Reclaimed Mine Sites: Forests and Plant Diversity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Characteristics
2.2. Study Design and Data Sampling
2.3. Data Analysis
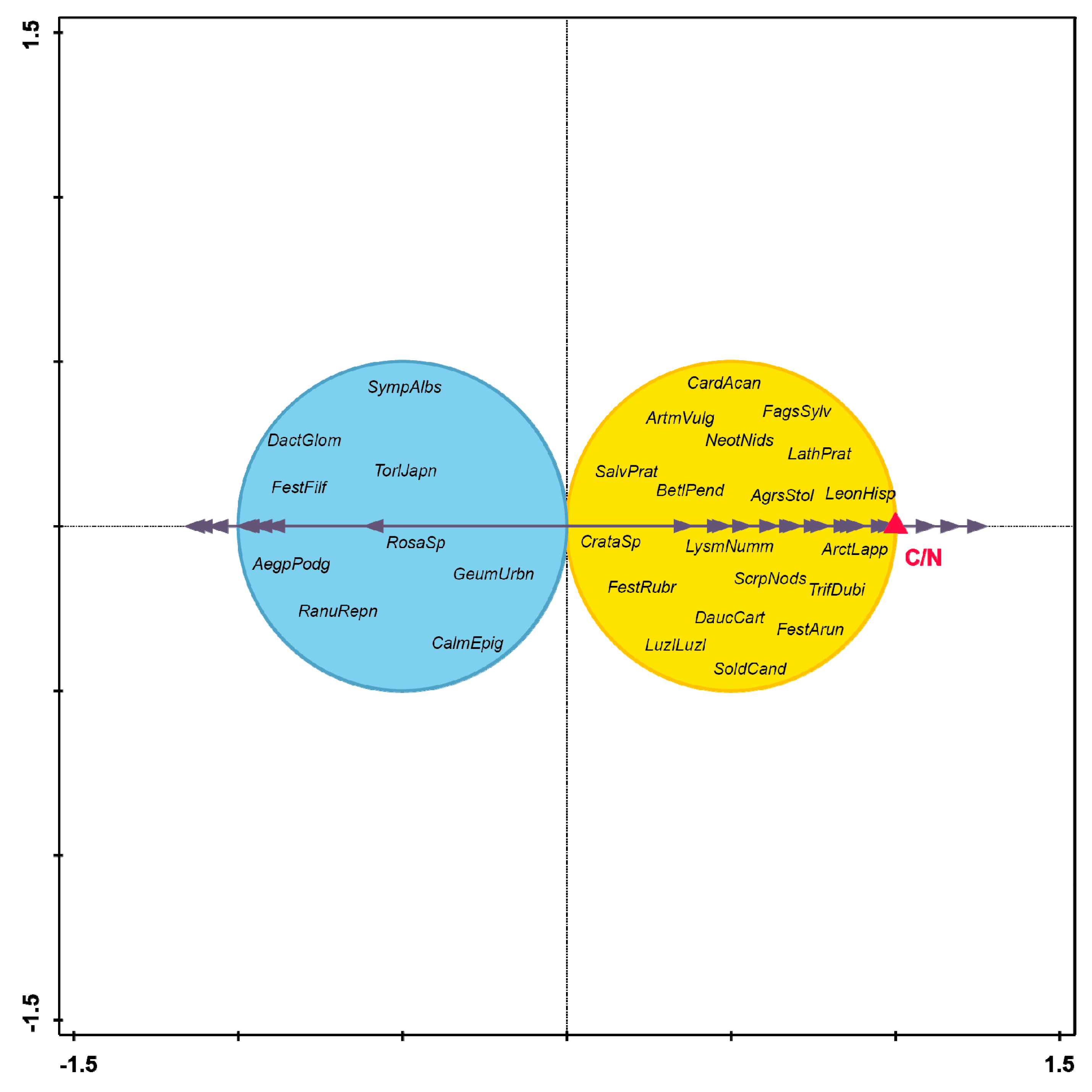
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tropek, R.; Kadlec, T.; Hejda, M.; Kocarek, P.; Skuhrovec, J.; Malenovsky, I.; Vodka, S.; Spitzer, L.; Banar, P.; Konvicka, M. Technical reclamations are wasting the conservation potential of post-mining sites. A case study of black coal spoil dumps. Ecol. Eng. 2012, 43, 13–18. [Google Scholar] [CrossRef]
- Hendrychová, M.; Kabrna, M. An analysis of 200-year-long changes in a landscape affected by large-scale surface coal mining: History, present and future. Appl. Geogr. 2016, 74, 151–159. [Google Scholar] [CrossRef]
- Van Grunsven, R.H.A.; van Der Putten, W.H.; Martijn Bezemer, T.; Berendse, F.; Veenendaal, E.M. Plant-soil interactions in the expansion and native range of a poleward shifting plant species. Glob. Chang. Biol. 2010, 16, 380–385. [Google Scholar] [CrossRef]
- Hickling, R.; Roy, D.B.; Hill, J.K.; Fox, R.; Thomas, C.D. The distributions of a wide range of taxonomic groups are expanding polewards. Glob. Chang. Biol. 2006, 12, 450–455. [Google Scholar] [CrossRef]
- Dhar, A.; Comeau, P.G.; Naeth, M.A.; Pinno, B.D.; Vassov, R. Plant community development following reclamation of oil sands mines using four cover soil types in northern Alberta. Restor. Ecol. 2020, 28, 82–92. [Google Scholar] [CrossRef]
- Jagodziński, A.M.; Wierzcholska, S.; Dyderski, M.K.; Horodecki, P.; Rusińska, A.; Gdula, A.K.; Kasprowicz, M. Tree species effects on bryophyte guilds on a reclaimed post-mining site. Ecol. Eng. 2018, 110, 117–127. [Google Scholar] [CrossRef]
- Brown, S.N.; Swab, R.M. To Establish a Healthy Forest: Restoration of the Forest Herb Layer on a Reclaimed Mine Site. Am. Midl. Nat. 2021, 186, 35–50. [Google Scholar] [CrossRef]
- Pyšek, P.; Danihelka, J.; Sádlo, J.; Chrtek, J., Jr.; Chytrý, M.; Jarošik, V.; Kaplan, Z.; Krahulec, F.; Moravcová, L.; Pergl, J.; et al. Catalogue of alien plants of the Czech Republic (2nd edition): Checklist update, taxonomic diversity and invasion patterns. Preslia 2012, 84, 155–255. [Google Scholar]
- Pergl, J.; Sádlo, J.; Petrusek, A.; Laštuvka, Z.; Musil, J.; Perglová, I.; Šanda, R.; Šefrová, H.; Šíma, J.; Vohralík, V.; et al. Black, Grey and Watch Lists of alien species in the Czech Republic based on environmental impacts and management strategy. NeoBiota 2016, 28, 1–37. [Google Scholar] [CrossRef]
- Řehounková, K.; Prach, K. Spontaneous vegetation succession in gravel-sand pits: A potential for restoration. Restor. Ecol. 2008, 16, 305–312. [Google Scholar] [CrossRef]
- Sun, D.; Müllerová, V.; Ardestani, M.M.; Frouz, J. Nitrogen fertilization and its legacy have inconsistent and often negative effect on plant growth in undeveloped post mining soils. Soil Tillage Res. 2019, 195, 104380. [Google Scholar] [CrossRef]
- Czech, B.; Krausman, P.R.; Devers, P.K. Economic Associations among Causes of Species Endangerment in the United States. Bioscience 2000, 50, 593–601. [Google Scholar] [CrossRef] [Green Version]
- Begon, M.; Harper, J.L.; Townsend, C.R. Ecology: Individuals, Populations and Communities; Vydavatelství Univerzity Palackého: Olomouc, Czech Republic, 1997; ISBN 80-7067-695-7. [Google Scholar]
- Grime, J.P. Plant Strategies, Vegetation Processes, and Ecosystem Properties; Wiley: Hoboken, NJ, USA, 2006; ISBN 047085040X. [Google Scholar]
- Hejcman, M.; Strnad, L.; Hejcmanová, P.; Pavlů, V. Response of plant species composition, biomass production and biomass chemical properties to high N, P and K application rates in Dactylis glomerata and Festuca arundinacea dominated grassland. Grass Forage Sci. 2012, 67, 488–506. [Google Scholar] [CrossRef]
- Miletić, Z.; Knežević, M.; Stajić, S.; Košanin, O.; Đorđević, I. Effect of European Black Alder Monocultures on The Characteristics of Reclaimed Mine Soil. Int. J. Environ. Res. 2012, 6, 703–710. [Google Scholar]
- Woś, B.; Smoliński, A.; Likus-Cieślik, J.; Pietrzykowski, M. The impact of alder litter on chemistry of Technosols developed from lignite combustion waste and natural sandy substrate: A laboratory experiment. Int. J. Phytoremediation 2020, 23, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Mudrák, O.; Frouz, J.; Velichová, V. Understory vegetation in reclaimed and unreclaimed post-mining forest stands. Ecol. Eng. 2010, 36, 783–790. [Google Scholar] [CrossRef]
- Elton, C.S. The Ecology of Invasions by Animals and Plants; Springer: Boston, MA, USA, 1958; ISBN 978-0-412-11430-4. [Google Scholar]
- Šebelíková, L.; Csicsek, G.; Kirmer, A.; Vítovcová, K.; Ortmann-Ajkai, A.; Prach, K.; Řehounková, K. Spontaneous revegetation versus forestry reclamation—Vegetation development in coal mining spoil heaps across Central Europe. L. Degrad. Dev. 2019, 30, 348–356. [Google Scholar] [CrossRef]
- Hodačová, D.; Prach, K. Spoil heaps from brown coal mining: Technical reclamation versus spontaneous revegetation. Restor. Ecol. 2003, 11, 385–391. [Google Scholar] [CrossRef]
- Frouz, J.; Mudrák, O.; Reitschmiedová, E.; Walmsley, A.; Vachová, P.; Šimáčková, H.; Albrechtová, J.; Moradi, J.; Kučera, J. Rough wave-like heaped overburden promotes establishment of woody vegetation while leveling promotes grasses during unassisted post mining site development. J. Environ. Manag. 2018, 205, 50–58. [Google Scholar] [CrossRef]
- Veselá, H.; Mudrák, O.; Frouz, J. The role of dead standing biomass of Calamagrostis epigejos in nutrient turnover during spontaneous succession. Sci. Total Environ. 2018, 644, 717–724. [Google Scholar] [CrossRef]
- Walmsley, A.; Vachová, P.; Vach, M. Topography of Spoil Heaps and Its Role in Plant Succession and Soil Fauna Presence. Sci. Agric. Bohem. 2017, 48, 30–38. [Google Scholar] [CrossRef] [Green Version]
- Vicentini, F.; Hendrychova, M.; Tajovskỳ, K.; Pižl, V.; Frouz, J. The effect of topography on long-term spontaneous development of soil and woody cover on graded and untreated overburden. Forests 2020, 11, 602. [Google Scholar] [CrossRef]
- Neuhäuslová, Z. Map of Potential Natural Vegetation of the Czech Republic; Academia: Prague, Czech Republic, 2001. [Google Scholar]
- Harabiš, F.; Tichanek, F.; Tropek, R. Dragonflies of freshwater pools in lignite spoil heaps: Restoration management, habitat structure and conservation value. Ecol. Eng. 2013, 55, 51–61. [Google Scholar] [CrossRef]
- Hendrychová, M.; Šálek, M.; Tajovský, K.; Řehoř, M. Soil Properties and Species Richness of Invertebrates on Afforested Sites after Brown Coal Mining. Restor. Ecol. 2012, 20, 561–567. [Google Scholar] [CrossRef]
- Rawlik, M.; Kasprowicz, M.; Jagodziński, A.M.; Kaźmierowski, C.; Łukowiak, R.; Grzebisz, W. Canopy tree species determine herb layer biomass and species composition on a reclaimed mine spoil heap. Sci. Total Environ. 2018, 635, 1205–1214. [Google Scholar] [CrossRef]
- Rawlik, M.; Kasprowicz, M.; Jagodziński, A.M. Differentiation of herb layer vascular flora in reclaimed areas depends on the species composition of forest stands. For. Ecol. Manag. 2018, 409, 541–551. [Google Scholar] [CrossRef]
- Roubíčková, A.; Mudrák, O.; Frouz, J. Effect of earthworm on growth of late succession plant species in postmining sites under laboratory and field conditions. Biol. Fertil. Soils. 2009, 45, 769–774. [Google Scholar] [CrossRef]
- Jačka, L.; Walmsley, A.; Kovář, M.; Frouz, J. Effects of different tree species on infiltration and preferential flow in soils developing at a clayey spoil heap. Geoderma 2021, 403, 115372. [Google Scholar] [CrossRef]
- Frouz, J.; Prach, K.; Pižl, V.; Hanel, L.; Starý, J.; Tajovský, K.; Materna, J.; Balík, V.; Kalcik, J.; Rehounkova, K. Interactions between soil development, vegetation and soil fauna during spontaneous succession in post mining sites. Eur. J. Soil Biol. 2008, 44, 109–121. [Google Scholar] [CrossRef]
- Józefowska, A.; Woś, B.; Pietrzykowski, M. Tree species and soil substrate effects on soil biota during early soil forming stages at afforested mine sites. Appl. Soil Ecol. 2016, 102, 70–79. [Google Scholar] [CrossRef]
- Roman, A.; Gafta, D. Proximity to successionally advanced vegetation patches can make all the difference to plant community assembly. Plant Ecol. Divers. 2013, 6, 269–278. [Google Scholar] [CrossRef]
- Hendrychová, M.; Svobodova, K.; Kabrna, M. Mine reclamation planning and management: Integrating natural habitats into post-mining land use. Resour. Policy 2020, 69, 101882. [Google Scholar] [CrossRef]
- Rojík, P. New stratigraphic subdivision of the Tertiary in the Sokolov Basin in Northwestern Bohemia. J. Czech. Geol. Soc. 2004, 49, 173–185. [Google Scholar]
- CHMI Czech Hydrometeorological Institute. Available online: https://www.chmi.cz/ (accessed on 4 September 2021).
- Kuráž, V.; Frouz, J.; Kuráž, M.; Mako, A.; Shustr, V.; Cejpek, J.; Romanov, O.V.; Abakumov, E.V. Changes in some physical properties of soils in the chronosequence of self-overgrown dumps of the Sokolov quarry-dump complex, Czechia. Eurasian Soil Sci. 2012, 45, 266–272. [Google Scholar] [CrossRef]
- Chytrý, M.; Danihelka, J.; Kaplan, Z.; Wild, J.; Holubová, D.; Novotný, P.; Řezníčková, M.; Rohn, M.; Dřevojan, P.; Grulich, V.; et al. Pladias Database of the Czech Flora and Vegetation Pladias-databáze české flóry a vegetace. Pladias. Database Czech Flora Veg. 2021, 93, 1–87. [Google Scholar] [CrossRef]
- Blanchet, F.G.; Legendre, P.; Borcard, D. Forward sellection of explanatory variables. Ecology 2008, 89, 2623–2632. [Google Scholar] [CrossRef]
- Šmilauer, P.; Lepš, J. Multivariate Analysis of Ecological Data using Canoco 5; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Borůvka, L.; Kozák, J.; Mühlhanselová, M.; Donátová, H.; Nikodem, A.; Němeček, K.; Drábek, O. Effect of covering with natural topsoil as a reclamation measure on brown-coal mining dumpsites. J. Geochemical Explor. 2012, 113, 118–123. [Google Scholar] [CrossRef]
- Mishra, S.K.; Hitzhusen, F.J.; Sohngen, B.L.; Guldmann, J.M. Costs of abandoned coal mine reclamation and associated recreation benefits in Ohio. J. Environ. Manag. 2012, 100, 52–58. [Google Scholar] [CrossRef]
- Plumlee, M.H.; Stanford, B.D.; Debroux, J.-F.; Hopkins, D.C.; Snyder, S.A. Costs of Advanced Treatment in Water Reclamation. Ozone Sci. Eng. 2014, 36, 485–495. [Google Scholar] [CrossRef]
- Walmsley, A.; Vachová, P.; Hlava, J. Tree species identity governs the soil macrofauna community composition and soil development at reclaimed post-mining sites on calcium-rich clays. Eur. J. For. Res. 2019, 138, 753–761. [Google Scholar] [CrossRef]
- Rahmonov, O.; Krzysztofik, R.; Środek, D.; Smolarek-Lach, J. Vegetation- and Environmental Changes on Non-Reclaimed Spoil Heaps in Southern Poland. Biology 2020, 9, 164. [Google Scholar] [CrossRef]
- Horodecki, P.; Jagodziński, A.M. Tree species effects on litter decomposition in pure stands on afforested post-mining sites. For. Ecol. Manag. 2017, 406, 1–11. [Google Scholar] [CrossRef]
- Wagner, S.; Litt, T.; Sánchez-Goñi, M.F.; Petit, R.J. History of Larix decidua Mill. (European larch) since 130 ka. Quat. Sci. Rev. 2015, 124, 224–247. [Google Scholar] [CrossRef]
- Zhang, H.; Chu, L.M. Plant Community Structure, Soil Properties and Microbial Characteristics in Revegetated Quarries. Ecol. Eng. 2011, 37, 1104–1111. [Google Scholar] [CrossRef]
- Burke, M.J.W.; Grime, J.P. An Experimental Study of Plant Community Invasibility. Ecology 1996, 77, 776–790. [Google Scholar] [CrossRef]
- Chytrý, M.; Pyšek, P. Kam se šíří zavlečené rostliny? 2. Invadovanost a invazibilita rostlinných společenstev. Živa 2009, 2, 60–63. (In Czech) [Google Scholar]
- Hobbs, R.J.; Humphries, S.E. An Integrated Approach to the Ecology and Management of Plant Invasions. Conserv. Biol. 1995, 9, 761–770. [Google Scholar] [CrossRef] [Green Version]
- Lonsdale, W.M. Global Patterns of Plant Invasions And the Concept Of Invasibility. Ecology 1999, 80, 1522–1536. [Google Scholar] [CrossRef]
- Vacek, Z.; Cukor, J.; Vacek, S.; Podrázský, V.; Linda, R.; Kovařík, J. Forest biodiversity and production potential of post-mining landscape: Opting for afforestation or leaving it to spontaneous development? Cent. Eur. For. J. 2018, 64, 116–126. [Google Scholar] [CrossRef]
- Zhang, W.Q.; Fleurial, K.; Sherr, I.; Vassov, R.; Zwiazek, J.J. Growth and physiological responses of tree seedlings to oil sands non-segregated tailings. Environ. Pollut. 2020, 259, 113945. [Google Scholar] [CrossRef]
- Prach, K.; Řehounková, K.; Lencová, K.; Jírová, A.; Konvalinková, P.; Mudrák, O.; Študent, V.; Vaněček, Z.; Tichý, L.; Petřík, P.; et al. Vegetation succession in restoration of disturbed sites in Central Europe: The direction of succession and species richness across 19 seres. Appl. Veg. Sci. 2014, 17, 193–200. [Google Scholar] [CrossRef]
- Šourková, M.; Frouz, J.; Šantrůčková, H. Accumulation of carbon, nitrogen and phosphorus during soil formation on alder spoil heaps after brown-coal mining, near Sokolov (Czech Republic). Geoderma 2005, 124, 203–214. [Google Scholar] [CrossRef]
- Cools, N.; Vesterdal, L.; De Vos, B.; Vanguelova, E.; Hansen, K. Tree species is the major factor explaining C:N ratios in European forest soils. For. Ecol. Manag. 2014, 311, 3–16. [Google Scholar] [CrossRef]
- Frouz, J.; Livečková, M.; Albrechtová, J.; Chroňáková, A.; Cajthaml, T.; Pižl, V.; Háněl, L.; Starý, J.; Baldrian, P.; Lhotáková, Z.; et al. Is the effect of trees on soil properties mediated by soil fauna? A case study from post-mining sites. For. Ecol. Manag. 2013, 309, 87–95. [Google Scholar] [CrossRef]
- Lorenz, M.; Thiele-Bruhn, S. Tree species affect soil organic matter stocks and stoichiometry in interaction with soil microbiota. Geoderma 2019, 353, 35–46. [Google Scholar] [CrossRef]
- Piekarska-Stachowiak, A.; Szary, M.; Ziemer, B.; Besenyei, L.; Woźniak, G. An application of the plant functional group concept to restoration practice on coal mine spoil heaps. Ecol. Res. 2014, 29, 843–853. [Google Scholar] [CrossRef] [Green Version]
- Vítovcová, K.; Tichý, L.; Řehounková, K.; Prach, K. Which landscape and abiotic site factors influence vegetation succession across seres at a country scale? J. Veg. Sci. 2020, 32, 12950. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Novo, L.A.B.; Pietrzykowski, M.; Maiti, S.K. Assessment of Forest Ecosystem Development in Coal Mine Degraded Land by Using Integrated Mine Soil Quality Index (IMSQI): The Evidence from India. Forest 2020, 11, 1310. [Google Scholar] [CrossRef]
- Kabrna, M.; Hendrychová, M.; Prach, K. Establishment of target and invasive plant species on a reclaimed coal mining dump in relation to their occurrence in the surroundings. Int. J. Min. Reclam. Environ. 2014, 28, 242–249. [Google Scholar] [CrossRef]
- Vach, M.; Vachová, P.; Walmsley, A.; Berka, M.; Albert, J.; Cienciala, E.; Braun Kohlová, M.; Máca, V.; Melichar, J. Stochastic evaluation of restoration procedures on post–mining land areas using a game theory approach. Land Degrad. Dev. 2021. [Google Scholar] [CrossRef]







| Type | Age | pH (CaCl2) | TC * [%] | TN * [%] | C/N [-] | D * [-] | Humus Thickness [cm] |
|---|---|---|---|---|---|---|---|
| Alnus | younger | 7.4 ab | 9.27 abcd | 0.57 ac | 16 ab | 0.92 b | 4.42 ab |
| older | 6.1 abc | 8.98 abcd | 0.58 ac | 16 ab | 0.48 ab | 9.55 c | |
| Larix | younger | 7.1 abc | 5.82 a | 0.26 b | 23 bc | 0.22 a | 3.19 ab |
| older | 6.8 abc | 10.87 cd | 0.39 ab | 28 cd | 0.65 ab | 7.33 ac | |
| L–L Deciduous | younger | 7.4 ab | 10.36 bcd | 0.75 c | 13 a | 0.34 ab | 10.00 c |
| older | 7.3 ab | 8.36 abc | 0.56 ac | 14 a | 0.23 a | 7.07 ac | |
| Picea | younger | 7.5 b | 6.40 abc | 0.41 ab | 15 ab | 0.06 a | 3.81 ab |
| older | 5.7 c | 6.16 ab | 0.40 ab | 15 ab | 0.36 ab | 3.59 ab | |
| Pinus | younger | 7.0 abc | 6.70 abc | 0.35 ab | 19 ab | 0.17 a | 2.95 ab |
| older | 5.9 ac | 13.29 d | 0.39 ab | 35 d | 0.24 a | 5.46 abc | |
| Succession | younger | 5.6 c | 6.24 ab | 0.48 ab | 12 a | 0.54 ab | 2.33 b |
| older | 7.4 ab | 6.32 ab | 0.41 ab | 15 ab | 0.55 ab | 3.92 ab | |
| F | (5;24) | 10.486 | 7.59 | 2.487 | 6.691 | 2.766 | 5.315 |
| p | 0.05 | <0.001 | <0.001 | 0.059 | <0.001 | 0.041 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vachova, P.; Vach, M.; Skalicky, M.; Walmsley, A.; Berka, M.; Kraus, K.; Hnilickova, H.; Vinduskova, O.; Mudrak, O. Reclaimed Mine Sites: Forests and Plant Diversity. Diversity 2022, 14, 13. https://doi.org/10.3390/d14010013
Vachova P, Vach M, Skalicky M, Walmsley A, Berka M, Kraus K, Hnilickova H, Vinduskova O, Mudrak O. Reclaimed Mine Sites: Forests and Plant Diversity. Diversity. 2022; 14(1):13. https://doi.org/10.3390/d14010013
Chicago/Turabian StyleVachova, Pavla, Marek Vach, Milan Skalicky, Alena Walmsley, Martin Berka, Kamil Kraus, Helena Hnilickova, Olga Vinduskova, and Ondrej Mudrak. 2022. "Reclaimed Mine Sites: Forests and Plant Diversity" Diversity 14, no. 1: 13. https://doi.org/10.3390/d14010013
APA StyleVachova, P., Vach, M., Skalicky, M., Walmsley, A., Berka, M., Kraus, K., Hnilickova, H., Vinduskova, O., & Mudrak, O. (2022). Reclaimed Mine Sites: Forests and Plant Diversity. Diversity, 14(1), 13. https://doi.org/10.3390/d14010013








