasty caves. A second cixiid species, Undarana rosella Hoch and Howarth, 1989, is found closer towards the entrance in Bayliss Cave and is considered troglophilic. Nymphs of cixiids drill into tree roots and suck xylem sap, which is a dilute, nutrient-poor food, unlike phloem sap. Two other hemipteran bugs are predatory assassin bugs (Reduviidae); the smaller species is Micropolytoxus cavicolus Malipatil and Howarth, 1990, and the larger one is an undescribed species of Peirates (Figure 7). Both reduviid species have reduced eyes and do not respond to light; they also have reduced hemelytra, lack hind wings, and like other troglobionts, they move slowly.
Moths are often missed in biological surveys of caves, partly because it is difficult to collect specimens suitable for identification and because cave biologists often assume that lepidopterans use caves only for temporary refuge. However, at least two species of moths have troglomorphic populations that are restricted to caves: a tineid in the Philippines and an erebid, Schrankia, in Hawaii [73], while the tineid Monopis is a common guanophile in many Australian caves. Erebid larvae, pupae and adults found living on tree roots in Undara caves were tentatively identified as a species of Schrankia (Figure 7). We encourage biologists to include moths and similarly overlooked ‘orphan’ taxa in surveys. Additional cavernicolous moths are predicted to occur in tropical caves.
In the Murronga Basalt flow, situated about 15 km south of Undara crater, Long Shot Cave is the most diverse with at least four, possibly six, troglobionts. It is worth noting that the Murronga caves harbour troglobionts and undescribed potential troglobionts that are: (1) the same morphological species is found in both Murronga and Undara caves, namely Solonaima baylissa; (2) distinct congeneric species are found in the Murronga and Undara flows, namely the cockroach, Nocticola species; and (3) belong to taxa not detected in Undara caves, namely phalangid harvestman, Zalmoxis lavacavernae Hunt, 1993; the emesine bugs, Ploiaria spp. The range of the troglobiontic cockroach Neotemnopteryx baylissensis spans the Undara and Silent Hill Basalt flows.
6. Discussion
6.1. The Challenge of Classifying Cavernicoles
As Pipan et al. [28] summarised, considerable confusion exists in the literature about the terms troglobiont and stygobiont—which should be used only for species unable to survive in surface habitats, irrespective of their morphology—and troglomorph, species with reduced eyes and pigment and elongated appendages. The latter are not necessarily restricted to caves, while some species without conspicuous troglomorphic features are only found in caves and other subterranean habitats [27].
In many cases, the assignment of ecological category, especially between troglobiont versus non-troglobiont, is fraught with uncertainty because the ecology of many species is so poorly known. The dilemma is especially acute among groups displaying slight troglomorphy or belonging to groups that are primitively troglomorphic, such as silverfish. Two species of nicoletiid silverfish that are recorded from Undara caves illustrate this difficulty. Metrinura subtropica Smith, 2006 was initially classified as a troglobiont by Howarth and Stone [2] based on its apparent troglomorphy, behavior, and its collection deep inside Bayliss Cave. When subsequently describing this species, and another congeneric species collected on the surface at Undara, Smith [52] noted that appendage lengths and sensory appendages of M. subtropica were within the normal range for the genus, and therefore, he considered that this species may be a troglophile. In contrast, members of the subfamily Atelurinae are generally found living as specialised inquilines of ants or termites, and Howarth and Stone [2] considered Bayliss specimens as a trogloxene since individuals appeared to be associated with the common ant Paratrechina sp. However, Smith [53] later found dense populations (about 30 individuals/m2) of Pseudogastrotheus undarae (Figure 7) deep inside Barkers Cave, in a zone with elevated carbon dioxide and without any obvious ant or other host present. Other species of Atelurinae have been collected without any obvious host, in soil and deep drill holes in iron-ore terrains in the Pilbara and Kimberley [74,75]. The presence of P. undarae deep inside the Undara caves and without hosts raises the question: Is the species troglobiontic?
While the Schiner–Racovitza system and its various derivative classification schemes are both useful, and sometimes confusing, the traditional focus of many biospeleologists on troglobionts and stygobionts, means that much cave biodiversity, biomass, and ecological function, is at risk of being under-recognised, and under-protected. In many cave ecosystems, most of the biomass and significant species richness, consists of trogloxenes, troglophiles and guanophiles. For these reasons we have chosen to list all species recorded from Undara lava caves and infer their ecological status based on expression of troglomorphy and behavior, as well as on their distribution in cave and surface collection records. However, taxonomic knowledge and field survey data in many cases is limited. With this proviso we caution that future field and taxonomic studies may determine that some taxa inferred to be troglobionts are in fact not, and vice versa.
Another dilemma facing biologists studying biodiversity is the question: What is a species? Several examples of the problem occur in this compilation of the Undara cave fauna. Isolated populations of morphologically similar individuals are often considered a single widespread species. However, more detailed study including behavior and molecular data can alter that view. For example, the pseudoscorpion previously known as Protochelifer cavernarum Beier, 1967 occurs in caves from southwest Australia to north Queensland, often associated with bat guano. It is recorded from the Undara and Murronga Basalts, as well as the Chillagoe karst. Subsequently, Moulds et al. [59] studied variation in DNA sequences of geographically dispersed Australian populations of P. cavernarum and found significant differences among the populations studied. They concluded that P. cavernarum was a complex of related locally endemic cave species.
Similarly, the cockroach, Paratemnopteryx stonei, is reported from Chillagoe and Undara. In describing the species, Roth [51] noted geographic variation calling the differences geographical races. Subsequently additional populations were collected from Broken River and Fanning River Caves. Slaney and Blair [21] analysed morphology and DNA, which confirmed the geographical differences. Later, Slaney [76] described the Fanning River population as a distinct species but left the other populations as races pending more detailed study. Other cavernicoles with geographically isolated populations may also prove to be complexes of locally endemic species.
6.2. Why Is Bayliss Cave So Rich in Troglobionts?
In terms of its species richness, Bayliss Cave stands out from all other caves in the Undara Basalt flow, and other flows in the McBride Province. There are two main reasons for the exceptional local richness in Bayliss Cave. First is size of the deep zone habitat. Its >550 m of highly suitable habitat is more than three times the size of the deep zone in the other known caves within the system. Second is the relative abundance and diversity of energy sources in the deep zone. The principal nutrients are numerous large tree root curtains, bat guano, and organic material. The latter filters in along roots, through cracks, or wanders into the cave. There is no leaflitter in Bayliss Cave as the constricted horizontal entrance limits inputs by gravity or air currents. These two factors, size and energy, allow colonization by greater numbers and diversity of cave-adapted animals from the pool of animals living in voids within the lava. The correlation between diversity and available energy agrees with the conclusions made by Brad et al. [77] in Movile Cave.
Two species of bats, Rhinolophus megaphyllus Gray, 1834 and Miniopterus species, also roost in the cave. Bats are also commonly found in most other McBride Province caves, including for example Barkers Cave, which contains many thousands of bats and a permanent lake to supply humidity. The bats and decomposing bat guano generate CO2. However, Barkers Cave does not contain the same diversity as Bayliss Cave. This may be because of its large open entrance and shorter length (560+ m) so that most of the cave is in the transition zone, and experiences greater fluctuations in temperature and humidity that are less optimum for troglobionts. Additionally, Barkers Cave does not have such extensive tree root curtains, the food source for troglobiontic planthoppers, weevils and possibly other invertebrates.
Based on the discoveries at Undara, Howarth and Stone [2] proposed that passages with elevated levels of carbon dioxide and high humidity would be found, generally, to harbour unique communities of obligate cave species, and that these bad air zones may be the typical habitat present in mesocavernous cracks and voids. Two other lava tubes in the McBride Province corroborate this thesis. In both Nasty Cave in Undara flows and the Two Ten/Long Shot cave system in the Murronga Lava Flow, the distribution of troglobionts is correlated with CO2 concentration [1].
The high-stress environment thesis has been sustained in the literature [19,78,79] and challenged by Humphreys [80] but never formally tested or refuted. In the years since the first observations that sparked this thesis were made in Bayliss Cave, several other karst areas in Australia with high carbon dioxide caves have been searched for troglobiont communities specifically associated with bad air zones, including: Camooweal (Queensland), Wellington and Bungonia (New South Wales), Cape Range, Roe Plains, Nullarbor Plain and Margaret River (Western Australia). Generally, bad air zones were observed to harbour troglobiont communities that were not noticeably richer or different to other deep zone habitats without bad air (S. Eberhard unpublished observations). On the Roe Plains and Nullarbor Plain, the bad air zones coincided exactly with deep zone humidity thus precluding disentanglement of these two variables by field observation. Additional research is required to resolve these conflicting observations on the role of carbon dioxide and other stressors on the biodiversity of caves.
6.3. Comparison with Other Subterranean Hotspots in Australia
Since the initial exciting discoveries made in Bayliss Cave more than 35 years ago, a great deal of research has been undertaken in other parts of Australia, especially in Western Australia where remarkably diverse stygobiont and troglobiont faunas have been revealed in arid and semi-arid zone limestone caves [81,82,83], calcrete and alluvial aquifers [84,85,86], and iron ore terrains [87,88]. Western Australia is now recognised as a globally significant hotspot for subterranean biodiversity [9]. It is therefore timely and appropriate to place the Undara cave system in context with other subterranean hotspots in Australia (Table 4).
In Australia, the terms ‘troglofauna’ and ‘stygofauna’ are commonly used, particularly in environmental impact assessments (EIA), to refer to all species collected from terrestrial and aquatic subterranean habitats, respectively. These terms do not distinguish ecological categories although they are sometimes misunderstood by non-specialists as equivalent in meaning to ‘troglobiont’ and ‘stygobiont’. Irrespective of terminological ambiguity, accurate determination of a species ecological status is not crucial in most Australian EIA contexts, where the focus is on species conservation and extinction risk. The latter is usually assessed based on the sampled and/or interpreted distribution range of species in relation to the proposed mining footprint or other potential impact area.
Many species of troglofauna collected from calcrete and iron-ore terrains in Western Australia have typically small ranges [87]. A high proportion of these are likely to be true troglobionts, based on their troglomorphy, and/or short-range distribution and apparent absence from surface collections; a few may be soil fauna [88] and a few may be typical surface species lacking troglomorphies but occupying subterranean environments as refugia from arid surface conditions, without being present in the surrounding surface environment [8,88]. Nonetheless many of these species remain undescribed and their ecology poorly known, thus distinguishing between edaphophiles, troglophiles and troglobionts in weakly troglomorphic, or non-troglomorphic, taxa is difficult.
In compiling a preliminary list of Australia’s subterranean hotspots (Table 4), we have therefore counted all recorded species, and specified the overall number of troglo/stygobionts if known. In selecting aquifer and cave/mesocavern systems to include, the geological continuity, hydrological connectivity, and overall areal extent have been taken into consideration. Some systems such as calcrete aquifers are compact and hydrogeologically well defined, whereas others such as Pilbara iron-ore formations and the Nullarbor karst (200,000 km2) are geologically continuous over many thousands of square kilometres, yet with few if any identifiable barriers to subterranean dispersal. These large regional-scale subterranean systems are excluded as beta-diversity will be dominating alpha diversity and obscuring localised hotspots. Table 4 is not intended to be comprehensive or definitive, rather its purpose is to establish a context and framework for recognising and comparing significant hotspots and regionally significant warm spots. Patterns and highlights emerging from Table 4 include:
- Undara remains the richest subterranean hotspot in humid tropical Australia, which far exceeds the richness of troglobionts recorded in other humid tropical Australian karsts such as Judburra-Gregory [89].
- In the arid Yilgarn and semi-arid Pilbara regions of Western Australia significantly richer subterranean assemblages have been documented in recent decades.
- The richest Western Australian hotspots are karstified calcrete aquifers [90,91,92,93,94,95], although mineralized iron-ore terrains [88,96,97] and ’hard-rock’ limestone karsts [81,82,83,98,99] also harbour very-rich assemblages.
- Most Western Australian hotspots in calcretes and iron-ore terrains do not contain enterable caves, and fauna can only be collected by sampling mesocaverns and microcaverns via constructed wells and drill holes.
- The Yeelirrie calcrete aquifer stands out as exceptionally rich with 70 stygofauna and 45 troglofauna species, the majority being short range endemics and almost certainly obligate subterranean species based on current knowledge of the groups represented [90,91,92].
- In Eastern Australia, the Jenolan karst in New South Wales recorded the highest species richness (136 taxa) however the majority of these are accidentals and troglophiles, and only 8 taxa are obligate cavernicoles [100].
- Overall species richness in warm temperate (New South Wales) [101,102,103] and cool temperate (Tasmania) [104] karst areas is comparable (median 54 taxa), and these karsts harbour a much lower proportion of obligate subterranean species compared with arid and semi-arid regions, although Tasmania stands out in terms of troglobiont richness in temperate latitudes (maximum 25 species at Precipitous Bluff, Tasmania) [104,105,106,107,108].
- The Peel Valley alluvial aquifer in New South Wales harbours the richest known stygofauna assemblage in eastern Australia; 54 species including 33 stygobionts [109].
- In terms of obligate species, the richest Australian localities are in arid and semi-arid climate regions, where most of the troglobionts and stygobionts are relictual with no close surface relatives. Molecular phylogenetic studies have shown that Quaternary aridification is the likely driving mechanism for troglo/stygogenesis in these regions [110].

Table 4.
Preliminary list of Australian hotspots (>30 obligate spp.) and regionally significant warm spots, grouped by geographic/climate region. Each locality is considered to represent a single subterranean ecosystem, characterised by geological and hydrological connectivity (excluding large-scale subterranean systems such as Nullarbor karst and Pilbara iron-ore ranges). Total species richness is the number of stygofauna and troglofauna (ecological categories combined) and the number in brackets (n) is the number of stygobionts (Sb) and troglobionts (Tb) where known. * For most localities in Western Australia (WA) the ecological status of many species remains unspecified however the majority are short-range endemics and almost certainly obligate subterranean species based on the groups represented.
Table 4.
Preliminary list of Australian hotspots (>30 obligate spp.) and regionally significant warm spots, grouped by geographic/climate region. Each locality is considered to represent a single subterranean ecosystem, characterised by geological and hydrological connectivity (excluding large-scale subterranean systems such as Nullarbor karst and Pilbara iron-ore ranges). Total species richness is the number of stygofauna and troglofauna (ecological categories combined) and the number in brackets (n) is the number of stygobionts (Sb) and troglobionts (Tb) where known. * For most localities in Western Australia (WA) the ecological status of many species remains unspecified however the majority are short-range endemics and almost certainly obligate subterranean species based on the groups represented.
| Geographic/Climate Region | Locality Name; Geology, Hydrology Type | Total spp. Richness (No. Sb/Tb) * | Stygofauna spp. (Sb) * | Troglofauna spp. (Tb) * | Sources |
|---|---|---|---|---|---|
| Queensland, humid tropical | Undara Basalt lava tubes | 77 (31) | 1 (1) | 76 (30) | [2], and text |
| Northern Territory, humid tropical | Judbarra-Gregory karst | 56 (7) | 3 (2) | 53 (5) | [89] |
| Yilgarn, WA, arid | Yeelirrie calcrete aquifer | 115 (*) | 70 (*) | 45 (*) | |
| Yilgarn, WA, arid | Uramurdah calcrete | 45 (*) | 36 (*) | 9 (*) | [90,91,92] |
| Yilgarn, WA, arid | Hinkler Well calcrete | 41 (*) | 32 (*) | 9 (*) | [95] |
| Yilgarn, WA, arid | Lake Violet calcrete | 39 (*) | 35 (*) | 4 (*) | [95] |
| Yilgarn, WA, arid | Barwidgee calcrete | 37 (*) | 28 (*) | 9 (*) | [95] |
| Pilbara, WA, arid/semi-arid | Ethel Gorge calcrete aquifer | 84 (45) | 84 (45) | 0 | [95] |
| Pilbara, WA, arid/semi-arid | Cape Range karst | 83 (*) | 42 (*) | 41 (*) | |
| Pilbara, WA, arid/semi-arid | Barrow Island karst | 74 (*) | 56 (*) | 18 (*) | [94] |
| Pilbara, WA, arid/semi-arid | Well PSS016, Robe River calcrete aquifer | 54 (*) | 54 (*) | 0 | [82,98] |
| Pilbara, WA, arid/semi-arid | Mesa A iron pisolite, Robe Valley | 24 (*) | 0 | 24 (*) | [83,99] |
| New South Wales, warm temperate | Jenolan karst | 136 (8) | 10 (2) | 126 (6) | [85,97] |
| New South Wales, warm temperate | Wombeyan karst | 55 (7) | 5 (2) | 50 (5) | [96] |
| New South Wales, warm temperate | Wee Jasper karst | 53 (7) | 5 (3) | 48 (4) | |
| New South Wales, warm temperate | Peel Valley alluvial aquifer | 54 (33) | 54 (33) | 0 | [100] |
| Tasmania, cool temperate | Ida Bay karst | 65 (18) | 17 (6) | 48 (12) | [101,102,103] |
| Tasmania, cool temperate | Junee Florentine karst | 60 (20) | 17 (8) | 43 (12) | [101,102,103] |
| Tasmania, cool temperate | Precipitous Bluff karst | 37 (25) | 11 (11) | 26 (14) | [109] |
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/d13070326/s1, Table S1: Troglophiles; Table S2: Trogloxenes, accidentals; Table S3: Environmental data.
Author Contributions
S.M.E. led the efforts to compile the data and completed the final formatting to comply with the journal style; S.M.E. and F.G.H. contributed equally to the conception, data analysis, and writing and editing of the drafts. All authors have read and agreed to the published version of the manuscript.
Funding
Funding for Field work in the 1980s for F.G.H. was provided by The Explorer’s Club, NY, USA, through sponsorship by Bro Nicholas Sullivan and by U.S. National Science Foundation Grant BSR-85-15183 to F.G.H.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We acknowledge and greatly appreciate the terrific help from staff at the Queensland Museum and the Australian Museum who tracked down specimen records and gave advice. At the Queensland Museum: Owen Seeman (Arachnida and Myriapoda), Marissa McNamara (Crustacea), Robert Raven (Arachnida), Susan Wright (Insecta). At the Australian Museum: Helen Smith (Arachnida, Insecta, Crustacea), Mandy Reid (Malacology). Peter Bannink (Chillagoe Caving Club) generously provided much information and material including his own unpublished specimen records, and drafted the excellent maps. For providing photographs and additional information we thank Arthur Clarke, Hannelore Hoch, Greg Middleton, Graeme Smith, Joe Sydney. We appreciate comments received from three anonymous reviewers, and the academic editors, which greatly improved the manuscript. This paper is dedicated to the memory of Fred Stone, a dear colleague, mentor, caver, and a kind and gentle man.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Howarth, F.G. Environmental Ecology of North Queensland Caves: Why are there so many troglobites in Australia? In Proceedings of the Tropicon 1988, 17th Biennial Australian Speleological Conference, Tinaroo, QLD, Australia, 27–31 December 1988; pp. 76–84. [Google Scholar]
- Howarth, F.G.; Stone, F.D. Elevated carbon dioxide levels in Bayliss Cave, Australia: Implications for the evolution of obligate cave species. Pac. Sci. 1990, 44, 207–218. [Google Scholar]
- Culver, D.C.; Sket, B. Hotspots of subterranean biodiversity in caves and wells. J. Cave Karst Stud. 2000, 62, 11–17. [Google Scholar]
- Hoch, H.; Howarth, F.G. The evolution of cave-adapted cixiid planthoppers in volcanic and limestone caves in north Queensland, Australia (Homoptera: Fulgoroidea). Mem. Biospeol. 1989, 16, 17–24. [Google Scholar]
- Hoch, H.; Howarth, F.G. Reductive evolutionary trends in two new cavernicolous species of a new Australian cixiid genus (Homoptera: Fulgroidea). Syst. Entomol. 1989, 14, 179–196. [Google Scholar] [CrossRef]
- Howarth, F.G. High-stress subterranean habitats and evolutionary change in cave-inhabiting arthropods. Am. Nat. 1993, 142, S65–S77. [Google Scholar] [CrossRef] [PubMed]
- Hamilton-Smith, E. The Arthropoda of Australian caves. J. Aust. Entomol. Soc. 1967, 6, 103–118. [Google Scholar] [CrossRef]
- Humphreys, W.F. The significance of the subterranean fauna in biogeographical reconstruction: Examples from Cape Range Peninsula, Western Australia. Rec. West. Aust. Mus. Suppl. 1993, 45, 165–192. [Google Scholar]
- Guzik, M.T.; Austin, A.D.; Cooper, S.; Harvey, M.; Humpherys, W.F.; Bradford, T.; Eberhard, S.; King, R.A.; Leys, R.; Muirhead, K.A.; et al. Is the Australian subterranean fauna uniquely diverse? Invertebr. Syst. 2010, 24, 407–418. [Google Scholar] [CrossRef]
- Vandel, A. Biospeleology—The Biology of Cavernicolous Animals; Elsevier: London, UK, 1965. [Google Scholar]
- Barr, T.C.J. Cave ecology and the evolution of troglobites. In Evolutionary Biology; Hecht, M.K., Steere, W.C., Eds.; Appleton-Century-Crofts: New York, NY, USA, 1968; Volume 2, pp. 35–102. [Google Scholar]
- Howarth, F.G. The tropical cave environment and the evolution of troglobites. In Proceedings of the 9th Congreso Internacional de Espeleologia, Barcelona, Spain, 9–15 August 1986; pp. 153–155. [Google Scholar]
- Leleup, N. Premier Partie. In Mission Zoologique Belge aux iles Galapagos et en Ecuador (N. et J. Leleup, 1964–1965) Resutats Scientifiques; Koninklijk Museum voor Midden-Africa—Musee Royal de l’Afrique Centrale: Tervueren, Belgium, 1968; pp. 9–34. [Google Scholar]
- Howarth, F.G. Cavernicoles in lava tubes on the island of Hawaii. Science 1972, 75, 325–326. [Google Scholar] [CrossRef] [PubMed]
- Elias-Gutierrez, M.; Martinez Jeronimo, F.; Ivanova, N.V.; Valdez-Moreno, M.; Hebert, P.D.N. DNA barcodes for Cladocera and Copepoda from Mexico and Guatemala highlights and new discoveries. Zootaxa 2008, 1839, 1–42. [Google Scholar] [CrossRef]
- Martínez, A.; González, B.C. Volcanic Anchialine Habitats of Lanzarote. In Cave Ecology; Moldovan, O.T., Kovác, L., Halse, S.A., Eds.; Ecological Studies, 235; Springer: Cham, Switzerland, 2018; pp. 195–228. [Google Scholar]
- Peck, S.B. The Invertebrate Fauna of Tropical American Caves, Part III: Jamaica, An Introduction. Int. J. Speleol. 1975, 7, 303–326. [Google Scholar] [CrossRef]
- Culver, D.C.; Deharveng, L.; Bedos, A.; Lewis, J.J.; Madden, M.; Reddell, J.R.; Sket, B.; Trontelj, P.; White, D. The mid-latitude biodiversity ridge in terrestrial cave fauna. Ecography 2006, 29, 120–128. [Google Scholar] [CrossRef]
- Deharveng, L.; Bedos, A. The cave fauna of southeast Asia. Origin, evolution, and ecology. In Subterranean Ecosystems; Wilken, H., Culver, D.C., Humphreys, W.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; pp. 603–632. [Google Scholar]
- Soulier-Perkins, A. Phylogenetic evidence for multiple invasions and speciation in caves: The Australian planthopper genus Solonaima (Hemiptera: Fulgoromorpha: Cixiidae). Syst. Entomol. 2005, 30, 281–288. [Google Scholar] [CrossRef]
- Slaney, D.P.; Blair, D. Molecules and Morphology are Concordant in Discriminating among Populations of Cave Cockroaches in the Genus Paratemnopteryx Saussure (Blattodea: Blattellidae). Ann. Entomol. Soc. Am. 2000, 93, 398–404. [Google Scholar] [CrossRef]
- Medeiros, M.J.; Davis, D.; Howarth, F.G.; Gillespie, R. Evolution of cave living in Hawaiian Schrankia (Lepidoptera: Noctuidae) with description of a remarkable new cave species. Zool. J. Linn. Soc. 2009, 156, 114–139. [Google Scholar] [CrossRef]
- Howarth, F.G.; Hoch, H.; Wessel, A. Adaptive shifts. In Encyclopedia of Caves, 3rd ed.; Culver, D.C., White, W., Eds.; Academic Press: Burlington, MA, USA, 2019; pp. 47–55. [Google Scholar]
- Howarth, F.G. A comparison of volcanic and karstic cave communities. In Proceedings of the 7th International Symposium on Vulcanospeleology, Santa Cruz de la Palma, Canary Islands, Spain, 4–11 November 1994; pp. 63–68. [Google Scholar]
- Howarth, F.G. Ecology of cave arthropods. Annu. Rev. Entomol. 1983, 28, 365–389. [Google Scholar] [CrossRef]
- Howarth, F.G.; Moldovan, O.T. Where cave animals live. In Cave Ecology; Moldovan, O.T., Kováč, Ľ., Halse, S., Eds.; Springer: Cham, Switzerland, 2018; pp. 23–37. [Google Scholar]
- Culver, D.; Pipan, T. The Biology of Caves and Other Subterranean Habitats, 2nd ed.; Oxford University Press: Oxford, UK, 2019; p. 301. [Google Scholar]
- Pipan, T.; Deharveng, L.; Culver, D.C. Hotspots of Subterranean Biodiversity. Diversity 2020, 12, 209. [Google Scholar] [CrossRef]
- Stone, F.D. Bayliss Lava Tube and the Discovery of a Rich Cave Fauna in Tropical Australia. In Proceedings of the 14th International Symposium on Vulcanospeleology, Undara National Park, Mount Surprise, QLD, Australia, 12–17 August 2010; pp. 47–58. [Google Scholar]
- Clarke, A. An Overview of Invertebrate Fauna Collections from the Undara Lava Tube System. In Proceedings of the 14th International Symposium on Vulcanospeleology, Undara National Park, Mount Surprise, QLD, Australia, 12–17 August 2010; pp. 59–76. [Google Scholar]
- Fontaine, B.; Perrad, A.; Bouchet, P.V.N.P. 21 years of shelf life between discovery and description of new species. Curr. Biol. 2012, 22, 943–944. [Google Scholar] [CrossRef]
- Bannink, P. List of invertebrates collected from the McBride Volcanic Province lava tubes and associated rainforest depressions. Unpublished Report. 1–12.
- Godwin, M.D. Undara and Associated Lava Fields of McBride Plateau, a Speleological Field Guide; Chillagoe Caving Club, Inc.: Cairns, Australia, 1993. [Google Scholar]
- Godwin, M.D.; Pearson, L.M. The Murronga lava flow. In Proceedings of the Cave Leeuwin Conference, Margaret River, WA, Australia, 30 December 1990–5 January 1991; pp. 34–54. [Google Scholar]
- Pearson, L.M. Field Guide to the Lava Tubes on the McBride Volcanic Province in North Queensland; Chillagoe Caving Club, Inc.: Cairns, Australia, 2010. [Google Scholar]
- Howarth, F.G.; Moldovan, O.T. The ecological classification of cave animals and their adaptations. In Cave Ecology; Moldovan, O.T., Kovác, L., Halse, S., Eds.; Ecological Studies; Springer: Cham, Switzerland, 2018; pp. 41–67. [Google Scholar]
- Cloudsley-Thompson, J.L. On the responses to environmental stimuli, and the sensory physiology of Millipedes (Diplopoda). Proc. Zool. Soc. Lond. 1951, 121, 253–277. [Google Scholar] [CrossRef]
- Griffin, T.J. The Geology, Mineralogy and Geochemistry of the McBride Basaltic Province, Northern Queensland. Ph.D. Thesis, James Cook University, Douglas, QLD, Australia, 1977. [Google Scholar]
- Cohen, B.E.; Mark, D.F.; Fallon, S.J.; Stephenson, P.J. Holocene-Neogene volcanism in northeastern Australia: Chronology and eruption history. Quat. Geochronol. 2017, 39, 79–91. [Google Scholar] [CrossRef]
- Griffin, T.G.; McDougall, I. Geochronology of the Cainozoic McBride Volcanic Province Northern Queensland. J. Geol. Soc. Aust. 1975, 22, 387–396. [Google Scholar] [CrossRef]
- Atkinson, A. The Undara lava tube system and its caves. Helictite 1990, 28, 3–14. [Google Scholar]
- Malipatil, M.B.; Howarth, F.G. Two new species of Micropolytaxus Elkins from Northern Australia (Hemiptera: Reduviidae: Saicinae). J. Aust. Entomol. Soc. 1990, 29, 37–40. [Google Scholar] [CrossRef]
- Atkinson, A.; Atkinson, V. Undara Volcano and Its Lava Tubes: A Geological Wonder of Australia in Undara Volcanic National Park, North Queensland; Atkinson, Anne & Vernon: Brisbane, Australia, 1995; pp. 1–96. [Google Scholar]
- Rein, T.; Kempe, S.; Dufresne, A. The “Cueva del Viento” on the Canaries, Spain. In Proceedings of the 17th International Vulcanspeleology Symposium Ocean View, Hawaii, HI, USA, 6–12 February 2016; pp. 1–8. [Google Scholar]
- Peterson, D.W.; Holcomb, R.T.; Tilling, R.I.; Christiansen, R.L. Development of lava tubes in the light of observations at Mauna Ulu, Kilauea Volcano, Hawaii. Bull. Volcanol. 1994, 56, 343–360. [Google Scholar] [CrossRef]
- Whitehead, P.W. The Regional Context of the McBride Basalt Province and the Formation of the Undara Lava Flows, Tubes, Rises and Depressions. In Proceedings of the 14th International Symposium on Vulcanospeleology, Undara National Park, Mount Surprise, QLD, Australia, 12–17 August 2010; pp. 9–18. [Google Scholar]
- Atkinson, A.; Griffin, T.J.; Stevenson, P.J. A major lava tube system, North Queensland. Bull. Volcanol. 1975, 39, 1–28. [Google Scholar] [CrossRef]
- Stephenson, P.J.; Griffin, T.J.; Sutherland, F.L.I.E.P. Cainozoic volcanism in north-eastern Australia. In Geology and Geophysics of North-Eastern Australia; Henderson, R.A., Stephenson, P.J., Eds.; Geological Association of Australia, Queensland Division: Douglas, QLD, Australia, 1980; pp. 349–374. [Google Scholar]
- Bureau of Meteorology. Climate Statistics for Australian Locations. Available online: http://www.bom.gov.au/climate/averages/tables/cw_030036.shtml (accessed on 25 April 2021).
- Bland, R.G.; Weinstein, P.; Slaney, D.P. Mouthpart sensilla of cave species of Australian Paratemnopteryx cockroaches (Blattaria: Blattellidae). Int. J. Insect Morphol. Embryol. 1998, 16, 291–300. [Google Scholar] [CrossRef]
- Roth, L.M. A revision of the Australian Parcoblattini (Blattaria: Blattellidae: Blattellinae). Mem. Qld. Mus. Nat. 1990, 28, 531–596. [Google Scholar]
- Smith, G.B. New species of Metrinura Mendes (Zygentoma: Nicoletiidae) from Queensland, Australia. Aust. J. Entomol. 2006, 45, 163–167. [Google Scholar] [CrossRef]
- Smith, G.B. New Atelurinae (Zygentoma: Nicoletiidae) from Northern Australia. Gen. Appl. Entomol. 2016, 44, 21–58. [Google Scholar]
- Stone, F.D.; (University of Hawaii). Personal Communication, 1985.
- Davies, V.T. The huntsman spiders Heteropoda Latreille and Yiinthi gen. nov. (Araneae: Heteropodidae) in Australia. Mem. Qld. Mus. 1994, 35, 75–122. [Google Scholar]
- Dyce, A.L.; Wellings, G. Phlebotomine sandflies (Diptera: Psychodidae) from caves in Australia. Parasitologia 1991, 33, 193–198. [Google Scholar]
- Bradbury, J.H.; (University of Adelaide). Personal Communication, 2000.
- Harvey, M.S. New cave-dwelling schizomids (Schizomida: Hubbardiidae) from Australia. Rec. West. Aust. Mus. Suppl. 2001, 64, 171–185. [Google Scholar] [CrossRef][Green Version]
- Moulds, T.A.; Murphy, N.; Adams, M.; Reardon, T.B.; Harvey, M.S.; Jennings, J.; Austin, A.D. Phylogeography of cave pseudoscorpions in southern Australia. J. Biogeogr. 2007, 34, 951–962. [Google Scholar] [CrossRef]
- Raven, R.J.; Stumkat, K.; Gray, M.R. Revisions of Australian ground-hunting spiders: I. Amauropelma gen. nov. (Araneomorphae: Ctenidae). Rec. West. Aust. Mus. Suppl. 2001, 64, 187–227. [Google Scholar] [CrossRef]
- Gray, M.R. Cavernicolous spiders (Araneae) from Undara, Queensland and Cape Range, Western Australia. Helictite 1989, 27, 87–89. [Google Scholar]
- Main, B.Y. Spiders; Collins: Sydney, Australia, 1984. [Google Scholar]
- Gray, M.R. Survey of the spider fauna of Australian caves. Helictite 1973, 11, 46–75. [Google Scholar]
- Baehr, B.C.; Jocqué, R. The new endemic Australian genus Nosterella and a review of Nostera (Araneae: Zodariidae), including eight new species. Mem. Qld. Mus. Nat. 2017, 60, 53–76. [Google Scholar]
- Slaney, D.P. New species of cave dwelling cockroaches in the genus Neotemnopteryx Princis (Blattaria: Blattellidae: Blattellinae). Mem. Qld. Mus. Nat. 2000, 46, 331–336. [Google Scholar]
- Stone, F.D. The cockroaches of North Queensland caves and the evolution of tropical troglobites. In Proceedings of the Tropicon 1988, 17th Biennial Australian Speleological Conference, Tinaroo, QLD, Australia, 27–31 December 1988; pp. 88–93. [Google Scholar]
- Escalona, H.; Oberprieler, R. Australian National Insect Collection; CSIRO: Canberra, Australia, 2021. [Google Scholar]
- Hoch, H.; Howarth, F.G. Six new cavernicolous cixiid planthoppers in the genus Solonaima from Australia (Homoptera: Fulgoroidea). Syst. Entomol. 1989, 14, 377–402. [Google Scholar] [CrossRef]
- Howarth, F.G. Bioclimatic and geologic factors governing the evolution and distribution of Hawaiian cave insects. Entomol. Gen. 1983, 8, 17–26. [Google Scholar] [CrossRef]
- Eberhard, S.M. Nowranie Caves and the Camooweal karst area, Queensland: Hydrology, geomorphology and speleogenesis, with notes on aquatic biota. Helictite 2003, 38, 27–38. [Google Scholar]
- Eberhard, S.M. Ecology and Hydrology of a Threatened Groundwater-Dependent Ecosystem: The Jewel Cave Karst System in Western Australia. Ph.D. Thesis, School of Environmental Science, Murdoch University, Perth, Australia, 2004. [Google Scholar]
- Wagner, W.H.; (University of Michigan, Ann Arbor, MI, USA). Personal Communication, 1988.
- Howarth, F.G.; Medeiros, M.J.; Stone, F.D. Hawaiian lava tube cave associated Lepidoptera from the collections of Francis G Howarth and Fred D Stone. Bish. Mus. Occas. Pap. 2020, 129, 37–54. [Google Scholar]
- Smith, G.B.; McRae, J.M. New species of subterranean silverfish (Zygentoma: Nicoletiidae: Atelurinae) from Western Australia’s semi-arid Pilbara region. Rec. West. Aust. Mus. 2014, 29, 105–127. [Google Scholar] [CrossRef][Green Version]
- Smith, G.B.; McRae, J.M. Further short range endemic troglobitic silverfish (Zygentoma: Nicoletiidae; Subnicoletiinae and Coletiniinae) from north-western Australia. Rec. West. Aust. Mus. 2016, 31, 41–55. [Google Scholar] [CrossRef][Green Version]
- Slaney, D.P. New species of Australian cockroaches in the genus Paratemnopteryx Saussure (Blattaria, Blattellidae, Blattellinae), and a discussion of some behavioural observations with respect to the evolution and ecology of cave life. J. Nat. Hist. 2001, 35, 1001–1012. [Google Scholar] [CrossRef]
- Brad, T.; Lepure, S.; Sarbu, S.M. The Chemoautotrophically Based Movile Cave Groundwater Ecosystem, a Hotspot of Subterranean Biodiversity. Diversity 2021, 13, 128. [Google Scholar] [CrossRef]
- Deharveng, L.; Bedos, A. Gaz carbonique. In Thai-Maros 85, Rapport Speleologique et Scientifique to Thailand and Sulawesi; Association Pyreneenne de Speleologie: Toulouse, France, 1986; pp. 144–152. [Google Scholar]
- Stone, F.D.; Howarth, F.G.; Hoch, H.; Asche, M. Root communities in lava tubes. In Encyclopedia of Caves, 2nd ed.; White, W.B., Culver, D.C., Eds.; Academic Press: Burlington, MA, USA, 2012; pp. 658–664. [Google Scholar]
- Humphreys, W.F. Where angels fear to tread: Developments in cave ecology. In Cave Ecology; Moldovan, O.T., Kovác, L., Halse, S., Eds.; Ecological Studies; Springer: Cham, Switzerland, 2018; pp. 497–532. [Google Scholar]
- Humphreys, W.F. (Ed.) The Biogeography of Cape Range, Western Australia; Records of the Western Australian Museum Supplement; Western Australian Museum: Perth, Australia, 1993; Volume 45, pp. 1–248. [Google Scholar]
- Poore, G.C.B.; Humphreys, W.F. Bunderanthura bundera gen. et sp. nov. from Western Australia, first anchialine Leptanthuridae (Isopoda) from the Southern Hemisphere. Rec. West. Aust. Mus. 2013, 28, 21–29. [Google Scholar] [CrossRef][Green Version]
- Humphreys, G.; Alexander, J.; Harvey, M.; Humphreys, W.F. The subterranean fauna of Barrow Island, northwestern Australia. Rec. West. Aust. Mus. Suppl. 2013, 83, 145–158. [Google Scholar] [CrossRef][Green Version]
- Eberhard, S.M.; Halse, S.A.; Humphreys, W.F. Stygofauna in the Pilbara region, north-west Western Australia: A review. J. R. Soc. West. Aust. 2005, 88, 167–176. [Google Scholar]
- Eberhard, S.M.; Halse, S.A.; Williams, M.; Scanlon, M.D.; Cocking, J.S.; Barron, H.J. Exploring the relationship between sampling efficiency and short range endemism for groundwater fauna in the Pilbara region, Western Australia. Freshw. Biol. 2009, 54, 885–901. [Google Scholar] [CrossRef]
- Humphreys, W.F. Groundwater calcrete aquifers in the Australian arid zone: The context to an unfolding plethora of stygal diversity. Rec. West. Aust. Mus. Suppl. 2001, 64, 233–234. [Google Scholar] [CrossRef]
- Halse, S.A. Research in calcretes and other deep subterranean habitats outside caves. In Cave Ecology; Moldovan, O.T., Kovác, L., Halse, S.A., Eds.; Ecological Studies; Springer: Cham, Switzerland, 2018; pp. 415–434. [Google Scholar]
- Halse, S.A.; Pearson, G.B. Troglofauna in the vadose zone: Comparison of scraping an dtrapping results and sampling adequacy. Subterr. Biol. 2014, 13, 17–34. [Google Scholar] [CrossRef]
- Moulds, T.; Bannink, P. Preliminary notes on the cavernicolous arthropod fauna of Judburra/Gregory karst area, northern Australia. Helictite 2012, 41, 75–85. [Google Scholar]
- Bennelongia Pty Ltd. Yeelirrie Subterranean Fauna Assessment; Report Prepared for Cameco Australia Pty Ltd.; 2015/236b; Bennelongia Pty Ltd.: Perth, Australia, 2015. [Google Scholar]
- Eberhard, S.M.; Watts, C.H.S.; Callan, S.K.; Leijs, R. Three new subterranean diving beetles (Coleoptera: Dytiscidae) from the Yeelirrie groundwater calcretes, Western Australia, and their distribution between several calcrete deposits including a potential mine site. Rec. West. Aust. Mus. 2016, 31, 27–40. [Google Scholar] [CrossRef]
- Subterranean Ecology Pty Ltd. Yeelirrie Subterranean Fauna Survey; Report Prepared for BHP Billiton Yeelirrie Development Company Pty Ltd.; 2010/14; Subterranean Ecology Pty Ltd.: Perth, Australia, 2011; p. 269. [Google Scholar]
- Subterranean Ecology Pty Ltd. Ethel Gorge Aquifer Threatened Ecological Community—Consolidated Taxonomy; Unpublished Report Prepared for BHP Billiton Iron Ore; Subterranean Ecology Pty Ltd.: Perth, Australia, 2013; p. 96. [Google Scholar]
- Tang, D.; Eberhard, S.M. Two new species of Nitocrella (Crustacea, Copepoda, Harpacticoida) from groundwaters of northwestern Australia expand the geographic range of the genus in a global hotspot of subterranean biodiversity. Subterr. Biol. 2016, 20, 51–76. [Google Scholar] [CrossRef]
- Toro Energy Limited. Extension to the Wiluna Uranium Project: Environmental Management Plan: Subterranean Fauna Management Plan; Toro Energy Limited: Perth, Australia, 2012. [Google Scholar]
- Biota Environmental Services Pty Ltd. Rio Tinto Regional Troglobitic Fauna Study; Unpublished Report Prepared for Rio Tinto Iron Ore; Biota Environmental Services Pty Ltd.: Perth, Australia, 2013. [Google Scholar]
- Halse, S.A.; Scanlon, M.D.; Cocking, J.S.; Barron, H.J.; Richardson, J.B.; Eberhard, S.M. Pilbara stygofauna: Deep groundwater of an arid landscape contains globally significant radiation of biodiversity. Rec. West. Aust. Mus. Suppl. 2014, 78, 443–483. [Google Scholar] [CrossRef]
- Bennelongia Pty Ltd. Stygofauna Survey—Exmouth Cape Aquifer: Scoping Document Describing Work Required to Determine Ecological Water Requirements for the Exmouth Cape Aquifer; Prepared for Department of Water; Bennelongia Pty Ltd.: Perth, Australia, 2008; p. 39. [Google Scholar]
- King, R.A.; Fagan-Jeffries, E.; Bradford, T.M.; Stringer, D.N.; Finston, T.; Halse, S.A.; Eberhard, S.M.; Humphreys, G.; Humphreys, W.F.; Austin, A.D.; et al. Cryptic Diversity down under: Defining species in the subterranean amphipod genus Nedsia Barnard and Williams (Hadzioidea: Eriopisidae) from the Pilbara, Western Australia. Invertebr. Syst. 2021. in review. [Google Scholar]
- Eberhard, S.; Smith, G.; Gibian, M.; Smith, H.; Gray, M. Invertebrate Cave Fauna of Jenolan. Proc. Linn. Soc. N. S. W. 2014, 136, 35–67. [Google Scholar]
- Eberhard, S.M.; Spate, A. Cave Invertebrate Survey: Toward an Atlas of NSW Cave Fauna; Report Prepared under the NSW Heritage Assistance Program NEP 94 765; NSW Heritage Assistance Program: Canberra, Australia, 1995; pp. 1–112. [Google Scholar]
- Thurgate, M.E.; Gough, J.S.; Clarke, A.; Serov, P.; Spate, A. Stygofauna diversity and distribution in Eastern Australian cave and karst areas. Rec. West. Aust. Mus. 2001, 64, 49–62. [Google Scholar] [CrossRef][Green Version]
- Thurgate, M.E.; Gough, J.S.; Spate, A.; Eberhard, S.M. Subterranean biodiversity in New South Wales: From rags to riches. Rec. West. Aust. Mus. Suppl. 2001, 64, 37–47. [Google Scholar] [CrossRef]
- Eberhard, S.M.; Richardson, A.M.M.; Swain, R. The Invertebrate Cave Fauna of Tasmania; Report to the National Estate Office, Canberra; Zoology Department, University of Tasmania: Hobart, Australia, 1991; p. 174. [Google Scholar]
- Ahyong, S.T. The Tasmanian Mountain Shrimps, Anaspides Thomson, 1894 (Crustacea, Syncarida, Anaspididae). Rec. Aust. Mus. 2016, 68, 313–364. [Google Scholar] [CrossRef]
- Eberhard, S.M.; Giachino, P.M. Tasmanian Trechinae and psydrinae (Coleoptera, Carabidae): A taxonomic and biogeographic synthesis, with description of new species and evaluation of the impact of Quaternary climate changes on evolution of the subterranean fauna. Subterr. Biol. 2011, 9, 1–72. [Google Scholar]
- Karanovic, I.; Eberhard, M.S.; Perina, G. Austromesocypris bluffensis sp. n. (Crustacea, Ostracoda, Cypridoidea, Scottiinae) from subterranean aquatic habitats in Tasmania, with a key to world species of the subfamily. ZooKeys 2012. [Google Scholar] [CrossRef] [PubMed]
- Ponder, W.F.; Clark, S.A.; Eberhard, S.M.; Studdert, J.B. A radiation of hydrobiid snails in the caves and streams at Precipitous Bluff, southwest Tasmania, Australia (Mollusca: Caenogastropoda: Rissooidea: Hydrobiidae s.l.). Zootaxa 2005, 1074, 1–66. [Google Scholar] [CrossRef]
- Tomlinson, M. A Framework for Determining Environmental Water Requirements for Alluvial Aquifer Ecosystems. Ph.D. Thesis, University of New England, Armidale, Australia, 2008. [Google Scholar]
- Humphreys, W.F. Australasian subterranean biogeography. In Handbook of Australasian Biogeography; Ebach, M.C., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 269–293. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).