Abstract
Coralligenous structuring species (CSS) form a group of marine megabenthic species with an engineering capacity. Since they are highly vulnerable to anthropogenic activities, they have been selected for the Marine Strategy Framework Directive (MSFD) monitoring programs. The pressure and impact of fishing gear and marine litter on these species were evaluated through the image analysis of 54 remotely operated vehicle (ROV) routes along the Campania coasts (Tyrrhenian Sea, Italy). CSS density was calculated as the number of colonies/100 m2. Anthropogenic pressure was estimated as the frequency of frames showing longline, nets, other gear, plastic objects, metal objects, and other litter; while the impact was expressed as the frequency showing necrosis/epibiosis, broken/upturned and covered/entangled colonies. Cnidaria dominate in the Napoli, Campanella and Capri areas, while Bryozoa dominate in Cilento N and Cilento S areas. Campanella and Capri appeared to be the least heterogeneous despite their higher CSS densities, which was possibly related to the dominance of a few species. These areas were the most affected by showing the highest numbers of fishing gear (longlines) and marine litter (metal objects) recorded, amongst which longlines are the most abundant. In addition, these fishing areas are either close to a large urban center or located along popular touristic routes. In all the areas, colonies with necrosis/epibiosis (CNE) impact are present with low-moderate values, while the category gears covering/entangling (GCE) impact prevails in the Campanella and Capri areas, and this is strictly connected to the high presence of fishing gear.
Keywords:
mesophotic reefs; ROV; anthropogenic pressure; megabenthos; mechanical damage; epibiosis; necrosis 1. Introduction
Coralligenous bioconstructions are heterogeneous structures of biogenic origin typical for the benthic Mediterranean habitats, developing under dim light conditions [1]. These mesophotic reefs occur at depths ranging from 30–40 m and extending to the limit of the photic zone, which may be up to 200 m depth [2,3,4,5]. Coralligenous assemblages mostly grow on hard substrates, but they can also develop on soft bottoms starting from the aggregation of free-living coralline algae, named rhodoliths [6,7]. Crustose coralline algae (mostly of the genera Mesophyllum, Pseudolithophyllum and Lithophyllum) build up a multi-layered substrate on which sponges (e.g., Chondrosia reniformis, Axinella spp., Ircinia spp., Sarcotragus spp., Petrosia ficiformis, Aplysina cavernicola, Spongia spp., Haliclona mediterranea, Oscarella lobularis and Agelas oroides), cnidarians (e.g., Leptopsammia pruvoti, Caryophyllia spp., Epizoanthus arenaceus, Eunicella cavolinii, E. singularis, Corallium rubrum and Paramuricea clavata), and bryozoans (e.g., Schizomavella spp., Pentapora fascialis, Myriapora truncata and Turbicellepora avicularis) grow in addition to other engineering taxa [1,8,9]. Indeed, they form complex three-dimensional structures, hosting more than 10% of the known Mediterranean marine benthic species and provide suitable habitats for several endemic, vulnerable and protected species [1,10,11,12]. In particular, some megabenthic species, quite easily recognizable by images, represent the coralligenous structuring species used for the Marine Strategy Framework Directive programs (MSFD, 2008/56/EC and its 2017 updated version) [13]. Moreover, coralligenous reefs are an important “hot spot” of species diversity in the Mediterranean Sea and supply a variety of valuable ecosystem services [14], boasting also their economic relevance [15,16] and offering important fisheries grounds and aesthetic seascapes for diving tourism [1,17,18]. To these latter ecosystem services, it is also important to add carbon stock and sequestration by carbonate structures producing the bioconstructions [19].
Mesophotic reefs are threatened by several anthropogenic impacts, such as fishing, pollution, introductions of alien species and climate change [20,21]. These impacts may lead to structural and functional changes, altering trophic webs and transferring organic and inorganic contaminants to the food chain, thus influencing ecosystem functioning and ultimately human health [22,23]. Fishing gear, such as trawling nets and longlines, has a high impact on benthic communities by entangling organisms, mechanically damaging the erect species, and increasing turbidity and sediment accumulation [24,25,26,27,28,29,30]. Specifically, the entangled colonies may be broken and upturned by gear reaching out to tissue necrosis and subsequent epibionts overgrown [31,32,33]. The recovery of coralligenous species from damage can negatively affect the regenerative process, the growth, the reproduction, the resistance to diseases, and the competitive ability of the colonies [34,35].
Marine litter may favor hitch-hiking organisms and the spreading of alien species [36], as well as producing toxic effects in marine animals [37]. In particular, plastic litter lost in the marine environment can fragmentize into smaller pieces, becoming microplastics (<5 mm diameter) [23] and they can then be ingested, causing problems both for feeding and digestive activities and favoring the spread of xenobiotics [38]. The litter ingestion by fishes also has potential consequences for human health [39,40,41]. Overall, the accumulation of lost fishing gear and litter is considered among the major causes of marine environment degradation, and for this reason, several studies are trying to characterize and assess their impacts on marine ecosystems [32,42,43,44,45,46].
In a classical ACI (after control impact) experimental design, the impacts due to anthropogenic activities have determined a decrease of β-diversity. This is explained by a decrease in the number of species adapted to natural edaphic factors and an increase of the most resilient ones [47]. Therefore, differences in species composition on a large scale can reveal impacts. Indeed, at a small scale (differences among replicates of a single station), the high heterogeneity of community species composition is associated with a less impacted environment [48]. Some European legislation for the protection of the marine environment and natural resources and sustainable use of marine waters, such as the MSFD, have measures and monitoring programs aimed at studying, protecting and restoring sensitive habitats in order to maintain the biodiversity in the Mediterranean Sea [49]. Particular attention has to be paid to some remarkable habitats that play an important role in marine ecosystems dynamics and biodiversity. Indeed, among coralligenous species, there are some regarded as vulnerable and considered for protection according to international agreements (Habitat Directive 92/43/CEE; SPA/BIO Protocol; Barcelona Convention; Berna Convention).
The aim of this study is to evaluate, by remotely operated vehicle (ROV) imaging technique, the effects of anthropogenic pressure type and abundance, represented by fishing gear and marine litter (D10—marine litter, MSFD), on coralligenous structuring species diversity (D6—seafloor integrity, MSFD), along the Campanian coasts (Tyrrhenian Sea, Italy). These coasts are characterized by high levels of urban population and maritime traffic for tourism and fishing activities, mostly represented by artisanal fishing, in which trawl nets, gill nets, and longlines are used as fishing gear [50], even though there are still not enough available data on the characterization and impact of fishing gear and marine litter on the coralligenous sensitive habitats [4,12,32,45,51,52]. Such information may be a useful tool for the implementation of marine resources management plans in the Mediterranean Sea, aimed at the reefs’ biodiversity and their associated species conservancy and the linked socioeconomic effects on fishing and tourism.
2. Materials and Methods
2.1. Study Area
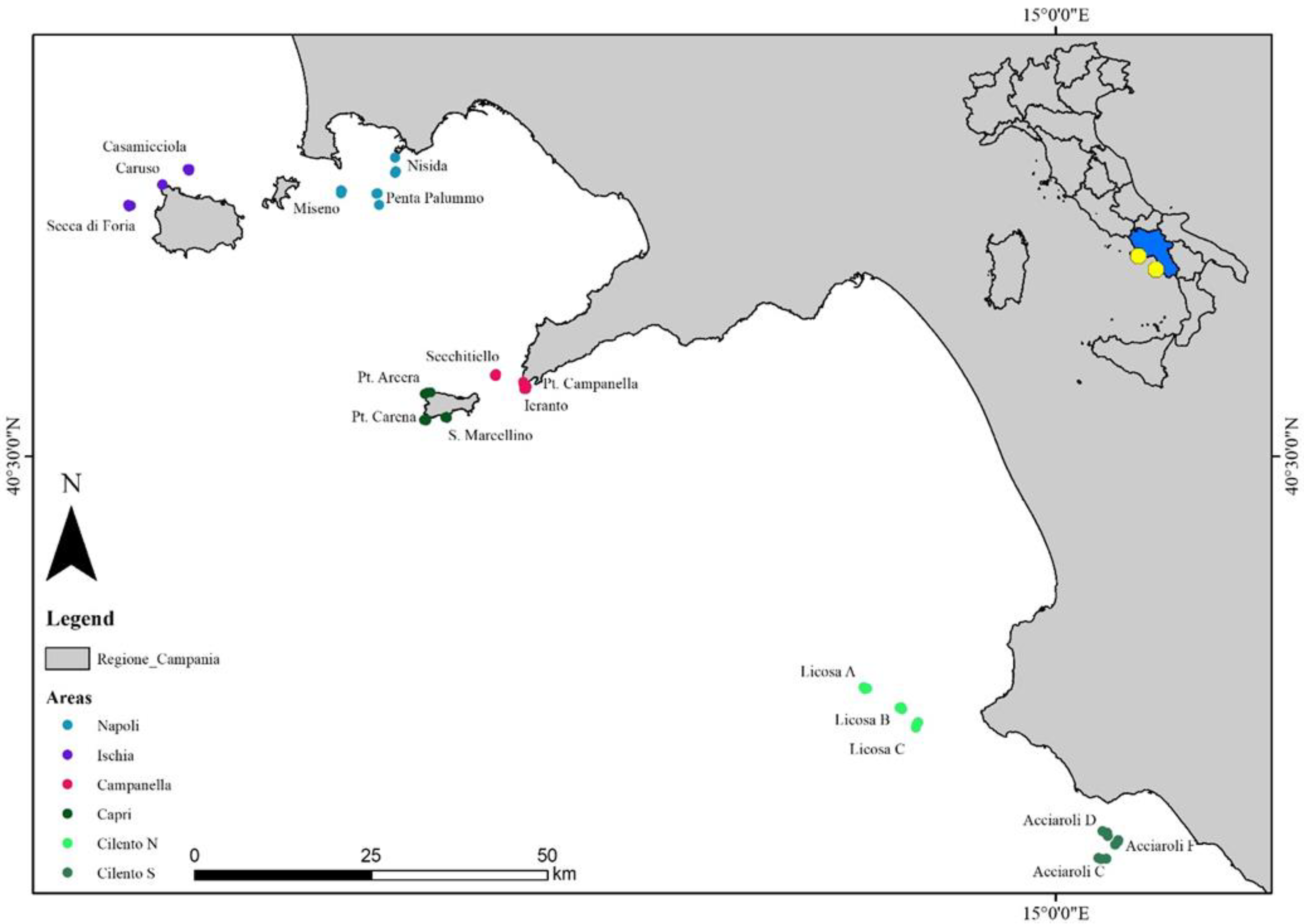
The study areas (Figure 1) are located along different parts of the Campanian coasts (south-central Tyrrhenian Sea, Italy). A total of six areas, and three randomly replicated sites for each area, were investigated: “the Napoli area” (Nisida, Penta Palummo and Miseno sites), between 20 m and 73 m depth; “the Ischia area” (Secca Forio, Caruso and Casamicciola sites), between 26 m and 60 m depth; “the Campanella area” (Ieranto, Secchitiello and Punta Campanella sites) between 34 m and 102 m depth; “the Capri area” (S. Marcellino, P. Carena and P. Arcera sites), between 30 m and 102 m depth; “the Cilento N area” (Licosa A, Licosa B and Licosa C sites), between 40 m and 95 m depth, and “the Cilento S area” (Acciaroli C, Acciaroli D and Acciaroli F sites), between 49 m and 78 m depth.
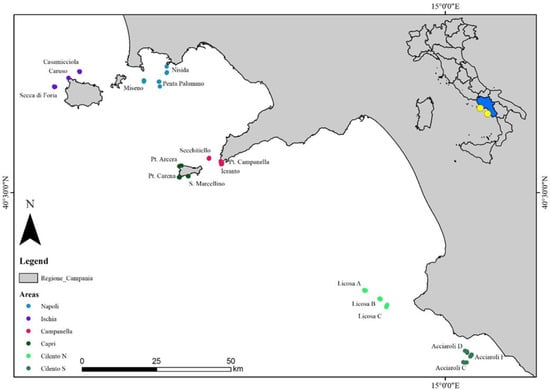
Figure 1.
Study areas with sampling sites.
The Napoli area consists of a sand/mud substrate with scattered coralligenous banks; Ischia island is mostly characterized by a hard bottom due to the presence of cliffs and rocky outcrops of volcanic origins; the sites located in the Campanella area are represented by two rocky cliffs (P. Campanella and Ieranto) and a shoal (Secchitiello); the Capri area also consists of steep rocky cliffs; finally, the Cilento N and Cilento S areas are characterized by a succession of rocky outcrops and coralligenous banks [53,54,55].
2.2. Field Activities
The investigation was carried out during several surveys in 2017 and 2018, aimed at assessing the ecological status of coralligenous habitats, following the MSFD protocols [56]). The study was carried out through a remotely operated vehicle (the ROV Ageotec, mod. Perseus), equipped with an HD camera (DVS-3000 high definition), a navigation camera with an underwater positioning Ultra Short Base Line System (USBL) interfaced with the on-board navigation system, two spotlights and two laser pointers providing a 14.5-cm scale for the measurement of the frame area and the size of the organisms. ROV moved at approximatively 0.3–0.4 knots and at a distance of about 0.5–1 m from the sea bottom, with an average visual width of 0.5 m.
Overall, 6 areas with 3 sites each (18 sites in total) were monitored; within each site three 300 m routes were performed (54 routes in total).
2.3. Data Management
The ROV videos were analyzed using the VisualSoft® software, rending the HD videos overlapped with the navigation data. The frames used for the analysis, were obtained by video defragmentation each 10 sec with the DVDVideoSoft® software. Taking into account the length of each route (around 300 m), a number of 74 images per route was chosen for uniformity between the different routes. Only high-quality images were analyzed.
The density of coralligenous structuring species identified by ROV-imaging technique (CSS, sensu MSFD protocols) was calculated as the number of colonies of each species on 100 m2 (n. CSS colonies/100 m2) and compared with CSS frequency (percentage times that CSS appeared in each route of the total images number with coralligenous habitat), in order to assess the spatial distribution of species [57]. Moreover, quantitative dominances (Di), as the ratio of a specific CSS density with total density, were also calculated for each area.
Anthropogenic pressure on coralligenous habitats was assessed by frequencies of coralligenous bioconstructions presenting fishing gear or marine litter, calculated as a percentage of frames. Likewise, the anthropogenic impacts were assessed as frequency, calculated as a percentage of frames with damaged coralligenous structuring species.
The main anthropogenic impacts on the coralligenous bioconstructions were represented by three categories of fishing gear: “longlines”, “nets” (such as trawl nets, gill nets or trammel nets) and “other gears” (pots, ropes, moorings, anchors, etc.), and three categories of marine litter: “plastic objects” (bottles, bags, containers, etc.), “metal objects” (such as cans and bins) and “other litter” (textile, glass, wood, tires, etc.).
Anthropogenic activities impacting structuring species colonies can be direct or indirect and they were classified into three categories [34]: the direct impacts are BUC (broken/upturned colonies) and GCE (gears covering/entangling), while the indirect impact is CNE (colonies with necrosis/epibiosis).
2.4. Statistical Analyses
The experimental design, with fishing gears and marine litter not causing damage to the coralligenous communities as the null hypothesis, used statistical analyses involving two factors: area (Ar: fixed, six levels) and site (Si: random, 18 levels) with n = 3 (routes per site). Prior to the analyses, abundances were square-root transformed to reduce the weight of very scanty CSS.
Univariate and multivariate analyses were performed on total densities and single species densities, respectively. In particular, following the Terlizzi et al. (2007) methodology [58], univariate analyses of variance PERMANOVA [59] based on Euclidean distance, was conducted on CSS densities, while the Bray–Curtis similarity was used for the remaining analyses [60]. Subsequently, a pairwise test (corresponding exactly to Gosset’s original t statistic in PRIMER v.6 + PERMANOVA (software) [61] was performed in order to detect differences among areas.
Multivariate patterns were visualized through the multivariate canonical analyses of principal coordinates (CAP) [62] of areas elements. Species showing a Pearson correlation index > 0.4 were also shown in CAP plot. Distance-based permutation multivariate analyses of variance (PERMANOVA) were carried out on densities in order to test for differences in assemblage CSS structure among areas and sites.
Permutation tests of multivariate dispersion, a β-diversity variation measure (PERMDISP [63]), were also performed to investigate small-scale heterogeneity (i.e., among replicates) of CSS assemblages.
Anthropogenic pressures patterns were visualized by the multivariate three-dimensional non-metric multidimensional scaling (3D-nMDS) of Areas centroids. CSS densities were related to them through the multivariate distance-based linear modelling (DistLM [64]), using Step-wise as section procedure and adjusted R2 as a selection criterion.
Finally, fishing gears (independent variable) being significantly correlated with CSS densities were related by linear regression (ordinary LS) to direct impacts (GCE + BUC), indirect impacts (CNE) and heterogeneities (dependent variables).
All the statistical analyses were carried out through PRIMER v.6 + PERMANOVA software [61], except the linear regressions, which were performed with Past4® software [65].
3. Results
In total, an area of 8851 m2 was investigated by ROV, with an average of about 164 m2 per route. A total of 12 CSS (Table 1) was detected for the density calculation, while the frequencies of species, anthropogenic pressures and impacts were calculated using 74 images per route, for a total of 3996 images of about 0.2 m2.

Table 1.
Coralligenous structural species (CSS) densities, present in each investigated area and identified by ROV-imaging technique.
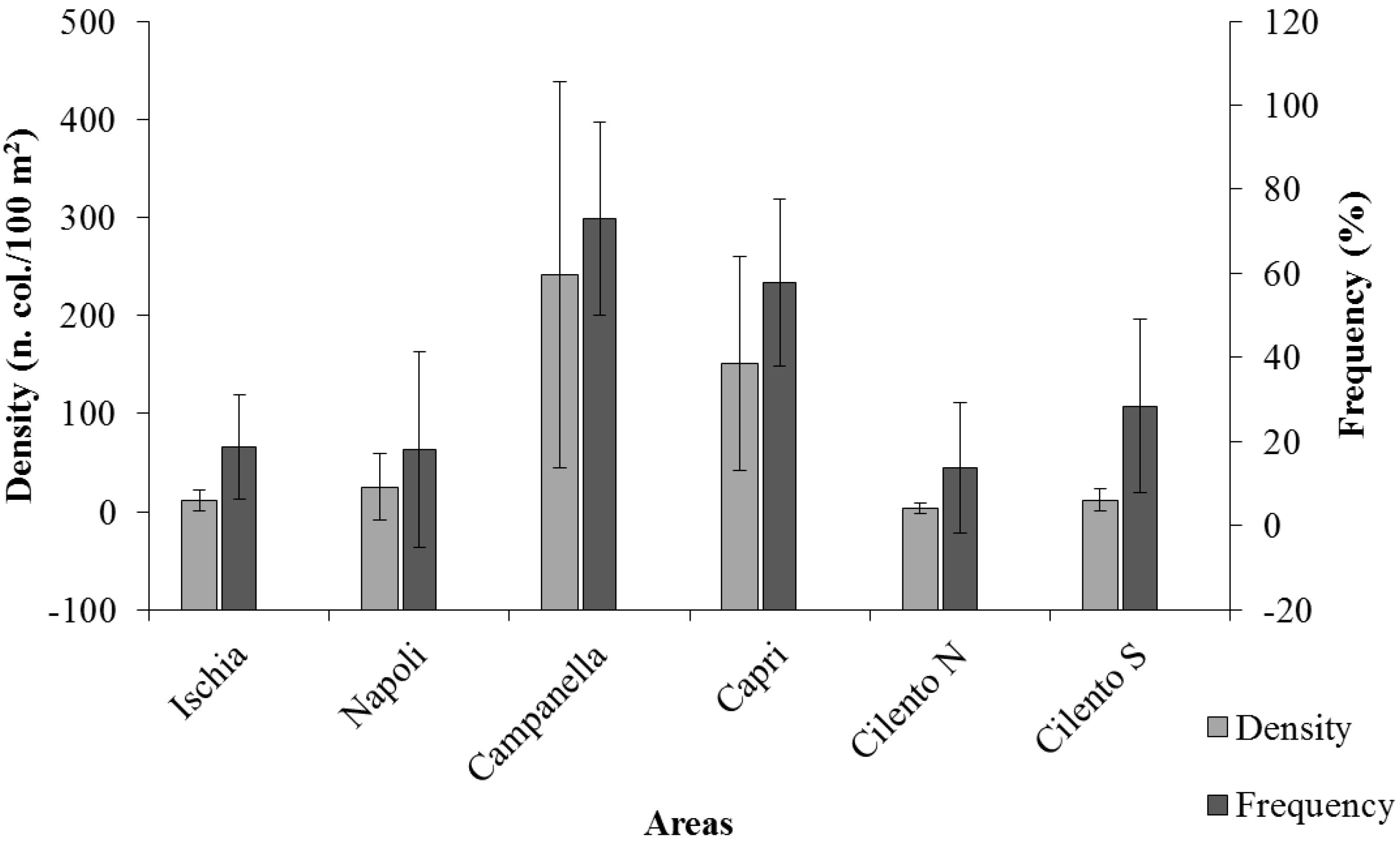
Only 39% of these images showed CSS: in particular, Ischia presented 6 CSS with a frequency of 18.63 ± 12.35%, Napoli presented 6 CSS with a frequency of 18.12 ± 23.24%, Campanella presented 7 CSS with a frequency of 72.98 ± 23.03%, Capri presented 6 CSS with a frequency of 57.79 ± 19.92%, Cilento N presented 6 CSS with a frequency of 13.75 ± 15.42%, Cilento S presented 7 CSS with a frequency of 28.38 ± 20.61% (Figure 2). Values ranged from a maximum of 88.67 ± 5.36% in a route for Campanella, to a minimum of 7.52 ± 6.75% in a route for Cilento S.
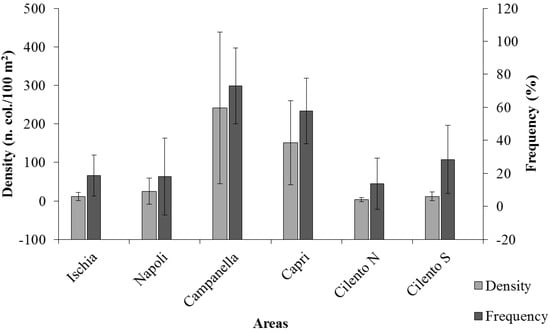
Figure 2.
Average values of density (n. col./100 m2) and frequency (%) of coralligenous structuring species (CSS) in each investigated area. Bars represent SD.
Average densities of total CSS (n. colonies/100 m2, Figure 2) show significant differences among areas (p = 0.0016): Ischia 11.63 ± 10.79 col./100 m2, Napoli 25.00 ± 34.22 col./100 m2, Campanella 241.56 ± 197.06 col./100 m2, Capri 150.92 ± 109.34 col./100 m2, Cilento N 3.21 ± 5.28 col./100 m2, and Cilento S 11.81 ± 10.98 col./100 m2. In particular, CSS range between a maximum value of 422.23 ± 213.21 col./100 m2 at Campanella, and a minimum value at Cilento S, with 0.70 ± 0.61 col./100 m2. Results of Pairwise tests, performed to detect differences among areas, are shown in Table 2.

Table 2.
Pairwise tests among investigated areas for CSS densities. The significant p-values are shown in bold.
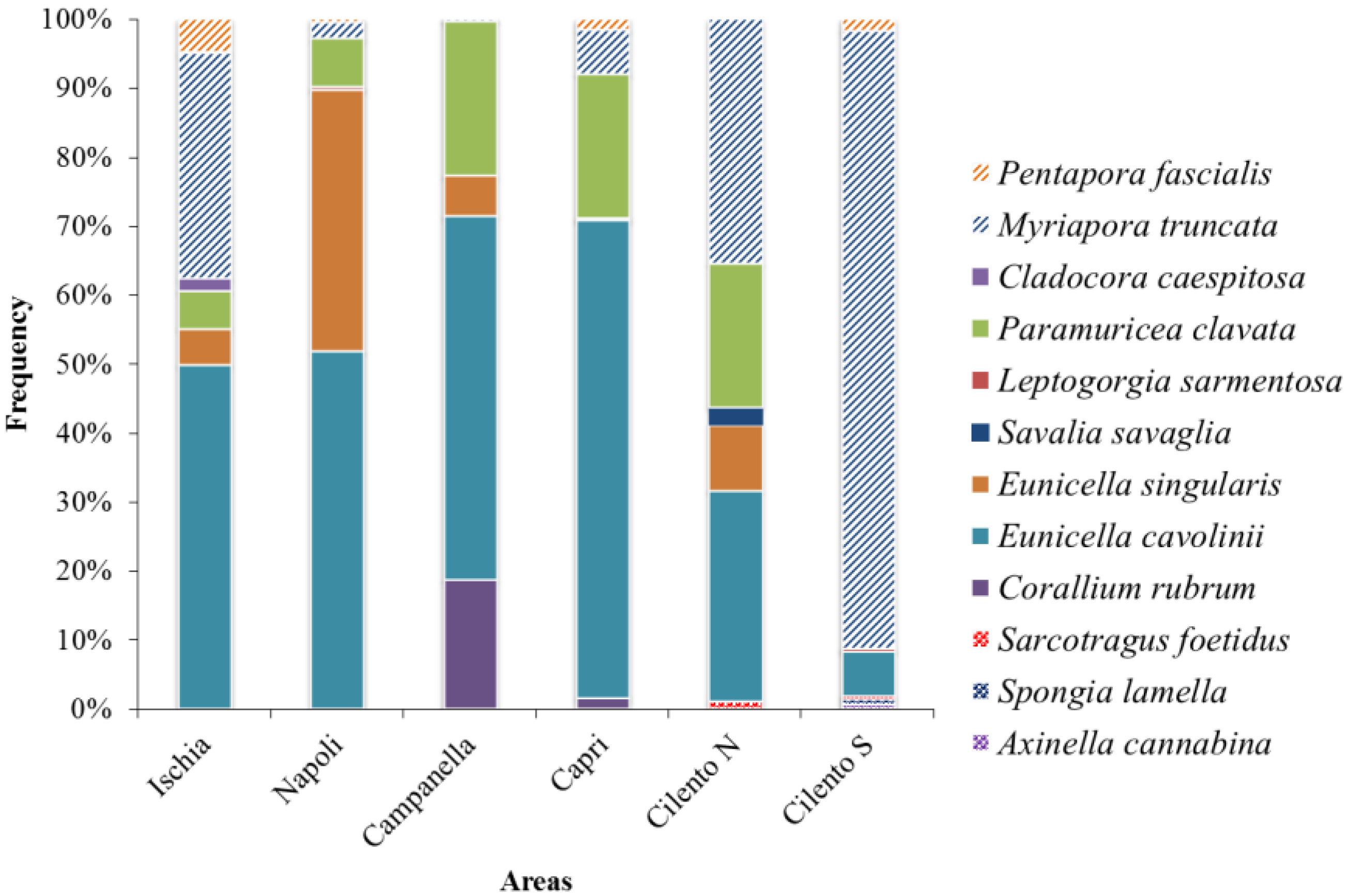
Figure 3 shows that the phylum Cnidaria mainly characterizes Ischia, Napoli, Campanella and Capri areas. In particular, E. cavolinii was a dominant species (respectively 50, 52, 53, and 69% of images containing CSS), followed by E. singularis in Napoli (38%), P. clavata and C. rubrum in Campanella (22% and 19%, respectively), and P. clavata in Capri (21%). In Ischia, the bryozoan M. truncata was the second dominant species (33%). This last species results the dominant species in Cilento N (35%), followed by E. cavolinii and P. clavata (31% and 21%, respectively), and Cilento S (90%).
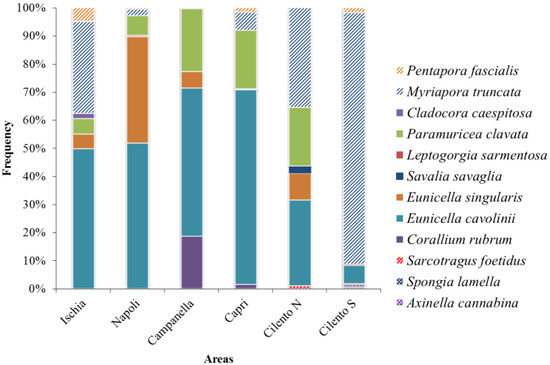
Figure 3.
Species Dominance (Di = frequency of i-th species on the total of CSS) in each area.
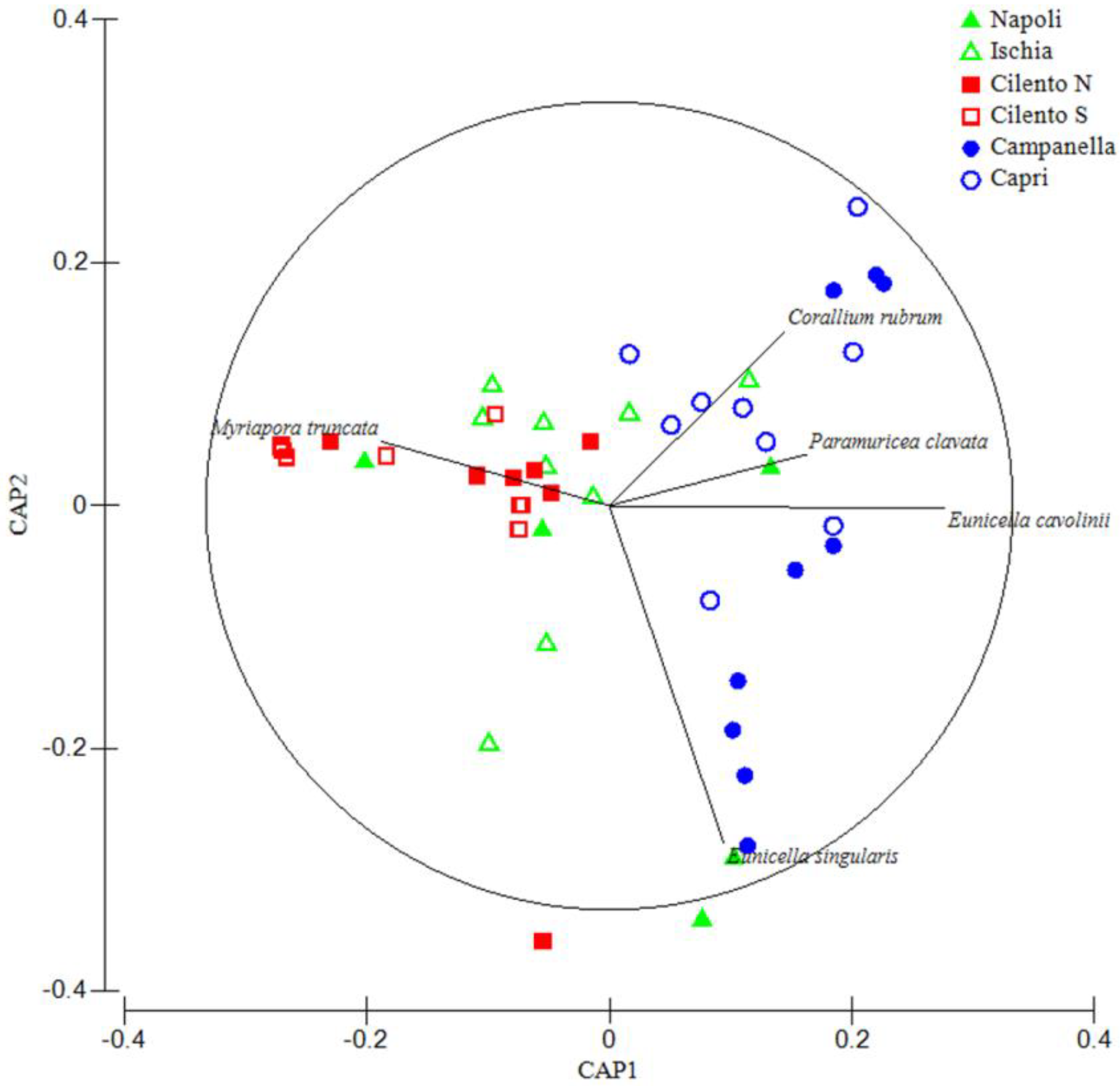
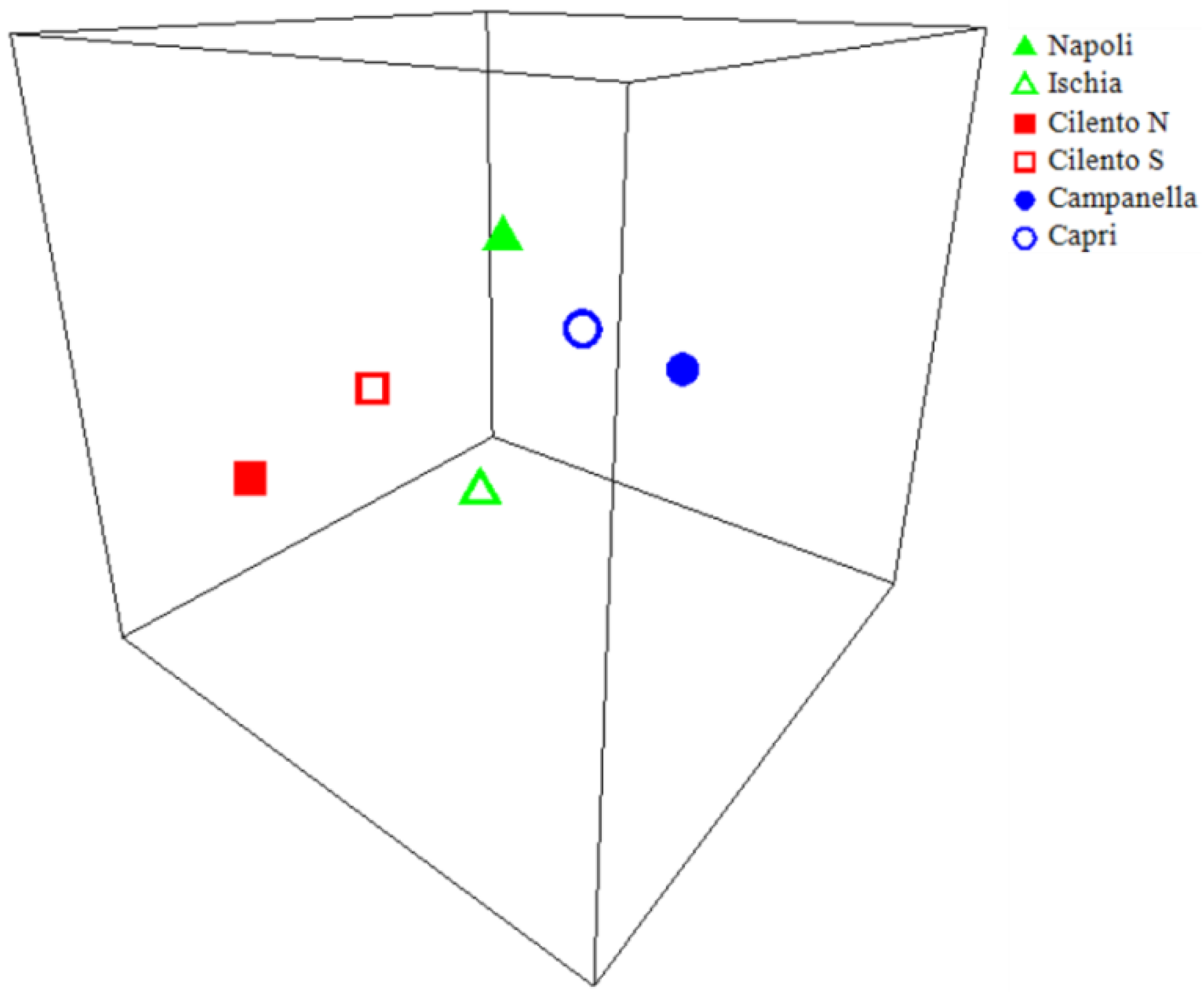
Such findings were confirmed by the CAP analysis (Figure 4), showing: a cluster composed by Campanella and Capri areas, polarized in the positive part of the CAP 1 axis, strongly related with cnidarian species, and a cluster composed by Cilento N and Cilento S areas, polarized in the negative part of the CAP 1 axis, strongly related with bryozoan species. Napoli and Ischia areas are scattered along the whole CAP 1 axis.
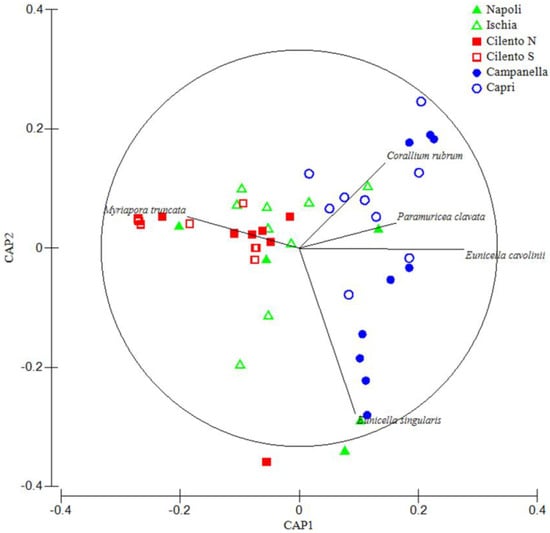
Figure 4.
The CAP analysis on CSS densities; straight lines are vectors of species whose orientation and length are proportional to the most correlated areas. Only species with a correlation Pearson index > 0.4 are shown. The circle represents a 95% confidence interval.
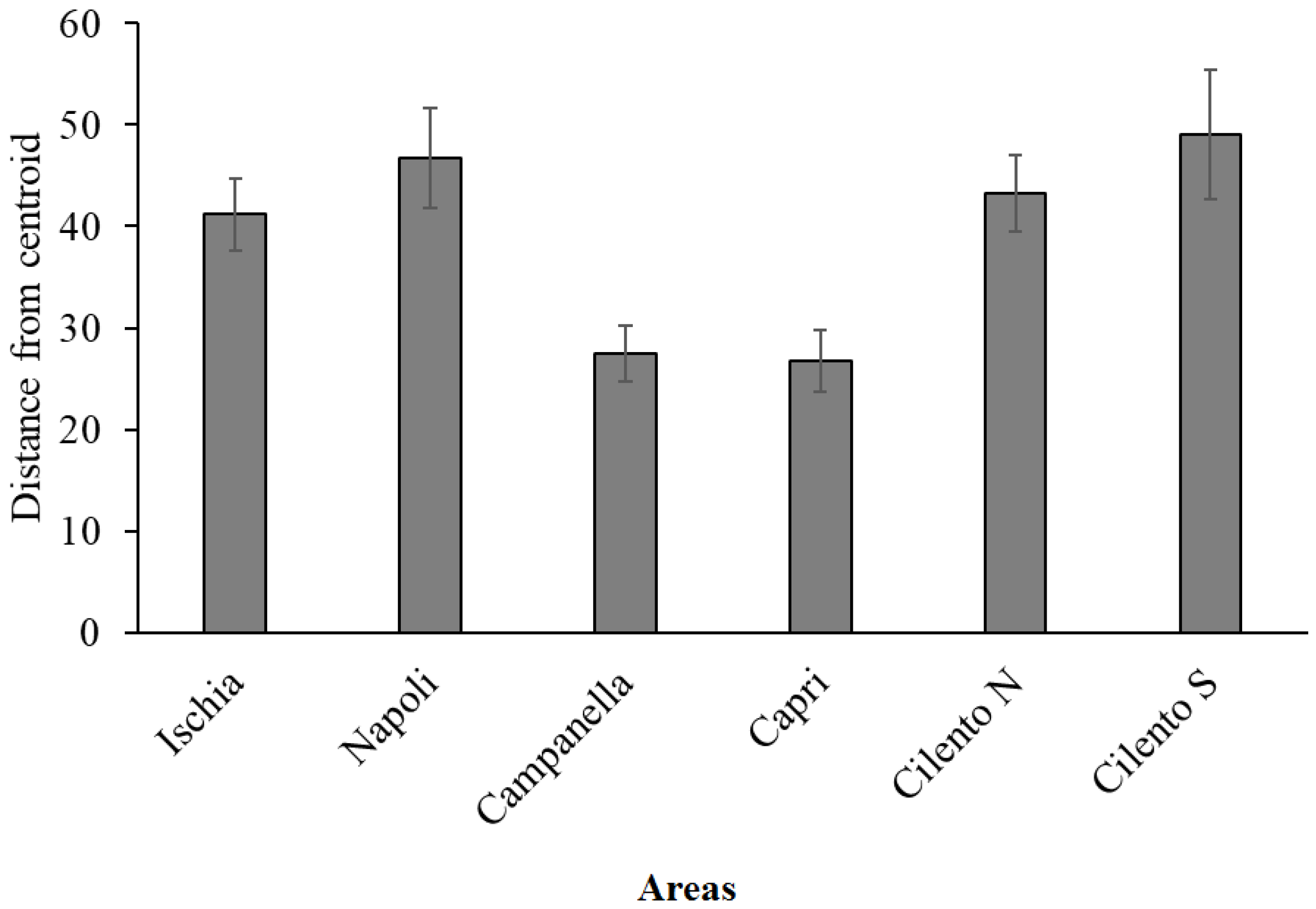
Multivariate PERMANOVA analysis on the species densities dataset showed significant differences both among sites (p = 0.0002) and areas (p = 0.0066); in particular, pairwise tests show that Cilento N and Cilento S were significantly different from Campanella and Capri (p < 0.05). Indeed, the PERMDISP analysis (p = 0.0076), and measure of β-diversity in terms of small-scale heterogeneities, showed the lowest values for Campanella and Capri and the highest values for the other areas (Figure 5).
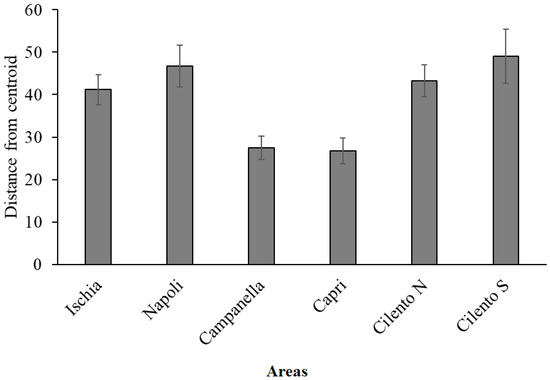
Figure 5.
Mean (± SE) multivariate dispersion (PERMDISP) of replicates around areas centroids, calculated on CSS densities. High distances correspond to high heterogeneity and consequently high β-diversity in each area.
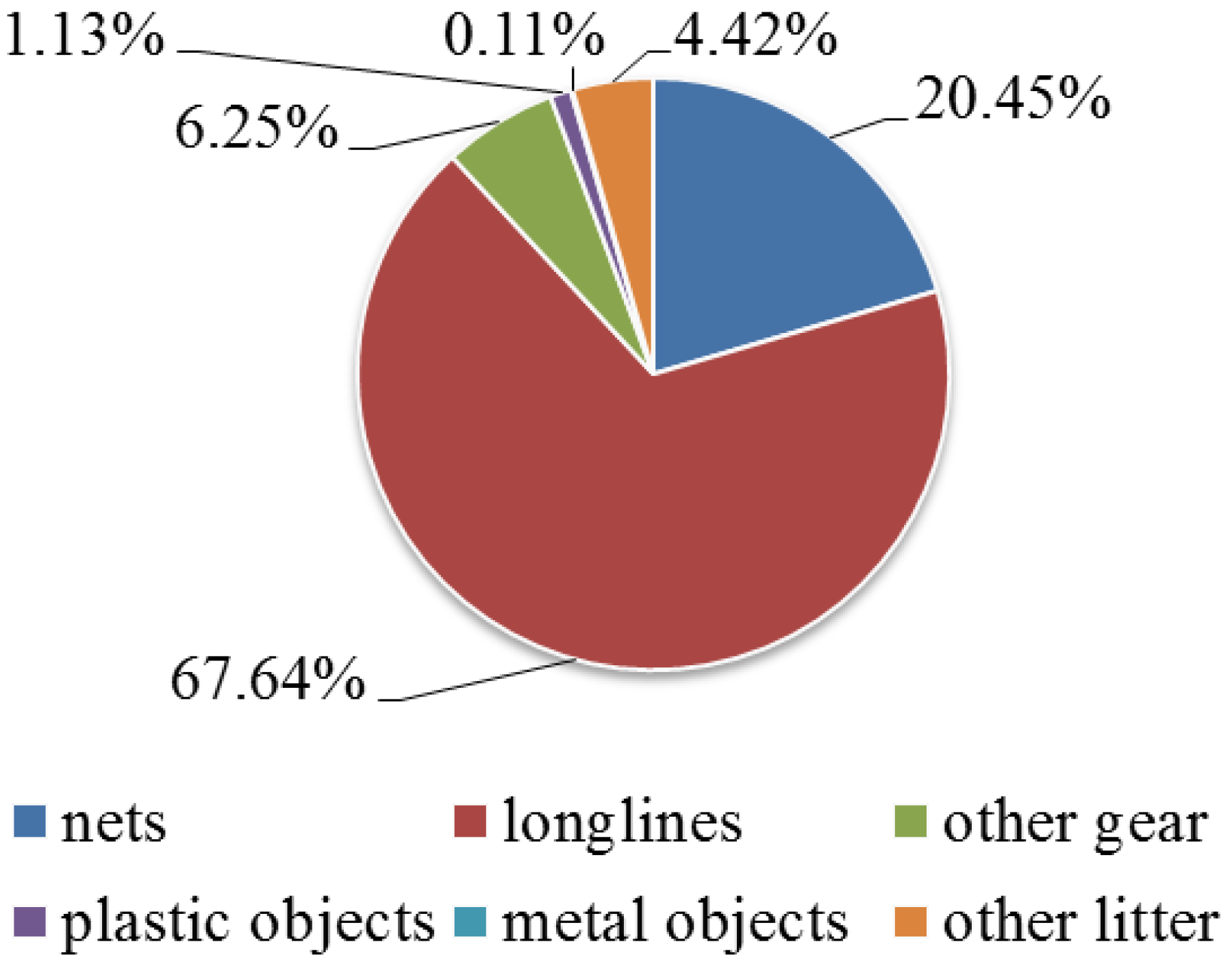
Anthropogenic pressure is mostly represented by longlines (67.6%), followed by nets (20.4%); while metal objects were the most frequent among marine litter (0.1%) (Figure 6). Capri is the most stressed area with the presence of gear and litter in the 32.7% of frames; while, Cilento N represents the less stressed site, with value of 4.6%. In particular, the medium values of fishing gear and marine litter for each area resulted: Ischia 14.2 ± 7.9%, Napoli 24.3 ± 26.3%, Campanella 30.3 ± 23%, Capri 32.7 ± 23.4%, Cilento N 4.5 ± 4.8%, and Cilento S 4.7 ± 4.8%.
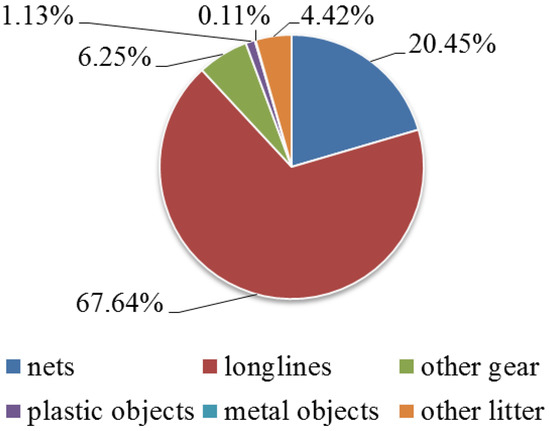
Figure 6.
Anthropogenic pressure is represented by frequencies of fishing gear and marine litter of total.
The nMDS ordination plot (Figure 7) performed on the different areas centroids show three evident clusters: Campanella–Capri, Cilento N–Cilento S, and in the middle Napoli–Ischia. Moreover, the distLM analysis highlights that longlines are the only gear significantly affecting coralligenous assemblages structures (p = 0.0004, r2 = 0.71), explaining 94.5% of the total variation.
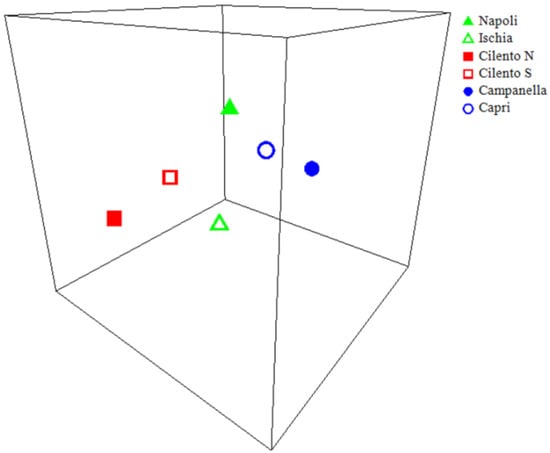
Figure 7.
3D-nMDS plot of anthropogenic pressure, from Euclidian distance matrixes. Points represent centroid of areas levels clusters in 3D multidimensional space, and the distance between any two points represents the difference between those two areas. The high quality of the ordination is indicated by a low-stress value (3D stress = 0.03).
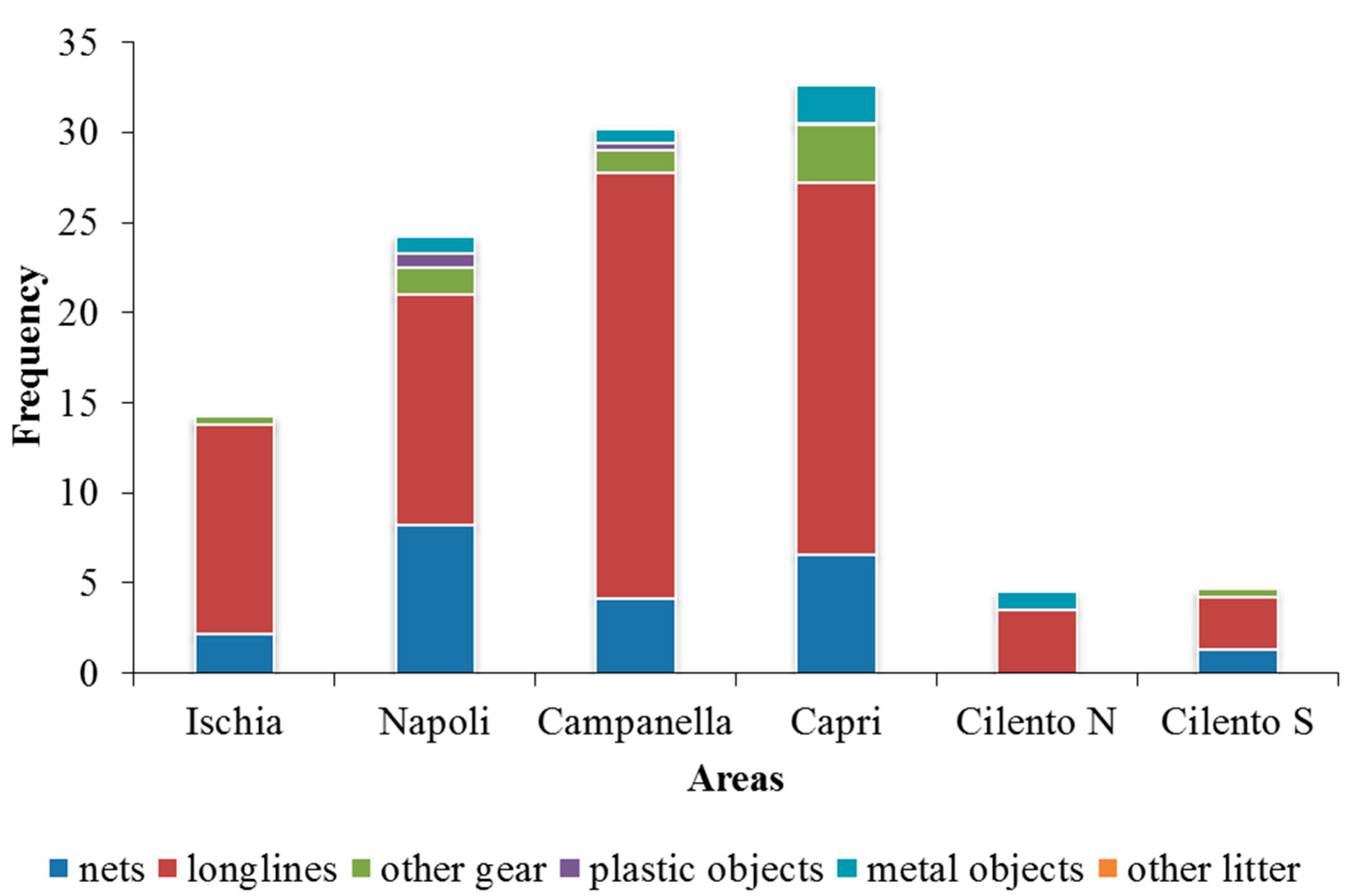
Longlines are the most frequent gear detected in each area (Ischia 11.7%, Napoli 12.8%, Campanella 23.6%, Capri 20.6%, Cilento N 3.3% and Cilento S 2.9%). Second to longlines, nets are also frequent in all areas (Ischia 2.2%, Napoli 8.3%, Campanella 4.1%, Capri 6.6% and Cilento S 1.3%), except in Cilento N where metal objects were the second most frequent ones (1%), but their relation with assemblages structure was not significant (Figure 8). Areas with the higher values of marine litter results were Capri (2.2%), Napoli (1.8%) and Campanella (1.2%). Among marine litter, metal objects were the most abundant (4.4%), followed by plastic objects (1.1%) and other litter (0.1%).
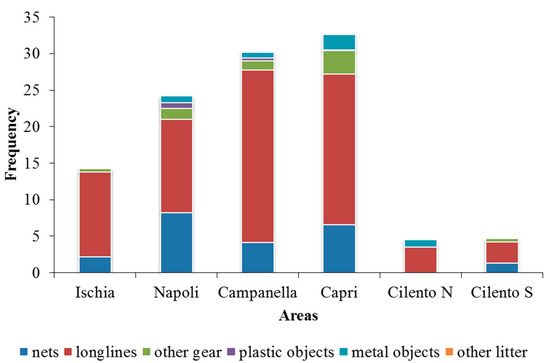
Figure 8.
Frequencies composition of fishing gear and marine litter in each area.
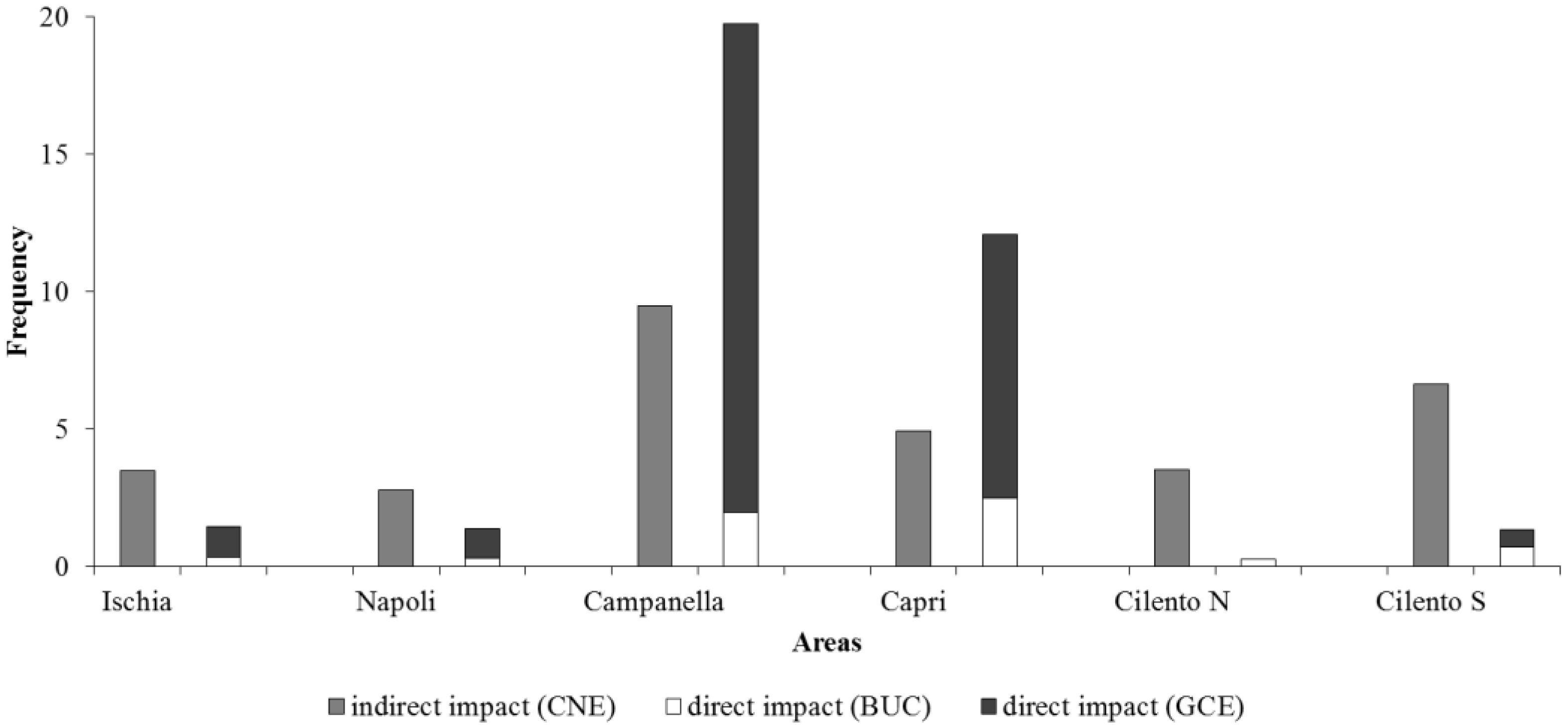
Anthropogenic impact types direct (BUC = broken/upturned colonies and GCE = gears covering/entangling), and indirect (CNE = colonies with necrosis/epibiosis) are shown in Figure 9.

Figure 9.
Examples of BUC (broken/upturned colonies, (A)), GCE (gears covering/entangling, (B)), and CNE (colonies with necrosis/epibiosis, (C)), black arrows show the necrotic parts).
In Figure 10, the frequencies of indirect and direct impacts detected in each area are shown. Campanella and Capri resulted in the most impacted areas (29.2% and 17% of frames), followed by Cilento S (7.9%), while Ischia, Napoli and Cilento N resulted in the less impacted areas (4.9%, 4.1% and 3.8%). In particular, CNE impact is the most frequent in Ischia, Napoli, Cilento N and Cilento S areas (3.5 ± 3.3%, 2.8 ± 4.7%, 3.5 ± 8.8% and 6.6 ± 5.2%, respectively). Instead, GCE is the most frequent impact in Campanella and Capri areas (17.8 ± 15.7% and 9.6 ± 6.7%, respectively).
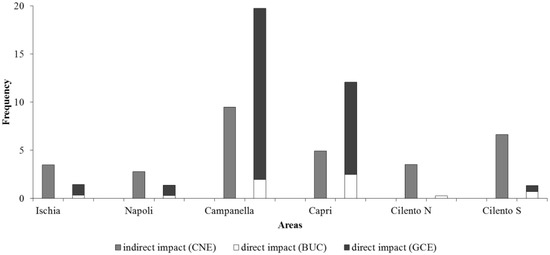
Figure 10.
Frequencies of indirect (CNE) and direct (BUC and GCE) impacts detected in each area.
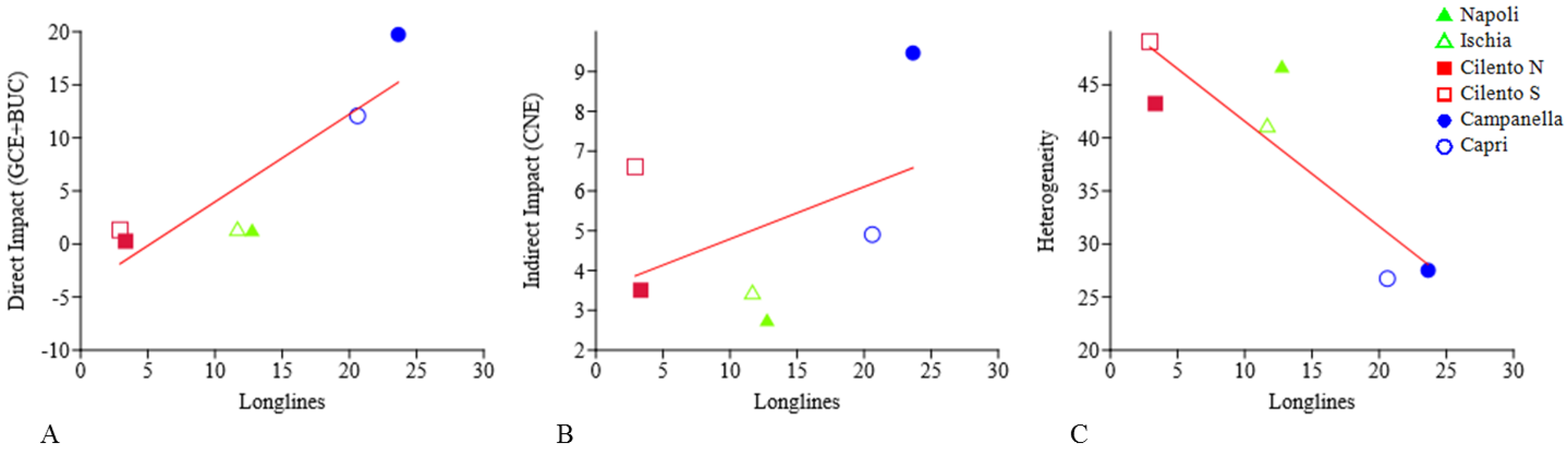
Linear regressions between longlines and direct impact, indirect impact, and heterogeneity are showed in Figure 11 and Table 3. In particular, a significant positive relation (p = 0.0086, r2 = 0.77) is detected between longlines and direct impact; while, no significant relation is detected between longlines and indirect impact. Finally, a significant negative relation (p = 0.035, r2 = 0.77) between longlines and heterogeneity.
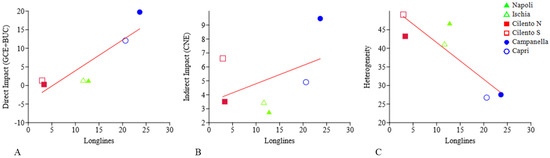
Figure 11.
Linear regressions between longlines and direct impact ((A), p = 0.0086, r2 = 0.77), indirect impact ((B), no correlation), and heterogeneity ((C), p = 0.035, r2 = 0.77).

Table 3.
Mean (± SD) of linear regression independent (longlines) and dependent variables (direct impact, indirect impact, and heterogeneity).
4. Discussion
Campanella and Capri represent the areas with the highest number of organisms. In all areas, however, as evident in Figure 2, density and frequency of CSS show a regular trend, typical of a random spatial species distribution, possibly due to the absence of limiting factors controlling species eco-physiology (e.g., temperature, water, substrate type) [66].
Although the species richness in terms of CSS number among areas is consistent, changes in the species composition were observed; in particular, the phylum Cnidaria dominates in Napoli, Campanella and Capri, while the phylum Bryozoa dominates in Cilento N and Cilento S. This result may also depend on the different abiotic factors among areas, such as the substrate type, the hydrodynamic regime, the depth range, or the water trophism; for example, large gorgonians show a higher preference for trophic and hydrodinamic waters [67]. Coralligenous assemblage’s structure and composition are strongly related to edaphic factors, which may act on their heterogeneity [51,68]. Some studies revealed that extreme environmental conditions may promote the growth of coralligenous assemblages and large suspension feeders [69]; indeed, the most abundant gorgonian species found in the study area grow in low-light conditions, induced probably also by the high turbidity and the prevalence of strong currents [57,70,71], that in the study areas may be near-bottom or upwelling, generating water turnover and promoting nutrient dispersion [72,73].
CSS densities in the Campanella and Capri areas are significantly different from those of the Cilento N and Cilento S areas, and appear to be less heterogeneous in terms of β-diversity, despite the higher densities values. This condition may be the result of high dominance values of few species, possibly due to anthropogenic impact [74]. Indeed, Campanella and Capri are mostly dominated by the large gorgonians E. cavolinii and P. clavata that are highly impacted, since their morphological structure may increase the likelihood to be entangled and damaged by fishing gear. On the contrary, Cilento areas are characterized by the presence of small bryozoans, such as M. truncata and P. fascialis that may decrease the likelihood to be entangled. Several studies have demonstrated that low diversity is linked to a high ecosystem impact. For example, some authors, i.e., [75], showed that impacted assemblages on artificial bottoms present fewer taxa and lower values of diversity, suggesting that those support a less diverse and more homogeneous assemblage than natural substrates. Moreover, communities characterized by high β-diversity should be more resistant to disturbance than others, acting as refuges for neighboring patches [76,77]. High diversity levels make communities able to persist and absorb fluctuations, increasing the resilience potential and reducing the fragility of marine communities and ecosystems [47].
Campanella and Capri are the most stressed areas with the highest numbers of fishing gear and marine litter, followed by Napoli and Ischia, while Cilento N and Cilento S are the least stressed ones with very low values. Generally, longlines dominate in all the sampled areas, followed by nets, with very high numbers in Campanella and Capri. Nets are more abundant in the Napoli area, where the coralligenous bioconstructions are present in the form of banks surrounded by muddy substrate. Longlines are widespread because of their dual-source from both professional and recreational fishing, while nets should be considered mainly professional gear [78]. Moreover, longlines can be used on different bottom types, while the nets are usually set on soft bottoms, even though they may get stuck on hard bottom, with relevant damages for the benthic communities [32,79,80].
Marine litter is the most abundant along the coastal areas of Capri, Napoli and Campanella; in particular, the Napoli area is close to a large urban center, while Capri and Campanella areas are located along popular touristic routes. Despite the highest impact of fishing gear, linked to the developed professional and recreational fishing activities, the use of the coasts for recreational tourism purposes may be responsible for the accumulation of anthropogenic debris, as well as the rivers input [44]. In addition, recreational beach activities can possibly contribute to marine litter accumulation along the coastal areas [81].
CSS colonies with necrosis or epibiosis are present in all areas, with the CNE impact results the highest in Ischia, Napoli, Cilento N and Cilento S. However, they appear lower than 5% in Ischia, Napoli and Cilento S, and between 5 and 10% in Campanella, Capri and Cilento N. These values may be considered respectively low and moderate since the impact is usually considered elevated for values higher than 10% [52,82]. Fishing gear may either eradicate the large colonies or get entangled in the ramifications, scraping their coenenchyma with consequent necrosis tissues and favoring the development of epibionts, which may lead to a burdening of the colonies and to a greater mechanical stress, increasing their resistance to water movement [28,80]. Necrosis, and more extensively, colonies mortality, may also be caused by different stressing factors such as water temperature increase, possibly linked to climate change [83]. The heatwave of 2003 in Europe caused an anomalous warming of seawater, which played a key role in the observed mass mortality event of fan corals [84,85], as well as climatic anomalies, which can generally cause biodiversity loss and ecological shifts [86,87]. Necrosis may also be caused by a high sedimentation rate, as a consequence of resuspension caused by trawl-fishing [88], altering metabolic functions, enhancing respiratory and interfering with the prey-capturing apparatus, and also compromising the settlement [89]. For this reason, the identification of the effective cause of this indirect impact can be difficult.
Direct impact, mostly represented by GCE, prevailed in Campanella and Capri areas, where it is strictly connected to the high presence of fishing gear. Erect or branched organisms among Cnidaria, Porifera and Bryozoa are the most endangered by fishing gear since they are often broken and upturned [28,90,91,92]. Direct impact was mostly caused by the presence of abundant longlines, while no relation was found between indirect impact and longlines. Moreover, an indirect relation between heterogeneity and longlines was detected, demonstrating that fishing activity may cause severe species diversity loss [93,94,95].
A better knowledge of the anthropogenic activities impact on benthic communities of mesophotic reefs is increasingly needed in order to improve the management tools to protect one of the most fragile and valuable Mediterranean habitats. This should be reached through sustainable fishing techniques, adequate protection measures and long-term monitoring programs. Environmental protection, also through Marine Protected Areas, should respond not only to an ecological purpose but also to socioeconomic dimensions of sustainability [96].
Author Contributions
Conceptualization, R.S. and G.F.R.; data curation, F.F., L.A. and L.D.; formal analysis, F.F.; investigation, F.F., L.D., F.D.S. and F.R.; methodology, L.A.; software, L.A.; writing–original draft, F.F.; writing–review and editing, R.S. and G.F.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
We sincerely thank the anonymous reviewers for their valuable comments and suggestions, which helped us to improve the quality of the manuscript. Thanks are due to the crew members and captain of R/V Cormorano V, ROV pilots and the informatics system technicians of Subonica Ltd. for field activities and data acquisition.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ballesteros, E. Mediterranean coralligenous assemblages: A synthesis of present knowledge. Oceanogr. Mar. Biol. Annu. Rev. 2006, 44, 123–195. [Google Scholar]
- Sheehan, P.M.; Fagerstrom, J.A. The evolution of reef communities. Palaios 1988, 3, 251. [Google Scholar] [CrossRef]
- Baker, E.K.; Puglise, K.A.; Harris, P.T. (Eds.) Mesophotic Coral Ecosystems—A Lifeboat for Coral Reefs? The United Nations Environment Programme: Nairobi, Kenya; GRID-Arendal: Arendal, Norway, 2016; p. 98. [Google Scholar]
- Ferrigno, F.; Russo, G.F.; Semprucci, F.; Sandulli, R. Unveiling the state of some underexplored deep coralligenous banks in the Gulf of Naples (Mediterranean Sea, Italy). Reg. Stud. Mar. Sci. 2018, 22, 82–92. [Google Scholar] [CrossRef]
- Corriero, G.; Pierri, C.; Mercurio, M.; Marzano, C.N.; Tarantini, S.O.; Gravina, M.F.; Lisco, S.; De Giosa, F.; Valenzano, E.; Giangrande, A.; et al. Mediterranean mesophotic coral reef built by non-symbiotic scleractinians. Sci. Rep. 2019, 9, 1–17. [Google Scholar] [CrossRef]
- Basso, D. Deep rhodolith distribution in the Pontian Islands, Italy: A model for the paleoecology of a temperate sea. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1998, 137, 173–187. [Google Scholar] [CrossRef]
- Rendina, F.; Kaleb, S.; Caragnano, A.; Ferrigno, F.; Appolloni, L.; Donnarumma, L.; Russo, G.F.; Sandulli, R.; Roviello, V.; Falace, A. Distribution and characterization of deep rhodolith beds off the Campania coast (SW Italy, Mediterranean Sea). Plants 2020, 9, 985. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.G.; Lawton, J.H.; Shachak, M. Organisms as ecosystems engineers. Nord. Soc. Oikos 1994, 69, 373–386. [Google Scholar] [CrossRef]
- Sartoretto, S. Vitesse de Croissance et Bioérosion des Concrétionnements “Coralligènes” de Méditerranée Nord-Occidentale. Rapport avec les Variations Holocènes du Niveau Marin. Ph.D. Thesis, Université d’Aix-Marseille, Marseille, France, 1996; p. 194. [Google Scholar]
- Boudouresque, C.F. Marine biodiversity in the Mediterranean: Status of species, populations and communities. Sci. Rep. Port-Cros Natl. Park 2004, 20, 97–146. [Google Scholar]
- Coll, M.; Piroddi, C.; Steenbeek, J.; Kaschner, K.; Ben Rais Lasram, F.; Aguzzi, J.; Ballesteros, E.; Bianchi, C.N.; Corbera, J.; Dailianis, T.; et al. The biodiversity of the Mediterranean Sea: Estimates, patterns, and threats. PLoS ONE 2010, 5, e11842. [Google Scholar] [CrossRef] [Green Version]
- Ferrigno, F.; Appolloni, L.; Rendina, F.; Donnarumma, L.; Russo, G.F.; Sandulli, R. Red coral (Corallium rubrum) populations and coralligenous characterization within “Regno di Nettuno MPA” (Tyrrhenian Sea, Italy). Eur. Zool. J. 2020, 87, 203–213. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Commission Decision (EU) 2017/848 of 17 May 2017 laying down criteria and methodological standards on good environmental status of marine waters and specifications and standardized methods for monitoring and assessment, and repealing Decision 2010/477/EU. Off. J. Eur. Union L 2017, 125, 43. [Google Scholar]
- Tonin, S. Economic value of marine biodiversity improvement in coralligenous habitats. Ecol. Indic. 2018, 85, 1121–1132. [Google Scholar] [CrossRef]
- Krieger, K.J.; Wing, B.L. Megafauna associations with deep water corals (Primnoa spp.) in the Gulf of Alaska. Hydrobiologia 2002, 471, 83–90. [Google Scholar] [CrossRef]
- Henry, L.A.; Roberts, J.M. Biodiversity and ecological composition of macrobenthos on cold-water coral mounds and adjacent off-mound habitat in the bathyal Porcupine Seabight, NE Atlantic. Deep-Sea Res. Part I Oceanogr. Res. Pap. 2007, 54, 654–672. [Google Scholar] [CrossRef]
- Lloret, J. Human health benefits supplied by Mediterranean marine biodiversity. Mar. Pollut. Bull. 2010, 60, 1640–1646. [Google Scholar] [CrossRef]
- Salomidi, M.; Katsanevakis, S.; Borja, A.; Braeckman, U.; Damalas, D.; Galpasoro, I.; Mifsud, R.; Mirto, S.; Pascual, M.; Pipitone, C.; et al. Assessment of goods and services, vulnerability, and conservation status of European seabed biotopes: A stepping stone towards ecosystem-based marine spatial management. Mediterr. Mar. Sci. 2012, 13, 49–88. [Google Scholar] [CrossRef] [Green Version]
- Buonocore, E.; Donnarumma, L.; Appolloni, L.; Miccio, A.; Russo, G.F.; Franzese, P.P. Marine natural capital and ecosystem services: An environmental accounting model. Ecol. Model. 2020, 424, 109029. [Google Scholar] [CrossRef]
- Walther, G.R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.C.; Fromentin, J.-M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Rendina, F.; Bouchet, P.J.; Appolloni, L.; Russo, G.F.; Sandulli, R.; Kolzenburg, R.; Putra, A.; Ragazzola, F. Physiological response of the coralline alga Corallina officinalis L. to both predicted long-term increases in temperature and short-term heatwave events. Mar. Environ. Res. 2019, 150, 104764. [Google Scholar] [CrossRef] [PubMed]
- Halpern, B.S.; Walbridge, S.; Selkoe, K.A.; Kappel, C.V.; Micheli, F.; D’Agrosa, C.; Bruno, J.F.; Casey, K.S.; Ebert, C.; Fox, H.E.; et al. A global map of human impact on marine ecosystems. Science 2008, 319, 948–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzo, L.; Musco, L.; Crocetta, F. Cohabiting with litter: Fish and benthic assemblages in coastal habitats of a heavily urbanized area. Mar. Pollut. Bull. 2021, 164, 112077. [Google Scholar] [CrossRef]
- Clark, M.R.; Koslow, J.A. Impacts of fisheries on seamounts. In Seamounts: Ecology, Fisheries and Conservation. Blackwell Publishing; Pitcher, T.J., Morato, T., Hart, P.J.B., Clark, M.R., Haggan, N., Santos, R.S., Eds.; Blackwell Publishing: Oxford, UK, 2007; pp. 413–441. [Google Scholar]
- Macfadyen, G.; Huntington, T.; Cappell, R. Abandoned, lost or Otherwise Discarded Fishing Gear UNEP Regional Seas Reports and Studies. FAO Fish. Aquac. Tech. Pap. 2009, 523, 115. [Google Scholar]
- Althaus, F.; Williams, A.; Schlacher, T.A.; Kloser, R.J.; Green, M.A.; Barker, B.A.; Bax, N.J.; Brodie, P.; Schlacher-Hoenlinger, M.A. Impacts of bottom trawling on deep-coral ecosystems of seamounts are long-lasting. Mar. Ecol. Prog. Ser. 2009, 397, 279–294. [Google Scholar] [CrossRef]
- Buhl-Mortensen, P.; Buhl-Mortensen, L. Impacts of bottom trawling and litter on the seabed in Norwegian waters. Front. Mar. Sci. 2018, 5, 42. [Google Scholar] [CrossRef] [Green Version]
- Betti, F.; Bavestrello, G.; Bo, M.; Ravanetti, G.; Enrichetti, F.; Coppari, M.; Cappanera, V.; Venturini, S.; Cattaneo-Vietti, R. Evidences of fishing impact on the coastal gorgonian forests inside the Portofino MPA (NW Mediterranean Sea). Ocean. Coast. Manag. 2020, 187, 105105. [Google Scholar] [CrossRef]
- Ballesteros, L.V.; Matthews, J.L.; Hoeksema, B.W. Pollution and coral damage caused by derelict fishing gear on coral reefs around Koh Tao, Gulf of Thailand. Mar. Pollut. Bull. 2018, 135, 1107–1116. [Google Scholar] [CrossRef]
- Figueroa-Pico, J.; Tortosa, F.S.; Carpio, A.J. Coral fracture by derelict fishing gear affects the sustainability of the marginal reefs of Ecuador. Coral Reefs 2020, 39, 819–827. [Google Scholar] [CrossRef]
- Angiolillo, M.; di Lorenzo, B.; Farcomeni, A.; Bo, M. Distribution and assessment of marine debris in the deep Tyrrhenian Sea (NW Mediterranean Sea, Italy). Mar. Pollut. Bull. 2015, 92, 149–159. [Google Scholar] [CrossRef]
- Ferrigno, F.; Appolloni, L.; Russo, G.F.; Sandulli, R. Impact of fishing activities on different coralligenous assemblages of Gulf of Naples (Italy). J. Mar. Biol. Assoc. UK 2018, 98, 41–50. [Google Scholar] [CrossRef]
- Giusti, M.; Canese, S.; Fourt, M.; Bo, M.; Innocenti, C.; Goujard, A.; Daniel, B.; Angeletti, L.; Taviani, M.; Aquilina, L.; et al. Coral forests and Derelict Fishing Gears in submarine canyon systems of the Ligurian Sea. Prog. Oceanogr. 2019, 178, 102186. [Google Scholar] [CrossRef]
- Cerrano, C.; Arillo, A.; Azzini, F.; Calcinai, B.; Castellano, L.; Muti, C.; Valisano, L.; Zega, G.; Bavestrello, G. Gorgonian population recovery after a mass mortality event. Aquat. Conserv. Mar. Freshw. Ecosyst. 2005, 15, 147–157. [Google Scholar] [CrossRef]
- Piazzi, L.; Gennaro, P.; Balata, D. Threats to macroalgal coralligenous assemblages in the Mediterranean Sea. Mar. Pollut. Bull. 2012, 64, 2623–2629. [Google Scholar] [CrossRef] [PubMed]
- Gregory, M.R. Environmental implications of plastic debris in marine settings-entanglement, ingestion, smothering, hangers-on, hitch-hiking and alien invasions. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2013–2025. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M. The Complex Mixture, Fate and Toxicity of Chemicals Associated With Plastic Debris in the Marine Environment. In Marine Anthropogenic Litter; Springer: Berlin/Heidelberg, Germany, 2015; pp. 117–140. [Google Scholar]
- Caruso, G. Microplastics as vectors of contaminants. Mar. Pollut. Bull. 2019, 146, 921–924. [Google Scholar] [CrossRef] [PubMed]
- Romeo, T.; Battaglia, P.; Pedà, C.; Consoli, P.; Andaloro, F.; Fossi, M.C. First evidence of presence of plastic debris in stomach of large pelagic fish in the Mediterranean Sea. Mar. Pollut. Bull. 2015, 95, 358–361. [Google Scholar] [CrossRef]
- Rummel, D.C.; Löder, G.J.M.; Fricke, F.N.; Lang, T.; Griebeler, E.M.; Janke, M.; Gerdts, G. Plastic ingestion by pelagic and demersal fish from the North Sea and Baltic Sea. Mar. Pollut. Bull. 2016, 102, 134–141. [Google Scholar] [CrossRef]
- Markic, A.; Niemand, C.; Bridson, J.H.; Mazouni-Gaertner, N.; Gaertner, J.C.; Eriksen, M.; Bowen, M. Double trouble in the South Pacific subtropical gyre: Increased plastic ingestion by fish in the oceanic accumulation zone. Mar. Pollut. Bull. 2018, 136, 547–564. [Google Scholar] [CrossRef]
- Gall, S.C.; Thompson, R.C. The impact of debris on marine life. Mar. Pollut. Bull. 2015, 92, 170–179. [Google Scholar] [CrossRef]
- Consoli, P.; Falautano, M.; Sinopoli, M.; Perzia, P.; Canese, S.; Esposito, V.; Battaglia, P.; Romeo, T.; Andaloro, F.; Galgani, F.; et al. Composition and abundance of benthic marine litter in a coastal area of the central Mediterranean Sea. Mar. Pollut. Bull. 2018, 136, 243–247. [Google Scholar] [CrossRef]
- Consoli, P.; Scotti, G.; Romeo, T.; Fossi, M.C.; Esposito, V.; D’Alessandro, M.; Battaglia, P.; Galgani, F.; Figurella, F.; Pragnell-Raasch, H.; et al. Characterization of seafloor litter on Mediterranean shallow coastal waters: Evidence from Dive Against Debris®, a citizen science monitoring approach. Mar. Pollut. Bull. 2020, 150, 110763. [Google Scholar] [CrossRef]
- Rendina, F.; Ferrigno, F.; Appolloni, L.; Donnarumma, L.; Sandulli, R.; Fulvio, G.F. Anthropic pressure due to lost fishing gears and marine litter on different rhodolith beds off the Campania Coast (Tyrrhenian Sea, Italy). Ecol. Quest. 2020, 31, 1–17. [Google Scholar] [CrossRef]
- Scotti, G.; Esposito, V.; D’Alessandro, M.; Panti, C.; Vivona, P.; Consoli, P.; Figurella, F.; Romeo, T. Seafloor litter along the Italian coastal zone: An integrated approach to identify sources of marine litter. Waste Manag. 2021, 124, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Appolloni, L.; Bevilacqua, S.; Sbrescia, L.; Sandulli, R.; Terlizzi, A.; Russo, G.F. Does full protection count for the maintenance of beta/diversity patterns in marine communities? Evidence from Mediterranean fish assemblages. Aquatic conservation. Mar. Freshw. Ecosyst. 2017, 27, 828–838. [Google Scholar] [CrossRef] [Green Version]
- Appolloni, L.; Zeppilli, D.; Donnarumma, L.; Baldrighi, E.; Chianese, E.; Russo, G.F.; Sandulli, R. Seawater Acidification Affects Beta-Diversity of Benthic Communities at a Shallow Hydrothermal Vent in a Mediterranean Marine Protected Area (Underwater Archaeological Park of Baia, Naples, Italy). Diversity 2020, 12, 464. [Google Scholar] [CrossRef]
- Fabri, M.C.; Vinha, B.; Allais, A.G.; Bouhier, M.E.; Dugornay, O.; Gaillot, A.; Arnaubec, A. Evaluating the ecological status of cold-water coral habitats using noninvasive methods: An example from Cassidaigne canyon, northwestern Mediterranean Sea. Prog. Oceanogr. 2019, 178, 102172. [Google Scholar] [CrossRef] [Green Version]
- Cataudella, S.; Spagnolo, M. Lo Stato Della Pesca e Dell’acquacoltura nei Mari Italiani; Ministero delle Politiche Agricole Alimentari e Forestali: Rome, Italy, 2011; 877p.
- Appolloni, L.; Ferrigno, F.; Russo, G.F.; Sandulli, R. β-Diversity of morphological groups as indicator of coralligenous community quality status. Ecol. Indic. 2020, 109, 105840. [Google Scholar] [CrossRef]
- Ferrigno, F.; Russo, G.F.; Sandulli, R. Coralligenous Bioconstructions Quality Index (CBQI): A synthetic indicator to assess the status of different types of coralligenous habitats. Ecol. Indic. 2017, 82, 271–279. [Google Scholar] [CrossRef]
- Russo, G.F. Il banco di Santa Croce. Ambient. e Mass Media mare e le coste 96, 1992.
- Russo, G.F. I fondali marini del Golfo di Napoli e del litorale ischitano: Particolarità e paradossi. Scheria 1995, 10, 58–72. [Google Scholar]
- Appolloni, L.; Russo, G.F. Underwater landscape in the Bay of Naples for maritime spatial planning. In La Baia Di Napoli—Strategie Integrate per La Conservazione e La Fruizione Del Paesaggio Culturale; Aveta, A., Marino, B.G., Amore, R., Eds.; Artstudio: Napoli, Italy, 2017; pp. 71–74. [Google Scholar]
- Available online: https://www.minambiente.it/sites/default/files/archivio/allegati/strategia_marina/ARPA/SM_ARPA_metodMOD_7_REV.pdf (accessed on 10 January 2018).
- Gori, A.; Rossi, S.; Berganzo, E.; Pretus, J.L.; Dale, M.R.; Gili, J.M. Spatial distribution patterns of the gorgonians Eunicella singularis, Paramuricea clavata, and Leptogorgia sarmentosa (Cape of Creus, Northwestern Mediterranean Sea). Mar. Biol. 2011, 158, 143–158. [Google Scholar] [CrossRef]
- Terlizzi, A.; Anderson, M.J.; Fraschetti, S.; Benedetti-Cecchi, L. Scales of spatial variation in Mediterranean subtidal sessile assemblages at different depths. Mar. Ecol. Prog. Ser. 2007, 332, 25–39. [Google Scholar] [CrossRef] [Green Version]
- Anderson, M.J. Permutation tests for univariate or multivariate analysis of variance and regression. Can. J. Fish. Aquat. Sci. 2001, 58, 626–639. [Google Scholar] [CrossRef]
- Anderson, M.J.; Ter Braak, C.J.F. Permutation tests for multi-factorial analysis of variance. J. Stat. Comput. Simul. 2003. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. Primer: User Manual/Tutorial; Prim. Ltd.: Plymouth, UK, 2015; p. 93. [Google Scholar]
- Anderson, M.J.; Willis, T.J. Canonical Analysis of Principal Coordinates: A Useful Method of Constrained Ordination for Ecology. Ecology 2003, 84, 511–525. [Google Scholar] [CrossRef]
- Anderson, M.J.; Ellingsen, K.E.; McArdle, B.H. Multivariate dispersion as a measure of beta diversity. Ecol. Lett. 2006, 9, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.J. DISTLM v. 5: A FORTRAN Computer Program to Calculate a Distance-Based Multivariate Analysis for a Linear Model; Department of Statistics, University: Auckland, UK, 2004; Volume 10, p. 2016. [Google Scholar]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Guisan, A.; Thuiller, W. Predicting species distribution: Offering more than simple habitat models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef]
- Di Camillo, C.G.; Ponti, M.; Bavestrello, G.; Krzelj, M.; Cerrano, C. Building a baseline for habitat-forming corals by a multi-source approach, including Web Ecological Knowledge. Biodivers. Conserv. 2018, 27, 1257–1276. [Google Scholar] [CrossRef]
- Donnarumma, L.; Sandulli, R.; Appolloni, L.; Ferrigno, F.; Rendina, F.; Di Stefano, F.; Russo, G.F. Bathymetrical and temporal variations in soft-bottom molluscan assemblages in the coastal area facing the Sarno River mouth (Mediterranean Sea, Gulf of Naples). Ecol. Quest. 2020, 31, 1–20. [Google Scholar] [CrossRef]
- Sini, M.; Garrabou, J.; Trygonis, V.; Koutsoubas, D. Coralligenous formations dominated by Eunicella cavolini (Koch, 1887) in the NE Mediterranean: Biodiversity and structure. Mediterr. Mar. Sci. 2019, 20, 174–188. [Google Scholar] [CrossRef] [Green Version]
- Irving, A.D.; Connell, S.D. Sedimentation and light penetration interact to maintain heterogeneity of subtidal habitats: Algal versus invertebrate dominated assemblages. Mar. Ecol. Prog. Ser. 2002, 245, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Balata, D.; Piazzi, L.; Cecchi, E.; Cinelli, F. Variability of Mediterranean coralligenous assemblages subject to local variation in sediment deposition. Mar. Environ. Res. 2005, 60, 403–421. [Google Scholar] [CrossRef]
- De Ruggiero, P.; Napolitano, E.; Iacono, R.; Pierini, S. A high-resolution modelling study of the circulation along the Campania coastal system, with a special focus on the Gulf of Naples. Cont. Shelf Res. 2016, 122, 85–101. [Google Scholar] [CrossRef]
- De Ruggiero, P.; Esposito, G.; Napolitano, E.; Iacono, R.; Pierini, S.; Zambianchi, E. Modelling the marine circulation of the Campania coastal system (Tyrrhenian Sea) for the year 2016: Analysis of the dynamics. J. Mar. Syst. 2020, 210, 103388. [Google Scholar] [CrossRef]
- Yang, H.Y.; Chen, B.; Piersma, T.; Zhang, Z.; Ding, C. Molluscs of an intertidal soft-sediment area in China: Does overfishing explain a high density but low diversity community that benefits staging shorebirds? J. Sea Res. 2016, 109, 20–28. [Google Scholar]
- Sedano, F.; Florido, M.; Rallis, I.; Espinosa, F.; Gerovasileiou, V. Comparing sessile benthos on shallow artificial versus natural hard substrates in the Eastern Mediterranean Sea. Mediterr. Mar. Sci. 2019, 20, 688–702. [Google Scholar] [CrossRef] [Green Version]
- Soininen, J. Species turnover along abiotic and biotic gradients: Patterns in space equal patterns in time? Bioscience 2010, 60, 433–439. [Google Scholar] [CrossRef] [Green Version]
- Ferrigno, F.; Bianchi, C.N.; Lasagna, R.; Morri, C.; Russo, G.F.; Sandulli, R. Corals in high diversity reefs resist human impact. Ecol. Indic. 2016, 70, 106–113. [Google Scholar] [CrossRef]
- Marengo, M.; Culioli, J.M.; Santoni, M.C.; Marchand, B.; Durieux, E.D.H. Comparative analysis of artisanal and recreational fisheries for Dentex dentex in a Marine Protected Area. Fish. Manag. Ecol. 2015, 22, 249–260. [Google Scholar] [CrossRef] [Green Version]
- Good, T.P.; June, J.A.; Etnier, M.A.; Broadhurst, G. Derelict fishing nets in Puget Sound and the Northwest Straits: Patterns and threats to marine fauna. Mar. Pollut. Bull. 2010, 60, 39–50. [Google Scholar] [CrossRef]
- Bo, M.; Bava, S.; Canese, S.; Angiolillo, M.; Cattaneo-Vietti, R.; Bavestrello, G. Fishing impact on deep Mediterranean rocky habitats as revealed by ROV investigation. Biol. Conserv. 2014, 171, 167–176. [Google Scholar] [CrossRef]
- Portman, M.E.; Brennan, R.E. Marine litter from beach-based sources: Case study of an Eastern Mediterranean coastal town. Waste Manag. 2017, 69, 535–544. [Google Scholar] [CrossRef] [Green Version]
- Kipson, S.; Linares, C.; Čižmek, H.; Cebrián, E.; Ballesteros, E.; Bakran-Petricioli, T.; Garrabou, J. Population structure and conservation status of the red gorgonian Paramuricea clavata (Risso, 1826) in the Eastern Adriatic Sea. Mar. Ecol. 2015, 36, 982–993. [Google Scholar] [CrossRef] [Green Version]
- Karvonen, A.; Rintamäki, P.; Jokela, J.; Valtonen, E.T. Increasing water temperature and disease risks in aquatic systems: Climate change increases the risk of some, but not all, diseases. Int. J. Parasitol. 2010, 40, 1483–1488. [Google Scholar] [CrossRef] [PubMed]
- Garrabou, J.; Coma, R.; Bensoussan, N.; Bally, M.; Chevaldonné, P.; Cigliano, M.; Diaz, D.; Harmelin, J.G.; Gambi, M.C.; Kersting, D.K.; et al. Mass mortality in Northwestern Mediterranean rocky benthic communities: Effects of the 2003 heat wave. Glob. Chang. Biol. 2009, 15, 1090–1103. [Google Scholar] [CrossRef]
- Turicchia, E.; Abbiati, M.; Sweet, M.; Ponti, M. Mass mortality hits gorgonian forests at Montecristo Island. Dis. Aquat. Org. 2018, 131, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Ponti, M.; Perlini, R.A.; Ventra, V.; Grech, D.; Abbiati, M.; Cerrano, C. Ecological shifts in Mediterranean coralligenous assemblages related to gorgonian forest loss. PLoS ONE 2014, 9, e102782. [Google Scholar] [CrossRef]
- Verdura, J.; Linares, C.; Ballesteros, E.; Coma, R.; Uriz, M.J.; Bensoussan, N.; Cebrian, E. Biodiversity loss in a Mediterranean ecosystem due to an extreme warming event unveils the role of an engineering gorgonian species. Sci. Rep. 2019, 9, 1–11. [Google Scholar]
- Martín, J.; Puig, P.; Palanques, A.; Masqué, P.; García-Orellana, J. Effect of commercial trawling on the deep sedimentation in a Mediterranean submarine canyon. Mar. Geol. 2008, 252, 150–155. [Google Scholar] [CrossRef] [Green Version]
- Tseng, L.C.; Dahms, H.U.; Hsu, N.J.; Hwang, J.S. Effects of sedimentation on the gorgonian Subergorgia suberosa (Pallas, 1766). Mar. Biol. 2011, 158, 1301–1310. [Google Scholar] [CrossRef]
- Clark, M.R.; Althaus, F.; Schlacher, T.A.; Williams, A.; Bowden, D.A.; Rowden, A.A. The impacts of deep-sea fisheries on benthic communities: A review. ICES J. Mar. Sci. 2016, 73 (Suppl. 1), i51–i69. [Google Scholar] [CrossRef]
- Lambert, G.I.; Murray, L.G.; Hiddink, J.G.; Hinz, H.; Lincoln, H.; Hold, N.; Cambiè, G.; Kaiser, M.J. Defining thresholds of sustainable impact on benthic communities in relation to fishing disturbance. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Casoli, E.; Piazzi, L.; Nicoletti, L.; Jona-Lasinio, G.; Cecchi, E.; Mancini, G.; Belluscio, A.; Ardizzone, G. Ecology, distribution and demography of erect bryozoans in Mediterranean coralligenous reefs. Estuar. Coast. Shelf Sci. 2020, 235, 106573. [Google Scholar] [CrossRef]
- Agardy, T. Effects of fisheries on marine ecosystems: A conservationist’s perspective. ICES J. Mar. Sci. 2000, 57, 761–765. [Google Scholar] [CrossRef] [Green Version]
- Pratchett, M.S.; Hoey, A.S.; Wilson, S.K.; Messmer, V.; Graham, N.A. Changes in biodiversity and functioning of reef fish assemblages following coral bleaching and coral loss. Diversity 2011, 3, 424–452. [Google Scholar] [CrossRef] [Green Version]
- Lenzen, M.; Moran, D.; Kanemoto, K.; Foran, B.; Lobefaro, L.; Geschke, A. International trade drives biodiversity threats in developing nations. Nature 2012, 486, 109–112. [Google Scholar] [CrossRef]
- Picone, F.; Buonocore, E.; Chemello, R.; Russo, G.F.; Franzese, P.P. Exploring the development of scientific research on Marine Protected Areas: From conservation to global ocean sustainability. Ecol. Inform. 2021, 61, 101200. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).