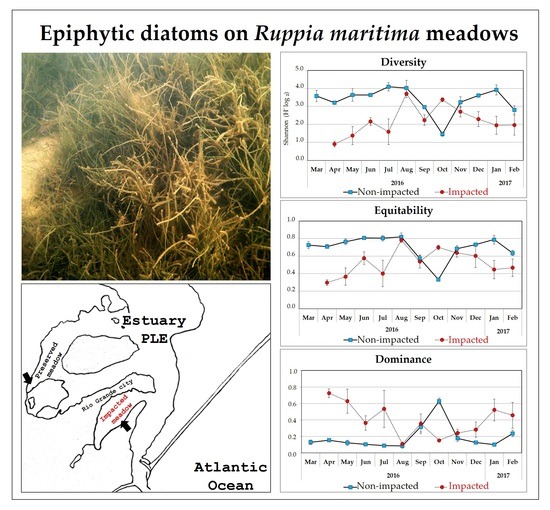
Diversity and Variation of Epiphytic Diatoms on Ruppia maritima L., Related to Anthropogenic Impact in an Estuary in Southern Brazil
Abstract
:1. Introduction
2. Materials and Methods
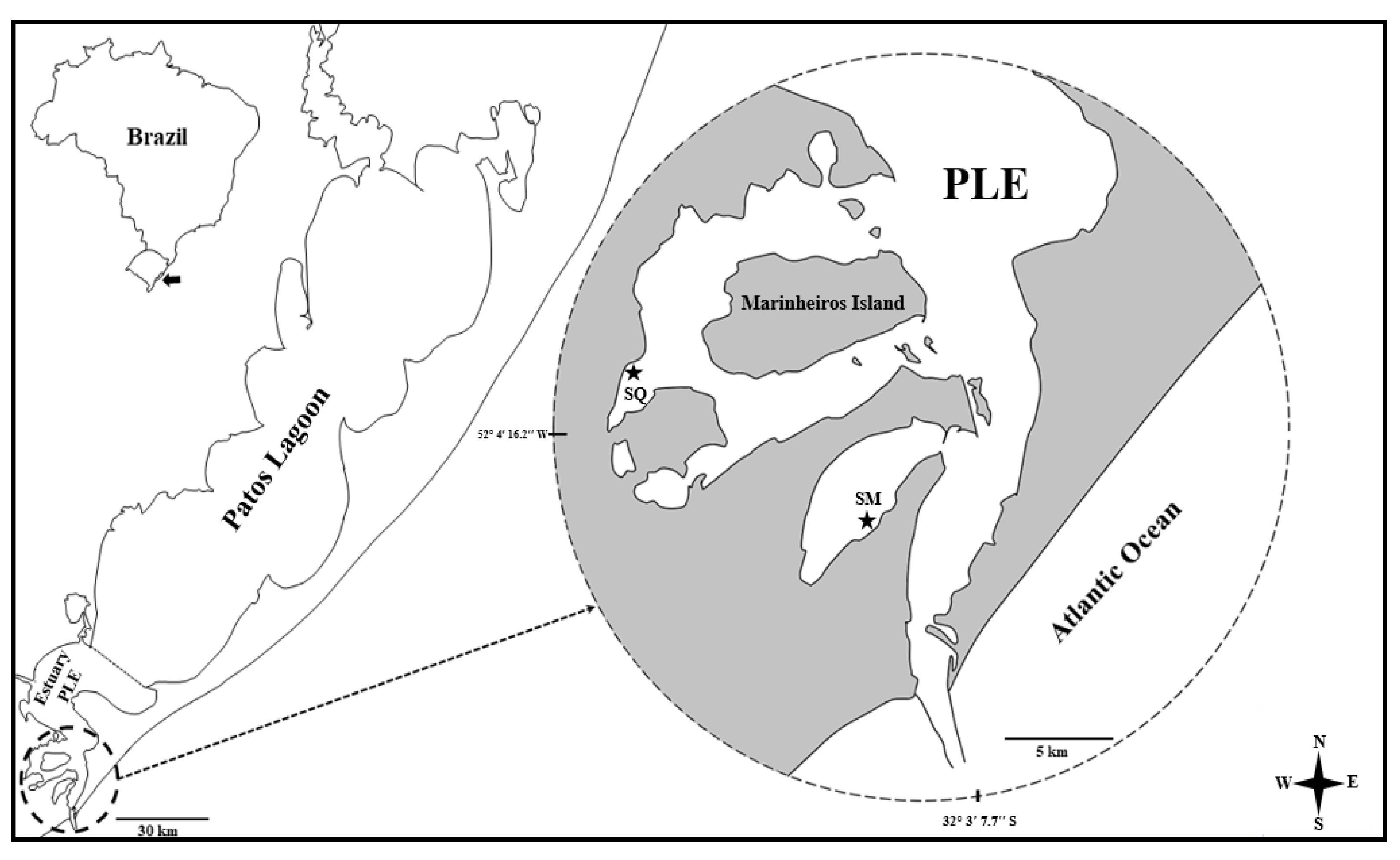
2.1. Study Area
2.2. Epiphytic Diatom Sampling and Laboratory Procedures
2.3. Data Analysis
3. Results
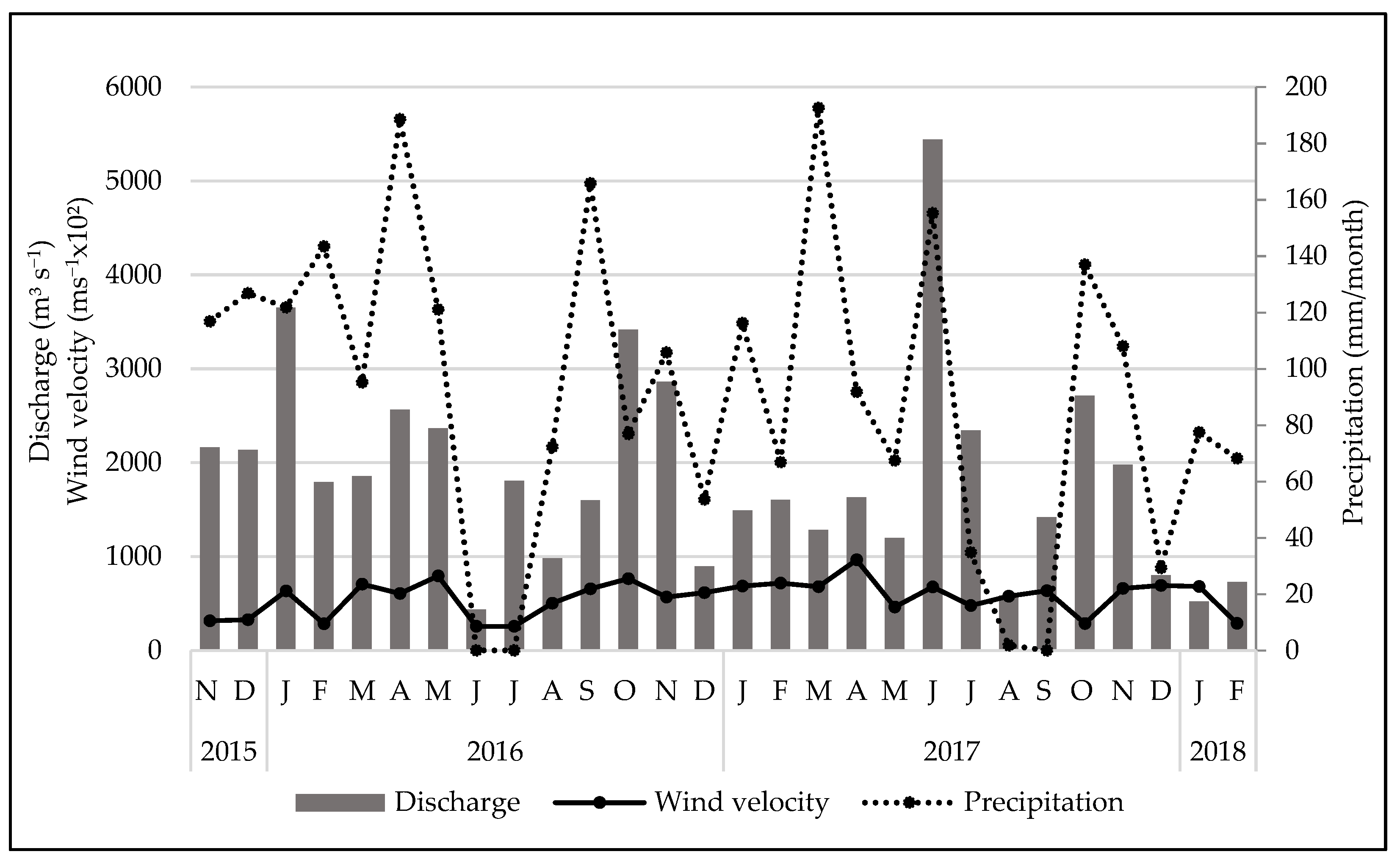
3.1. Environmental Variables
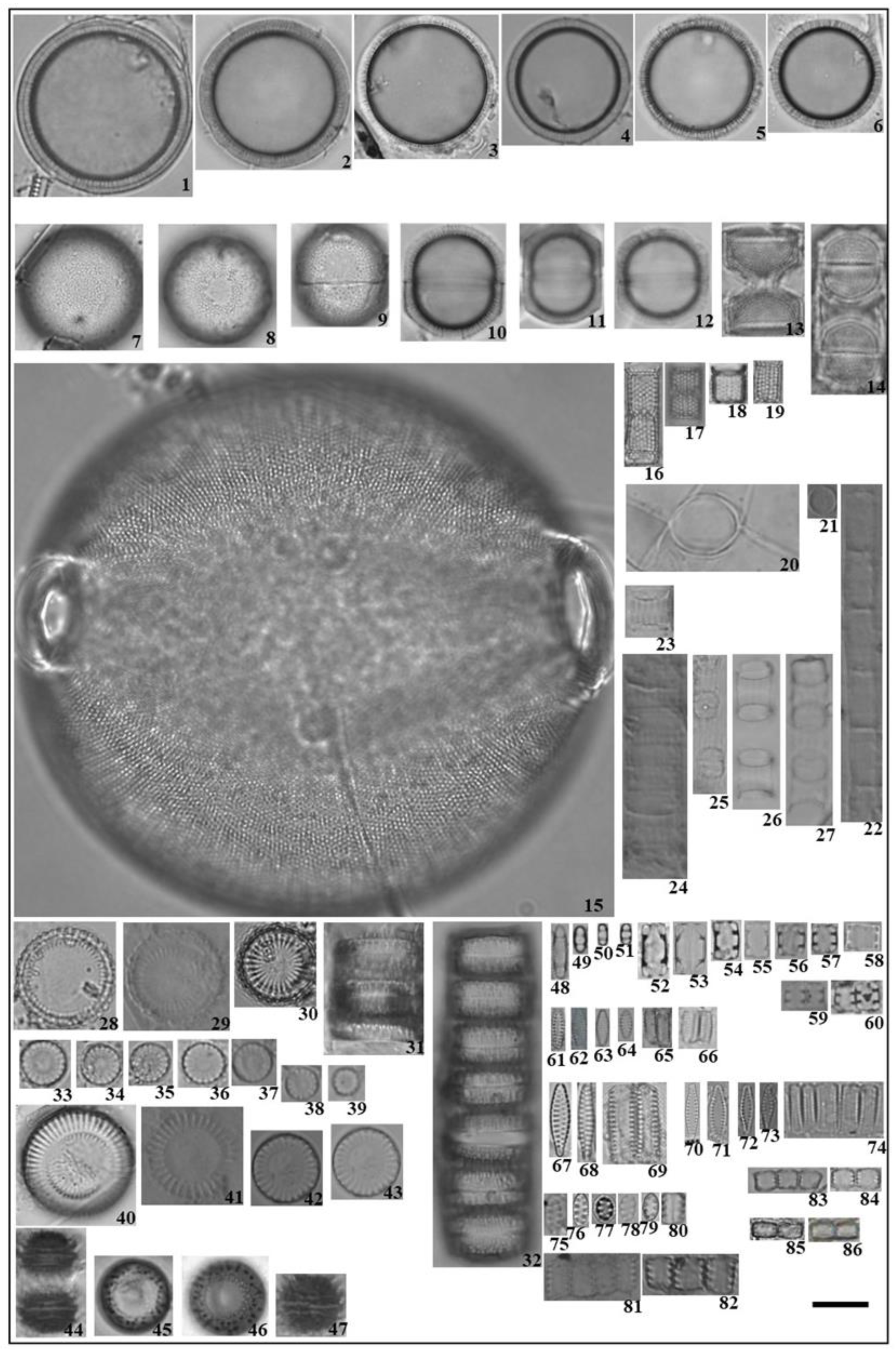
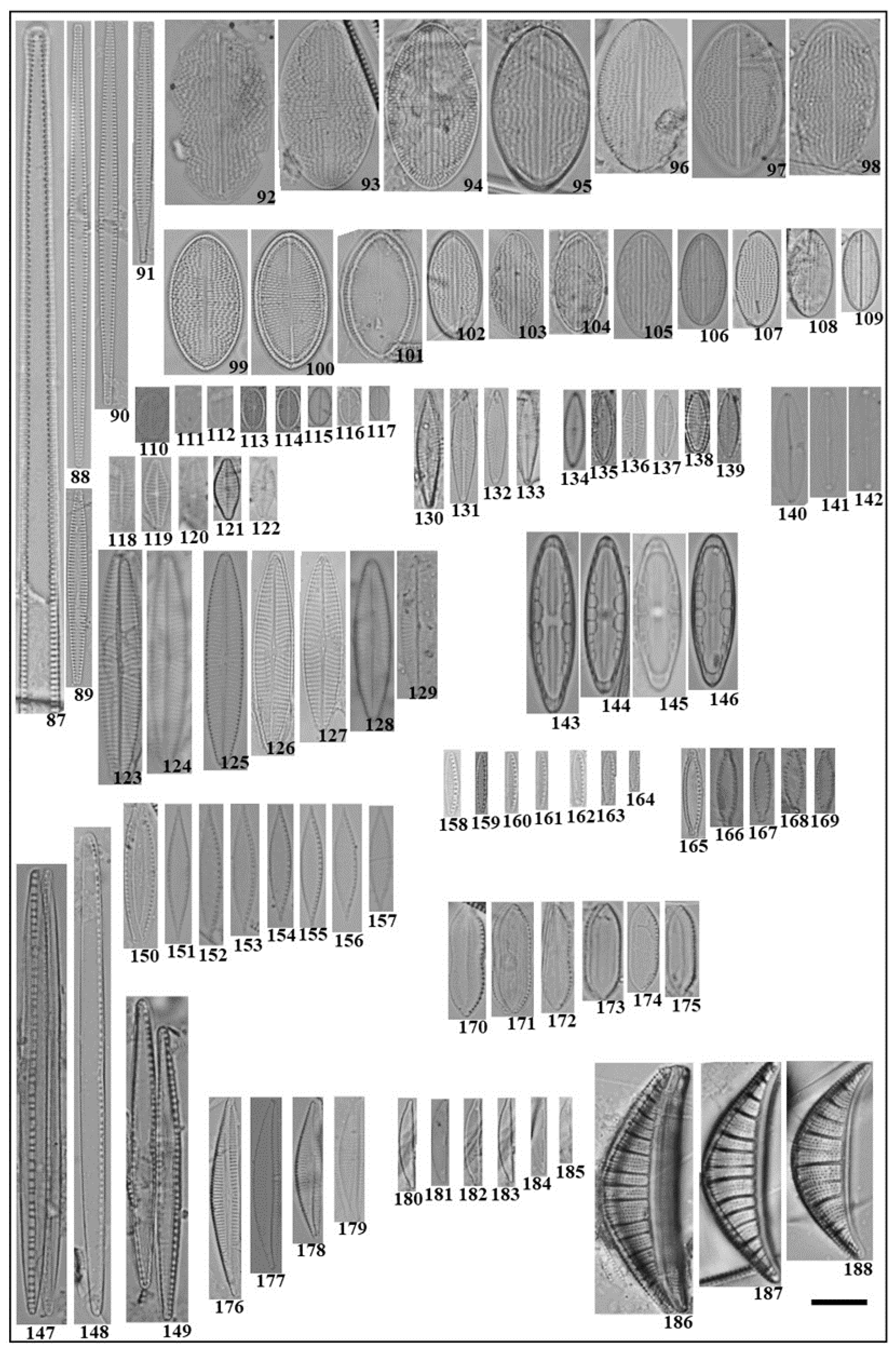
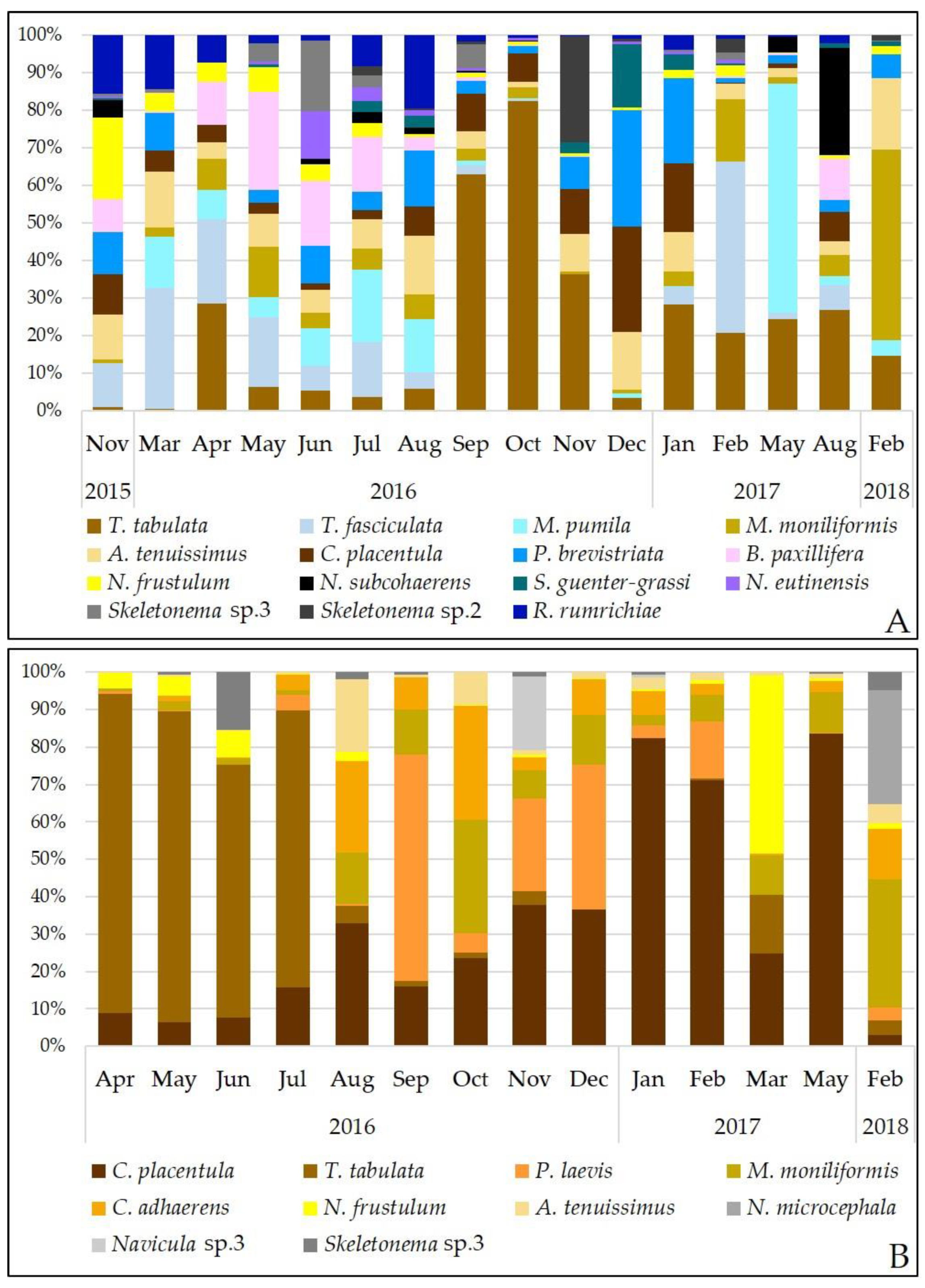
3.2. Community Composition and Abundance
3.3. Spatial and Temporal Variability
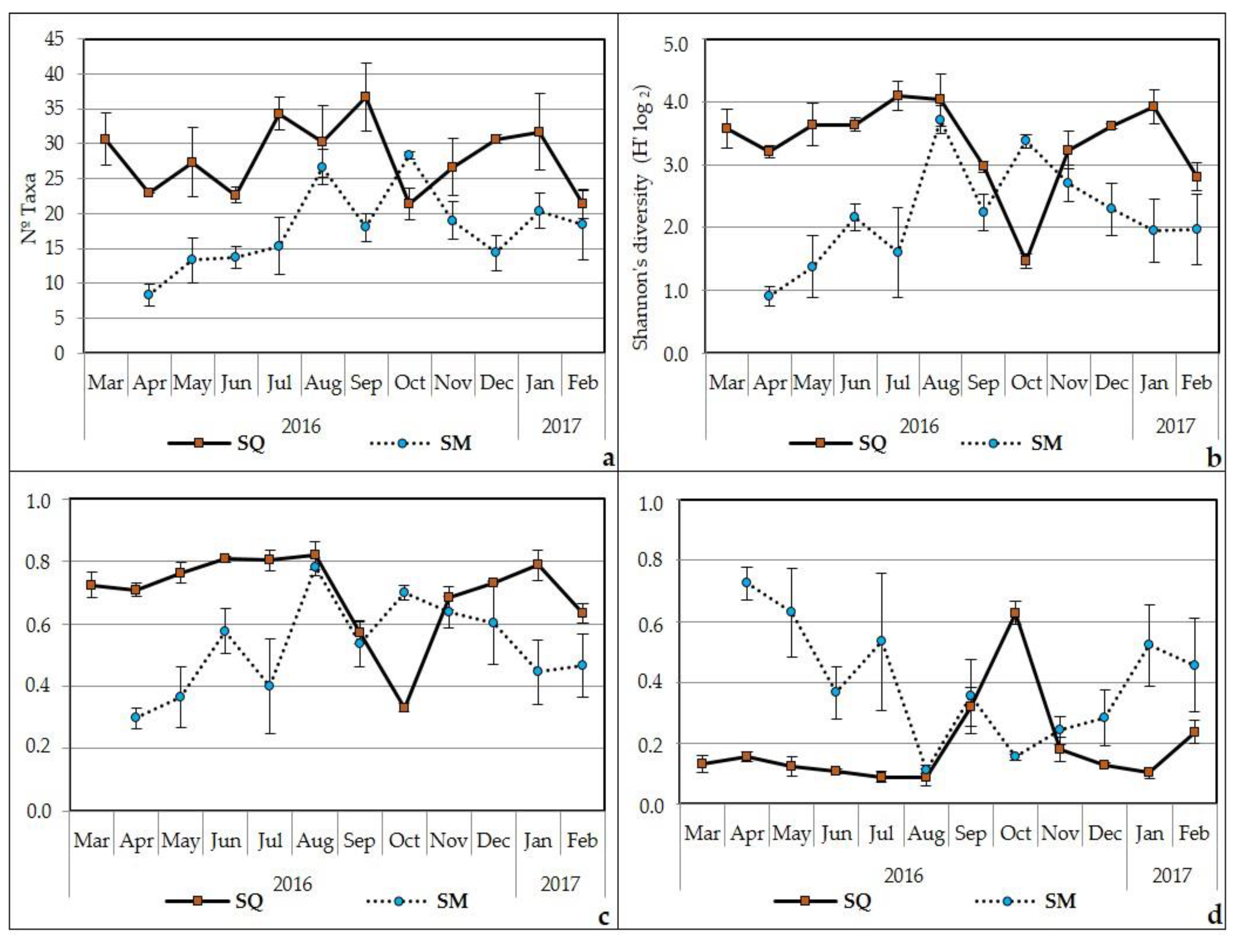
Community Attributes: Diversity, Equitability and Dominance
3.4. Correspondence between Epiphytic Diatom Assemblages and Environmental Variables
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Michael, T.S.; Shin, H.W.; Hanna, R.; Spafford, D.C. A review of epiphyte community development: Surface interactions and settlement on seagrass. J. Environ. Biol. 2008, 29, 629–638. [Google Scholar] [PubMed]
- Brandt, L.A.; Koch, E.W. Periphyton as a UV-B filter on seagrass leaves: A result of different transmittance in the UV-B and PAR ranges. Aquat. Bot. 2003, 76, 317–327. [Google Scholar] [CrossRef]
- Aho, K.; Beck, E. Effects of epiphyte cover on seagrass growth rates in two tidal zones. Ecology 2011, 2011, 43–44. [Google Scholar]
- Kitting, C.L.; Fry, B.; Morgan, M.D. Detection of inconspicuous epiphytic alga supporting food webs in seagrass meadows. Oecologia 2011, 62, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Orth, R.J.; Van Montfrans, J. Epiphyte-seagrass relationships with an emphasis on the role of micrograzing: A review. Aquat. Bot. 1984, 18, 43–69. [Google Scholar] [CrossRef]
- Connolly, R.M.; Hindell, J.S.; Gorman, D. Seagrass and epiphytic algae support nutrition of a fisheries species, Sillago schomburgkii, in adjacent intertidal habitats. Mar. Ecol. Prog. Ser. 2005, 286, 69–79. [Google Scholar]
- Moncreiff, C.A.; Sullivan, M.J.; Daehnick, A.E. Primary production dymanics in seagrass beds of Mississippi Sound: The contributions of seagrass, epiphytic algae, sand microflora, and phytoplankton. Mar. Ecol. Prog. Ser. 1992, 87, 161–171. [Google Scholar] [CrossRef]
- Kennedy, H.; Beggins, J.; Duarte, C.C.; Fourqurean, J.W.; Holmer, M.; Marbà, N.; Middelburg, J.J. Seagrass sediments as a global carbon sink: Isotopic constraints. Glob. Biogeochem. Cycles 2010, 24, GB4026. [Google Scholar] [CrossRef]
- Duffy, J.E. Biodiversity and the functioning of seagrass ecosystems. Mar. Ecol. Prog. Ser. 2006, 311, 233–250. [Google Scholar] [CrossRef]
- Harlin, M.M. Transfer of products between epiphytic marine algae and host plants. J. Phycol. 1973, 9, 243–248. [Google Scholar]
- Licursi, M.; Gómez, N.; Donadelli, J. Ecological optima and tolerances of coastal benthic diatoms in the freshwater-mixohaline zone of the Río da Plata estuary. Mar. Ecol. Prog. Ser. 2010, 418, 105–117. [Google Scholar] [CrossRef]
- Orth, R.J.; Carruthers, T.J.B.; Dennison, W.C.; Duarte, C.M.; Fourqurean, J.W.; Heck, K.L.; Hughes, A.R.; Kendrick, G.A.; Kenworthy, W.J.; Olyarnik, S.; et al. A Global Crisis for Seagrass Ecosystems. BioScience 2006, 56, 987–996. [Google Scholar] [CrossRef]
- Copertino, S.M.; Creed, J.C.; Lanari, M.O.; Magalhães, K.; Barros, K.; Lana, P.C.; Sordo, L.; Horta, P.A. Seagrass and Submerged Aquatic vegetation (VAS) Habitats of the Coast of Brazil: State of knowledge, conservation and main threats. Braz. J. Oceanogr. 2016, 62, 53–80. [Google Scholar] [CrossRef]
- Frankovich, T.A.; Armitage, A.R.; Wachnicka, A.H.; Gaiser, E.E.; Fourqurean, J.W. Nutrient effects on seagrass epiphyte community structure in Florida bay. J. Phycol. 2009, 45, 1010–1020. [Google Scholar] [CrossRef]
- Mc Intyre, H.L.; Geider, R.J.; Miller, D.C. Microphytobenthos: The Ecological Role of the “Secret Garden” of Unvegetated, Shallow-Water Marine Habitats. I. Distribution, Abundance and Primary Production. Estuaries 1996, 19, 186–201. [Google Scholar] [CrossRef]
- Trobajo, R.; Sullivan, M.J. Apllied diatom studies in estuaries and shallow coastal environments. In Applications for the Environmental and Earth Sciences; Smol, J.P., Stoermer, E.F., Eds.; Cambridge University Press: Cambridge, UK, 2010; pp. 309–323. [Google Scholar]
- Vos, P.C.; Boer, P.L.; Misdorp, R. Sediment stabilizations by benthic diatoms in intertidal sandy shoals; qualitative and quantitative observations. In Tide-influenced Sedimentary Environments and Facies; Boer, P.L., Gelder, A., Nio, S.D., Eds.; D.Reidel: Dordrecht, The Netherlands, 1988; pp. 511–526. [Google Scholar]
- Passy, S.I. Diatom ecological guilds display distinct and predictable behavior along nutrient and disturbance gradients in running waters. Aquat. Bot. 2007, 86, 171–178. [Google Scholar] [CrossRef]
- Lange, K.; Liess, A.; Piggott, J.J.; Townsend, C.R.; Matthaei, C.D. Light, nutrients and grazing Interact to determine stream diatom community composition and functional group structure. Fresh. Biol. 2011, 56, 264–278. [Google Scholar] [CrossRef]
- Pan, J.; Cuadrado, D.G.; Bournod, C.N. Diatom-driven recolonization of microbial mat-dominated siliciclastic tidal flat sediments. FEMS Microbiol. Ecol. 2017, 93, fix111. [Google Scholar] [CrossRef]
- Sterrenburg, F.A.S.; Erftemeijera, P.L.A.; Nienhuis, P.H. Diatoms as Epiphytes on Seagrasses in South Sulawesi (Indonesia) Comparison with Growth on Inert Substrata. Bot. Mar. 1995, 38, 1–7. [Google Scholar] [CrossRef]
- Frankovich, T.A.; Gaiser, E.E.; Zienan, J.C.; Wachninka, A.H. Spatial and temporal distributions of epiphytic diatoms growing on Thalassia testudinum Banks ex König: Relationship to water quality. Hydrobiologia 2006, 569, 259–271. [Google Scholar] [CrossRef]
- Stanca, E.; Parsons, M.L. Examining the dynamic nature of epiphytic microalgae in the Florida Keys: What factors influence community composition? J. Exp. Mar. Biol. Ecol. 2021, 538, 151538. [Google Scholar] [CrossRef]
- Majewska, R.; Zgrundo, A.; Lemke, P.; De Stefano, M. Benthic diatoms of the Vistula River estuary (Northern Poland): Seasonality, substrata preferences, and the influence of water chemistry. Phycol. Res. 2012, 60, 1–19. [Google Scholar] [CrossRef]
- Snoeijs, P. Distribution of epiphytic diatom species composition, diversity and biomass on different macroalgal hosts along seasonal and salinity gradients in the Baltic Sea. Diatom Res. 1994, 9, 189–211. [Google Scholar] [CrossRef]
- Snoeijs, P. Effects of salinity on epiphytic diatom communities on Pilayella litoralis (Phaeophyceae) in the Baltic Sea. Ecoscience 1995, 2, 382–394. [Google Scholar] [CrossRef]
- Pedrini, A.G.; Silveira, I.C.A. Composição taxonômica e estimativa da biomassa das macroalgas epífitas em Ruppia maritima L. na Lagoa de Marapendi, Rio de Janeiro, RJ, Brasil. Atas Soc. Bot. Bras. 1985, 3, 45–60. [Google Scholar]
- Pacobahyba, L.D.; Eskinazi-Leça, E.; Silva-Cunha, M.G.G. Diatomáceas (Bacillariophyceae) epífitas na fanerógama marinha Halodule wrightii Aschers coletada no ambiente costeiro de Itamaracá-PE. Trop. Oceanogr. 1993, 22, 39–64. [Google Scholar]
- Ferreira, S.; Seeliger, U. The colonization Process of algal epiphytes on Ruppia maritma L. Bot. Mar. 1985, 28, 245–249. [Google Scholar] [CrossRef]
- Odebrecht, C.; Secchi, E.R.; Abreu, P.C.A.; Muelbert, J.H.; Uiblein, F. Biota of the Patos Lagoon estuary and adjacent marine coast: Long-term changes induced by natural and human-related factors. Mar. Biol. Res. 2017, 13, 3–8. [Google Scholar] [CrossRef]
- Baumgarten, M.G.Z.; Niencheski, L.F.; Martins, B.A. Saco do Justino (RS-Brasil): Amônio e Fosfato na coluna d’água e na coluna intersticial de uma enseada não contaminada. Atlântica 2005, 27, 113–129. [Google Scholar]
- Baumgarten, M.G.Z.; Niencheski, L.F.H. A coluna sedimentar como reservatório e fonte de nutrientes em enseadas estuarinas. Trop. Oceanogr. 2010, 38, 88–104. [Google Scholar] [CrossRef]
- Copertino, M.; Seeliger, U. Habitas de Ruppia maritima e de macroalgas. In O estuário da Lagoa dos Patos: Um século de transformações; Seeliger, U.C., Odebrecht, C., Castello, J.P., Eds.; FURG: Rio Grande, Brazil, 2010; pp. 91–98. [Google Scholar]
- Belarmino, E.; Nóbrega, M.F.; Grimm, A.M.; Copertino, M.S.; Vieira, J.P.; Garcia, A.M. Long-term trends in the abundance of an estuarine fish and relationships with El Niño climatic impacts and seagrass meadows reduction. Estuar. Coast. Shelf Sci. 2021, 261, 107576. [Google Scholar] [CrossRef]
- Garcia, A.M.; Claudino, M.C.; Mont’Alverne, R.; Pereyra, P.E.R.; Copertino, M.; Vieira, J.P. Temporal variability in assimilation of basal food sources by an omnivorous fish at Patos Lagoon Estuary revealed by stable isotopes (2010–2014). Mar. Biol. Res. 2017, 13, 98–107. [Google Scholar] [CrossRef]
- Lanari, M.; Possamai, B.; Copertino, M.S.; Garcia, A.M. Seasonal and El Niño Southern Oscillation-driven variations in isotopic and elemental patterns among estuarine primary producers: Implications for ecological studies. Hydrobiologia 2021, 848, 593–611. [Google Scholar] [CrossRef]
- Odebrecht, C.; Bergesch, M.; Medeanic, S.; Abreu, P.C. As comunidades de microalgas. In O estuário da Lagoa dos Patos: Um século de transformações; Seeliger, U.C., Odebrecht, C., Castello, J.P., Eds.; FURG: Rio Grande, Brazil, 2010; pp. 51–63. [Google Scholar]
- Abreu, P.C.; Hartmann, C.; Odebrecht, C. Nutrient-rich saltwater and its influence on the phytoplankton of the patos lagoon estuary, Southern Brazil. Estuar. Coast. Shelf Sci. 1995, 40, 219–229. [Google Scholar] [CrossRef]
- Abreu, P.C.; Odebrecht, C.; Niencheski, L.F. Nutrientes dissolvidos. In O Estuário da Lagoa dos Patos: Um Século de Transformações; Seeliger, U.C., Odebrecht, C., Castello, J.P., Eds.; FURG: Rio Grande, Brazil, 2010; pp. 43–48. [Google Scholar]
- Sullivan, M.J. Structural characteristics of a diatom community epiphytic on Ruppia maritima. Hydrobiologia 1977, 53, 81–86. [Google Scholar] [CrossRef]
- Reyes-Velasquez, G. Studies on the diatom flora living on Thalassia testudinum Konig in Biscayne Bay, Florida. Bull. Mar. Sci. 1970, 20, 105–134. [Google Scholar]
- Sullivan, M.J. Epiphytic diatoms of three seagrass species in Mississippi Sound. Bull. Mar. Sci. 1979, 29, 459–464. [Google Scholar]
- Dunton, K.H. Production ecology of Ruppia maritima L. s.1 and Halodule wrightii Aschers. In two subtropical estuaries. Exp. Mar. Biol. Ecol. 1990, 143, 147–164. [Google Scholar]
- Gordon, N.; Adams, J.B.; Bate, G.C. Epiphytes of the St. Lucia Estuary and their response to water level and salinity changes during a severe drought. Aquat. Bot. 2008, 88, 66–76. [Google Scholar]
- Chung, M.H.; Lee, K.S. Species Composition of the Epiphytic Diatoms on the Leaf Tissues of Three Zostera Species Distributed on the Southern Coast of Korea. Algae 2008, 23, 75–81. [Google Scholar] [CrossRef]
- Sullivan, M.J. Taxonomic notes on epiphytic diatoms of Mississippi Sound, U.S.A. Nova Hedwigia, Beih 1979, 64, 241–249. [Google Scholar]
- Dhib, A.; Brahim, M.B.; Turki, S.; Aleya, L. Contrasting key roles of Ruppia cirrhosa in a southern Mediterranean lagoon: Reservoir for both biodiversity and harmful species and indicator of lagoon health status. Mar. Poll. Bull. 2013, 76, 116–127. [Google Scholar] [CrossRef]
- Majewska, R.; D’Alelio, D.; De Stefano, M. Cocconeis Ehrenberg (Bacillariophyta), a genus dominating diatom communities associated with Posidonia oceanica Delile (monocotyledons) in the Mediterranean Sea. Aquat. Bot. 2014, 112, 48–56. [Google Scholar] [CrossRef]
- Mabrouk, L.; Asma, H.; Bradai, M.N. Temporal and bathymetric variation of epiphyte cover and leaf biomass in a Southern Posidonia oceanica (L.) meadow: The case of Mahdia coast, Tunisia. Mar. Ecol. 2016, 38, e12394. [Google Scholar] [CrossRef]
- Marques, W.C.; Moller, O.O. Variabilidade temporal em longo período da descarga fluvial e níveis de água da Lagoa dos Patos, Rio Grande do Sul, Brasil. Rev. Bras. Rec. Hidr. 2008, 13, 155–163. [Google Scholar] [CrossRef]
- Moller, O.; Fernandes, E. Hidrologia e hidrodinâmica. In O Estuário da Lagoa dos Patos: Um Século de Transformações; Seeliger, U., Odebrecht, C., Castello, J.P., Eds.; FURG: Rio Grande, Brazil, 2010; pp. 17–27. [Google Scholar]
- Simonsen, R. The diatom plankton of the Indian Ocean Expedition of R/V “Meteor” 1964–65. Meteor Forsch.-Ergeb. 1974, 19, 1–107. [Google Scholar]
- Lobo, E.; Leighton, G. Estruturas comunitarias de las fitocenosis planctonicas e los sistemas de desembocaduras de rios u esteros de la zona central de Chile. Rev. Biol. Mar. 1986, 22, 1–29. [Google Scholar]
- Baumgarten, M.G.Z.; Wallner-Kersanach, M.; Niencheski, L.F.H. Manual de Análises em Oceanografia Química; Editora da FURG: Rio Grande, Brazil, 2010; p. 174. [Google Scholar]
- Bernardino, A.F.; Barros, F.; Perez, L.F.; Netto, S.A.; Colling, L.A.; Pagliosa, P.R.; Lana, P.C.; Maia, R.C.; Christofoletti, R.A.; Filho, J.S.R.; et al. Monitoramento de ecossistemas bentônicos estuarinos. In Protocolos Para o Monitoramento de Habitats Bentônicos Costeiros—Rede de Monitoramento de Habitats Bentônicos Costeiros—ReBentos; Instituto Oceanográfico da Universidade de São Paulo-SP: São Paulo, Brasil, 2015; pp. 134–154. [Google Scholar]
- The Jamovi Project. Jamovi. (Version 1.6), Computer Software. 2021. Available online: http://www.jamovi.org (accessed on 1 November 2020).
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Paleontol. Eleyronica 2001, 4, 9. [Google Scholar]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA + for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymonth, UK, 2008; p. 214. [Google Scholar]
- Melo, A.S.; Hepp, L.U. Ferramentas estatísticas para análises de dados provenientes de biomonitoramento. Oecol. Bras. 2008, 12, 463–486. [Google Scholar] [CrossRef]
- Garcia, M.; Talgatti, D. Morfologia e distribuição de Catenula adhaerens Mereschkowsky (Bacillariophyceae) no sul do Brasil. Iheringia, Sér. Bot. 2011, 66, 99–108. [Google Scholar]
- Snoeijs, P. Studies in the Tabularia fasciculata complex. Diatom Res. 1992, 7, 313–344. [Google Scholar] [CrossRef]
- Round, F.E.; Crawford, R.M.; Mann, D.G. The Diatoms. Biology and Morphology of the Genera; Cambridge University Press: Cambridge, UK, 1990; p. 747. [Google Scholar]
- Sabanci, F.C. Species of Mastogloia (Bacillariophyceae)—New for the Aegean coast of Turkey. Mediterr. Mar. Sci. 2013, 14, 129–140. [Google Scholar] [CrossRef]
- Bergesch, M.; Odebrecht, C.; Abreu, P.C.O. Microalgas do estuário da Lagoa dos Patos: Interação entre o sedimento e a coluna de água. Oecol. Bras. 1995, 1, 273–289. [Google Scholar] [CrossRef]
- Torgan, L.C.; Raupp, S.V. Melosira moniliformis (O.F Müller) C. Agardth var. moniliformis (Bacillariophyta) do estuário da Laguna dos Patos, Rio Grande do Sul, Brasil. Iheringia, Sér. Bot. 2001, 56, 185–196. [Google Scholar]
- Yamamoto, M.; Chiba, T.; Tuji, A. Salinity responses of benthic diatoms inhabiting tidal flats. Diatom Res. 2017, 32, 243–250. [Google Scholar] [CrossRef]
- Lebreton, B.; Richard, P.; Redenac, G.; Borges, M.; Bréret, M.; Arnaud, C.; Blanchard, G.F. Are epiphytes a significant component of intertidal Zostera noltii beds? Aquat. Botany. 2009, 91, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Bauer, D.E.; Gómez, N.; Hualde, P.R. Biofilms coating Schoenoplectus californicus as indicators of water quality in the Río de la Plata Estuary (Argentina). Environ. Minit. Assess. 2007, 133, 309–320. [Google Scholar] [CrossRef]
- Rosa, V.C.; Garcia, M. Diatomáceas (Bacillariophyceae) epífitas em Acrostichum danaeifolium (Pteridaceae) no Arroio Pseudônimo, Pelotas, Rio Grande do Sul, Brasil. Sitientibus Sér. Ciên. Biol. 2013, 13, 1–14. [Google Scholar] [CrossRef]
- Silva, J.G.; Torgan, L.C.; Cardoso, L.S. Diatomáceas (Bacillariophyceae) em marismas no sul do Brasil. Acta Bot. Bras. 2010, 24, 935–947. [Google Scholar] [CrossRef]
- Baumgarten, M.G.Z. A eutrofização das águas de uma enseada do estuário da Lagoa dos Patos (RS) protegida pela legislação ambiental. Fepam em Rev. 2010, 3, 34–42. [Google Scholar]
- Brasil. Conselho Nacional do Meio Ambiente, Resolução CONAMA n° 357 de 2005. Diário Oficial da União da República Federativa do Brasil, 18 de Março de 2005. Available online: http://www.icmbio.gov.br (accessed on 1 June 2020).
- Aminot, A.; Chaussepied, M. Manuel des Analyses Chimiques em Milieu Marin; CNEXO: Brest, France, 1993; p. 395. [Google Scholar]
- Marreto, R.N.; Baumgarten, M.G.Z.; Wallner-Kersanach, M. Trophic quality of Waters in the Patos Lagoon estuary: A comparison between its margins and the port channel located in Rio Grande, RS, Brazil. Acta Limnol. Bras. 2017, 29, e11. [Google Scholar] [CrossRef]
- Gough, S.B.; Gough, L.P. Comment on “primary production of algae growing on natural and artificial aquatic plants: A study of interactions between epiphytes and their substrate” (Cattaneo and Kalff). Limnol. Oceanogr. 1981, 26, 987–988. [Google Scholar] [CrossRef]
- Letáková, M.; Fránková, M.; Paulícková, A. Ecology and applications of freshwater epiphytic diatoms—Review. Cryptogam. Algol. 2018, 39, 3–22. [Google Scholar] [CrossRef]
- Liess, A.; Lange, K.; Schulz, F.; Piggott, J.J.; Matthaei, C.D.; Townsend, C.R. Light, nutrients and grazing Interact to determine diatom species richness via changes to productivity, nutrient state and grazer activity. J. Ecol. 2009, 97, 236–336. [Google Scholar] [CrossRef]
- Huang, D.; Chu, T.; Sheng, Q.; Chen, J.; Wu, J. Variable bottom-up and top-down effects on diversity of different prey assemblages in an estuarine saltmarsh. Mar. Ecol. Prog. Ser. 2013, 472, 15–25. [Google Scholar] [CrossRef]
- Virta, L.; Soininen, J.; Norkko, A. Biodiversity Loss Threatens the Current Functional Similarity of Beta Diversity in Benthic Diatom Communities. Microb. Ecol. 2021, 81, 293–303. [Google Scholar] [CrossRef]
- Eminson, D.F. A comparison of diatom epiphytes their diversity and density, attached to Myriophyllum spicatum L. in Norfolk dykes and broads. Br. Phycol. 1978, 13, 57–64. [Google Scholar] [CrossRef]
- Frankovich, T.A.; Fourqurean, J.W. Seagrass epiphyte loads along a nutrient availability gradient, Florida Bay, USA. Mar. Ecol. Prog. Ser. 1997, 159, 37–50. [Google Scholar] [CrossRef]
- López-Fuerte, O.F.; Siqueiros-Beltrones, D.A.; Hernandez-Almeida, O.U. Epiphytic diatoms of Thalassia testudinum (Hydrocharitaceae) from the Mexican Caribbean. Mar. Biodivers. Rec. 2013, 6, 1–11. [Google Scholar] [CrossRef]
- Mannino, A.M. Temporal and spatial variation of the algal community in a Southern Mediterranean shallow system. Cryptogam. Algol. 2010, 31, 255–272. [Google Scholar]
- Chen, C.P.; Gao, Y.H.; Lin, P. Geographical and seasonal patterns of epiphytic diatoms on a subtropical mangrove (Kandelia candel) in southern China. Ecol. Indic. 2010, 10, 143–147. [Google Scholar] [CrossRef]
- Rosa, V.C.; Garcia, M. Ecological guilds of epiphytic diatoms (Bacillariophyta) on Acrostichum danaeifolium Längst. & Fisch in a subtropical wetland in southern Brazil. Acta Limnol. Bras. (Online) 2015, 27, 311–321. [Google Scholar]
- Lepoint, G.; Havelange, S.; Gobert, S.; Bouquegneau, J.M. Fauna vs flora contribution to the leaf epiphytes biomass in a Posidonia oceanica seagrass bed (Revellata Bay, Corsica). Hydrobiologia 1999, 394, 63–67. [Google Scholar] [CrossRef]
- Ersanli, E.; Gonulol, A. Epiphytic diatoms on Cladophora rivularis (Linnaeus) Hoek (Chlorophyta) and Potamogeton pectinatus Linnaeus (Spermatophyta) in Lake Simenit (Samsun—Turkey). Diatom Res. 2007, 22, 27–44. [Google Scholar] [CrossRef]
- John, J. The distribution of epiphytic diatoms in the Swan River Estuary, Western Australia, in relation to hydrological factors. In Proceedings of the 10th International Diatom Symposium, Joensuu, Finland, 28 August–2 September 1988; pp. 335–344. [Google Scholar]
- Siqueiro-Beltrones, D.A.; Castrejon, E.S. Structure of Benthic Diatom Assemblages from a Mangrove Environment in a Mexican Subtropical Lagoon. Biotropica 1999, 31, 48–70. [Google Scholar]
- Rosa, L.C.; Bemvenuti, C.E. Temporal variability of the estuarine macrofauna of the Patos Lagoon, Brazil. Rev. Biol. Mar. Oceanogr. 2006, 41, 1–9. [Google Scholar] [CrossRef]
- Torgan, L.C.; Becker, V.; Santos, C.B. Skeletonema potamos (Bacillariophyta) in Patos Lagoon, Southern Brazil: Taxonomy and distribution. Rev. Peru. Biol. 2009, 16, 093–096. [Google Scholar]
- Roubeix, V.; Lancelot, C. Effect of salinity on growth, cell size and silification of an euryhaline freshwater diatom: Cyclotella meneghiniana Kütz. Transitional Waters Bull. 2008, 1, 31–38. [Google Scholar]
- Lobo, E.A.; Wetzel, C.E.; Ector, L.; Katoh, K.; Blanco, S.; Mayama, S. Response of epilithic diatom communities to environmental gradients in subtropical temperate Brazilian rivers. Limnetica 2010, 29, 323–340. [Google Scholar] [CrossRef]
- Zgrundo, A.; Lemke, P.; Pniewski, F.; Cox, E.J.; Latala, A. Morphological and molecular phylogenetic studies on Fistulifera saprophila. Diatom Res. 2013, 28, 431–443. [Google Scholar] [CrossRef]
- Khairy, H.M.; Hussein, N.R.; Faragallah, H.M.; Dorgham, M.M. The phytoplankton communities in two eutrophic areas on the Alexandria coast, Egipt. Rev. Biol. Mar. Oceanogr. 2014, 49, 267–277. [Google Scholar] [CrossRef]
- McQuoid, M.R.; Nordberg, K. The diatom Paralia sulcata as an environmental indicator species in coastal sediments. Estuar. Coast. Shelf Sci. 2003, 56, 339–354. [Google Scholar] [CrossRef]
- Gómez, N.; Licursi, M. The Pampean Diatom Index (IDP) for assessment of rivers and streams in Argentina. Aquat. Ecol. 2001, 35, 173–181. [Google Scholar] [CrossRef]
- Coleman, V.L.; Burkholder, J.M. Response of microalgal epiphyte communities to nitrate enrichment in a eelgrass (Zostera marina) meadow. J. Phycol. 1995, 31, 36–43. [Google Scholar] [CrossRef]
- Aykut, T.O.; Balkis-Ozdelice, N.; Durmus, T.; Solak, C.N. Evaluation of the Relationship between Epiphytic Diatoms and Water Quality Parameters in the Büyükçekmece Reservoir. Eur. J. Biol. 2021, 80, 54–68. [Google Scholar] [CrossRef]
- Hillebrand, H.; Worm, B.; Lotze, H.K. Marine microbenthic community structure regulated by nitrogen loading and grazing pressure. Mar. Ecol. Prog. Ser. 2000, 204, 27–38. [Google Scholar] [CrossRef]
- Gotoh, T. Diatoms of Brackish Water, Lake Shinjiand Lake Nakaumi, I. The Genus Mastogloia Thwaites. Acta Phytotax. Geobot. 1990, 41, 143–154. [Google Scholar]
- Lionard, M.; Muylaert, K.; Gansbeke, D.V.; Vyverman, W. Influence of changes in salinity and ligth intensity on growth of phytoplankton communities from the Schelde river and estuary (Belgium/The Netherlands). Hydrobiologia 2005, 540, 105–115. [Google Scholar] [CrossRef]


| Variables/Periods and Sites | Temperature | Salinity | Depth | Transparency (Secchi Disk) | Total Suspended Solids | Phosphate | Nitrogen (TAN) | Nitrate + Nitrite | Organic Matter (Sediment) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SQ | SM | SQ | SM | SQ | SM | SQ | SM | SQ | SM | SQ | SM | SQ | SM | SQ | SM | SQ | SM | ||
| 2015 | November | 24.20 | 20.40 | 0.20 | 1.18 | 0.65 | 0.43 | 0.42 | 0.13 | 7.33 | 74.00 | 0.02 | 0.08 | 0.36 | 0.67 | 0.68 | 2.12 | 0.48 | 0.74 |
| December | 21.70 | 26.50 | 1.60 | 6.80 | 0.55 | 0.63 | 0.38 | 0.20 | 32.00 | 42.83 | 0.01 | 0.03 | 0.50 | 1.28 | 0.54 | 0.39 | 0.70 | 0.50 | |
| 2016 | January | 27.60 | 24.80 | 0.12 | 6.61 | 0.99 | 0.53 | 0.35 | 0.23 | 23.33 | 68.93 | 0.02 | 0.05 | 0.07 | 0.27 | 0.32 | 0.39 | 0.73 | 0.33 |
| February | 28.50 | 27.00 | 0.00 | 6.00 | 0.28 | 0.58 | 0.28 | 0.58 | 20.67 | 16.00 | 0.01 | 0.04 | 0.70 | 2.78 | 0.73 | 0.49 | 0.54 | 0.67 | |
| March | 23.50 | 24.00 | 17.00 | 15.00 | 0.38 | 0.37 | 0.38 | 0.37 | 38.33 | 21.87 | 0.01 | 0.07 | 3.16 | 4.85 | 0.63 | 0.49 | 0.92 | 0.73 | |
| April | 13.00 | 11.00 | 19.00 | 8.00 | 0.53 | 0.55 | 0.53 | 0.55 | 19.33 | 20.93 | 0.02 | 0.06 | 4.01 | 4.79 | 0.60 | 1.31 | 0.83 | 0.90 | |
| May | 14.00 | 12.00 | 15.00 | 10.00 | 0.64 | 1.17 | 0.64 | 0.75 | 38.89 | 26.00 | 0.01 | 0.04 | 2.70 | 1.59 | 0.48 | 0.56 | 0.72 | 1.40 | |
| June | 13.00 | 12.00 | 5.00 | 5.00 | 0.65 | 0.61 | 0.65 | 0.49 | 29.33 | 66.67 | 0.01 | 0.07 | 0.71 | 1.69 | 0.60 | 0.61 | 1.45 | 1.12 | |
| July | 11.00 | 9.00 | 0.00 | 10.00 | 0.51 | 0.81 | 0.51 | 0.81 | 17.67 | 9.00 | 0.01 | 0.01 | 1.59 | 3.58 | 0.47 | 0.59 | 1.17 | 1.83 | |
| August | 16.00 | 14.00 | 1.00 | 6.00 | 0.52 | 0.77 | 0.25 | 0.77 | 40.00 | 24.00 | 0.01 | 0.05 | 0.71 | 2.66 | 0.68 | 0.68 | 0.78 | 0.93 | |
| September | 19.00 | 17.00 | 6.00 | 6.00 | 0.63 | 0.68 | 0.63 | 0.25 | 19.67 | 10.83 | 0.01 | 0.07 | 2.08 | 1.66 | 0.60 | 0.67 | 0.82 | 1.38 | |
| October | 19.00 | 20.00 | 9.00 | 10.00 | 0.86 | 0.89 | 0.32 | 0.34 | 30.00 | 10.00 | 0.01 | 0.06 | 2.54 | 3.74 | 0.56 | 0.61 | 0.79 | 1.34 | |
| November | 19.00 | 19.50 | 1.00 | 5.00 | 0.65 | 0.53 | 0.12 | 0.53 | 70.00 | 6.00 | 0.04 | 0.05 | 1.09 | 1.67 | 3.44 | 1.04 | 0.59 | 0.91 | |
| December | 22.50 | 22.50 | 0.00 | 11.00 | 0.80 | 0.76 | 0.12 | 0.52 | 98.00 | 16.22 | 0.02 | 0.06 | 0.55 | 2.62 | 1.15 | 0.81 | 0.47 | 0.89 | |
| 2017 | January | 25.50 | 24.00 | 5.00 | 8.00 | 0.58 | 0.74 | 0.58 | 0.72 | 15.33 | 29.67 | 0.01 | 0.04 | 2.24 | 2.13 | 0.90 | 0.68 | 0.56 | 1.35 |
| February | 22.00 | 22.00 | 9.00 | 10.00 | 0.64 | 0.86 | 0.64 | 0.86 | 5.60 | 8.40 | 0.01 | 0.05 | 3.54 | 3.79 | 0.78 | 0.68 | 0.90 | 1.45 | |
| March | ND | 19.50 | ND | 7.00 | ND | 0.97 | ND | 0.97 | ND | 23.33 | ND | 0.06 | ND | 3.15 | ND | 0.57 | ND | 1.18 | |
| April | ND | 18.50 | ND | 25.00 | ND | 0.81 | ND | 0.38 | ND | 25.33 | ND | 0.11 | ND | 2.05 | ND | 0.90 | ND | ND | |
| May | 15.00 | 15.50 | 18.00 | 20.00 | 0.55 | 0.70 | 0.55 | 0.70 | 20.67 | 10.27 | 0.01 | 0.07 | 5.00 | 5.30 | 0.65 | 0.69 | 1.05 | 1.99 | |
| June | 13.00 | 17.00 | 0.00 | 0.00 | 1.29 | 0.81 | 0.35 | 0.81 | 29.67 | 19.33 | 0.02 | 0.07 | 0.98 | 0.70 | 1.34 | 1.01 | ND | ND | |
| July | 17.20 | 16.50 | 0.00 | 0.00 | 0.80 | 0.80 | 0.20 | 0.36 | 71.67 | 49.67 | 0.03 | 0.11 | 0.83 | 0.93 | 1.91 | 1.51 | ND | ND | |
| August | 15.50 | 15.00 | 4.00 | 6.00 | 0.85 | 0.60 | 0.85 | 0.60 | 7.00 | 14.00 | 0.01 | 0.07 | 0.52 | 3.11 | 1.16 | 0.93 | 0.78 | 1.08 | |
| September | 17.50 | 17.50 | 0.00 | 5.00 | 0.79 | 0.86 | 0.32 | 0.52 | 18.00 | 28.00 | 0.07 | 0.11 | 1.03 | 2.26 | 1.65 | 1.44 | ND | ND | |
| October | 18.50 | 18.50 | 0.00 | 0.00 | 1.30 | 0.98 | 0.15 | 0.17 | 154.00 | 105.33 | 0.06 | 0.04 | 0.60 | 0.61 | 1.95 | 1.57 | ND | ND | |
| November | 21.00 | 19.80 | 17.00 | 20.00 | 0.91 | 0.99 | 0.91 | 0.55 | 15.67 | 23.67 | 0.02 | 0.06 | 2.75 | 2.72 | 1.17 | 1.00 | 0.51 | 0.81 | |
| December | 21.50 | 22.00 | 1.00 | 6.00 | 0.69 | 0.89 | 0.69 | 0.15 | 15.67 | 74.67 | 0.03 | 0.05 | 0.44 | 1.77 | 0.89 | 0.97 | ND | ND | |
| 2018 | January | 23.00 | 24.00 | 5.00 | 17.00 | 0.90 | ND | 0.90 | ND | 7.50 | 21.00 | 0.01 | 0.04 | 2.11 | 3.62 | 0.71 | 0.63 | ND | ND |
| February | 25.50 | 26.00 | 10.00 | 15.00 | 0.50 | 0.90 | 0.50 | 0.23 | 15.00 | 23.67 | 0.01 | 0.01 | 0.70 | 4.96 | 0.80 | 0.65 | 0.78 | 1.56 | |
| Test | Student’s t | Mann–Whitney | Student’s t | Student’s t | Mann–Whitney | Mann–Whitney | Mann–Whitney | Mann–Whitney | Mann–Whitney | ||||||||||
| p-value | 0.756 | 0.025 | 0.505 | 0.629 | 0.840 | <0.001 | 0.01 | 0.77 | 0.008 | ||||||||||
| PERMANOVA Results | ||||||||
|---|---|---|---|---|---|---|---|---|
| Spatial Variation | Temporal Variation | |||||||
| Sites | Abundance | Composition | Monthly | Abundance | Composition | |||
| SQ × SM | p = 0.0001 | p = 0.0001 | p = 0.0001 | p = 0.0001 | ||||
| Comparisons (Monte Carlo test p-value) | Comparisons (Monte Carlo test p-value) | |||||||
| SQ × SM | April 2016 | 0.0010 | 0.0115 | SQ | SM | SQ | SM | |
| SQ × SM | May 2016 | 0.0084 | 0.0568 | April × May 2016 | 0.0155 | 0.1127 | 0.0485 | 0.2005 |
| SQ × SM | June 2016 | 0.0015 | 0.0049 | May × June 2016 | 0.0180 | 0.0326 | 0.0455 | 0.0999 |
| SQ × SM | July 2016 | 0.0052 | 0.0183 | June × July 2016 | 0.0181 | 0.0115 | 0.0168 | 0.0307 |
| SQ × SM | August 2016 | 0.0187 | 0.0216 | July × August 2016 | 0.0691 | 0.0131 | 0.0892 | 0.0904 |
| SQ × SM | September 2016 | 0.0061 | 0.0201 | August × September 2016 | 0.0228 | 0.0135 | 0.0529 | 0.1170 |
| SQ × SM | October 2016 | 0.0061 | 0.0076 | September × October 2016 | 0.0394 | 0.0216 | 0.0321 | 0.1540 |
| SQ × SM | November 2016 | 0.0104 | 0.0236 | October × November 2016 | 0.0243 | 0.0106 | 0.0625 | 0.0165 |
| SQ × SM | December 2016 | 0.0050 | 0.0128 | November × December 2016 | 0.0341 | 0.0412 | 0.1373 | 0.0281 |
| SQ × SM | January 2017 | 0.0400 | 0.0565 | December × January 2017 | 0.0523 | 0.1226 | 0.1529 | 0.1506 |
| SQ × SM | February 2017 | 0.0126 | 0.0446 | January × February 2017 | 0.0298 | 0.2053 | 0.0622 | 0.2385 |
| SQ × SM | May 2017 | 0.0078 | 0.0298 | February × May 2017 | 0.0039 | 0.1874 | 0.0127 | 0.6075 |
| SQ × SM | February 2018 | 0.0063 | 0.0105 | May × February 2018 | 0.0051 | 0.0196 | 0.0139 | 0.0735 |
| Month | Average Dissimilarity | Taxa and Contribution (%) | ||
|---|---|---|---|---|
| April 2016 | AD = 52.56 | T. fasciculata (12.51) | M. pumila (10.6) | R. rumrichiae (9.75) |
| May 2016 | AD = 48.57 | B. paxillifera (7.86) | T. fasciculata (7.39) | A. tenuissimus (6.69) |
| June 2016 | AD = 46.37 | N. eutinensis (10.28) | M. pumila (9.54) | P. brevistriata (8.74) |
| July 2016 | AD = 71.71 | B. paxillifera (6.17) | M. pumila (6.17) | T. fasciculata (5.41) |
| August 2016 | AD = 45.41 | R. rumrichiae (6.14) | N. cryptotenella (6.01) | M. pumila (5.9) |
| September 2016 | AD = 67.21 | P. laevis (8.26) | T. tabulata (6.91) | P. brevistriata (4.39) |
| October 2016 | AD = 61.63 | T. tabulata (8.81) | C. adhaerens (7.09) | P. sulcata (6.44) |
| November 2016 | AD = 61.15 | Skeletonema sp.2 (8.44) | P. laevis (6.04) | Navicula sp.3 (6.02) |
| December 2016 | AD = 66.15 | P. laevis (8.25) | P. brevistriata (7.9) | S. guenter-grassii (6.76) |
| January 2017 | AD = 52.80 | T. tabulata (8.52) | T. fasciculata (4.88) | P. brevistriata (4.5) |
| February 2017 | AD = 54.17 | C. placentula (11.85) | T. fasciculata (10.38) | T. tabulata (9.07) |
| May 2017 | AD = 70.73 | M. pumila (12.65) | T. tabulata (9.64) | C. placentula (9.36) |
| February 2018 | AD = 53.12 | N. microcephala (9.81) | P. laevis (5.44) | P. sulcata (5.39) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Rosa, V.C.; Copertino, M. Diversity and Variation of Epiphytic Diatoms on Ruppia maritima L., Related to Anthropogenic Impact in an Estuary in Southern Brazil. Diversity 2022, 14, 787. https://doi.org/10.3390/d14100787
da Rosa VC, Copertino M. Diversity and Variation of Epiphytic Diatoms on Ruppia maritima L., Related to Anthropogenic Impact in an Estuary in Southern Brazil. Diversity. 2022; 14(10):787. https://doi.org/10.3390/d14100787
Chicago/Turabian Styleda Rosa, Vanessa Corrêa, and Margareth Copertino. 2022. "Diversity and Variation of Epiphytic Diatoms on Ruppia maritima L., Related to Anthropogenic Impact in an Estuary in Southern Brazil" Diversity 14, no. 10: 787. https://doi.org/10.3390/d14100787
APA Styleda Rosa, V. C., & Copertino, M. (2022). Diversity and Variation of Epiphytic Diatoms on Ruppia maritima L., Related to Anthropogenic Impact in an Estuary in Southern Brazil. Diversity, 14(10), 787. https://doi.org/10.3390/d14100787