Floristic Groups, and Changes in Diversity and Structure of Trees, in Tropical Montane Forests in the Southern Andes of Ecuador
Abstract
1. Introduction
2. Materials and Methods
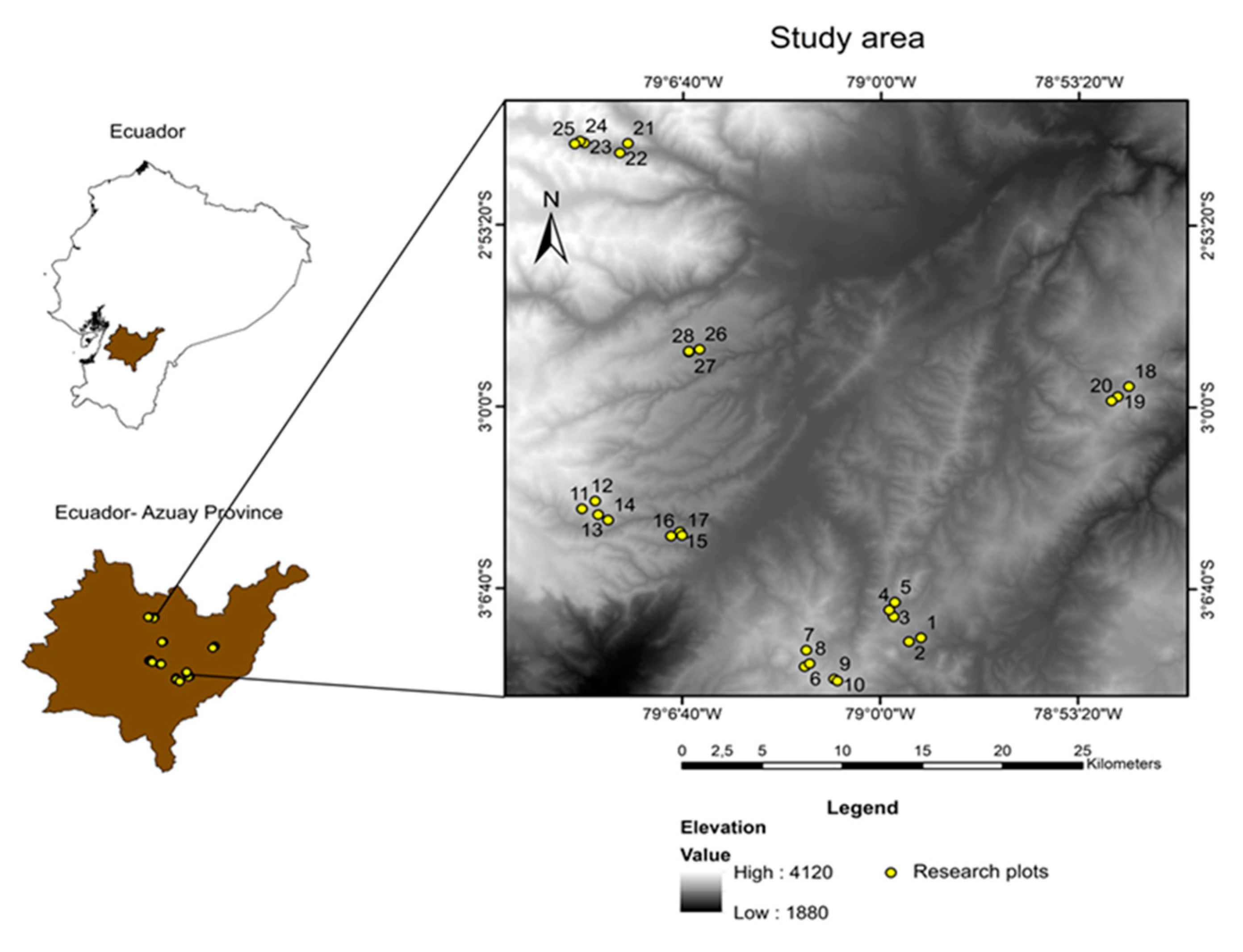
2.1. Study Area
2.2. Vegetation Survey and Data Collection
2.3. Identification of Floristic Groups and Indicator Species
2.4. Relationship of Floristic Groups and Predictor Variables
3. Results
3.1. Floristics Groups and Indicator Species
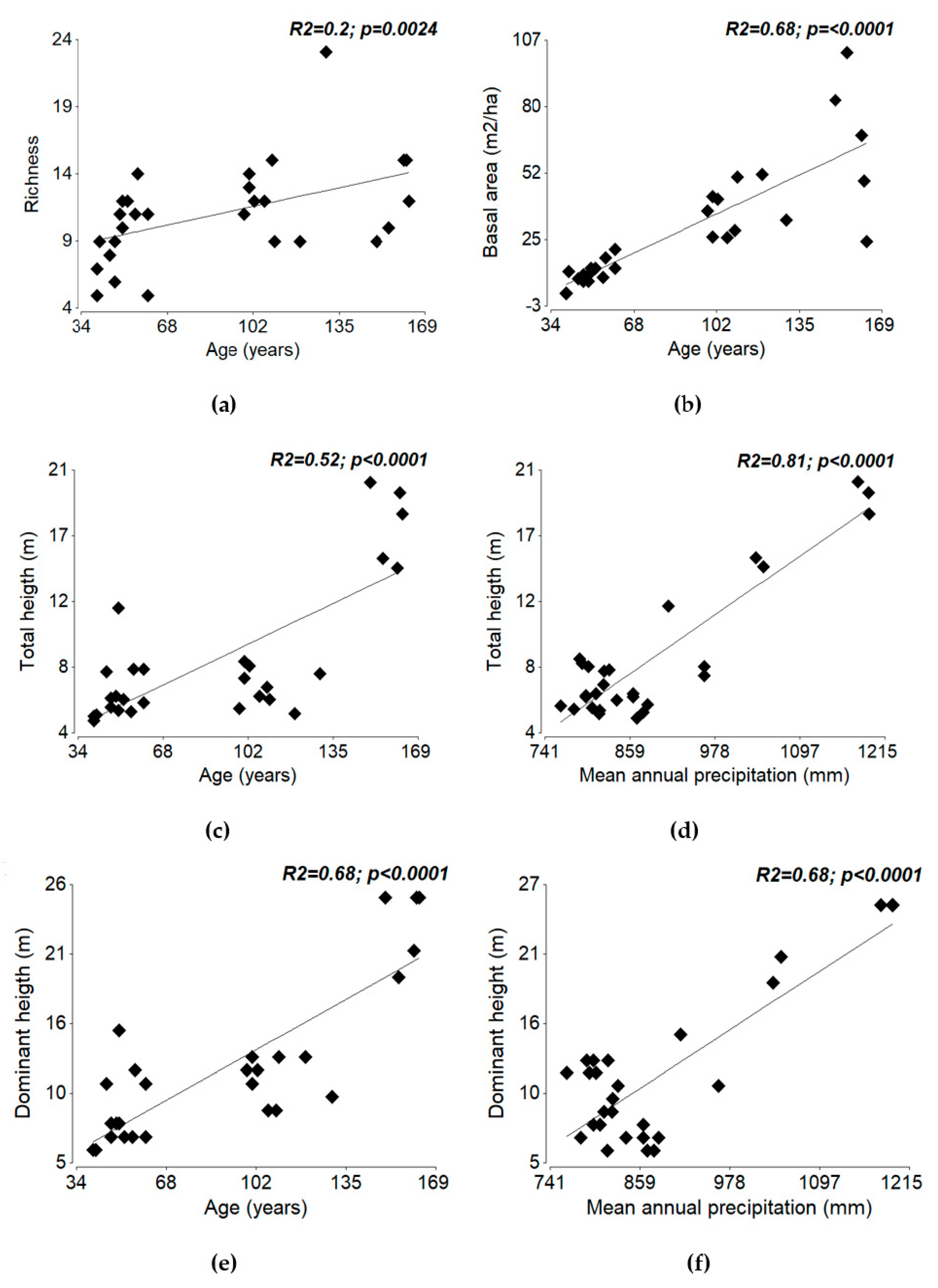
3.2. Floristic Groups and Relationship with Predictor Variables
4. Discussion
4.1. Floristic Groups at Local Scales
4.2. Floristic Group Composition, Diversity and Structure, and Their Relationship with Predictor Variables
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Báez, S.; Ambrose, K.; Hofstede, R. Ecological and social bases for the restoration of a High Andean cloud forest: Preliminary results and lessons from a case study in northern Ecuador. In Tropical Montane Cloud Forests: Science for Conservation and Management; Bruijnzeel, L., Scatena, F., Hamilton, L., Eds.; Cambridge University Press: Cambridg, UK, 2011; pp. 628–643. [Google Scholar]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- De la Cruz-Amo, L.; Bañares-de-Dios, G.; Cala, V.; Granzow-de la Cerda, Í.; Espinosa, C.I.; Ledo, A.; Salinas, N.; Macía, M.J.; Cayuela, L. Trade-offs among aboveground, belowground, and soil organic carbon stocks along altitudinal gradients in Andean tropical montane forests. Front. Plant Sci. 2020, 11, 106. [Google Scholar] [CrossRef]
- Rozendaal, D.M.; Chazdon, R.L.; Arreola-Villa, F.; Balvanera, P.; Bentos, T.V.; Dupuy, J.M.; Hernández-Stefanoni, J.L.; Jakovac, C.C.; Lebrija-Trejos, E.E.; Lohbeck, M. Demographic drivers of aboveground biomass dynamics during secondary succession in neotropical dry and wet forests. Ecosystems 2017, 20, 340–353. [Google Scholar] [CrossRef]
- Werner, F.A.; Homeier, J. Is tropical montane forest heterogeneity promoted by a resource-driven feedback cycle? Evidence from nutrient relations, herbivory and litter decomposition along a topographical gradient. Funct. Ecol. 2015, 29, 430–440. [Google Scholar] [CrossRef]
- Blundo, C.; Malizia, L.R.; Blake, J.G.; Brown, A.D. Tree species distribution in Andean forests: Influence of regional and local factors. J. Trop. Ecol. 2012, 28, 83–95. [Google Scholar] [CrossRef]
- Homeier, J.; Breckle, S.; Günter, S.; Rollenbeck, R.T.; Leuschner, C. Tree diversity, forest structure and productivity along altitudinal and topographical gradients in a species-rich ecuadorian montane rain forest. Biotropica 2010, 42, 140–148. [Google Scholar] [CrossRef]
- Moser, G.; Röderstein, M.; Soethe, N.; Hertel, D.; Leuschner, C. Altitudinal changes in stand structure and biomass allocation of tropical mountain forests in relation to microclimate and soil chemistry. In Gradients in a Tropical Mountain Ecosystem of Ecuador; Beck, E., Bendix, J., Kottke, I., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 229–242. [Google Scholar]
- Girardin, C.A.; Farfan-Rios, W.; Garcia, K.; Feeley, K.J.; Jørgensen, P.M.; Murakami, A.A.; Cayola Pérez, L.; Seidel, R.; Paniagua, N.; Fuentes Claros, A.F. Spatial patterns of above-ground structure, biomass and composition in a network of six Andean elevation transects. Plant Ecol. Divers. 2014, 7, 161–171. [Google Scholar] [CrossRef]
- Báez, S.; Malizia, A.; Carilla, J.; Blundo, C.; Aguilar, M.; Aguirre, N.; Aquirre, Z.; Álvarez, E.; Cuesta, F.; Duque, Á. Large-scale patterns of turnover and basal area change in Andean forests. PLoS ONE 2015, 10, e0126594. [Google Scholar] [CrossRef] [PubMed]
- Rezende, V.L.; de Miranda, P.L.; Meyer, L.; Moreira, C.V.; Linhares, M.F.; de Oliveira-Filho, A.T.; Eisenlohr, P.V. Tree species composition and richness along altitudinal gradients as a tool for conservation decisions: The case of Atlantic semideciduous forest. Biodivers. Conserv. 2015, 24, 2149–2163. [Google Scholar] [CrossRef]
- Veintimilla, D.; Ngo Bieng, M.A.; Delgado, D.; Vilchez-Mendoza, S.; Zamora, N.; Finegan, B. Drivers of tropical rainforest composition and alpha diversity patterns over a 2520 m altitudinal gradient. Ecol. Evol. 2019, 9, 5720–5730. [Google Scholar] [CrossRef]
- Chain-Guadarrama, A.; Finegan, B.; Vilchez, S.; Casanoves, F. Determinants of rain-forest floristic variation on an altitudinal gradient in southern Costa Rica. J. Trop. Ecol. 2012, 28, 463–481. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, B.; Li, D.; Mallik, A.U.; Xiang, W.; Ding, T.; Wen, S.; Lu, S.; Huang, F.; He, Y. Effects of topography and spatial processes on structuring tree species composition in a diverse heterogeneous tropical karst seasonal rainforest. Flora 2017, 231, 21–28. [Google Scholar] [CrossRef]
- Vlam, M.; Baker, P.J.; Bunyavejchewin, S.; Zuidema, P.A. Temperature and rainfall strongly drive temporal growth variation in Asian tropical forest trees. Oecologia 2014, 174, 1449–1461. [Google Scholar] [CrossRef]
- Castellanos-Castro, C.; Newton, A.C. Environmental heterogeneity influences successional trajectories in Colombian seasonally dry tropical forests. Biotropica 2015, 47, 660–671. [Google Scholar] [CrossRef]
- Duque, A.; Phillips, J.F.; von Hildebrand, P.; Posada, C.A.; Prieto, A.; Rudas, A.; Suescún, M.; Stevenson, P. Distance decay of tree species similarity in protected areas on terra firme forests in Colombian Amazonia. Biotropica 2009, 41, 599–607. [Google Scholar] [CrossRef]
- Hubbell, S.P. The Unified Neutral Theory of Biodiversity and Biogeography (MPB-32); Princeton University Press: Princeton, NJ, USA, 2011. [Google Scholar]
- Legendre, P. Studying beta diversity: Ecological variation partitioning by multiple regression and canonical analysis. J. Plant Ecol. 2008, 1, 3–8. [Google Scholar] [CrossRef]
- Curatola Fernández, G.F.; Obermeier, W.A.; Gerique, A.; López Sandoval, M.F.; Lehnert, L.W.; Thies, B.; Bendix, J. Land cover change in the Andes of Southern Ecuador—Patterns and drivers. Remote Sens. 2015, 7, 2509–2542. [Google Scholar] [CrossRef]
- Zuluaga, G.; Colorado, J.; Rodewald, A. Response of mixed-species flocks to habitat alteration and deforestation in the Andes. Biol. Conserv. 2015, 188, 72–81. [Google Scholar] [CrossRef]
- Chacón, G.; Gagnon, D.; Paré, D. Comparison of soil properties of native forests, Pinus patula plantations and adjacent pastures in the Andean highlands of southern Ecuador: Land use history or recent vegetation effects? Soil Use Manag. 2009, 25, 427–433. [Google Scholar] [CrossRef]
- Tapia-Armijos, M.F.; Homeier, J.; Espinosa, C.I.; Leuschner, C.; de la Cruz, M. Deforestation and forest fragmentation in South Ecuador since the 1970s–losing a hotspot of biodiversity. PLoS ONE 2015, 10, e0133701. [Google Scholar] [CrossRef]
- Jadán, O.; Cedillo, H.; Zea, P.; Peralta, A.; Quichimbo, P.; Vaca, C. Relación entre deforestación y variables topográficas en un contexto agrícola ganadero, cantón Cuenca. Bosques Latid. Cero 2016, 6, 1–13. [Google Scholar]
- Tovar, C.; Seijmonsbergen, A.C.; Duivenvoorden, J.F. Monitoring land use and land cover change in mountain regions: An example in the Jalca grasslands of the Peruvian Andes. Landsc. Urban Plan. 2013, 112, 40–49. [Google Scholar] [CrossRef]
- Hoffmann, C.; Márquez, J.R.G.; Krueger, T. A local perspective on drivers and measures to slow deforestation in the Andean-Amazonian foothills of Colombia. Land Use Policy 2018, 77, 379–391. [Google Scholar] [CrossRef]
- Rubiano, K.; Clerici, N.; Norden, N.; Etter, A. Secondary forest and shrubland dynamics in a highly transformed landscape in the Northern Andes of Colombia (1985–2015). Forests 2017, 8, 216. [Google Scholar] [CrossRef]
- Yepes, A.P.; del Valle, J.I.; Jaramillo, S.L.; Orrego, S.A. Recuperación estructural en bosques sucesionales andinos de Porce (Antioquia, Colombia). Rev. Biol. Trop. 2010, 58, 427–445. [Google Scholar]
- Jadán, O.; Cedillo, H.; Pillacela, P.; Guallpa, D.; Gordillo, A.; Zea, P.; Díaz, L.; Bermúdez, F.; Arciniegas, A.; Quizhpe, W. Regeneración de árboles en ecosistemas naturales y plantaciones de Pinus patula (Pinaceae) dentro de un gradiente altitudinal andino (Azuay, Ecuador). Rev. Biol. Trop. 2019, 67, 182–195. [Google Scholar] [CrossRef]
- Jadán, O.; Toledo, C.; Tepán, B.; Cedillo, H.; Peralta, Á.; Zea, P.; Castro, P.; Vaca, C. Comunidades forestales en bosques secundarios alto-andinos (Azuay, Ecuador). Bosque 2017, 38, 141–154. [Google Scholar] [CrossRef][Green Version]
- INAMHI. Anuario Metereológico; Instituto Nacional de Metereología e Hidrología: Quito, Ecuador, 2014.
- Pinos-Arévalo, N.J. Prospectiva del uso del suelo y cobertura vegetal en el ordenamiento territorial-Caso cantón Cuenca. In Estoa. Revista de la Facultad de Arquitectura y Urbanismo de la Universidad de Cuenca; Universidad de Cuenca: Cuenca, Ecuador, 2016; Volume 5, pp. 7–19. [Google Scholar]
- MAGAP. Manual de Procedmientos de Geopedología. Proyecto de Levantamiento de Cartografía Temática a Escala 1:25000, Lotes 1 y 2; Ministerio de Agricultura y Ganaderia: Quito, Ecuador, 2015.
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.; O’hara, R.; Simpson, G.; Solymos, P.; Stevens, M.; Wagner, H. Community Ecology Package; R Package Version; Vegan: Vienna, Austria, 2013; Volume 2. [Google Scholar]
- Bakker, J.D. Increasing the utility of indicator species analysis. J. Appl. Ecol. 2008, 45, 1829–1835. [Google Scholar] [CrossRef]
- De Cáceres, M.; Legendre, P.; Wiser, S.K.; Brotons, L. Using species combinations in indicator value analyses. Methods Ecol. Evol. 2012, 3, 973–982. [Google Scholar] [CrossRef]
- Chao, A.; Gotelli, N.J.; Hsieh, T.; Sander, E.L.; Ma, K.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Hsieh, T.; Ma, K.; Chao, A. iNEXT Online: Interpolation and Extrapolation (Version 1.0) [Software]. 2013. Available online: http://chaostatnthuedutw/blog/software-downlod/ (accessed on 2 September 2014).
- Kuhn, M.; Wing, J.; Weston, S.; Williams, A.; Keefer, C.; Engelhardt, A.; Cooper, T.; Mayer, Z. Caret: Classification and Regression Training. R Package Version 6.0–84. 2019. Available online: https://CRAN.R-project.org/package=caret (accessed on 1 March 2020).
- Gelviz-Gelvez, S.M.; Sánchez-Montaño, L.R.; Lopez-Toledo, L.; Barragán, F. The andean forest soil seed bank in two successional stages in northeastern Colombia. Bot. Sci. 2016, 94, 727. [Google Scholar] [CrossRef][Green Version]
- González, W.; Llambí, L.D.; Smith, J.K.; Gámez, L.E. Dinámica sucesional del componente arbóreo en la zona de transición bosque-Páramo en Los Andes Tropicales. Ecotrópicos 2011, 24, 60–79. [Google Scholar]
- Homeier, J.; Werner, F.; Gradstein, S.; Breckle, S.; Richter, M. Potential vegetation and floristic composition of Andean forests in South Ecuador, with a focus on the RBSF. Ecol. Stud. 2008, 198, 87. [Google Scholar]
- Sarmiento, F.O. Human impacts on the cloud forests of the upper Guayllabamba river basin, Ecuador, and suggested management responses. In Tropical Montane Cloud Forests; Hamilton, L., Juvik, J., Scatena, F., Eds.; Springer: Berlin/Heidelberg, Germany, 1995; pp. 284–295. [Google Scholar]
- Goodale, U.M.; Ashton, M.S.; Berlyn, G.P.; Gregoire, T.G.; Singhakumara, B.; Tennakoon, K.U. Disturbance and tropical pioneer species: Patterns of association across life history stages. For. Ecol. Manag. 2012, 277, 54–66. [Google Scholar] [CrossRef]
- Jørgensen, P.M.; León-Yánez, S. Catalogue of the Vascular Plants of Ecuador; Missouri Botanical Garden St. Louis: Quito, Ecuador, 1999; Volume 75. [Google Scholar]
- Quichimbo, P.; Tenorio, G.; Borja, P.; Cárdenas, I.; Crespo, P.; Célleri, R. Efectos sobre las propiedades físicas y químicas de los suelos por el cambio de la cobertura vegetal y uso del suelo: Páramo de Quimsacocha al sur del Ecuador. Suelos Ecuat. 2012, 42, 138–153. [Google Scholar]
- Liu, K.-b.; Colinvaux, P. Forest changes in the Amazon Basin during the last glacial maximum. Nature 1985, 318, 556–557. [Google Scholar] [CrossRef]
- Schmidt, S.B.; Husted, S. The biochemical properties of manganese in plants. Plants 2019, 8, 381. [Google Scholar] [CrossRef]
- Imai, N.; Tanaka, A.; Samejima, H.; Sugau, J.B.; Pereira, J.T.; Titin, J.; Kurniawan, Y.; Kitayama, K. Tree community composition as an indicator in biodiversity monitoring of REDD+. For. Ecol. Manag. 2014, 313, 169–179. [Google Scholar] [CrossRef]
- Moralez_Salazar, M.C.; Robin, L.; Gutiérrez, M.O.; Malavasi, E.O.; Bonilla, M.G. Diversidad y estructura horizontal en los bosques tropicales del Corredor Biológico de Osa, Costa Rica. Rev. For. Mesoam. Kurú 2012, 9, 19–28. [Google Scholar]
- Fukami, T.; Lee, W.G. Alternative stable states, trait dispersion and ecological restoration. Oikos 2006, 113, 353–356. [Google Scholar] [CrossRef]
- Zanini, L.; Ganade, G. Restoration of Araucaria forest: The role of perches, pioneer vegetation, and soil fertility. Restor. Ecol. 2005, 13, 507–514. [Google Scholar] [CrossRef]
- Guariguata, M.R.; Ostertag, R. Neotropical secondary forest succession: Changes in structural and functional characteristics. For. Ecol. Manag. 2001, 148, 185–206. [Google Scholar] [CrossRef]
- Fischer, J.; Lindenmayer, D.B. Landscape modification and habitat fragmentation: A synthesis. Glob. Ecol. Biogeogr. 2007, 16, 265–280. [Google Scholar] [CrossRef]
- Liebsch, D.; Marques, M.C.; Goldenberg, R. How long does the Atlantic Rain Forest take to recover after a disturbance? Changes in species composition and ecological features during secondary succession. Biol. Conserv. 2008, 141, 1717–1725. [Google Scholar] [CrossRef]
- Rowland, L.; Malhi, Y.; Silva-Espejo, J.E.; Farfán-Amézquita, F.; Halladay, K.; Doughty, C.; Meir, P.; Phillips, O.L. The sensitivity of wood production to seasonal and interannual variations in climate in a lowland Amazonian rainforest. Oecologia 2014, 174, 295–306. [Google Scholar] [CrossRef]
- Baribault, T.W.; Kobe, R.K.; Finley, A.O. Tropical tree growth is correlated with soil phosphorus, potassium, and calcium, though not for legumes. Ecol. Monogr. 2012, 82, 189–203. [Google Scholar] [CrossRef]




| Species-Code | Floristic Group | p |
|---|---|---|
| Hesperomeles ferruginea (Pers.) Benth. - Hesfe | FG1 | 0.01 |
| Myrsine dependens (Ruiz & Pav.) Spreng. - Myrde | <0.0001 | |
| Gaiadendron punctatum (Ruiz & Pav.) G. Don - Gaipu | FG2 | 0.04 |
| Gynoxys azuayensis Cuatrec. - Gynaz | <0.0001 | |
| Gynoxys hallii Hieron. - Gynha | 0.002 | |
| Hedyosmum cumbalense H. Karst. - Hedcu | <0.0001 | |
| Ocotea infrafoveolata van der Werff - Ocoin | <0.0001 | |
| Ageratina dendroides (Spreng.) R.M. King & H. Rob. - Agede | FG3 | 0.02 |
| Hedyosmum goudotianum Solms - Hedgo | <0.0001 | |
| Hedyosmum luteynii Todzia - Hedlu | <0.0001 | |
| Hedyosmum racemosum (Ruiz & Pav.) G. Don - Hedra | <0.0001 | |
| Meriania tomentosa (Cogn.) Wurdack - Merto | 0.02 | |
| Nectandra sp. - Necsp | 0.03 | |
| Piper andreanum C. DC. - Pipan | <0.0001 |
| Floristic Groups | Diversity | Observed | Estimated | S. E. |
|---|---|---|---|---|
| FG1 | q = 0 | 41 | 49.3 | 6.9 |
| q = 1 | 16.1 | 17 | 0.9 | |
| q = 2 | 8.6 | 8.7 | 0.7 | |
| FG2 | q = 0 | 50 | 68.3 | 12.2 |
| q = 1 | 20.4 | 21.8 | 1.1 | |
| q = 2 | 12.7 | 13 | 0.7 | |
| FG3 | q = 0 | 34 | 43.3 | 6.8 |
| q = 1 | 16.4 | 19 | 1.7 | |
| q = 2 | 10.1 | 10.7 | 1.2 |
| Predictor Variables | NMDS Axis 1 | NMDS Axis 2 | r2 | p |
|---|---|---|---|---|
| Age since abandonment | 0.9 | −0.05 | 0.7 | 0.001 |
| Mean monthly precipitation | 0.5 | 0.8 | 0.7 | 0.001 |
| Spatial correlation | 0.8 | −0.6 | 0.2 | 0.066 |
| Manganese | 0.5 | 0.8 | 0.4 | 0.006 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jadán, O.; Donoso, D.A.; Cedillo, H.; Bermúdez, F.; Cabrera, O. Floristic Groups, and Changes in Diversity and Structure of Trees, in Tropical Montane Forests in the Southern Andes of Ecuador. Diversity 2021, 13, 400. https://doi.org/10.3390/d13090400
Jadán O, Donoso DA, Cedillo H, Bermúdez F, Cabrera O. Floristic Groups, and Changes in Diversity and Structure of Trees, in Tropical Montane Forests in the Southern Andes of Ecuador. Diversity. 2021; 13(9):400. https://doi.org/10.3390/d13090400
Chicago/Turabian StyleJadán, Oswaldo, David A. Donoso, Hugo Cedillo, Fernando Bermúdez, and Omar Cabrera. 2021. "Floristic Groups, and Changes in Diversity and Structure of Trees, in Tropical Montane Forests in the Southern Andes of Ecuador" Diversity 13, no. 9: 400. https://doi.org/10.3390/d13090400
APA StyleJadán, O., Donoso, D. A., Cedillo, H., Bermúdez, F., & Cabrera, O. (2021). Floristic Groups, and Changes in Diversity and Structure of Trees, in Tropical Montane Forests in the Southern Andes of Ecuador. Diversity, 13(9), 400. https://doi.org/10.3390/d13090400






