Assessing Ecological Indicators for Remnant Vegetation Strips as Functional Biological Corridors in Chilean Vineyards
Abstract
:1. Introduction
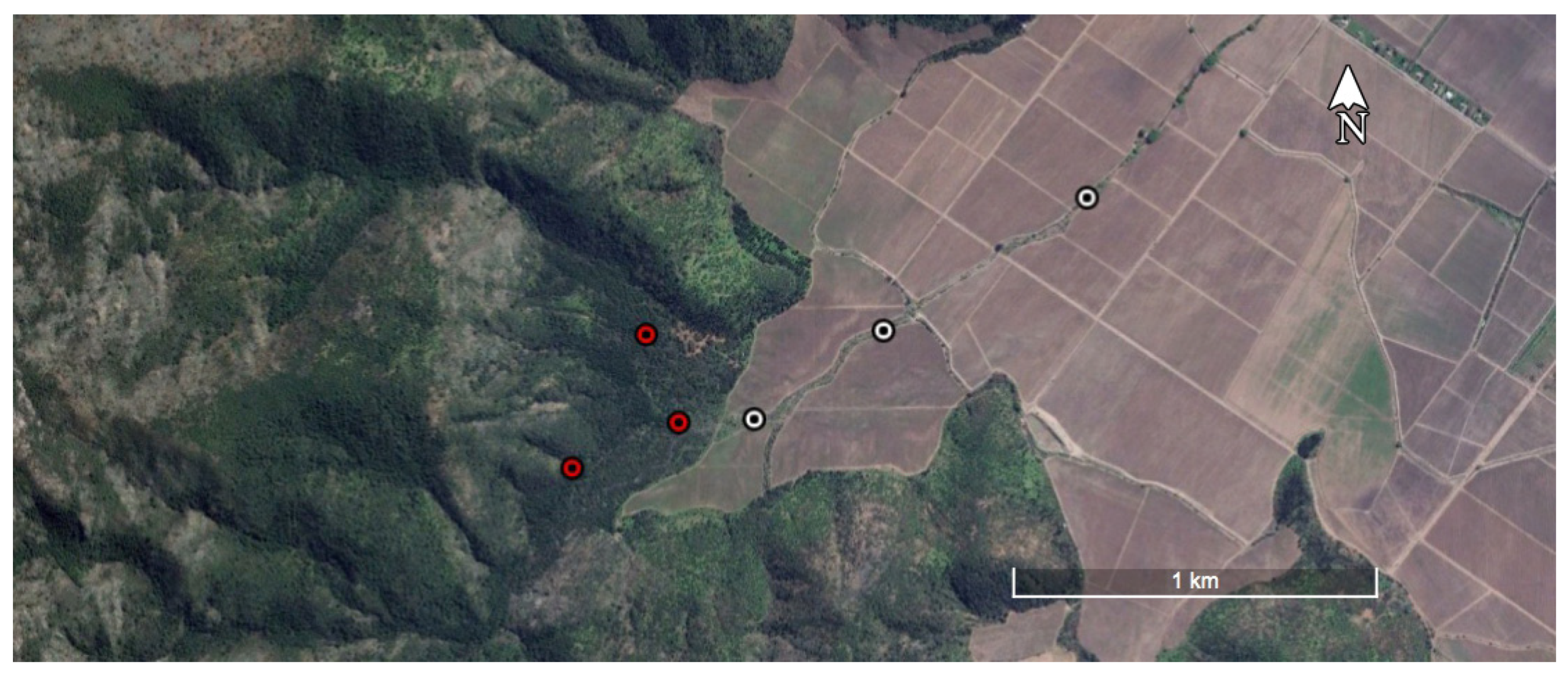
2. Materials and Methods
2.1. Data Analysis
2.1.1. Corridors vs. Remnant Natural Areas
2.1.2. Effect of Corridor Features on Ecological Indicators
3. Results
3.1. Corridor Characterization
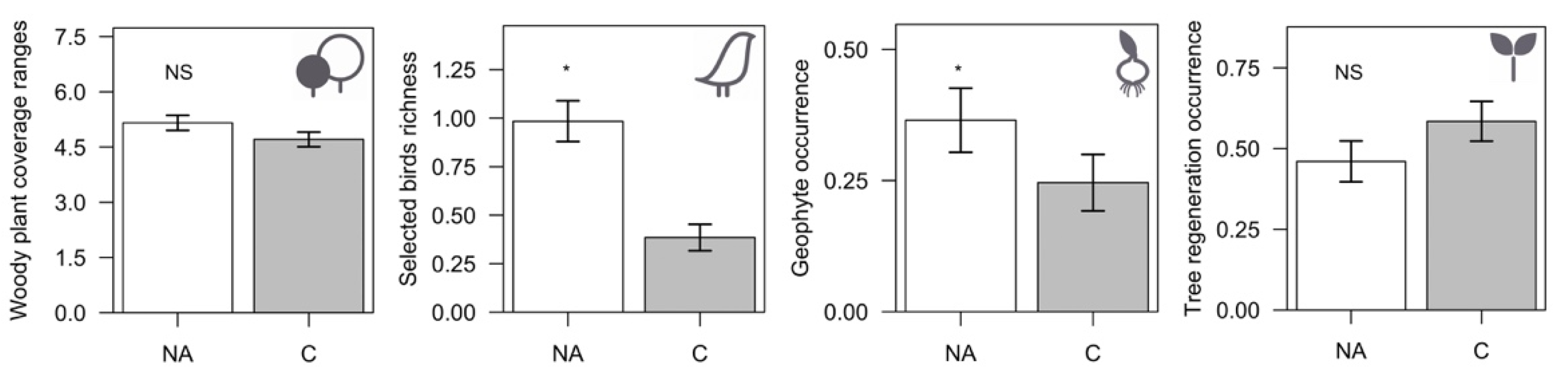
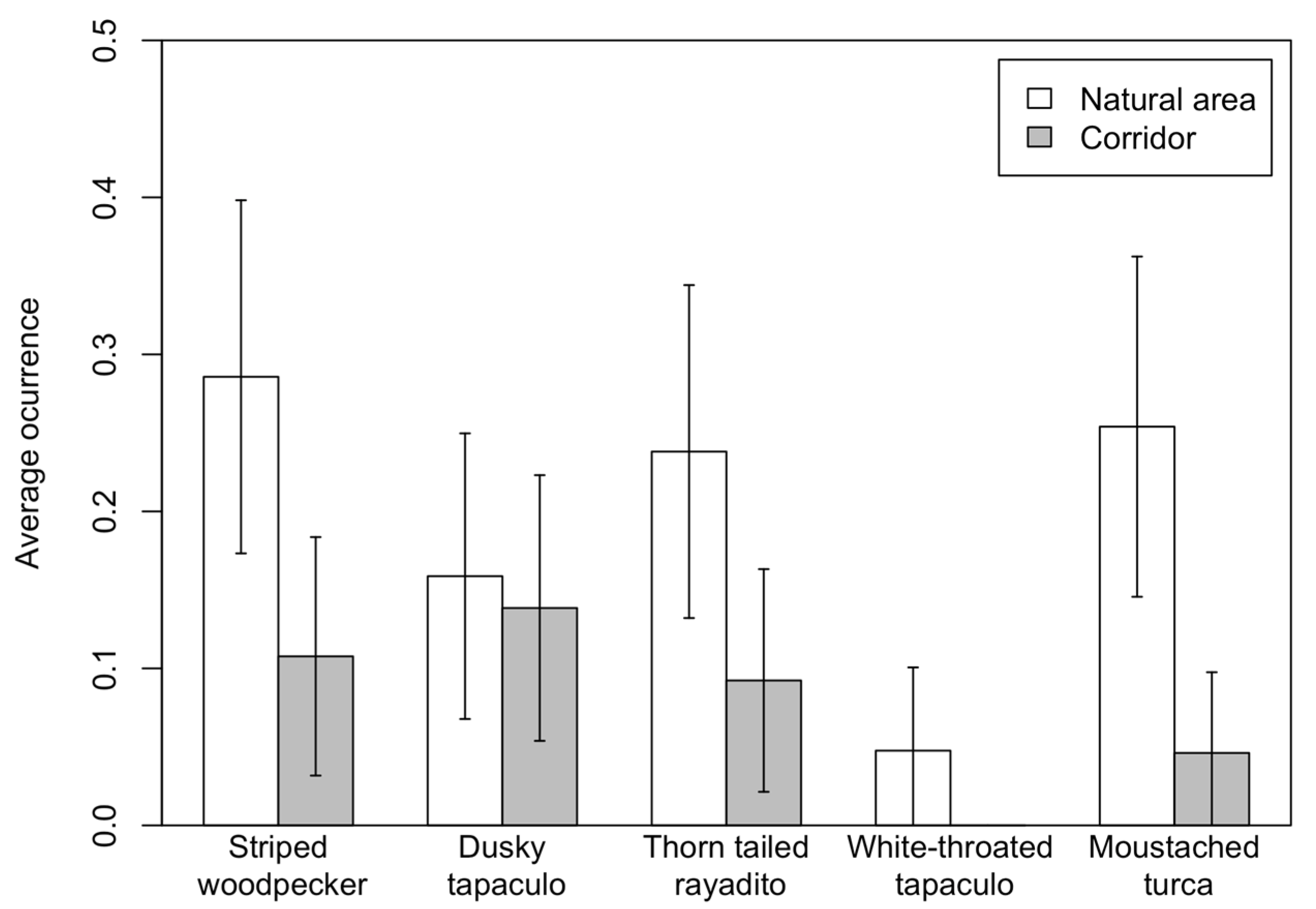
3.2. Corridors vs. Remnant Natural Areas
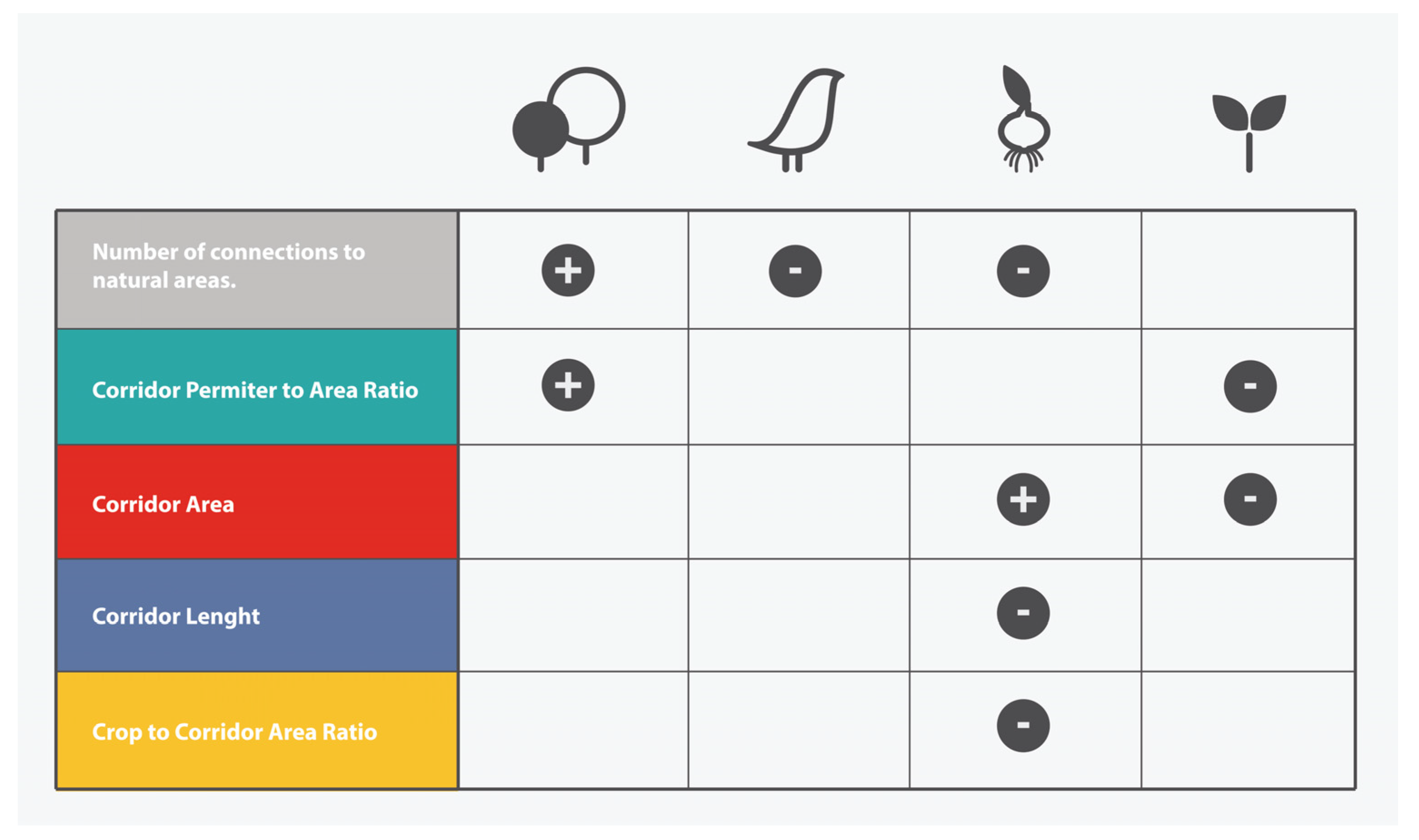
3.3. Effect of Corridor Attributes on Ecological Indicators
4. Discussion
- (1)
- Maintain and restore vegetation strip quality (habitat continuity and width) by reducing impact barriers, such as gaps without plant cover, and preventing corridors from ending in a cultivated area or road, thus avoiding ecological traps for wildlife [29].
- (2)
- Move from a single farm scale towards a broader landscape scale for agricultural planning and decision making [62]. Connect different natural areas with management that goes beyond private property limits [10]. The pattern of alternating valleys and ridges trending east–west, connecting the Andes and Coastal ranges, that shape the landscapes in central Chile, favor the design and implementation of large-scale corridors. The frequency of creeks, irrigation canals, stream hedgerows, riparian buffers, along with the conservation of scattered remnant vegetation patches could be used favorably to provide connections among natural areas conserved in central Chile, at the scale of basins or valleys. These natural areas often occur within the land owned by wineries worldwide [18,19].
- (3)
- Implement protections (herbivory exclusions and watering in extremely dry summers) for seedlings and saplings in vegetation corridors to maintain or enhance the contribution of propagule dispersal to the production of vegetation, which, in turn, facilitates the distribution of other organisms across productive areas, such as arthropod natural enemies [63].
- (4)
- Use our selected ecological indicators to evaluate conservation and management impacts, considering that: (a) Birds, geophytes, and woody plant cover are, according to our results, acceptable indicators of corridor quality, compared to the surrounding natural, unmodified, ecosystems. This requires that productive and natural areas be considered integrated and connected components within management planning; and (b) On the other hand, geophyte and tree seedling occurrences (that were shown to be associated with most vegetation strip traits), could be considered good indicators of the ecological status of corridors, and they can be used to monitor the success of different models of corridor design. These ecological variables can be easily measured by farmers, thus avoiding specialized technical assistance, and can provide useful insights and guidance to land managers.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loos, J.; Abson, D.J.; Chappell, M.J.; Hanspach, J.; Mikulcak, F.; Tichit, M.; Fischer, J. Putting meaning back into “sustainable intensification”. Front. Ecol. Environ. 2014, 12, 356–361. [Google Scholar] [CrossRef]
- Geertsema, W.; Rossing, W.A.; Landis, D.A.; Bianchi, F.J.; Van Rijn, P.C.; Schaminée, J.H.; Tscharntke, T.; Van Der Werf, W. Actionable knowledge for ecological intensification of agriculture. Front. Ecol. Environ. 2016, 14, 209–216. [Google Scholar] [CrossRef]
- Grass, I.; Loos, J.; Baensch, S.; Batáry, P.; Librán-Embid, F.; Ficiciyan, A.; Klaus, F.; Riechers, M.; Rosa, J.; Tiede, J.; et al. Land-sharing/-sparing connectivity landscapes for ecosystem services and biodiversity conservation. People Nat. 2019, 1–11. [Google Scholar] [CrossRef]
- Dainese, M.; Martin, E.A.; Aizen, M.A.; Albrecht, M.; Bartomeus, I.; Bommarco, R.; Carvalheiro, L.G.; Chaplin-Kramer, R.; Gagic, V.; Garibaldi, L.A.; et al. A global synthesis reveals biodiversity-mediated benefits for crop production. Sci. Adv. 2019, 5, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Chaplin-Kramer, R.; Sharp, R.P.; Mandle, L.; Sim, S.; Johnson, J.; Butnar, I.; Milà, I.; Canals, L.; Eichelberger, B.A.; Ramler, I.; et al. Spatial patterns of agricultural expansion determine impacts on biodiversity and carbon storage. Proc. Natl. Acad. Sci. USA 2015, 112, 7402–7407. [Google Scholar] [CrossRef] [Green Version]
- Kehoe, L.; Romero-Muñoz, A.; Polaina, E.; Estes, L.; Kreft, H.; Kuemmerle, T. Biodiversity at risk under future cropland expansion and intensification. Nat. Ecol. Evol. 2017, 1, 1129–1135. [Google Scholar] [CrossRef]
- IPBES. The Regional Assessment Report on Biodiversity and Ecosystem Services for the Americas; Secretariat of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services: Bonn, Germany, 2018; p. 656. [Google Scholar]
- Myers, N.; Mittermeier Russell, A.; Mittermeier Cristina, G.; da Fonseca Gustavo, A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Armesto, J.J.; Manuschevich, D.; Mora, A.; Smith-Ramirez, C.; Rozzi, R.; Abarzúa, A.M.; Marquet, P.A. From the Holocene to the Anthropocene: A historical framework for land cover change in southwestern South America in the past 15,000 years. Land Use Policy 2010, 27, 148–160. [Google Scholar] [CrossRef]
- Cox, R.L.; Underwood, E.C. The importance of conserving biodiversity outside of protected areas in mediterranean ecosystems. PLoS ONE 2011, 6, e0014508. [Google Scholar] [CrossRef]
- Magrach, A.; Sanz, M.J. Environmental and social consequences of the increase in the demand for ‘superfoods’ world-wide. People Nat. 2020, 2, 267–278. [Google Scholar] [CrossRef]
- Fisher, B.; Naidoo, R.; Ricketts, T. A Field Guide to Economics for Conservationists; Roberts and Company Publishers: Greenwood Village, CO, USA, 2015; 190p. [Google Scholar]
- Castañeda, L.E.; Godoy, K.; Manzano, M.; Marquet, P.A.; Barbosa, O. Comparison of soil microbial communities inhabiting vineyards and native sclerophyllous forests in central Chile. Ecol. Evol. 2015, 5, 3857–3868. [Google Scholar] [CrossRef]
- Simonetti, J.A.; Mella, J.E. Park size and the conservation of Chilean mammals. Rev. Chil. Hist. Nat. 1997, 70, 213–220. [Google Scholar]
- Schulz, J.J.; Cayuela, L.; Echeverria, C.; Salas, J.; Rey Benayas, J.M. Monitoring land cover change of the dryland forest landscape of Central Chile (1975–2008). Appl. Geogr. 2010, 30, 436–447. [Google Scholar] [CrossRef] [Green Version]
- Lovell, S.T.; Johnston, D.M. Creating multifunctional landscapes: How can the field of ecology inform the design of the landscape? Front. Ecol. Environ. 2009, 7, 212–220. [Google Scholar] [CrossRef]
- Agnoletti, M.; Santoro, A. Cultural values and sustainable forest management: The case of Europe. J. For. Res. 2015, 20, 438–444. [Google Scholar] [CrossRef]
- Santoro, A.; Venturi, M.; Agnoletti, M. Agricultural heritage systems and landscape perception among tourists. The case of Lamole, Chianti (Italy). Sustainability 2020, 12, 3509. [Google Scholar] [CrossRef]
- Pulpón, Á.R.R.; Ruiz, M.D.C.C. Potential of vineyard landscapes for sustainable tourism. Geosciences 2019, 9, 472. [Google Scholar] [CrossRef] [Green Version]
- Garibaldi, L.A.; Gemmill-Herren, B.; D’Annolfo, R.; Graeub, B.E.; Cunningham, S.A.; Breeze, T.D. Farming Approaches for Greater Biodiversity, Livelihoods, and Food Security. Trends Ecol. Evol. 2017, 32, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Pretty, J. Intensification for redesigned and sustainable agricultural systems. Science 2018, 362. [Google Scholar] [CrossRef] [Green Version]
- Gerten, D.; Heck, V.; Jägermeyr, J.; Bodirsky, B.L.; Fetzer, I.; Jalava, M.; Kummu, M.; Lucht, W.; Rockström, J.; Schaphoff, S.; et al. Feeding ten billion people is possible within four terrestrial planetary boundaries. Nat. Sustain. 2020, 3, 200–208. [Google Scholar] [CrossRef]
- Viers, J.H.; Williams, J.N.; Nicholas, K.A.; Barbosa, O.; Kotzé, I.; Spence, L.; Webb, L.B.; Merenlender, A.; Reynolds, M. Vinecology: Pairing wine with nature. Conserv. Lett. 2013, 6, 287–299. [Google Scholar] [CrossRef]
- Garibaldi, L.A.; Carvalheiro, L.G.; Leonhardt, S.D.; Aizen, M.A.; Blaauw, B.R.; Isaacs, R.; Kuhlmann, M.; Kleijn, D.; Klein, A.M.; Kremen, C.; et al. From research to action: Enhancing crop yield through wild pollinators. Front. Ecol. Environ. 2014, 12, 439–447. [Google Scholar] [CrossRef] [Green Version]
- Márquez-García, M.; Jacobson, S.K.; Barbosa, O. Wine with a Bouquet of Biodiversity: Assessing Agricultural Adoption of Conservation Practices in Chile. Environ. Conserv. 2019, 46, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Caro, T. Conservation by Proxy: Indicator, Umbrella, Keystone, Flagship, and Other Surrogate Species; Island Press: Washington, DC, USA, 2010. [Google Scholar]
- Bal, P.; Tulloch, A.I.T.; Addison, P.F.E.; McDonald-Madden, E.; Rhodes, J.R. Selecting indicator species for biodiversity management. Front. Ecol. Environ. 2018, 16, 589–598. [Google Scholar] [CrossRef]
- Failing, L.; Gregory, R. Ten common mistakes in designing biodiversity indicators for forest policy. J. Environ. Manag. 2003, 68, 121–132. [Google Scholar] [CrossRef] [Green Version]
- Hilty, J.A.; Merenlender, A.M. Use of Riparian Corridors and Vineyards by Mammalian Predators in Northern California. Conserv. Biol. 2004, 18, 126–135. [Google Scholar] [CrossRef]
- Schulz, J.J.; Schröder, B. Identifying suitable multifunctional restoration areas for Forest Landscape Restoration in Central Chile. Ecosphere 2017, 8. [Google Scholar] [CrossRef]
- Tewksbury, J.J.; Levey, D.J.; Haddad, N.M.; Sargent, S.; Orrock, J.L.; Weldon, A.; Danielson, B.J.; Brinkerhoff, J.; Damschen, E.I.; Townsend, P. Corridors affect plants, animals, and their interactions in fragmented landscapes. Proc. Natl. Acad. Sci. USA 2002, 99, 12923–12926. [Google Scholar] [CrossRef] [Green Version]
- Haddad, N.M.; Bowne, D.R.; Cunningham, A.; Danielson, B.J.; Levey, D.J.; Sargent, S.; Spira, T. Corridor use by diverse taxa. Ecology 2003, 84, 609–615. [Google Scholar] [CrossRef]
- Deslauriers, M.R.; Asgary, A.; Nazarnia, N.; Jaeger, J.A.G. Implementing the connectivity of natural areas in cities as an indicator in the City Biodiversity Index (CBI). Ecol. Indic. 2018, 94, 99–113. [Google Scholar] [CrossRef]
- Butler, S.J.; Freckleton, R.P.; Renwick, A.R.; Norris, K. An objective, niche-based approach to indicator species selection. Methods Ecol. Evol. 2012, 3, 317–326. [Google Scholar] [CrossRef]
- Morelli, F.; Pruscini, F.; Santolini, R.; Perna, P.; Benedetti, Y.; Sisti, D. Landscape heterogeneity metrics as indicators of bird diversity: Determining the optimal spatial scales in different landscapes. Ecol. Indic. 2013, 34, 372–379. [Google Scholar] [CrossRef]
- Noss, R.F. Indicators for Monitoring Biodiversity: A Hierarchical Approach. Conserv. Biol. 1990, 4, 355–364. [Google Scholar] [CrossRef]
- Altamirano, T.A.; Ibarra, J.T.; Martin, K.; Bonacic, C. The conservation value of tree decay processes as a key driver structuring tree cavity nest webs in South American temperate rainforests. Biodivers. Conserv. 2017, 26, 2453–2472. [Google Scholar] [CrossRef]
- Sieving, K.E.; Willson, M.F.; De Santo, T.L. Habitat Barriers to Movement of Understory Birds in Fragmented South-Temperate Rainforest. Auk 1996, 113, 944–949. [Google Scholar] [CrossRef]
- Sekercioglu, Ç.H.; Ehrlich, P.R.; Daily, G.C.; Aygen, D.; Goehring, D.; Sandí, R.F. Disappearance of insectivorous birds from tropical forest fragments. Proc. Natl. Acad. Sci. USA 2002, 99, 263–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steel, Z.L.; Steel, A.E.; Williams, J.N.; Viers, J.H.; Marquet, P.A.; Barbosa, O. Patterns of bird diversity and habitat use in mixed vineyard-matorral landscapes of Central Chile. Ecol. Indic. 2017, 73, 345–357. [Google Scholar] [CrossRef]
- Fuentes, E.R.; Otaiza, R.D.; Alliende, M.C.; Hoffmann, A.; Fuentes, E.R.; Otaiza, R.D.; Alliende, M.C.; Hoffmann, A.; Poiani, A. Shrub clumps of the Chilean matorral vegetation: Structure and possible maintenance mechanisms. Oecologia 1984, 62, 405–411. [Google Scholar] [CrossRef]
- Arroyo, M.T.K.; Cavieres, L.; Marticorena, C.; Muñoz-Schick, M. Convergence in the Mediterranean Floras in Central Chile and California: Insights from Comparative Biogeography. In Ecology and Biogeography of Mediterranean Ecosystems in Chile, California, and Australia; Arroyo, M.T.K., Zedler, P.H., Fox, M.D., Eds.; Springer: New York, NY, USA, 1995; pp. 43–88. ISBN 978-1-4612-2490-7. [Google Scholar]
- Root-Bernstein, M.; Bennett, M.; Armesto, J.J.; Ebensperger, L.A. Small mammals as indicators of cryptic plant species diversity in the central Chilean plant endemicity hotspot. Glob. Ecol. Conserv. 2014, 2, 277–288. [Google Scholar] [CrossRef] [Green Version]
- Contreras, L.; Gutiérrez, J.R. Effects of the subterranean herbivorous rodent Spalacopus cyanus on herbaceous vegetation in arid coastal Chile. Oecologia 1991, 87, 106–109. [Google Scholar] [CrossRef]
- Braun-Blanquet, J. Pflanzensoziologie. Grundzüge der Vegetationskunde, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 1964; p. 865. [Google Scholar]
- Zuur, A.F.; Saveliev, A.A.; Ieno, E.N. Zero-Inflated Models and Generalized Linear Mixed Models with R; Highland Statistics Ltd.: Newburgh, UK, 2012. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach; Springer: New York, NY, USA, 2002. [Google Scholar]
- Jackman, S. Pscl: Classes and Methods for R Developed in the Political Science Computational Laboratory. United States Studies Centre, University of Sydney. Sydney, New South Wales, Australia. R Package Version 1.5.2. 2017. Available online: https://github.com/atahk/pscl/ (accessed on 16 July 2021).
- Hothorn, T.; Zeileis, A.; Farebrother, R.W.; Cummins, C.; Millo, G.; Mitchell, M. lmtest: Testing Linear Regression Models. 2018. Available online: https://cran.r-project.org/web/packages/lmtest/index.html (accessed on 16 July 2021).
- Calcagno, V.; Mazancourt, C. de glmulti: An R Package for Easy Automated Model Selection with (Generalized) Linear Models. J. Stat. Softw. 2015, 34, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Barton, K. MuMIn: Multi-Model Inference. R Package Version 1.43.6. 2019. Available online: https://cran.r-project.org/web/packages/MuMIn/index.html (accessed on 16 July 2021).
- Wanger, T.C.; DeClerck, F.; Garibaldi, L.A.; Ghazoul, J.; Kleijn, D.; Klein, A.M.; Kremen, C.; Mooney, H.; Perfecto, I.; Powell, L.L.; et al. Integrating agroecological production in a robust post-2020 Global Biodiversity Framework. Nat. Ecol. Evol. 2020, 4, 1150–1152. [Google Scholar] [CrossRef] [PubMed]
- Beier, P. A rule of thumb for widths of conservation corridors. Conserv. Biol. 2019, 33, 976–978. [Google Scholar] [CrossRef]
- Castellón, T.D.; Sieving, K.E. Landscape history, fragmentation, and patch occupancy: Models for a forest bird with limited dispersal. Ecol. Appl. 2006, 16, 2223–2234. [Google Scholar] [CrossRef]
- Becerra, P.I.; González-Rodríguez, V.; Smith-Ramírez, C.; Armesto, J.J. Spatio-temporal variation in the effect of herbaceous layer on woody seedling survival in a Chilean mediterranean ecosystem. J. Veg. Sci. 2011, 22, 847–855. [Google Scholar] [CrossRef]
- Holmgren, M.; Segura, A.M.; Fuentes, E.R. Limiting mechanisms in the regeneration of the Chilean matorral—Experiments on seedling establishment in burned and cleared mesic sites. Plant Ecol. 2000, 147, 49–57. [Google Scholar] [CrossRef]
- Holmgren, M. Exotic herbivores as drivers of plant invasion and switch to ecosystem alternative states. Biol. Invasions 2002, 4, 25–33. [Google Scholar] [CrossRef]
- Holmgren, M.; Celis-Diez, J.L.; Armesto, J.J. Box 5.10 Tree-seedling establishment in fragmented Mediterranean forests of central Chile. In Principles and Practice of Forest Landscape Restoration: Case studies from the drylands of Latin America, Newton, A.C., Tejedor, N., Eds.; IUCN: Gland, Switzerland, 2011; p. 153. ISBN 978-2-8317-1340-3. [Google Scholar]
- Schiappacasse, F.; Peñailillo, P.; Yáñez, P.; Bridgen, M. Propagation studies on chilean geophytes. Acta Hortic. 2005, 673, 121–126. [Google Scholar] [CrossRef]
- Vergara, P.M.; Armesto, J.J. Responses of Chilean forest birds to anthropogenic habitat fragmentation across spatial scales. Landsc. Ecol. 2009, 24, 25–38. [Google Scholar] [CrossRef]
- Fischer, J.; Brosi, B.; Daily, G.C.; Ehrlich, P.R.; Goldman, R.; Goldstein, J.; Lindenmayer, D.B.; Manning, A.D.; Mooney, H.A.; Pejchar, L.; et al. Should agricultural policies encourage land sparing or wildlife-friendly farming? Front. Ecol. Environ. 2008, 6, 380–385. [Google Scholar] [CrossRef]
- DeClerck, F.; Estrada-Carmona, N.; Garbach, K.; Martinez-Salinas, A. Biodiversity and Ecosystem Services of Agricultural Landscapes. In Agroecology for Food Security and Nutrition, Proceedings of the FAO International Symposium, Rome, Italy, 18–19 September 2014; FAO: Rome, Italy, 2014; pp. 140–157. [Google Scholar]
- Nicholls, C.I.; Altieri, M.A.; Parella, M. The effects of a vegetational corridor on the abundance and dispersal of insect biodiversity within a northern California organic vineyard. Landsc. Ecol. 2001, 16, 133–146. [Google Scholar] [CrossRef]
- Garratt, M.P.D.; Senapathi, D.; Coston, D.J.; Mortimer, S.R.; Potts, S.G. The benefits of hedgerows for pollinators and natural enemies depends on hedge quality and landscape context. Agric. Ecosyst. Environ. 2017, 247, 363–370. [Google Scholar] [CrossRef] [Green Version]
- Kremen, C.; Merenlender, A.M. Landscapes that work for biodiversity and people. Science 2018, 362. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Forestier, J.; Abades, S.; Pohl, N.; Barbosa, O.; Godoy, K.; Svensson, G.L.; Undurraga, M.I.; Bravo, C.; García, C.; Root-Bernstein, M.; et al. Assessing Ecological Indicators for Remnant Vegetation Strips as Functional Biological Corridors in Chilean Vineyards. Diversity 2021, 13, 447. https://doi.org/10.3390/d13090447
Díaz-Forestier J, Abades S, Pohl N, Barbosa O, Godoy K, Svensson GL, Undurraga MI, Bravo C, García C, Root-Bernstein M, et al. Assessing Ecological Indicators for Remnant Vegetation Strips as Functional Biological Corridors in Chilean Vineyards. Diversity. 2021; 13(9):447. https://doi.org/10.3390/d13090447
Chicago/Turabian StyleDíaz-Forestier, Javiera, Sebastián Abades, Nélida Pohl, Olga Barbosa, Karina Godoy, Gabriella L. Svensson, María I. Undurraga, Camila Bravo, Camila García, Meredith Root-Bernstein, and et al. 2021. "Assessing Ecological Indicators for Remnant Vegetation Strips as Functional Biological Corridors in Chilean Vineyards" Diversity 13, no. 9: 447. https://doi.org/10.3390/d13090447
APA StyleDíaz-Forestier, J., Abades, S., Pohl, N., Barbosa, O., Godoy, K., Svensson, G. L., Undurraga, M. I., Bravo, C., García, C., Root-Bernstein, M., Armesto, J. J., & Celis-Diez, J. L. (2021). Assessing Ecological Indicators for Remnant Vegetation Strips as Functional Biological Corridors in Chilean Vineyards. Diversity, 13(9), 447. https://doi.org/10.3390/d13090447






