Abstract
Located in Northern Pyrenees, in the Arbas massif, France, the system of the Coume Ouarnède, also known as Réseau Félix Trombe—Henne Morte, is the longest and the most complex cave system of France. The system, developed in massive Mesozoic limestone, has two distinct resurgences. Despite relatively limited sampling, its subterranean fauna is rich, composed of a number of local endemics, terrestrial as well as aquatic, including two remarkable relictual species, Arbasus caecus (Simon, 1911) and Tritomurus falcifer Cassagnau, 1958. With 38 stygobiotic and troglobiotic species recorded so far, the Coume Ouarnède system is the second richest subterranean hotspot in France and the first one in Pyrenees. This species richness is, however, expected to increase because several taxonomic groups, like Ostracoda, as well as important subterranean habitats, like MSS (“Milieu Souterrain Superficiel”), have not been considered so far in inventories. Similar levels of subterranean biodiversity are expected to occur in less-sampled karsts of central and western Pyrenees.
1. Introduction
Stretching at the border between France and Spain, the Pyrenees are known as one of the subterranean hotspots of the world [1]. This remarkable diversity is unevenly distributed along the Pyrenean range, reaching its highest value on the northern slope of central and western Pyrenees.
The Arbas massif is located on the northern slope of central Pyrenees, about 70 km south of Toulouse, at the limit of the departments of Haute-Garonne in the west and Ariège in the east (Figure 1). Extending the Lestelas massif to the west, it develops north of the Bouigane valley, east of the Ger valley, and south of rolling hills of Comminges. It ranges from 500 m to 1608 m in altitude.
Figure 1.
Location of the Arbas massif, in central French Pyrenees.
The massif is formed by relatively complex series of Mesozoic limestones, generally overlaid by massive Urgonian limestones [2]. Under high rainfall exceeding 2000 mm a year, the massif is mostly covered by beech forest, with small stands of fir locally. Water transfers are very rapid due to a well-organized drainage and high surface karstification [2]. Not under threat and not under formal protection measures, the biological richness of the massif is nevertheless remarkable, and has been labeled as Zone Naturelle d’Intérêt Écologique, Faunistique et Floristique (ZNIEFF) Massifs d’Arbas, Paloumère et Cornudère (national ID: 730011048).
The main cave system of the massif is the Réseau Félix Trombe—Henne Morte, from the name of the French engineer and caver Félix Trombe, also known for his pioneer works on solar energy. This large system is commonly called Coume Ouarnède or Coumo d’Hyouernedo by cavers. It was explored for years by the famous speleologist Norbert Casteret who often referred to these explorations in his writings (see [3] for a list of references) and remains a well-known cave system for speleologists worldwide.
Various taxon-centered studies since more than one century ago progressively brought to light that the Coume Ouarnède system hosted a high diversity of cave animals. In the only global synthesis of its fauna, published in 1982, 29 cave-restricted species were listed from the system, including 18 stygobionts and 11 troglobionts [4]. Today, that is, 40 years later, there are 38 cave-restricted species recorded, including 21 stygobionts and 17 troglobionts, making of the Coume Ouarnède the richest cave fauna of the Pyrenees, which is itself a major European hotspot. The richness of the Pyrenean range can be explained by its biogeographical history [5]. The richness of the Coume Ouarnède can be explained by the great extent of the cave system and the diversity of its subterranean habitats. It may also result from the relatively good knowledge we have of its fauna, as the system is close to the renowned Subterranean Laboratory of Moulis where a number of biospeologists from all over the world have worked for decades.
In the framework of the special issue of the journal “Diversity” that deals with world hotspots of subterranean biodiversity, the Coume Ouarnède clearly deserves some attention. We shall provide in this paper an updated and comprehensive checklist of its subterranean fauna, put in its ecological and biogeographical context.
2. The Coume Ouarnède System
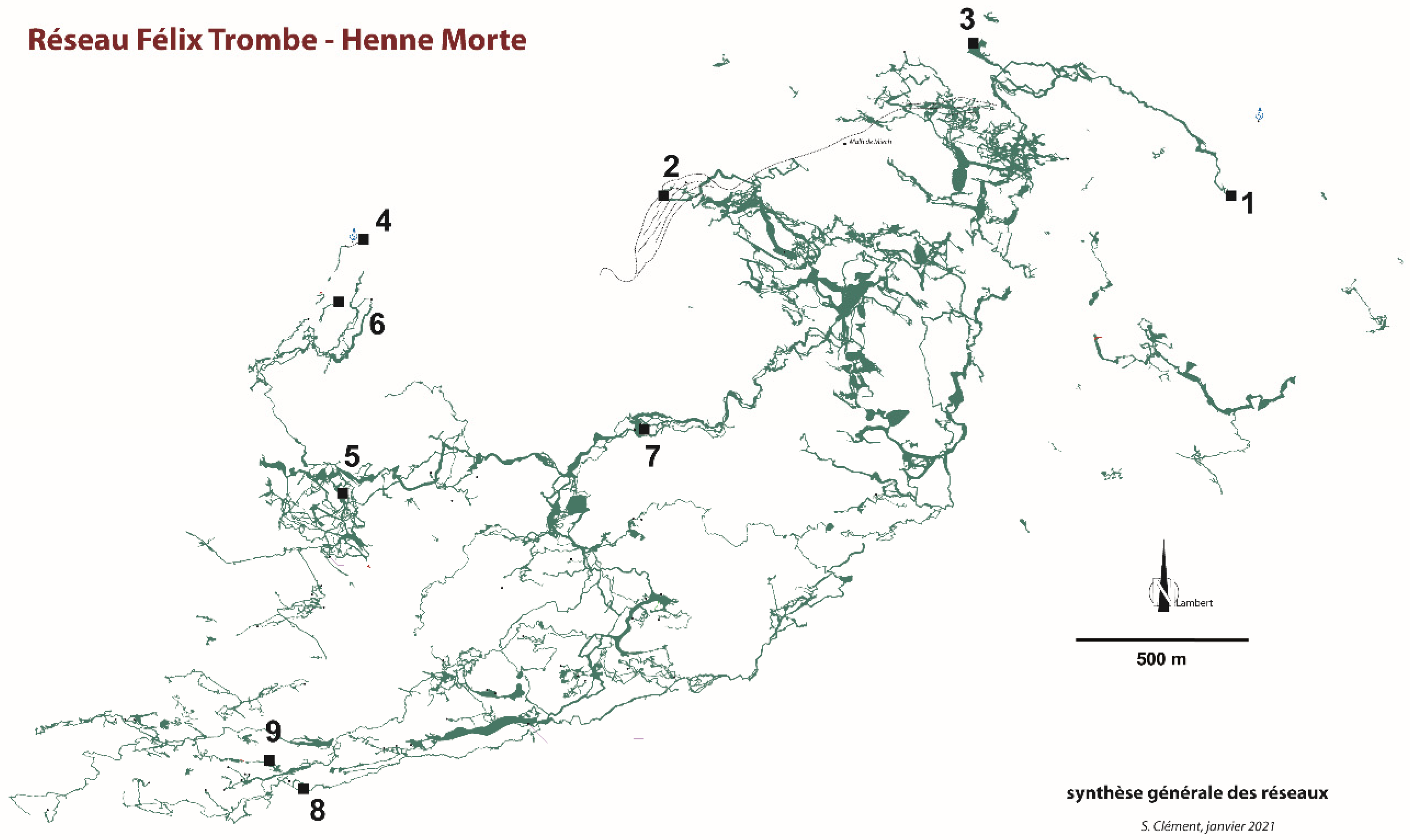
The Coume Ouarnède system is the longest subterranean system of France, and one of the most famous sites regarding French speleology. Today, it has a development of more than 112 km for a depth of 1020 m, with 57 inter-connected caves, while the massif of Arbas has about 500 caves in total (Figure 2 and Figure 3) ([6]; S. Clément pers. comm. 05.2021). The network offers a great variety of geomorphological features, with countless fossil and active galleries and shafts, some of large dimensions (Pont de Gerbaut, Pène Blanque), and subterranean rivers [7].
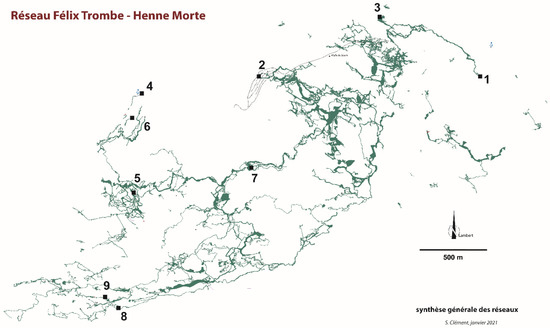
Figure 2.
Coume Ouarnède. Synthesis of the networks (modified from [7] and S. Clément pers. comm.). 1–9: Localities with three or more listed species, by decreasing species richness. 1: Goueil di Her; 2: Grotte de Pène Blanque; 3: Poudac Gran; 4: Hount deras Hechos; 5: Henne Morte; 6: Puits du Mistral; 7: Gouffre du Pont de Gerbaut; 8: Trou Mile; 9: Gouffre Raymonde.
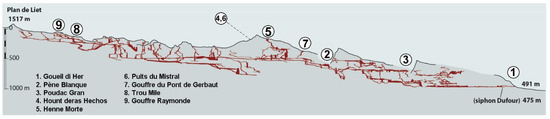
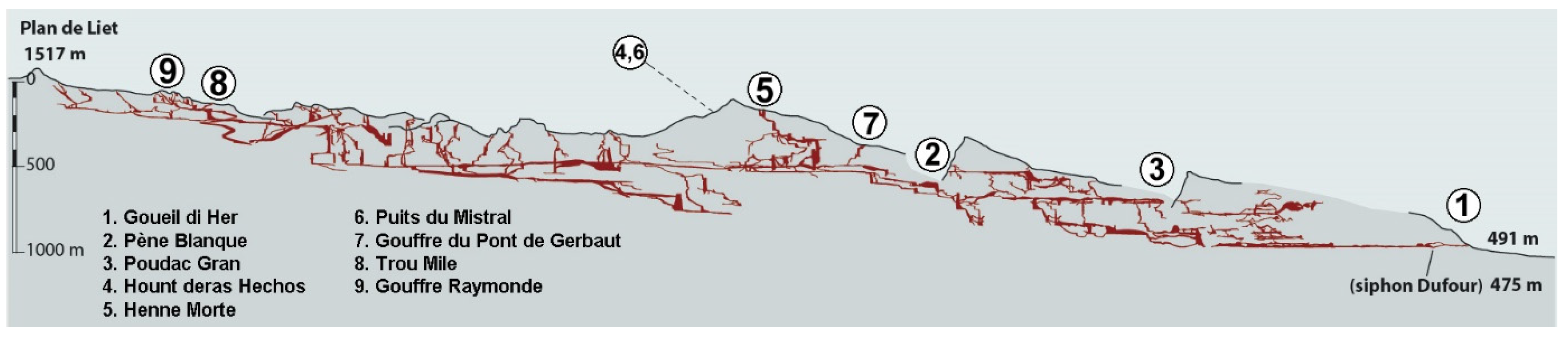
Figure 3.
A transverse view of the system, with the location of the nine caves from which more than three taxa are listed (modified from [7]). Cave numbers as in Figure 2.
The Coume Ouarnède system has two distinct resurgences: the Goueil di Her at Arbas, and the Hount deras Hechos at Herran, 2.5 km to the west (“Hount-des-Heretchos” sensu [8,9], that is, «the source of the ash trees» after [10]). The Goueil di Her is the resurgence of the main hydrological system, the Réseau Félix Trombe. The Hount deras Hechos is the resurgence of the smaller Henne Morte system, regarded as a secondary derivation of the main system [2,7]. The two systems are interconnected.
3. Methods
Data synthetized here are drawn from the literature, that is, species lists [4,9,11,12,13,14] and taxonomic literature. Most specimens were collected in nine caves or cave complexes of the system: Gouffre de la Henne Morte (and spring nearby), Gouffre du Pont de Gerbaut, Poudac Gran, Gouffre Raymonde, Grotte de Pène Blanque, Goueil di Her, Hount deras Hechos (cave and spring), Puits du Mistral, and Trou Mile (Figure 2 and Figure 3). These caves are now interconnected, except Poudac Gran. Most of the collections in the Goueil di Her, the richest of these caves, were made between the entrance and the first sump.
Species names and species validity have been checked from [15] and public databases [16,17]. Species ecological status has been inferred from the taxonomic literature, from [18], and from [19] for spiders.
Abbreviations and terms defining species ecology that are used in the text are defined below:
Endogean or euedaphic species: living or assumed to live only in deep soil, often common at cave entrances.
Eutroglophile: a species with permanent populations inside and outside caves.
Hyporheic species: living or assumed to live only in interstitia of sediment beneath and alongside streams.
MSS: Milieu Souterrain Superficiel (often translated as “Superficial Underground Compartment” in the literature).
Stygobiont: a species living or assumed to live only in groundwater.
Subtroglophile: a species spending a part of its life cycle in caves.
Troglobiont or troglobiotic species: living or assumed to live only in caves or in the MSS.
Troglophile or troglophilic species: living inside as well as outside caves.
Only troglobionts, stygobionts and the most important troglophiles are considered in this paper. Guano and shallow subterranean habitats have not been sampled, though these habitats are present and promising in the massif d’Arbas [20].
4. The History of Biological Explorations
The Goueil di Her cave, the eye of Hell (“uèlh d’in hèrn”) in the Gascon dialect, is the main resurgence of the Coume Ouarnède system. The first explorations of the cave are related in [21]. In 1908, 30 years later, Jeannel visited the cave, and did the first biological collections, during a speleological expedition conducted by E.A. Martel which aimed at studying the hydrological characteristics of Pyrenees [22,23]. He introduced the cave in a dramatic way [23]: «Près d´Arbas se trouve une étrange caverne, le Goueil di Her, redouté des habitants du pays, parce qu´après les pluies une puissante rivière souterraine jaillit sous pression hors de la grotte, produisant une détonation qui s´entend à plusieurs kilomètres». “Near Arbas is a strange cave, the Goueil di Her, feared by the inhabitants of the country, because after the rains a powerful subterranean river gushes under pressure out of the cave, producing a detonation which can be heard several kilometers away”. During subsequent trips, Jeannel, Racovitza and several biologists continued to regularly sample this cave (Figure 4).

Figure 4.
René Jeannel at the entrance of the Goueil di Her cave, modified from [11].
A summary of their collections was published in the “Enumérations des Grottes Visitées” [22,24,25]. In 1908, Jeannel visited several caves of the Arbas massif, and sampled their fauna (Gouffre du Pont de Gerbaut, Grotte de Pène Blanque, Poudac Gran, Hount deras Hechos, Grotte de Gourgue) [22]. In particular, he returned repeatedly to the Goueil di Her (1908, 1910, 1912). An important integrative work was done by F. Trombe and his collaborators (1945–1947), based on a compilation of data in the fields of speleology, karstology, climatology, hydrology, ecology, and biospeology of the Comminges area, with a strong focus on the Arbas massif karst [12,26] Some years later, the Pène Blanque cave fauna was the subject of another contribution [13]. The last and most important investigations for aquatic subterranean fauna were done by Lescher-Moutoué, Gourbault and Rouch, who studied the composition, distribution and ecology of the stygobiotic fauna and characterized abiotic parameters of the Goueil di Her habitats in great detail [9,14]. A synthesis of our knowledge on the hydrology and biospeology of the massif was published by Bou [4,27]. Aside from these fundamental works, data available in the literature are few, based on punctual samples in a small number of caves.
5. The Fauna
The Goueil di Her was ranked among the world hotspots of subterranean biodiversity, with 26 cave-restricted species taxa (14 aquatics, 12 terrestrial species) in [28,29], then with 29 species [30]. This last number is actually an underestimation of the biological richness of the cave, and of the whole system. Our knowledge of the Coume Ouarnède terrestrial biodiversity relies on disparate sampling surveys in a few caves and pits, that often focused on peculiar groups only, mostly beetles; conversely, aquatic fauna has been the object of an intensive taxonomic and ecological study in karst and hyporheic habitats, but limited to the first part of the Goueil di Her cave itself and to the hyporheic zone of the stream that emerges from this cave [9].
Although the system counts more than 50 entrances, faunistic records come from a few of them: nine caves have three cave species or more, but among them, only two were investigated thoroughly, and provided more than seven cave-restricted species: the Pène Blanque cave (eight troglobionts, no stygobionts) and the Goueil di Her, the main resurgence of the system and by far the richest (17 troglobionts and 13 stygobionts). Fourteen stygobionts were collected in the hyporheic of streamlet under Goueil di Her. Faunistic data are detailed in Table 1 and Table 2.

Table 1.
List of stygobionts and troglobionts of the Coume Ouarnède system (CO), Grotte de Lestelas (L), Grotte de Gourgue (G). *, ectoparasite; hab, habitats; c, cave; h, hyporheic; s, spring; x, species present in CO, L or G.

Table 2.
List of troglophiles of the Coume Ouarnède system (CO), Grotte de Lestelas (L), Grotte de Gourgue (G). ecol, ecology; endo, endogean; TPeu, eutroglophiles; TPsub, subtroglophiles; x, species present in CO, L or G.
5.1. Stygobiotic Taxa
The works of Lescher-Moutoué, Gourbault, and Rouch are the main sources of information about stygobionts of the system [9,14]. They mainly focused on crustacea of Goueil di Her cave and of the hyporheic of the stream down to 2 km from the resurgence, that is, a limited number of habitats compared to those of the whole system. In most cases, Copepoda were the dominant group in abundance and diversity, rarely surpassed by Ostracoda for abundance. The taxonomic coverage of the collected fauna is globally good, but Ostracoda remain unidentified and only two species of Gastropoda were mentioned. Below, we browse the most interesting stygobiotic species of the Coume Ouarnède system.
5.1.1. Tricladida
Plagnolia vandeli, the only representative of the genus Plagnolia, is a blind white flatworm endemic of a few karsts in central northern Pyrenees. Metabolism of the species has been shown to be strongly reduced compared to epigean species and is associated with a considerable lengthening of all biological processes, such as regeneration and life expectancy [31].
5.1.2. Gastropoda
Both listed species, Moitessieria simoniana and Islamia moquiniana, are wide-range endemics of subterranean aquifers, the former in eastern Pyrenees and Montagne Noire, the later in eastern Pyrenees [32].
5.1.3. Amphipoda
Five species of Amphipoda in three different genera are present in the system. Parasalentinella rouchi, from the hyporheic zone of the Arbas and Escalette streams, is a small species known from the hyporheic of a few stations in Ariège and eastern Haute-Garonne [33]; it is present in this habitat downstream of Goueil di Her resurgence, where it sometimes occurs together with Salentinella petiti.
Niphargus are present but less common in Pyrenees than in most other French regions [34]. Three species of Niphargus have been collected in the system, in three different habitats: N. pachypus in the hyporheic downstream of the resurgence [4], a species of the group longicaudatus Costa, 1851, probably N. robustus, inside the Goueil di Her [4,34] and N. foreli at a spring in the upper part of the system [35]. Further sampling as well as taxonomic work would be necessary to confirm these findings.
5.1.4. Isopoda
Proasellus racovitzai was described from the Goueil di Her where it coexists with Stenasellus virei hussoni. It is considered endemic of this cave [36]. Stenasellus virei hussoni (Figure 5), a “carnivore facultative omnivore” after [37], is widespread in caves of the northern part of central Pyrenees. This typical stygobiont has a life span of 15–20 years [38].

Figure 5.
Stenasellus virei hussoni, a stygobiotic species common in hypogean waters of the Coume Ouarnède system (specimen from the Tute de Jovis cave, in the Sourroque massif, east of the Arbas massif; body 10 mm long; reproduced with permission from S. Huang).
5.1.5. Copepoda
The Coume Ouarnède is especially rich in Copepoda, with nine stygobiotic species recorded so far, of which six occur at the Goueil di Her. A large number of epigean species are associated to these strictly hypogean species, for example 11 troglophilic or trogloxenic species for four troglobiotic ones among harpacticoids are cited from Goueil di Her [14]. Copepoda are by far dominant in number and diversity in all hypogean compartments.
5.2. Terrestrial Taxa
5.2.1. Acari
Rhagidiidae, a family of tiny predatory mites, comprises the largest number of troglobionts among Acari of temperate caves. Troglocheles vandeli is a troglomorphic mite only known so far from its type locality, the Trou Mile in Arbas [39]. The remarkable blind, slender, and transparent mite mentioned in [40] in the Goueil di Her might be this species. Given the rarity of cave Rhagidiidae, the presence of a second troglobiotic species of this family, Rhagidia (Deharvengiella) troglomorphica Zacharda, 1987, in the Grotte de la Buhadère, a cave of the Source Bleue system adjacent to the southwestern part of the Arbas massif, may indicate that these rare mites may be more diversified than expected.
5.2.2. Araneae
The Coume Ouarnède system, as well as the Arbas and surrounding massifs, are riche in troglophilic spiders, but very poor in troglobiotic ones. A single troglobiotic spider, Leptoneta microphthalma, is recorded from system, and present in several caves of the massif d’Arbas and surrounding karsts. It has six reduced eyes. L. infuscata, a troglophilic but mostly cave dwelling species, is also frequent in the caves of the region [41].
5.2.3. Opiliones
Arbasus caecus is a relictual species of harvestmen, the only species in the genus Arbasus (Figure 6). The superfamily Travunioidea, to which Arbasus belongs, is a north temperate lineage known from North America, Europe and East Asia (Japan, Korea) and counts four families and 24 genera, some of them troglobiotic [42]. The peculiar morphology of Arbasus makes difficult its attribution to a family, and it is regarded as a representative of either Travuniidae [43] or Cladonychiidae [42]. Genetic data are still lacking to confirm this attribution.

Figure 6.
Arbasus caecus, a monospecific genus of harvestmen Travuniidae described from Pène Blanque cave (specimen from Artigouli cave, Estadens; body 2 mm long; reproduced with permission from C. Vanderbergh).
First described in the genus Phalangodes by Simon [44], Arbasus caecus was subsequently included in a new genus, Arbasus, by Roewer in his revision of European Opiliones [45]. Contrary to what was mentioned by Bou [4], the species was not described from Gourgue cave, where it is present, but on a single specimen collected in the Pène Blanque cave, probably by R. Jeannel [22,44]. Since then, the species was found in Goueil di Her, as well as a few other caves in karsts surrounding the Arbas massif: Riusec cave of the Source Bleue system [12], Cap de Payssas cave at Juzet-d’Izaut [46], the Lestelas cave [47] of the Caussanous system [2], and more recently, the Artigouli cave at Estadens (C. Vanderbergh pers. comm.), a cave of the small Peyrein system northwest of Arbas [48].
5.2.4. Chilopoda
One of the three troglophilic Lithobius, L. troglodytes, has a strong affinity for cave habitats as more than 70% of its 73 French records listed by Iorio in 2014 were collected in caves [49]. The two other species are more abundant outside, rather than inside caves.
5.2.5. Diplopoda
All three species listed in Table 1 are detritivores. Four Blaniulidae (two species and four subspecies) have been reported in various caves of the Coume Ouarnède system in the literature. A taxonomic re-examination of this material would be necessary to validate these forms. We provisionally listed only the two species, Blaniulus lorifer and B. troglobius, regardless of their subspecies. The third species, Spelaeoglomeris jeanneli, is a small pill-millipede only known from the Arbas massif (Goueil di Her, Grotte de Gourgue, Grotte de Paloumère and Poudac Gran). This species is the only troglobiont endemic of the Arbas massif [4,11,50].
5.2.6. Isopoda Oniscida
The species Scotoniscus macromelos, endemic of central Pyrenees, is split into nine parapatric subspecies, all strictly troglobiotic. S. macromelos macromelos is the easternmost form, endemic of a cluster of karstic massifs including Arbas and Lestelas [51,52,53].
5.2.7. Collembola
Collembola are usually the dominant arthropod species in caves. They are detritivore, but troglobiotic species mostly ingest clay, like many deep soil species, perhaps feeding on the micro-organisms it contains, while many litter species ingest fungi mycelium.
Three cave-restricted species are known from the system. At least two additional ones from two genera are expected to occur, as they are known in caves of other systems of the region: a Micronychiurus and a blind Pseudosinella. No species from the guano has been cited from the system, but they probably exist, and a guanobiotic endemic Hypogastruridae, like those known from the Grotte de Mont de Chac and Grotte de Payssa nearby can also be expected.
Pseudosinella theodoridesi. It is a common species in the caves of Ariège and Haute-Garonne. Its eyes and pigmentation are reduced, but present. Its appendages are, however, clearly elongated. Several forms differing by eye reduction have been recognized in the caves of the region [54], but their taxonomic status is unclear.
Oncopodura tricuspidata. Described from the grotte de Pène Blanque, this troglomorphic species has populations in several caves of central Pyrenees, which are likely to be undescribed species [55].
Tritomurus falcifer. This highly troglomorphic species is blind, depigmented, and has long antennae and elongate claw, in contrast to all other western European Tomoceridae. It is geographically isolated from the two other species of its genus which are located in caves of the Dinarides [56] and can be qualified as relictual. The species is the only troglobiont of the Coume Ouarnède system to live in hygropetric habitats [57], where it moves relatively slowly, often associated to Aphaenops ehlersi; though equipped with a long furca, “the large Collembola, special to” the Goueil di Her “jump with difficulty and prefer to run away: they walk by sweeping the ground with their very long antennae which strike the roughness of the ground while folding back.” [40]. It is known from the Goueil di Her and Trou Mile in the system, but also from a few caves in the surroundings of the Arbas massif, that is, Grotte du Béguet, Grotte de la Buhadère and Grotte de Riusec.
5.2.8. Coleoptera
The system is rich in 11 species of Coleoptera, among which six are troglobitic (genera Aphaenops and Speonomus, both endemic of the Pyrenees), three are endogean and two are troglophilic. Though narrow endemics, all Coume Ouarnède beetle species in their current acceptation have distribution areas exceeding the Coume Ouarnède and even the Arbas massif. None of the species are endemic of the Arbas massif itself, but all the troglobiotic ones have a narrow distribution in Arbas and surrounding massifs, like the Leiodidae Speonomus (Machaeroscelis) infernus arbasanus (Figure 7) [58].

Figure 7.
Speonomus (Machaeroscelis) infernus arbasanus, a hypogean Leiodidae present in the Arbas massif (specimen from Lespugue cave, Saleich; body 2.5 mm long; reproduced with permission from S. Huang).
The genus Aphaenops, endemic of the Pyrenees, is composed of many troglobiotic and a few endogean species. Five species are quoted from the system, all strictly troglobiotic. These Aphaenops are easily recognizable morphologically and were, until recently, distributed in different subgenera. Three of them are common (A. ehlersi, A. t. tiresias, A. cerberus bruneti) in the Goueil di Her cave, while the two other ones are rare.
Aphaenops (formerly Hydraphaenops) ehlersi (Figure 8) has a particularly interesting ecology, very different from the other species of the genus present in the Goueil di Her. During a visit of the cave just after a storm and a flood in July 1914, Jeannel observed numerous exemplars of “Hydraphaenops” (actually A. ehlersi) walking on wet clay around the sump [59], that were usually not present or rare in this habitat. He was convinced that he found here the real way of life of the Hydraphaenops-like species, which are always extremely rare in most of caves of Pyrenees. He hypothesized the existence of a terrestrial phreatic habitat restricted to the deep parts of the karsts, to which some species are subservient, coming out to the riverbanks of subterranean rivers only after flooding [60,61].

Figure 8.
Aphaenops ehlersi, specimen from Goueil di Her (body 4.2 mm long; reproduced with permission from C. Vanderbergh).
Aphaenops (formerly Arachnaphaenops) tiresias is a wide-range endemic that is present in several karsts in the Pyrenees of Ariège and Haute-Garonne.
Aphaenops (formerly Cerbaphaenops) cerberus is widespread in Ariège and Haute-Garonne, where it is differentiated in many genetically divergent populations, some taxonomically recognized as subspecies, the splits between the populations reflecting largely the fragmentation of the karst itself [62]. The Aphaenops cerberus bruneti populations of Coume Ouarnède and of Lestelas, locus typicus of the subspecies are slightly different morphologically. Because of morphological similarities, it was suggested that the Coume Ouarnède population, the westernmost of A. cerberus, might actually belong to the species A. jauzioni Faille, Déliot, and Quéinnec, 2007 which was described from Grotte d’Artigouli, a cave in a small isolated limestone outcrop 5 km northwest from Goueil di Her [63,64].
Aphaenops (formerly Cephalaphaenops) bucephalus is known from many caves of Ariège and Haute-Garonne, where it is always rare [65] (Figure 9).

Figure 9.
Aphaenops bucephalus, a species endemic from the Ariège and Haute-Garonne departments, always rare in its distribution area, and known by only a few records in Goueil di Her cave (specimen from Mount cave, Juzet-d’Izaut; body 5.6 mm long; reproduced with permission from S. Huang).
Aphaenops crypticola is quoted from a single record under the name A. parallelus Coiffait, 1954, and its identification needs confirmation [66].
One species of the Coume Ouarnède system initially described as distinct subspecies (A. tiresias ssp. proserpina Jeannel, 1909) was recently synonymized with its nominal subspecies. Another one, A. ehlersi ssp. longiceps Jeannel, 1926, is no more recognized as a valid subspecies. As a result, none of the recognized Aphaenops of the Coume Ouarnède is currently restricted to the system, but all remain relatively narrow regional endemics.
Among the three endogean species found in the system, Geotrechus orpheus consorranus belongs to a genus very close to Aphaenops, but with as many endogean forms as troglobiotic ones. All Geotrechus are blind and depigmented, but none exhibit strong appendage elongation. The species found in the Coume Ouarnède, often endogean, is also present in Grotte de Lestelas. However, this last cave also has another species of the same genus, G. trophonius trophonius, which is rare.
5.2.9. Laboulbeniomycetes
Pyrenean Trechinae frequently carry on their integument a species of fungi of the Laboulbeniaceae family. Laboulbeniomycetes are a group of ascomycete fungi that utilizes arthropods for nutrition and/or dispersal. The species Rhachomyces aphaenopsis is known on a large number of Pyrenean cave Trechinae (see [67] for a complete list of the hosts) and was found on three species of Trechinae occurring in the Coume Ouarnède system: Aphaenops cerberus bruneti, A. tiresias tiresias and A. ehlersi [67,68].
6. Species Richness in a Regional Context
The Coume Ouarnède system is the second hotspot of subterranean biodiversity of France; it is second to Cent Fonts spring in total species richness and second to Grotte de Lestelas in number of troglobionts (Table 3). Aside from its 21 stygobionts and 17 troglobionts, the system is also home to 20 troglophiles, including three species of endogean beetles (Table 2).

Table 3.
Number of obligate subterranean species of the richest caves and aquifers of France, and of three rich caves close to the Coume Ouarnède system (Grotte de la Buhadère, Grotte de Lestelas and Grotte de Gourgue). Tb, number of troglobionts; Sb, number of stygobionts; *, caves close to Coume Ouarnède.
The Coume Ouarnède system shares most of its species with three karstic systems geographically close to it [2], that is, the Grotte de Gourgue, 200 m from Goueil di Her, but not related to the Coume Ouarnède system; the Lestelas cave of the Cassaunous system; and the Buhadère cave of the Source Bleue system. The differences in faunistic richness are primarily due to undersampling of these three caves, in particular for aquatics. None of the three cited caves have been sampled for microcrustaceans, which account for more than half of the total number of species in the Coume Ouarnède system. More generally, inventories of the hyporheic fauna would be required to precise the distribution limits and the degree of endemicity of the microfauna of subterranean aquifers in most parts of the Pyrenees. The few stygobionts encountered in the three caves are present in the Coume Ouarnède, except Echinophrya stenaselli, ectoparasite on Stenasellus pleopods [72].
The troglobiont dataset is less uneven. In the Grotte de Gourgue, very close to the Goueil di Her, all troglobionts, but one, are shared with the Coume Ouarnède system. The missing one is a rare Pseudoscorpion, Neobisium (Blothrus) sp. The Grotte de Lestelas, more thoroughly sampled, shares 11 species with the Coume Ouarnède, but has 8 species not found in this system. Among them, two species have vicariants in the Coume Ouarnède. Tomocerus problematicus is replaced by Tritomurus falcifer (though very different morphologically, they are mutually exclusive in the region). Speonomus (M.) infernus infernus is represented by a different subspecies, S. (M.) infernus arbasanus in the Coume Ouarnède. The other six species, that live in habitats undersampled in the Coume Ouarnède system, could be potentially found in the system, like Pseudoscorpiones or Ischyropsalis pyrenaea, a Pyrenean endemic, which occurs in several caves close to the system: Lestelas, Lespugue among others [46]. Comparisons with Grotte de la Buhadère would require deeper taxonomic investigations, but this cave has at least two rare troglobiotic micro-arthropods that could be potentially present in the Coume Ouarnède system: a Rhagidiidae and a new Oncopodura springtail of a species group different from O. tricuspidata. Discoveries of additional troglobionts in the Coume Ouarnède system is therefore foreseeable.
Conversely, several of the troglobionts (Spelaeoglomeris jeanneli for instance) present in the Coume Ouarnède system and lacking in Lestelas, are susceptible to be found in this last cave. Given the lack of all-taxa inventories in most caves of Pyrenees, it is likely that caves of richness similar to that of the Coume Ouarnède exist in the central and western Pyrenees. In the same line, some groups which are widespread in subterranean ecosystems of the Pyrenean range have not been reported from the Coume Ouarnède system nor from the three caves of its surroundings mentioned above, such as Diplura, frequent troglobionts in Pyrenean caves [73], or Ostracoda among stygobionts. This last group is widespread and diversified in groundwaters of central Pyrenees, but its species have been rarely identified.
At the scale of France, between-site comparisons are more biased. The highest species richness known so far, that of Cent-Fonts spring, and the third and fourth richest ones, those of Triadou and of the Baget, do not compare with the Coume Ouarnède dataset as they are focused on interstitial aquatic fauna based on exceptional sampling efforts during years. The current pattern at country level is that hotspots of subterranean diversity are located in two regions: central and western Pyrenees on one hand for both stygobionts and troglobionts, and southeastern Massif Central on the other hand for stygobionts. Within these regions, the richest caves are clearly also the most heavily sampled.
7. Conclusions and Perspectives
The faunistic inventory presented in this paper has to be put in perspective. Its limitation is due to several ecological, spatial, taxonomical and methodological gaps. Guano and shallow subterranean habitats have been only marginally sampled or not sampled at all. A single site, the Goueil di Her, has been the object of thorough all-taxa sampling; however, its cave habitats, though highly interesting, are quite unusual among the caves of the region due to frequent flooding of all the sampled passages. Taxonomic coverage has been insufficient for snails and springtails, while Ostracoda and Diplura have not been identified. At least, mostly basic sampling techniques have been used for terrestrial and aquatic fauna except at Goueil di Her. Overall, this may explain why several cave species encountered in the surroundings of the Arbas massif have not yet been recorded from the Coume Ouarnède system. Filling these gaps would significantly increase the subterranean biodiversity of the system. On the other hand, sampling surrounding karsts, finetuning distribution limits, and testing genetic differentiation of populations of stygobionts would obviously help in understanding the observed geographical patterns. In this respect, investigations are currently being carried out, that focus on the major biodiversity issues raised above, that is, sampling a few targeted caves located in the upper Coume Ouarnède system and shallow subterranean habitats of its basin, and caves in the three surrounding systems identified by the BRGM [2]. The basic sampling methods were completed by bait-based techniques in oligotrophic and aquatic habitats.
In spite of these limitations, this work confirms the richness of the Coume Ouarnède system and of its most interesting cave, the Goueil di Her. Springtails and beetles are dominant, like in all central and western Pyrenean karsts (Table 1) [18], and these two groups clearly illustrate its difference with non-Pyrenean regions of France in terms of global subterranean species richness. The presence of two relictual troglomorphic species in the Coume Ouarnède fauna is a second illustration of its exceptional biodiversity. This raises fascinating questions about the origin and age of this fauna, as much as similarly isolated relicts do not exist in the karsts of central Pyrenees surrounding the Arbas and its satellite massifs.
Author Contributions
Both authors A.F. and L.D. participated in all phases of the realization and writing of the paper. Both authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Christian Vanderbergh and Sunbin Huang for providing pictures of some hypogean taxa. We are particularly grateful to Sylvestre Clément (Speleo-Club du Comminges, Arbas) for sharing with us his deep knowledge of the Coume Ouarnède system and for providing an up-to-date general topography of the system.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Culver, D.C.; Deharveng, L.; Bedos, A.; Lewis, J.J.; Madden, M.; Reddell, J.R.; Sket, B.; Trontelj, P.; White, D. The mid-latitude biodiversity ridge in terrestrial cave fauna. Ecography 2006, 29, 120–128. [Google Scholar] [CrossRef]
- BRGM. Couledoux Arbas—Estelas. In Atlas des Potentialités Aquifères des Formations Pyrénéennes Projet Potapyr—BRGM/RP-66912-FR, (Technical Document); BRGM: Orléans, France, 2017; pp. 1–27. [Google Scholar]
- Chabert, C. Bibliographie. In La Coumo d’Hyouernedo—Réseau Félix Trombe—Henne Morte—Massif d’Arbas; Duchêne, M., Drillat, P.A., Eds.; Groupe Spéléologique des Pyrénées: Toulouse, France, 1982; pp. 335–340. [Google Scholar]
- Bou, C. Biospéologie. In La Coumo d’Hyouernedo—Réseau Félix Trombe—Henne Morte—Massif d’Arbas; Duchêne, M., Drillat, P.A., Eds.; Groupe Spéléologique des Pyrénées: Toulouse, France, 1982; pp. 195–204. [Google Scholar]
- Jeannel, R. Le peuplement des Pyrénées. Rev. Fr. Entomol. 1947, 14, 53–104. [Google Scholar]
- Clément, S.; Vennarecci, P. Réseau Félix Trombe—Henne Morte. Massif d’Arbas—Pyrénées Centrales. Synthèse Topo explo du Karst d’Arbas; CDS Haute-Garonne: Toulouse, France, 2003; pp. 1–351. [Google Scholar]
- Clément, S. La Coume Ouarnède, le plus grand réseau souterrain de France. In Grottes et karsts de France; Audra, P., Ed.; Karstologia Mémoires: Lyon, France, 2010; Volume 19, pp. 278–279. [Google Scholar]
- Trombe, F. L’exploration du Gouffre de la Henne Morte, commune d’Arbas (Haute-Garonne). Ann. Spéléol. 1948, 3, 25–48. [Google Scholar]
- Lescher-Moutoué, F.; Gourbault, N. Recherches sur les eaux souterraines. Etude écologique du peuplement des eaux souterraines de la zone de circulation permanente d’un massif karstique. Ann. Spéléol. 1970, 25, 765–848. [Google Scholar]
- Martel, E.A. Rapport sur l’exploration souterraine hydrologique des Pyrénées en 1908. In Ann. Minist. Agriculture Paris; Imprimerie Nationale: Paris, France, 1910; Volume 38, pp. 1–96. [Google Scholar]
- Jeannel, R. Faune cavernicole de la France; Lechevalier: Paris, France, 1926; pp. 1–334. [Google Scholar]
- Dresco, E.; Nègre, J. Recherches souterraines dans les Pyrénées Centrales Années 1945 à 1947. Chapitre IV. Résultats Biospéléologiques. Ann. Spéléol. 1947, 2, 149–164. [Google Scholar]
- Derouet, L.; Dresco, E. Etudes sur la grotte de Pèneblanque. I. Faune et Climats. Notes Biospéol. 1955, 10, 123–131. [Google Scholar]
- Rouch, R. Recherches sur les eaux souterraines. 14—Peuplement par les Harpacticides d’un drain situé dans la zone de circulation permanente. Ann. Spéléol. 1971, 26, 107–133. [Google Scholar]
- INPN. Muséum National d’Histoire Naturelle. Available online: https://inpn.mnhn.fr (accessed on 5 July 2021).
- World Register of Marine Species, WoRMS Editorial Board. Available online: https://www.marinespecies.org (accessed on 15 June 2021).
- World Spider Catalog. Version 22.5. Natural History Museum Bern. Available online: http://wsc.nmbe.ch (accessed on 5 July 2021).
- Juberthie, C. France. In Encyclopaedia Biospeologica, Tome 1; Juberthie, C., Decu, V., Eds.; Société de Biospéologie: Moulis, France, 1994; pp. 665–692. [Google Scholar]
- Mammola, S.; Cardoso, P.; Ribera, C.; Pavlek, M.; Isaia, M. A synthesis on cave-dwelling spiders in Europe. J. Zoolog. Syst. Evol. Res. 2018, 56, 301–316. [Google Scholar] [CrossRef]
- Culver, D.C.; Pipan, T.; Gottstein, S. Hypotelminorheic—A unique freshwater habitat. Subterr. Biol. 2006, 4, 1–7. [Google Scholar]
- Filhol, C.; Jeanbernat, E.; Timbal-Lagrave, E. Exploration scientifique du Massif d’Arbas; Imprimerie de Louis & Jean Matthieu Douladoure: Toulouse, France, 1874; Volume 2, pp. 367–477. [Google Scholar]
- Jeannel, R.; Racovitza, E.G. Biospeologica XVI. Enumération des grottes visitées, 1908–1909 (Troisième série). Arch. Zool. Exp. Gen. 1910, 5ème série, tome V, 489–536. [Google Scholar]
- Jeannel, R. Quarante années d’Explorations souterraines. Notes Biospéologiques 1950, 6, 1–94. [Google Scholar]
- Jeannel, R.; Racovitza, E.G. Biospeologica XXIV. Enumération des grottes visitées, 1909–1911 (Quatrième série). Arch. Zool. Exp. Gen. 1912, 5ème série, tome IX, 501–667. [Google Scholar]
- Jeannel, R.; Racovitza, E.G. Biospeologica LIV. Enumération des grottes visitées, 1918–1927 (Septième série). Arch. Zool. Exp. Gen. 1929, 68, 293–608. [Google Scholar]
- Trombe, F. Recherches souterraines dans les Pyrénées centrales. Ann. Spéléol. 1947, 2, 67–164. [Google Scholar]
- Bou, C. Hydrogéologie. In La Coumo d’Hyouernedo—Réseau Félix Trombe—Henne Morte—Massif d’Arbas; Duchêne, M., Drillat, P.A., Eds.; Groupe Spéléologique des Pyrénées: Toulouse, France, 1982; pp. 205–214. [Google Scholar]
- Culver, D.C.; Sket, B. Hotspots of subterranean biodiversity in caves and wells. J. Caves Karst Stud. 2000, 62, 11–17. [Google Scholar]
- Deharveng, L. Diversity in the Tropics. In Encyclopedia of Caves; Culver, D.C., White, W.D., Eds.; Elsevier: London, UK, 2005; pp. 166–170. [Google Scholar]
- Deharveng, L.; Bedos, A. Chapter 18—Diversity Patterns in the Tropics. In Encyclopedia of Caves; White, W.B., Culver, D.C., Pipan, T., Eds.; Academic Press—Elsevier: Amsterdam, The Netherlands, 2019; pp. 146–162. [Google Scholar]
- Gourbault, N. Recherches sur les Triclades Paludicoles hypogés. In Mémoires du Muséum National d’Histoire Naturelle; Éditions du Muséum: Paris, France, 1972; Volume 73, pp. 1–249. [Google Scholar]
- Bertrand, A. Atlas préliminaire de répartition géographique des mollusques stygobies de la faune de France (Mollusca: Rissoidea: Caenogastropoda). Doc. Malacol. 2004, h.s. 2, 1–81. [Google Scholar]
- Bou, C. Recherches sur les eaux souterraines 16. Parasalentinella rouchi n. g., n. sp., des eaux souterraines des Pyrénées françaises (Amphipoda, Gammaridae). Ann. Spéléol. 1971, 26, 481–494. [Google Scholar]
- Ginet, R. Stations de Niphargus pyrénéens. Notes Biospéol. 1956, 11, 17–22. [Google Scholar]
- Balazuc, J. Les Amphipodes troglobies et phréatobies de la faune gallo-rhénane. Arch. Zool. Exp. Gen. 1954, 91, 153–193. [Google Scholar]
- Henry, J.P.; Magniez, G. Observations sur un Aselle obscuricole de France: Proasellus racovitzai n. sp. (Crustacea Isopoda Asellota). Int. J. Speleol. 1972, 4, 171–188. [Google Scholar] [CrossRef]
- Racovitza, E.G. Biospeologica LXX. Asellides (Première série). Arch. Zool. Exp. Gen. 1950, 87, 1–94. [Google Scholar]
- Henry, J.P.; Magniez, G. Introduction pratique à la systématique des organismes des eaux continentales françaises. 4: Crustacés Isopodes (principalement Asellotes). Bull. Soc. Linn. Lyon 1983, 52, 319–357. [Google Scholar] [CrossRef]
- Zacharda, M. New taxa of Rhagidiidae (Acari: Prostigmata) from Pyrenean caves. Can. J. Zool. 1987, 65, 2051–2056. [Google Scholar] [CrossRef]
- Jeannel, R.; Racovitza, E.G. Biospeologica XXXIII. Enumération des grottes visitées 1911–1913 (cinquième série). Arch. Zool. Exp. Gen. 1914, 53, 325–558. [Google Scholar]
- Dresco, E. Etude des Leptoneta. Leptoneta convexa Sim. et microphtalma Sim. (Araneae, Leptonetidae). Bull. Soc. Hist. Nat. Toulouse 1980, 116, 146–149. [Google Scholar]
- Derkarabetian, S.; Starrett, J.; Tsurusaki, N.; Ubick, D.; Castillo, S.; Hedin, M. A stable phylogenomic classification of Travunioidea (Arachnida, Opiliones, Laniatores) based on sequence capture of ultraconserved elements. Zookeys 2018, 760, 1–36. [Google Scholar] [CrossRef]
- Kury, A.B.; Mendes, A.C. Taxonomic status of the European genera of Travuniidae (Arachnida: Opiliones: Laniatores). Munis Entomol. Zool. 2007, 2, 1–14. [Google Scholar]
- Simon, E. Biospeologica XXIII. Araneae et Opiliones (3ème série). Arch. Zool. Exp. Gen. 1911, 49, 177–206. [Google Scholar]
- Roewer, C.F.R. Biospeologica LXII. Opiliones (fünfte série)—Zugleich eine Revision aller bisher bekannten europäischen Laniatores. Arch. Zool. Exp. Gen. 1935, 78, 1–96. [Google Scholar]
- Juberthie, C.; Jauzion, I.; Gers, C. Faune de la grotte du Cap de Payssas. Mém. Biospéol. 1988, 15, 215. [Google Scholar]
- Delfosse, E. Catalogue préliminaire des Opilions de France métropolitaine (Arachnida Opiliones). Bull. Phyllie 2004, 20, 34–52. [Google Scholar]
- BRGM. Sauveterre-de-Comminges Gar-Cagire. In Atlas des Potentialités Aquifères des Formations Pyrénéennes Projet Potapyr—BRGM/RP-66912-FR, (Technical Document); BRGM: Orléans, France, 2017; pp. 1–24. [Google Scholar]
- Iorio, E. Catalogue biogéographique et taxonomique des chilopodes (Chilopoda) de France métropolitaine [Biogeographic and taxonomic catalogue of the centipedes (Chilopoda) of metropolitan France. Mém. Soc. Linn. Bordx. 2014, 15, 1–372. [Google Scholar]
- Manfredi, P. Contributo alla conscenza dei Miriapodi cavernicoli della Francia (Diplopodi). In Premier Congrès International de Spéléologie, Paris, France, 1953, Tome III; CNRS: Paris, France, 1956; pp. 283–288. [Google Scholar]
- Vandel, A. Faune de France 64—Isopodes Terrestres (Première Partie); Lechevalier: Paris, France, 1960; pp. 1–416. [Google Scholar]
- Deharveng, L.; Dalens, H.; Bedos, A.; Souqual, M.C. Les Isopodes Terrestres Endémiques de l’Europe de l’Ouest. 2012. Available online: http://endemica.mnhn.fr (accessed on 10 July 2021).
- Séchet, E.; Noël, F. Catalogue commenté des Crustacés Isopodes terrestres de France métropolitaine (Crustacea, Isopoda, Oniscidea). Mém. Soc. Linn. Bordeaux 2015, 16, 1–156. [Google Scholar]
- Christiansen, K.A.; Bouillon, M. An Evolutionary and Ecological Analysis of the Terrestrial Arthropods of Caves in the Central Pyrenees, Part One: Ecological Analysis with Special Reference to Collembola. NSS Bull. 1978, 40, 103–113. [Google Scholar]
- Deharveng, L. Collemboles cavernicoles—VIII—Contribution à l’étude des Oncopoduridae. Bull. Soc. Entomol. Fr. 1988, 92, 133–147. [Google Scholar]
- Yu, D.; Deharveng, L.; Lukic, M.; Wei, Y.; Hu, F.; Liu, M. Molecular phylogeny and trait evolution in an ancient terrestrial arthropod lineage: Systematic revision and implications for ecological divergence (Collembola, Tomocerinae). Mol. Phylogenet. Evol. 2021, 154, 106995. [Google Scholar] [CrossRef]
- Lukic, M.; Houssin, C.; Deharveng, L. A new relictual and highly troglomorphic species of Tomoceridae (Collembola) from a deep Croatian cave. ZooKeys 2010, 69, 1–16. [Google Scholar]
- Coiffait, H. Enumération des grottes visitées 1950–1957 (9ème série). Biospeologica LXXVII. Arch. Zool. Exp. Gen. 1959, 97, 209–465. [Google Scholar]
- Jeannel, R.; Racovitza, E.G. Biospeologica XXXIX. Enumération des grottes visitées 1913–1917 (sixième série). Arch. Zool. Exp. Gen. 1918, 57, 203–470. [Google Scholar]
- Jeannel, R. Les Fossiles Vivants des Cavernes; Gallimard: Paris, France, 1943; p. 321. [Google Scholar]
- Jeannel, R. Le sous-genre Hydraphaenops Jeannel (Coleoptera Trechidae). Notes Biospéol. 1948, 3, 17–27. [Google Scholar]
- Faille, A.; Tänzler, R.; Toussaint, E.F.A. On the way to speciation: Shedding light on the karstic phylogeography of the micro-endemic cave beetle Aphaenops cerberus in the Pyrenees. J. Hered. 2015, 106, 692–699. [Google Scholar] [PubMed]
- Faille, A. Endémisme et Adaptation à la Vie Cavernicole Chez les Trechinae Pyrénéens (Coleoptera: Caraboidea). Approches Moléculaire et Morphométrique. Ph.D. Thesis, Muséum National d’Histoire Naturelle, Paris, France, 2006; pp. 1–318. [Google Scholar]
- Faille, A.; Déliot, P.; Queinnec, E. A new cryptic species of Aphaenops (Coleoptera, Trechinae) from french Pyrenean cave: Congruence between morphometrical and geographical data confirm species isolation. Ann. Soc. Entomol. Fr. 2007, 43, 363–370. [Google Scholar] [CrossRef]
- Deliot, P.; Delay, B. Variabilité biométrique et morphologique d’Aphaenops bucephalus (Coléoptère Trechinae). Mém. Biospéol. 1983, 10, 285–294. [Google Scholar]
- Coiffait, H. Coléoptères troglobies pyrénéens nouveaux ou peu connus. Ann. Spéléol. 1969, 24, 557–561. [Google Scholar]
- Santamaria, S.; Faille, A. Rhachomyces (Ascomycota, Laboulbeniales) parasites on cave-inhabiting Carabidae beetles from Pyrenees. N. Hedwig. 2007, 85, 159–186. [Google Scholar] [CrossRef]
- Lepesme, P. Catalogue des Laboulbéniales de la collection François Picard. Bull. Mus. Natl. Hist. Nat. 1941, 13, 481–488. [Google Scholar]
- Olivier, M.J.; Martin, D.; Bou, C.; Prié, V. Interprétation du suivi hydrobiologique de la faune stygobie réalisé sur le système karstique des Cent Fonts lors du pompage d’essai. In Système karstique des Cent Fonts Simulation de Scénarios d’Exploitation et de Gestion de la Ressource, Rapport Final; BRGM: Montpellier, France, 2006; pp. 235–265. [Google Scholar]
- Faille, A.; Bourdeau, C.; Deharveng, L. Weak impact of tourism activities on biodiversity in a subterranean hotspot of endemism and its implications for the conservation of cave fauna. Insect Conserv. Divers. 2015, 8, 205–215. [Google Scholar] [CrossRef]
- Deharveng, L.; Bedos, A. The cave fauna of Southeast Asia. Origin, evolution and ecology. In Ecosystems of the World 30. Subterranean Ecosystems; Wilkens, H., Culver, D.C., Humphreys, W.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; pp. 603–632. [Google Scholar]
- Matjasic, J. Une nouvelle Choanophrya (Ciliata Suctoria) sur Stenasellus virei. Ann. Spéléol. 1963, 18, 267–270. [Google Scholar]
- Sendra, A.; Antić, D.; Barranco, P.; Borko, Š.; Christian, E.; Delić, T.; Fadrique, F.; Faille, A.; Galli, L.; Gasparo, F.; et al. Flourishing in subterranean ecosystems: Euro-Mediterranean Plusiocampinae and Tachycampoids (Diplura, Campodeidae). Eur. J. Taxon. 2020, 591, 1–138. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).