Bee Guilds’ Responses to Urbanization in Neotropics: A Case Study †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Bee Sampling
2.3. Environmental Conditions
2.4. Data Analysis
3. Results
3.1. Bee Community
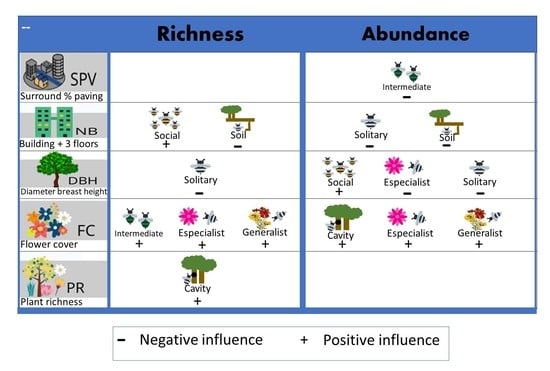
3.2. Environmental Influence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations, Department of Economic and Social Affairs. Population Division; World Population Prospects 2019: Press Release. (ST/ESA/SER.A/352); New York, NY, USA, 2018; p. 27. Available online: https://www.un.org/development/desa/en/news/population/2018-revision-of-world-urbanization-prospects.html (accessed on 13 April 2020).
- McKinney, M.L. Urbanization, biodiversity, and conservation. Bioscience 2002, 52, 883–8902. [Google Scholar] [CrossRef]
- Vanbergen, A.J.; Garratt, M.P. the Insect Pollinators Initiative Threats to an ecosystem service: Pressures on pollinators. Front. Ecol. Environ. 2013, 11, 251–259. [Google Scholar] [CrossRef] [Green Version]
- Fortel, L.; Henry, M.; Guilbaud, L.; Guirao, A.L.; Kuhlmann, M.; Mouret, H.; Vaissière, B.E. Decreasing abundance, increasing diversity and changing structure of the wild bee community (Hymenoptera: Anthophila) along an urbanization gradient. PLoS ONE 2014, 9, e104679. [Google Scholar] [CrossRef] [PubMed]
- Ollerton, J.; Erenler, H.; Edwards, M.; Crockett, R. Extinctions of Aculeate pollinators in Britain and the role of large-scale agricultural changes. Science 2014, 346, 1360–1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamblin, A.L.; Youngsteadt, E.; Frank, S.D. Wild bee abundance declines with urban warming, regardless of floral density. Urban Ecosyst. 2018, 21, 419–428. [Google Scholar] [CrossRef]
- Roubik, D.W. Ecology and Natural History of the Tropical Bees; Cambridge University: New York, NY, USA, 1989; p. 514. [Google Scholar]
- Cane, J.H.; Sipes, S. Characterizing floral specialization by bees: Analytical methods and a revised lexicon for oligolecty. In Plant-Pollinator Interactions: From Specialization to Generalization; Waser, N.M., Ollerton, J., Eds.; University of Chicago Press: Chicago, IL, USA, 2006; pp. 99–122. [Google Scholar]
- Morato, E.F.; Martins, R.P. An overview of proximate factors affecting the nesting behavior of solitary wasps and bees (Hymenoptera: Aculeata) in preexisting cavities in wood. Neotrop. Entomol. 2006, 35, 285–298. [Google Scholar] [CrossRef] [Green Version]
- Steneck, R.; Dethier, M. A Functional Group Approach to the Structure of Algal-Dominated Communities. Oikos 1994, 69, 476–498. [Google Scholar] [CrossRef] [Green Version]
- Threlfall, C.G.; Walker, K.; Williams, N.S.G.; Hahs, A.K.; Mata, L.; Stork, N.; Livesley, S.J. The conservation value of urban green space habitats for Australian native bee communities. Biol. Conserv. 2015, 187, 240–248. [Google Scholar] [CrossRef]
- McFrederick, Q.S.; LeBuhn, G. Are urban parks refuges for bumble bees Bombus spp. (Hymenoptera: Apidae)? Biol. Conserv. 2006, 129, 372–382. [Google Scholar] [CrossRef]
- Dearborn, D.C.; Kark, S. Motivations for Conserving Urban Biodiversity. Conserv. Biol. 2010, 24, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Seto, K.C.; Güneralp, B.; Hutyra, L.R. Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proc. Natl. Acad. Sci. USA 2012, 109, 16083–16088. [Google Scholar] [CrossRef] [Green Version]
- De Palma, A.; Abrahamczyk, S.; Aizen, M.A.; Albrecht, M.; Basset, Y.; Bates, A.; Purvis, A. Predicting bee community responses to land-use changes: Effects of geographic and taxonomic biases. Sci. Rep. 2016, 6, 31153. [Google Scholar] [CrossRef] [Green Version]
- Wenzel, A.; Grass, I.; Belavadi, V.V.; Tscharntke, T. How urbanization is driving pollinator diversity and pollination—A systematic review. Biol. Conserv. 2020, 241, 108321. [Google Scholar] [CrossRef]
- Normandin, É.; Vereecken, N.J.; Buddle, C.M.; Fournier, V. Taxonomic and functional trait diversity of wild bees in different urban settings. PeerJ 2017, 5, e3051. [Google Scholar] [CrossRef] [Green Version]
- Instituto Brasileiro de Geografia e Estatística. IBGE. Available online: http://ibge.gov.br (accessed on 20 May 2020).
- Köppen, W.; Geiger, R. Klimate der Erde; Verlag Justus Perthes: Gotha, Germany, 1928. [Google Scholar]
- Pedlowski, M.A.; Carneiro da Silva, V.A.; Adell, J.J.C.; Heynen, N.C. Urban forest and environmental inequality in Campos dos Goytacazes, Rio de Janeiro, Brazil. Urban Ecosyst. 2002, 6, 9–20. [Google Scholar] [CrossRef]
- Michener, C.D. The Bees of the World, 2nd ed.; Johns Hopkins University: Baltimore, MD, USA, 2007; p. 953. [Google Scholar]
- Martins, A.C.; Goncalves, R.B.; Melo, G.A.R. Changes in wild bee fauna of a grassland in Brazil reveal negative effects associated with growing urbanization during the last 40 years. Zoologia 2013, 30, 157–176. [Google Scholar] [CrossRef]
- Lorenzi, H. Árvores brasileiras: Manual de Identificação e Cultivo de Plantas Arbóreas Nativas do Brasil, 2nd ed.; Editora Plantarum: Nova Odessa, SP, Brasil, 1992; Volume 1, p. 384. [Google Scholar]
- Lorenzi, H. Árvores brasileiras: Manual de Identificação e Cultivo de Plantas Arbóreas Nativas do Brasil, 2nd ed.; Editora Plantarum: Nova Odessa, SP, Brasil, 2002; Volume 2, p. 368. [Google Scholar]
- Pereira, M.E.C.; Jasmim, J.M.; Pereira, M.H. Guia de Vegetação de Praças; Editora; EdUENF: Campos dos Goytacazes, Brasil, 2021; p. 70. [Google Scholar]
- Brazil Flora, G. Brazilian Flora 2020 Project—Projeto Flora do Brasil 2020. Version 393.292. Instituto de Pesquisas Jardim Botanico do Rio de Janeiro. Checklist Dataset. Available online: https://doi.org/10.15468/1mtkaw accessed via GBIF.org (accessed on 19 July 2021). Available online: http://floradobrasil.jbrj.gov.br/ (accessed on 15 April 2019).
- RCPol. Rede de Catálogos Polínicos Online. Available online: http://chave.rcpol.org.br/ (accessed on 24 May 2019).
- Geslin, B.; Gauzens, B.; Thébault, E.; Dajoz, I. Plant pollinator networks along a gradient of urbanisation. PLoS ONE 2013, 8, e63421. [Google Scholar] [CrossRef] [PubMed]
- Magurram, A.E. Ecological Diversity and its Measurements; Princeton University Press: Princeton, NJ, USA, 2004; p. 99. [Google Scholar]
- Hammer, Q.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics software package for education and data analysis. Paleontol. Electron. 2001, 4, 9. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: http://www.R-project.org (accessed on 25 March 2020).
- Taura, H.M.; Laroca, S. A associação de abelhas silvestres de um biótopo urbano de Curitiba (Brasil), com comparações espaço temporais: Abundância relativa, fenologia, diversidade e exploração de recursos. Acta Biológica Parana. 2001, 30, 135–137. [Google Scholar] [CrossRef] [Green Version]
- Zanette, L.R.S.; Martins, R.P.; Ribeiro, S.P. Effects of urbanization on neotropical wasp and bee assemblages in a Brazilian metropolis. Landsc. Urban Plan. 2005, 71, 105–121. [Google Scholar] [CrossRef]
- Taura, H.M.; Laroca, S.; Barbosa, J.F.; Rodrigues, J. Melissocenótica (Hymenoptera, Anthophila) no Parque Florestal dos Pioneiros, Maringá, PR. (sul do Brasil)—I. Abundância relativae diversidade. Acta Biológica Parana. 2007, 36, 47–65. [Google Scholar] [CrossRef]
- Cardoso, M.C.; Gonçalves, R.B. Reduction by half: The impact on bees of 34 years of urbanization. Urban Ecosyst. 2018, 21, 1–7. [Google Scholar] [CrossRef]
- McKinney, M.L. Urbanization as a major cause of biotic homogenization. Biol. Conserv. 2006, 127, 247–260. [Google Scholar] [CrossRef]
- McKinney, M.L.; Lockwood, J.L. Biotic homogenization: A few winners replacing many losers in the next mass extinction. Trends Ecol. Evol. 1999, 14, 450–453. [Google Scholar] [CrossRef]
- McKinney, M.L. Do exotics homogenize or differentiate communities? Roles of sampling and exotic species richness. Biol. Invasions. 2004, 6, 495–504. [Google Scholar] [CrossRef]
- Horsák, M.; Lososová, Z.; Čejka, T.; Juřičková, L.; Chytrỳ, M. Diversity and biotic homogenization of urban land-snail faunas in relation to habitat types and macroclimate in 32 central European cities. PLoS ONE 2013, 8, e71783. [Google Scholar] [CrossRef]
- Theodorou, P.; Radzevičiūtė, R.; Kahnt, B.; Soro, A.; Grosse, I.; Paxton, R.J. Genome-wide single nucleotide polymorphism scan suggests adaptation to urbanization in an important pollinator, the red-tailed bumblebee (Bombus lapidarius L.). Proc. R. Soc. B Biol. Sci. 2018, 285, 20172806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, N.M.; Cariveau, D.; Winfree, R.; Kremen, C. Bees in disturbed habitats use, but do not prefer, alien plants. Basic Appl. Ecol. 2011, 12, 332–341. [Google Scholar] [CrossRef]
- Depra, M.S.; Evans, D.M.; Gaglianone, M.C. Pioneer herbaceous plants contribute to the restoration of pollination interactions in restinga habitats in tropical Atlantic Forest. Restor. Ecol. under review.
- Rollin, O.; Bretagnolle, V.; Decourtye, A.; Aptel, J.; Michel, N.; Vaissière, B.E.; Henry, M. Differences of floral resource use between honey bees and wild bees in an intensive farming system. Agric. Ecosyst. Environ. 2013, 179, 78–86. [Google Scholar] [CrossRef]
- Ramalho, M. Stingless bees and mass flowering trees in the canopy of Atlantic Forest: A tight relationship. Acta. Bot. Brasilica. 2004, 18, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Scheper, J.; Reemer, M.; van Kats, R.; Ozinga, W.A.; van der Linden, G.T.; Schaminee, J.H.; Siepel, H.; Kleijn, D. Museum specimens reveal loss of pollen host plants as key factor driving wild bee decline in The Netherlands. Proc. Natl. Acad. Sci. USA 2014, 111, 17552–17557. [Google Scholar] [CrossRef] [Green Version]
- Senapathi, D.; Goddard, M.A.; Kunin, W.E.; Baldock, K.C.R. Landscape impacts on pollinator communities in temperate systems: Evidence and knowledge gaps. Funct. Ecol. 2017, 31, 26–37. [Google Scholar] [CrossRef] [Green Version]
- Williams, N.M.; Kremen, C. Resource distributions among habitats determine solitary bee offspring production in a mosaic landscape. Ecol. Appli. 2007, 17, 910–921. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, S.; Gathof, A.K.; Grossmann, A.J.; Kowarik, I.; Fischer, L.K. Wild bees in urban grasslands: Urbanisation, functional diversity and species traits. Landsc. Urban Plan. 2020, 196, 103731. [Google Scholar] [CrossRef]



| Urban Green Area (UGA) | Abundance | Bee Guilds | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | U1 | U2 | U3 | U4 | U5 | U6 | U7 | U8 | U9 | U10 | U11 | U12 | Total | Rel.% | NB | NS | TS |
| APIDAE | |||||||||||||||||
| Apini | |||||||||||||||||
| Apis mellifera L. | 64 | 11 | 19 | 8 | 12 | 24 | 98 | 56 | 22 | 46 | 15 | 6 | 381 | 32.7 | E | C | G |
| Centridini | |||||||||||||||||
| Centris analis Lep. | 2 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0.4 | S | C | G |
| Centris tarsata Sm. | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 3 | 1 | 0 | 6 | 0.5 | S | C | G |
| Emphorini | |||||||||||||||||
| Melitoma segmentaria (Fab.) | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9 | 0.8 | S | SO | S |
| Euglossini | |||||||||||||||||
| Euglossa cordata (L.) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.1 | I | C | G |
| Euglossa sp | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 4 | 0.3 | I | C | G |
| Eulaema flavescens (Fr.) | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.1 | I | SO | G |
| Eulaema nigrita Lep. | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0.3 | I | SO | G |
| Exomalopsini | |||||||||||||||||
| Exomalopsis analis Spinola | 4 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 7 | 0.6 | I | SO | G |
| Exomalopsis auropilosa Spinola | 0 | 0 | 0 | 0 | 0 | 1 | 5 | 1 | 0 | 0 | 0 | 0 | 7 | 0.6 | I | SO | G |
| Meliponini | |||||||||||||||||
| Nannotrigona testaceicornis (Lep.) | 0 | 0 | 0 | 0 | 52 | 0 | 0 | 0 | 0 | 0 | 12 | 0 | 64 | 5.5 | E | C | G |
| Plebeia droryana (Fr.) | 0 | 4 | 17 | 7 | 21 | 49 | 0 | 0 | 15 | 0 | 31 | 6 | 150 | 12.9 | E | C | G |
| Plebeia sp | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.1 | E | C | G |
| Tetragonisca angustula (Latr.) | 0 | 15 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 16 | 1.4 | E | C | G |
| Trigona spinipes (Fab.) | 73 | 24 | 0 | 0 | 7 | 6 | 15 | 10 | 110 | 32 | 9 | 21 | 307 | 26.4 | E | C | G |
| Xylocopini | |||||||||||||||||
| Xylocopa frontalis Ol. | 0 | 2 | 0 | 0 | 4 | 2 | 0 | 0 | 9 | 0 | 1 | 2 | 20 | 1.7 | I | C | G |
| Xylocopa nigrocincta Brèthes | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 4 | 0.3 | I | C | G |
| Xylocopa ordinaria Sm. | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0.3 | I | C | G |
| Xylocopa suspecta Moure & Camargo | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0.1 | I | C | G |
| COLLETIDAE | |||||||||||||||||
| Hylaeini | |||||||||||||||||
| Hylaeus tricolor (Schr.) | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 5 | 0 | 0 | 6 | 0.5 | S | SO | G |
| Colletes sp | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0.1 | S | SO | G |
| HALICTIDAE | |||||||||||||||||
| Augochlorini | |||||||||||||||||
| Augochlora (Oxytoglossella) thalia Sm. | 4 | 5 | 11 | 2 | 0 | 0 | 0 | 4 | 0 | 2 | 4 | 2 | 34 | 2.9 | S | SO | G |
| Augochlora (Augochlora) esox Vachal | 3 | 2 | 0 | 1 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 2 | 12 | 1.0 | S | SO | G |
| Augochlora sp3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 3 | 4 | 0.3 | S | SO | G |
| Augochlora sp4 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 7 | 0.6 | S | SO | G |
| Augochlora sp5 | 2 | 1 | 1 | 3 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 4 | 13 | 1.1 | S | SO | G |
| Augochlora sp6 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.1 | S | SO | G |
| Augochlora sp7 | 1 | 3 | 2 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 3 | 13 | 1.1 | S | SO | G |
| Augochlora sp8 | 3 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 0 | 0 | 0 | 2 | 9 | 0.8 | S | SO | G |
| Augochlora sp9 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 3 | 0.3 | S | SO | G |
| Augochloropsis sp1 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 4 | 0.3 | I | SO | G |
| Augochloropsis sp2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0.1 | I | SO | G |
| Augochloropsis sp3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.1 | I | SO | G |
| Augochloropsis sp4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0.1 | I | SO | G |
| Halictini | |||||||||||||||||
| Dialictus sp1 | 5 | 1 | 1 | 1 | 0 | 1 | 13 | 19 | 4 | 2 | 0 | 4 | 51 | 4.4 | I | SO | G |
| MEGACHILIDAE | |||||||||||||||||
| Megachilini | |||||||||||||||||
| Megachile affabilis Mitchell | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0.1 | S | C | G |
| Megachile neoxanthoptera Cock. | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0.3 | S | C | G |
| Anthidiini | |||||||||||||||||
| Dicranthidium seabrai Urban | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 0.2 | S | C | G |
| Dicrantidium sp | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 6 | 0.5 | S | C | G |
| Total sampled | 183 | 74 | 53 | 25 | 99 | 92 | 136 | 100 | 164 | 102 | 80 | 55 | 1163 | ||||
| Species Richness | 20 | 14 | 8 | 9 | 7 | 14 | 8 | 11 | 8 | 13 | 12 | 11 | |||||
| Diversity (Shannon H′) | 1.760 | 2.065 | 1.482 | 1.821 | 1.322 | 1.545 | 1.072 | 1.500 | 1.122 | 1.591 | 1.781 | 2.073 | |||||
| Dominance (Berger–Parker) | 0.399 | 0.324 | 0.359 | 0.320 | 0.552 | 0.521 | 0.715 | 0.560 | 0.671 | 0.451 | 0.400 | 0.382 | |||||
| Urban Green Areas | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| U1 | U2 | U3 | U4 | U5 | U6 | U7 | U8 | U9 | U10 | U11 | U12 | |
| PV | 61.3 | 92.5 | 50.4 | 37.3 | 35.9 | 27.8 | 0 | 63.0 | 90.3 | 19.4 | 8.7 | 70.9 |
| NT | 34 | 36 | 33 | 22 | 46 | 78 | 22 | 33 | 15 | 63 | 369 | 17 |
| DBH | 23 | 27 | 16 | 22 | 26 | 35 | 63 | 30 | 53 | 22 | 32 | 32 |
| PR | 13 | 11 | 12 | 6 | 13 | 14 | 17 | 9 | 10 | 14 | 18 | 14 |
| FC | 60.8 | 47.5 | 10.6 | 6.5 | 42.6 | 33.7 | 82.5 | 22 | 22.7 | 26.7 | 21.3 | 11.7 |
| SPV | 63 | 96 | 87 | 71 | 92 | 97 | 45 | 51 | 72 | 84 | 75 | 89 |
| NB | 12 | 0 | 11 | 14 | 60 | 37 | 4 | 0 | 8 | 0 | 7 | 10 |
| Variables | Model | FC | DBH | PR | SPV | NB | Intercept | df | AICc | ΔAICc | Weight | AdjR2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abundance | ||||||||||||
| Eusocial | 2 | 0.029 | 3.186 | 3 | 123.0 | 0.00 | 0.405 | 0.396 | ||||
| Intermediate | 11 | −0.289 | 4.301 | 3 | 76.8 | 0.00 | 0.318 | 0.477 | ||||
| Solitary | 19 | −0.052 | −0.038 | 4.350 | 4 | 82.0 | 0.00 | 0.329 | 0.069 | |||
| Soil | 17 | −0.032 | 3.102 | 3 | 88.4 | 0.00 | 0.355 | 0.436 | ||||
| Cavity | 2 | 0.015 | 3.830 | 3 | 127.6 | 0.00 | 0.276 | 0.318 | ||||
| Specialist | 4 | 1.447 | −1.521 | −50.88 | 4 | 17.8 | 0.00 | 0.217 | 0.993 | |||
| Generalist | 2 | 0.015 | 4.096 | 3 | 127.1 | 0.00 | 0.285 | 0.345 | ||||
| Richness | ||||||||||||
| Eusocial | 17 | 0.012 | 0.937 | 3 | 47.3 | 1.77 | 0.134 | 0.151 | ||||
| Intermediate | 3 | 0.101 | 0.851 | 3 | 49.2 | 1.51 | 0.168 | 0.169 | ||||
| Solitary | 2 | −0.029 | 2.486 | 3 | 65.8 | 0.00 | 0.179 | 0.264 | ||||
| Soil | 17 | −0.015 | 2.034 | 3 | 65.7 | 0.41 | 0.215 | 0.239 | ||||
| Cavity | 129 | 0.079 | 0.661 | 3 | 57.6 | 0.00 | 0.143 | 0.282 | ||||
| Specialist | 2 | 0.047 | −46.38 | 3 | 14.6 | 2.27 | 0.095 | 0.250 | ||||
| Generalist | 2 | 0.002 | 2.311 | 3 | 66.7 | 3.23 | 0.083 | 0.035 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guimarães Alves, S.; Gaglianone, M.C. Bee Guilds’ Responses to Urbanization in Neotropics: A Case Study. Diversity 2021, 13, 365. https://doi.org/10.3390/d13080365
Guimarães Alves S, Gaglianone MC. Bee Guilds’ Responses to Urbanization in Neotropics: A Case Study. Diversity. 2021; 13(8):365. https://doi.org/10.3390/d13080365
Chicago/Turabian StyleGuimarães Alves, Sônia, and Maria Cristina Gaglianone. 2021. "Bee Guilds’ Responses to Urbanization in Neotropics: A Case Study" Diversity 13, no. 8: 365. https://doi.org/10.3390/d13080365
APA StyleGuimarães Alves, S., & Gaglianone, M. C. (2021). Bee Guilds’ Responses to Urbanization in Neotropics: A Case Study. Diversity, 13(8), 365. https://doi.org/10.3390/d13080365