Genetic Assessment of Remnant Sub-Populations of Sterlet (Acipenser ruthenus Linnaeus, 1758) in the Upper Danube
Abstract
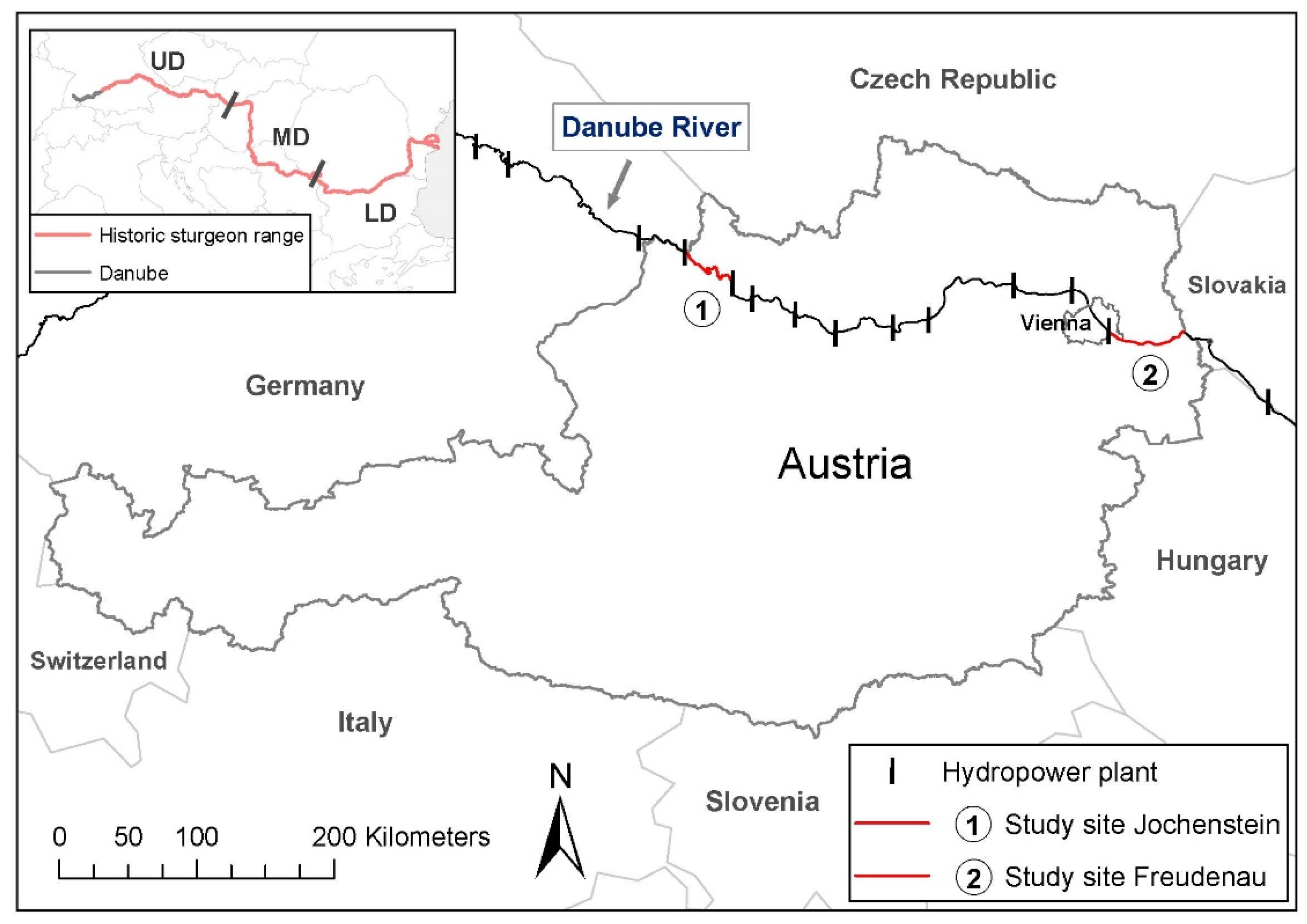
:1. Introduction
2. Materials and Methods
3. Results
3.1. Hybrids & Non-Native Species
3.2. Population Genetic Analysis
3.3. Reproductive Population Sizes Jochenstein & Freudenau
3.4. Relationship
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Two-Thirds of Sturgeon Species Now Critically Endangered with One Confirmed Extinct. Available online: https://wwf.panda.org/wwf_news/press_releases/?6080466/sturgeon-slipping-towards-extinction (accessed on 24 August 2022).
- Habersack, H.; Hein, T.; Stanica, A.; Liska, I.; Mair, R.; Jäger, E.; Hauer, C.; Bradley, C. Challenges of river basin management: Current status of, and prospects for, the River Danube from a river engineering perspective. Sci. Total Environ. 2016, 543, 828–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedrich, T.; Reinartz, R.; Gessner, J. Sturgeon re-introduction in the Upper and Middle Danube River Basin. J. Appl. Ichthyol. 2019, 35, 1059–1068. [Google Scholar] [CrossRef] [Green Version]
- Hensel, K.; Holčík, J. Past and Current Status of Sturgeons in the Upper and Middle Danube River. Environ. Biol. Fishes 1997, 48, 185–200. [Google Scholar] [CrossRef]
- Friedrich, T. Danube Sturgeons: Past and Future. In Riverine Ecosystem Management; Schmutz, S., Szendzimir, J., Eds.; Springer: Cham, Switzerland, 2018; pp. 507–518. [Google Scholar] [CrossRef] [Green Version]
- Reinartz, R.; Lippold, S.; Lieckfeldt, D.; Ludwig, A. Population genetic analyses of Acipenser ruthenus as a prerequisite for the conservation of the uppermost Danube population. J. Appl. Ichthyol. 2011, 27, 477–483. [Google Scholar] [CrossRef]
- Wolfram, G.; Mikschi, E. Rote Liste der Fische (Pisces) Österreichs; Böhlau Verlag: Wien, Austria, 2007; pp. 61–198. [Google Scholar]
- Gessner, J.; Freyhof, J.; Kottelat, M.; Friedrich, T. Acipenser ruthenus. The IUCN Red List of Threatened Species 2022: E.T227A135062526. Available online: https://www.iucnredlist.org/species/227/135062526 (accessed on 24 August 2022).
- Friedrich, T.; Schmall, B.; Ratschan, C.; Zauner, G. Die Störarten der Donau: Teil 3: Sterlet, “Stierl” (Acipenser ruthenus) und aktuelle Schutzprojekte im Donauraum. Osterr. Fischerei 2014, 67, 167–183. [Google Scholar]
- Neuburg, J.; Friedrich, T. First description of a remnant sterlet (Acipenser ruthenus Linnaeus, 1758) population in the eastern Austrian Danube. unpublished.
- Ludwig, A.; Lippold, S.; Debus, L.; Reinartz, R. First evidence of hybridization between endangered sterlets (Acipenser ruthenus) and exotic Siberian sturgeons (Acipenser baerii) in the Danube River. Biol. Invasions 2009, 11, 753–760. [Google Scholar] [CrossRef] [Green Version]
- Koščo, J.; Košuthová, L.; Košuth, P.; Pekárik, L. Non-native fish species in Slovak waters: Origins and present status. Biologia 2010, 65, 1057–1063. [Google Scholar] [CrossRef]
- King, T.; Lubinski, B.; Spidle, A. Microsatellite DNA variation in Atlantic sturgeon (Acipenser oxyrinchus oxyrinchus) and cross-species amplification in the Acipenseridae. Conserv. Genet. 2001, 2, 103–119. [Google Scholar] [CrossRef]
- May, B.; Krueger, C.C.; Kincaid, H.L. Genetic variation at microsatellite loci in sturgeon: Primer sequence homology in Acipenser and Scaphirhynchus. Can. J. Fish Aquat. Sci. 1997, 54, 1542–1547. [Google Scholar] [CrossRef]
- Mc Quown, E.C.; Sloss, B.L.; Sheehan, R.J.; Rodzen, J.; Tranah, G.J.; May, B. Microsatellite Analysis of Genetic Variation in Sturgeon: New Primer Sequences for Scaphirhynchus and Acipenser. Trans. Am. Fish Soc. 2000, 129, 1380–1388. [Google Scholar] [CrossRef]
- Structure Software. Available online: https://web.stanford.edu/group/pritchardlab/structure.html (accessed on 24 August 2022).
- Jones, O.; Wang, J. COLONY: A program for parentage and sibship inference from multilocus genotype data. Mol. Ecol. Resour. 2010, 10, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Individual identification from genetic marker data: Developments and accuracy comparisons of methods. Mol. Ecol. Resour. 2016, 16, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Cvijanović, G.; Adnadjević, T.; Lenhardt, M.; Marić, S. New data on sterlet (Acipenser ruthenus L.) genetic diversity in the middle and Lower Danube sections, based on mitochondrial DNA analyses. Genetika 2015, 47, 1051–1062. [Google Scholar] [CrossRef]
- Otis, D.L.; Burnham, K.P.; White, G.C.; Anderson, D.R. Statistical Inference from Capture Data on Closed Animal Populations. Wildl. Monogr. 1978, 62, 3–135. [Google Scholar]
- Schwarz, C.J.; Bailey, R.E.; Irvine, J.R.; Dalziel, F.C. Estimating salmon spawning escapement using capture-recapture methods. Can. J. Fish. Aquat. Sci. 1993, 50, 1181–1197. [Google Scholar] [CrossRef]
- Schwarz, C.J.; Arnason, A.N. A General Methodology for the Analysis of Capture-Recapture Experiments in Open Populations. Biometrics 1996, 52, 860–873. [Google Scholar] [CrossRef]
- Ratschan, C.; Zauner, G.; Jung, M. Der Sterlet im oberen Donautal–Identifikation der Laichhabitate mittels Telemetrie. Ezb-TB Zauner GmbH. Tech. Büro Für Gewässerökologie Und Fischereiwirtschaft 2017, 18–60. [Google Scholar]
- Eichhorn, H.; Friedrich, T. Migration patterns of sterlets in the Nationalpark Donauauen. Unpublished (in preparation)
- Guy, C.S.; Treanor, H.B.; Kappenman, K.M.; Scholl, E.A.; Ilgen, J.E.; Webb, M.A.H. Ampliando el paradigma de manejo de la regulación de ríos: La olvidada zona muerta como obstáculo para la recuperación del esturión pálido. Fisheries 2015, 40, 6–14. [Google Scholar] [CrossRef]
- Pekárik, L.; Čiamporová-Zaťovičová, Z.; Arendt, D.; Čiampor, F. Current stocking program of the sterlet (Acipenser ruthenus, L.) can negatively shape its genetic variability in the Middle DanubeKnowl. Manag. Aquat. Ecosyst. 2019, 420, 19. [Google Scholar] [CrossRef]


| Year | Country | Location | Number | Incl. in Population Assessment | Intraspec. Hybrids | Volga Genotype | Interspec. Hybrids |
|---|---|---|---|---|---|---|---|
| 2011 | AT | Jochenstein | 1 | 1 | |||
| 2012 | AT | Jochenstein | 1 | 1 | |||
| 2013 | AT | Jochenstein | 16 | 11 | 3 | 1 | 1 |
| 2014 | AT | Jochenstein | 30 | 19 | 6 | 6 | |
| AT | Freudenau | 3 | 1 | ||||
| 2015 | AT | Jochenstein | 25 | 16 | 4 | 6 | 1 |
| AT | Freudenau | 1 | |||||
| 2016 | AT | Jochenstein | 8 | 5 | 1 | 2 | |
| HU, GE, CZ, IT | CAPTIVE | 18 | / | 5 | 5 | ||
| SK, RO | WILD | 8 | / | ||||
| 2017 | AT | Jochenstein | 4 | 3 | 3 | ||
| 2018 | AT | Jochenstein | 24 | 23 | 2 | ||
| AT | Freudenau | 9 | 5 | 3 | 1 | ||
| BG | WILD | 30 | / | 1 | 1 | ||
| 2019 | AT | Freudenau | 8 | 6 | 2 | ||
| 2020 | AT | Jochenstein | 9 | 8 | 1 | ||
| AT | Freudenau | 8 | 7 | 1 | |||
| BG | CAPTIVE | 40 | / | 3 | 2 | ||
| 2021 | AT | Jochenstein | 14 | 14 | |||
| AT | Freudenau | 8 | 7 | 2 | |||
| Total samples analyzed | 265 | / | 37 | 23 | 2 | ||
| Total samples Jochenstein | 132 | 101 | 20 | 15 | 2 | ||
| Total samples Freudenau | 37 | 29 | 9 | 1 | |||
| TOTAL samples Aquaculture | 58 | / | 8 | 6 | |||
| TOTAL samples wild comparison | 38 | / | 1 | 1 | |||
| Abbreviations: AT (Austria); BG (Bulgaria); CZ (Czech Republic); GE (Germany); HU (Hungary); IT (Italy); SK (Slovakia) | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Friedrich, T.; Lieckfeldt, D.; Ludwig, A. Genetic Assessment of Remnant Sub-Populations of Sterlet (Acipenser ruthenus Linnaeus, 1758) in the Upper Danube. Diversity 2022, 14, 893. https://doi.org/10.3390/d14100893
Friedrich T, Lieckfeldt D, Ludwig A. Genetic Assessment of Remnant Sub-Populations of Sterlet (Acipenser ruthenus Linnaeus, 1758) in the Upper Danube. Diversity. 2022; 14(10):893. https://doi.org/10.3390/d14100893
Chicago/Turabian StyleFriedrich, Thomas, Dietmar Lieckfeldt, and Arne Ludwig. 2022. "Genetic Assessment of Remnant Sub-Populations of Sterlet (Acipenser ruthenus Linnaeus, 1758) in the Upper Danube" Diversity 14, no. 10: 893. https://doi.org/10.3390/d14100893
APA StyleFriedrich, T., Lieckfeldt, D., & Ludwig, A. (2022). Genetic Assessment of Remnant Sub-Populations of Sterlet (Acipenser ruthenus Linnaeus, 1758) in the Upper Danube. Diversity, 14(10), 893. https://doi.org/10.3390/d14100893







