Hard Nut to Crack. Acorn Hardness Implications on Oviposition of the Acorn Weevil Curculio glandium Marsham, 1802 (Coleoptera: Curculionidae)
Abstract
:1. Introduction
2. Material and Methods
2.1. Experiment 1. Acorn Development and Hardness Change during Reproduction of C. glandium
2.1.1. Differences along the Season
2.1.2. Mother Tree Differences
2.2. Experiment 2. Female Ovipositional Behavior on Acorns
2.3. Experiment 3. Female Choice Test—Cracked vs. Uncracked Acorns
2.4. Experiment 4. Changes in Egg Output during the Season and Female Fecundity
2.5. Statistical Analysis
- a parametric, one-way ANOVA with a post-hoc Tukey’s HSD test was used to analyze significant differences (p < 0.05) in hardness and masses of acorns; the effects of oak fruit development during the season and mother tree differences on four variables (Force 1, Force 2, Deformation 1, Mass) were checked (Experiment 1);
- a non-parametric Mann–Whitney U-test was used to determine significant differences (p < 0.05) in the amount of time spent on creating egg channels in cracked and uncracked acorns (Experiment 2);
- a non-parametric Cochran Q test was used to settle significant differences (p < 0.05) in the choice of cracked (coded as 0) or uncracked (coded as 1) acorns as ovipositional sites (Experiment 3);
- a non-parametric Kruskal–Wallis ANOVA with a post-hoc Dunn’s test was performed to evaluate whether females differed significantly (p < 0.05) in reproductive capacity from 29 July to 20 August and, after the hardening of acorns, from 23 August to 17 September (Experiment 4);
- a non-parametric Mann–Whitney U-test was applied to assess the significant difference (p < 0.05) in the number of eggs laid into two typical acorns given to females prior to fruit hardening, as well as in the number of eggs laid in pierced or unpierced fruits after hardening (Experiment 4).
3. Results
3.1. Experiment 1. Acorn Development and Hardness Change during Reproduction of C. glandium
3.2. Experiment 2. Female Ovipositional Behavior on Acorns
3.3. Experiment 3. Female Choice Test—Cracked vs. Uncracked Acorns
3.4. Experiment 4. Changes in Egg Output during the Season and Female Fecundity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonal, R.J.; Espelta, M.; Vogler, A.P. Complex selection on life-history traits and the maintenance of variation in exaggerated rostrum length in acorn weevils. Oecologia 2011, 167, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Dobrosavljevic, J.; Markovic, C.; Milanovic, S.; Vujicic, P.; Srbulovic, B.; Bojic, S. Influence of Curculio glandium (Marsham, 1802)(Coleoptera, Curculionidae) on Turkey Oak (Quercus cerris L., 1753) (Fagales, Fagaceae) Acorn Germination. In Proceedings of the IX International Agricultural Symposium “Agrosym 2018”, Sarajevo, Bosnia and Herzegovina, 4–7 October 2018. [Google Scholar]
- Espelta, J.M.; Bonal, R.; Sánchez-Humanes, B. Pre-dispersal acorn predation in mixed oak forests: Interspecific differences are driven by the interplay among seed phenology, seed size and predator size. J. Ecol. 2009, 97, 1416–1423. [Google Scholar] [CrossRef]
- Muñoz, A.; Bonal, R.; Espelta, J.M. Acorn–weevil interactions in a mixed-oak forest: Outcomes for larval growth and plant recruitment. Forest Ecol. Manag. 2014, 322, 98–105. [Google Scholar] [CrossRef]
- Wanat, M.; Mokrzycki, T. The Checklist of the Weevils (Coleoptera: Curculionoidea) of Poland Revisited. Ann. Zool. 2018, 68, 1–48. [Google Scholar] [CrossRef]
- Hughes, J.; Vogler, A.P. Ecomorphological adaptation of acorn weevils to their oviposition site. Evolution 2004, 58, 1971–1983. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Jafarpour, M.; Reut, M.; Shams Moattar, B.; Darvizeh, A.; Gorb, S.N.; Rajabi, H. Excavation mechanics of the elongated female rostrum of the acorn weevil Curculio glandium (Coleoptera; Curculionidae). Appl. Phys. A 2021, 127, 348. [Google Scholar] [CrossRef]
- Csóka, G.; Csókáné Hirka, A. Direct effects of carpophagous insects on the germination ability and early abscission of oak acorns. Acta Silv. Lignaria Hung. 2006, 2, 57–67. [Google Scholar]
- Oltean, I.; Stana, A. Curculio glandium, a pest which reduces the quality of the seed at Quercus petraea in Hilly Tree From Os Almaş, Ds Zalău. Bull. USAMV-CN 2007, 63, 123–126. [Google Scholar]
- Venner, S.; Pélisson, P.F.; Bel-Venner, M.C.; Débias, F.; Rajon, E.; Menu, F. Coexistence of insect species competing for a pulsed resource: Toward a unified theory of biodiversity in fluctuating environments. PLoS ONE 2011, 6, e18039. [Google Scholar] [CrossRef]
- Pélisson, P.F.; Bel-Venner, M.C.; Rey, B.; Burgevin, L.; Martineau, F.; Fourel, F.; Lecuyer, C.; Menu, F.; Venner, S. Contrasted breeding strategies in four sympatric sibling insect species: When a proovigenic and capital breeder copes with a stochastic environment. Funct. Ecol. 2012, 26, 198–206. [Google Scholar] [CrossRef]
- Reut, M.; Chrabąszcz, M.; Moniuszko, H. Timing Is Everything. Temporal and Spatial Niche Segregation in Curculio spp. (Coleoptera: Curculionidae) Associated with Oak Trees. Insects 2021, 12, 687. [Google Scholar] [CrossRef] [PubMed]
- Reut, M.; Moniuszko, H. Atypical Oviposition in Curculio glandium Marsham (Coleoptera: Curculionidae)—A Response to Unfavorable Environmental Conditions? Coleopt. Bull. 2022, 76, 36–38. [Google Scholar] [CrossRef]
- Addicott, F.T. Abscission; University of California Press: Berkeley, CA, USA, 1982; pp. 1–355. [Google Scholar]
- Bogdziewicz, M.; Espelta, J.M.; Muñoz, A.; Aparicio, J.M.; Bonal, R. Effectiveness of predator satiation in masting oaks is negatively affected by conspecific density. Oecologia 2018, 186, 983–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koenig, W.D.; Knops, J.M. The behavioral ecology of masting in oaks. In Oak Forest Ecosystems: Ecology and Management for Wildlife; Mc Shea, W.J., Healy, W.M., Eds.; Johns Hopkins University Press: Baltimore, MD, USA, 2002; pp. 129–148. [Google Scholar]
- Rohlfs, D.A. A Study of Acorn Feeding Insects: Filbert Weevil (Curculio Occidentis (Casey)) and Filbertworm (Cydia Latiferreana (Walsingham)) on Garry Oak (Quercus Garryana) (Dougl.) in the Southeastern Vancouver Island Area. Doctoral Dissertation, University of British Columbia, Vancouver, BC, Canada, 1999. [Google Scholar]
- Holtz, B.A. Plant protection for pistachio. HortTechnology 2002, 12, 626–632. [Google Scholar] [CrossRef] [Green Version]
- Valentini, N.; Moraglio, S.T.; Rolle, L.G.C.; Tavella, L.; Botta, R. Nut and kernel growth and shell hardening in eighteen hazelnut cultivars (Corylus avellana L.). Hort. Sci. 2015, 42, 149–158. [Google Scholar] [CrossRef] [Green Version]
- Guidone, L.; Valentini, N.; Rolle, L.; Me, G.; Tavella, L. Early nut development as a resistance factor to the attacks of Curculio nucum (Coleoptera: Curculionidae). Ann. Appl. Biol. 2007, 150, 323–329. [Google Scholar] [CrossRef]
- Calcote, V.R.; Hyder, D.E. Pecan weevil preference for various pecan cultivars. J. Econ. Entomol. 1981, 74, 223–226. [Google Scholar] [CrossRef]
- Calcote, V.R. Pecan Weevil: Feeding and initial oviposition as related to nut development. J. Econ. Entomol. 1975, 68, 4–6. [Google Scholar] [CrossRef]
- Rakić, S.; Povrenović, D.; Tešević, V.; Simić, M.; Maletić, R. Oak acorn, polyphenols and antioxidant activity in functional food. J. Food Eng. 2006, 74, 416–423. [Google Scholar] [CrossRef]
- Sekeroglu, N.; Ozkutlu, F.; Kilic, E. Mineral composition of acorn coffees. Indian J. Pharm. Educ. Res. 2017, 51, 504–507. [Google Scholar] [CrossRef] [Green Version]
- Smreczyński, S. The Key for Identification of Polish Insects. Beetles–Coleoptera. Part XIX (98f). Weevils–Curculionidae. Subfamily–Curculioninae; Państwowe Wydawnictwo Naukowe: Warsaw, Poland, 1972; p. 195. [Google Scholar]
- Valentini, N.; Rolle, L.; Stévigny, C.; Zeppa, G. Mechanical behaviour of hazelnuts used for table consumption under compression loading. J. Sci. Food Agric. 2006, 86, 1257–1262. [Google Scholar] [CrossRef]
- Koyuncu, M.A.; Ekinci, K.; Savran, E. Cracking characteristics of walnut. Biosyst. Eng. 2004, 87, 305–311. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Ji, Y.C.; Wen, X.J.; Li, Q.; Ren, Y.; Wen, J.B. Oviposition behaviour of Eucryptorrhynchus brandti (Coleoptera: Curculionidae: Cryptorrhychinae) on Ailanthus altissima (Mill.) Swingle (Sapindales: Simaroubaceae). Biocontrol Sci. Technol. 2017, 27, 1153–1167. [Google Scholar] [CrossRef]
- Bystrowski, C.; Wójcik, G. Use of neonicotinoid insecticides for the protection of pedunculate oak (Quercus robur L.) acorns in the seed orchard in the Lezajsk Forest District. Leśne Pr. Badaw. 2009, 70, 271. [Google Scholar] [CrossRef] [Green Version]
- Pelisson, P.F.; Bernstein, C.; Francois, D.; Menu, F.; Venner, S. Dispersal and dormancy strategies among insect species competing for a pulsed resource. Ecol. Entomol. 2013, 38, 470–477. [Google Scholar] [CrossRef]
- Keesey, I.W. The Chemical Ecology of the Lesser Chestnut Weevil: Behavioral and Electrophysiological Responses of Curculio sayi (Coleoptera: Curculionidae) to Host-Plant Volatile Organic Compounds. Doctoral Dissertation, University of Missouri, Columbia, MO, USA, 2011. [Google Scholar]
- Forrester, G.J. The Population Ecology of Acorn Weevils and Their Influence on Natural Regeneration of Oak. Doctoral Dissertation, University of London, London, UK, 1990. [Google Scholar]
- Doležal, P.; Kleinová, L.; Davídková, M. Adult Feeding Preference and Fecundity in the Large Pine Weevil, Hylobius abietis (Coleoptera: Curculionidae). Insects 2021, 12, 473. [Google Scholar] [CrossRef]
- Easterbrook, M.A.; Fitzgerald, J.D.; Pinch, C.; Tooley, J.; Xu, X.M. Development times and fecundity of three important arthropod pests of strawberry in the United Kingdom. Ann. App. Biol. 2003, 143, 325–331. [Google Scholar] [CrossRef]
- Faleiro, J.; Rangnekar, P.A.; Satarkar, V.R. Age and fecundity of female red palm weevils Rhynchophorus ferrugineus (Olivier) (Coleoptera: Rhynchophoridae) captured by pheromone traps in coconut plantations of India. Crop Prot. 2003, 22, 999–1002. [Google Scholar] [CrossRef]
- Addesso, K.M.; McAuslane, H.J.; Stansly, P.A.; Schuster, D.J. Host-marking by female pepper weevils, Anthonomus eugenii. Entomol. Exp. Appl. 2007, 125, 269–276. [Google Scholar] [CrossRef]
- Addesso, K.M.; Alborn, H.T.; Bruton, R.R.; McAuslane, H.J. A multicomponent marking pheromone produced by the pepper weevil, Anthonomus eugeni (Coleoptera: Curculionidae). Chemoecology 2021, 31, 247–258. [Google Scholar] [CrossRef]
- Campbell, J.F. Influence of seed size on exploitation by the rice weevil, Sitophilus oryzae. J. Insect Behav. 2002, 15, 429–445. [Google Scholar] [CrossRef]
- Bonal, R.; Muñoz, A. Seed growth suppression constrains the growth of seed parasites: Premature acorn abscission reduces Curculio elephas larval size. Ecol. Entomol. 2008, 33, 31–36. [Google Scholar] [CrossRef]

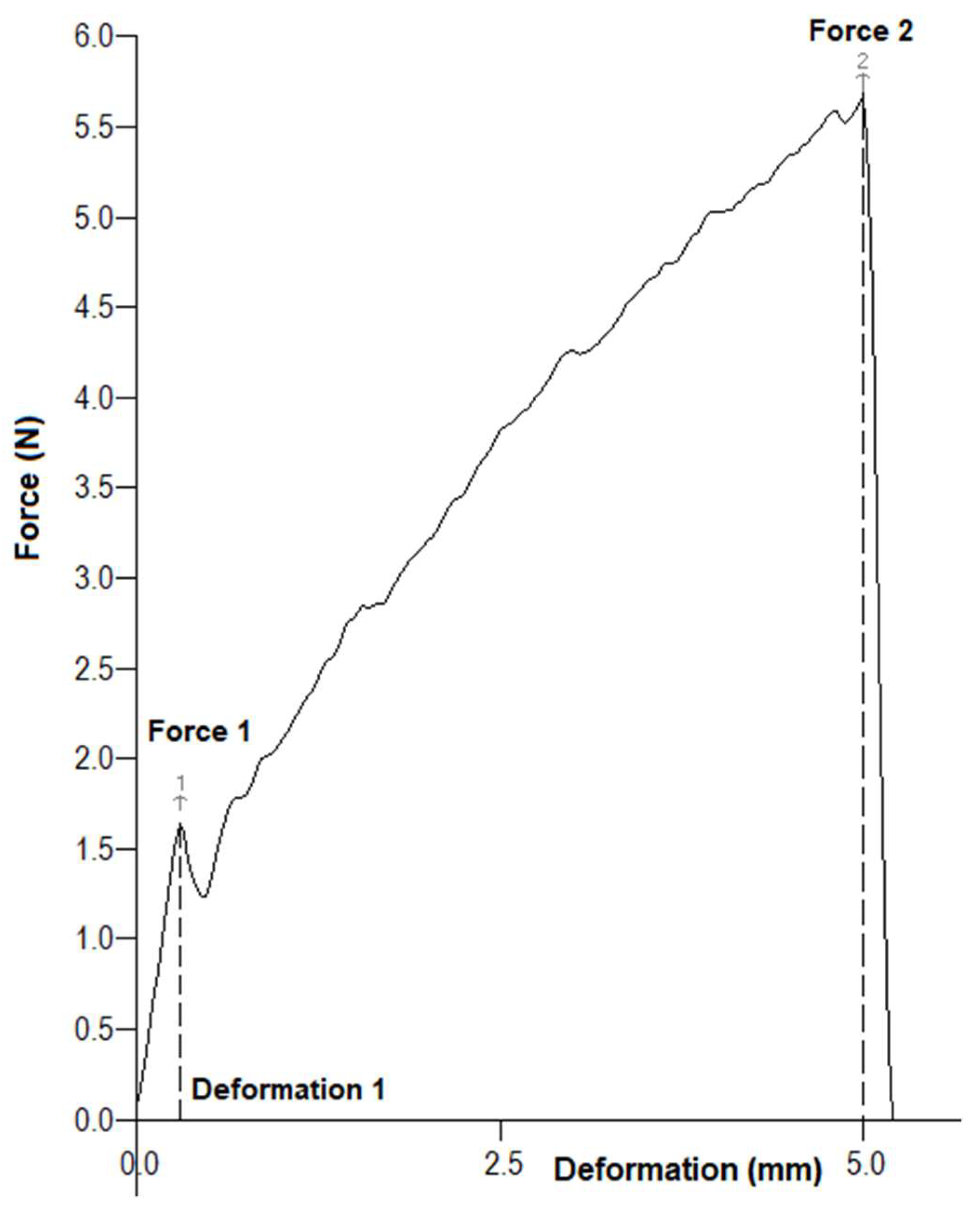



| Parameter | Tree 1 | Tree 2 | Tree 3 |
|---|---|---|---|
| Force 1 [N] | 2.65 ± 0.17 a | 1.87 ± 0.21 b | 3.15 ± 0.22 c |
| Force 2 [N] | 8.44 ± 0.78 a | 6.41 ± 0.45 b | 7.89 ± 1.19 ab |
| Deformation 1 [mm] | 0.335 ± 0.036 a | 0.329 ± 0.036 a | 0.335 ± 0.036 a |
| Mass [g] | 9.02 ± 0.65 a | 3.00 ± 0.88 b | 4.46 ± 0.44 c |
| Behavior | Description |
|---|---|
| Climbing on acorn | Climbs on acorn with antennas close to its surface |
| Chewing through outer skin | Breaks through epidermis with mandibles |
| Shallow drilling | Egg channel creation start; female does not insert whole rostrum yet and moves frequently around acorn |
| Deep drilling | Drilling continues; almost entire snout is inside hole and female’s movements stop |
| Hole localization | Female turns around and tries to find hole with ovipositor |
| Egg deposition | Placing egg inside drilled channel |
| Successful deposition | Egg placed inside acorn |
| Unsuccessful deposition | Female not able to place egg correctly |
| Egg loss | Egg placed on acorn surface and then consumed |
| Walking | Wanders around without particular purpose |
| Leaving | Female abandons acorn |
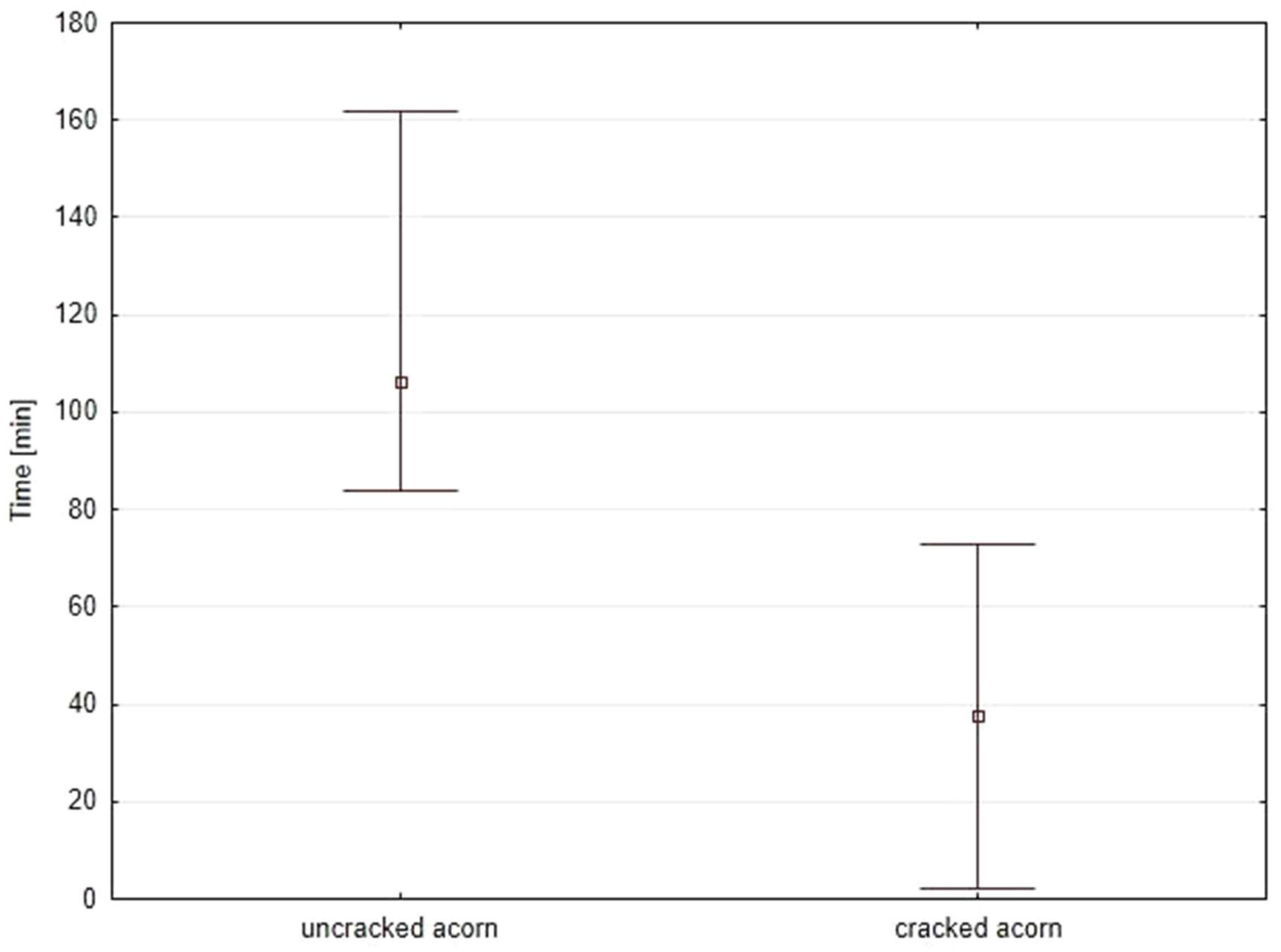
| Event | uncracked acorns (n = 30) min–max: 84–197 mean: 107 SD: 99 | cracked acorns (n = 30) min–max: 2–88 mean: 41 * SD: 62 |
| creating an egg channel | ||
| searching for the hole with an ovipositor | uncracked and cracked acorns (n = 60) min–max: 0.05–11 mean: 0.47 SD: 1.97 | |
| ovipositor insertion and the first egg deposition | uncracked and cracked acorns (n = 60) min–max: 0.45–3.33 mean: 0.82 SD: 0.5 | |
| interval between the first and the second egg deposition | uncracked and cracked acorns (n = 60) min–max: 21–167 mean: 62 SD: 78 | |
| interval between the second and the third egg deposition | uncracked and cracked acorns (n = 60) min–max: 28–145 mean: 68 SD: 72 | |
| interval between the third and the fourth egg deposition | uncracked and cracked acorns (n = 60) min–max: 29–205 mean: 132 SD: 65 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reut, M.; Jakubczyk, E.; Chrabąszcz, M.; Moniuszko, H. Hard Nut to Crack. Acorn Hardness Implications on Oviposition of the Acorn Weevil Curculio glandium Marsham, 1802 (Coleoptera: Curculionidae). Diversity 2022, 14, 922. https://doi.org/10.3390/d14110922
Reut M, Jakubczyk E, Chrabąszcz M, Moniuszko H. Hard Nut to Crack. Acorn Hardness Implications on Oviposition of the Acorn Weevil Curculio glandium Marsham, 1802 (Coleoptera: Curculionidae). Diversity. 2022; 14(11):922. https://doi.org/10.3390/d14110922
Chicago/Turabian StyleReut, Michał, Ewa Jakubczyk, Mariusz Chrabąszcz, and Hanna Moniuszko. 2022. "Hard Nut to Crack. Acorn Hardness Implications on Oviposition of the Acorn Weevil Curculio glandium Marsham, 1802 (Coleoptera: Curculionidae)" Diversity 14, no. 11: 922. https://doi.org/10.3390/d14110922
APA StyleReut, M., Jakubczyk, E., Chrabąszcz, M., & Moniuszko, H. (2022). Hard Nut to Crack. Acorn Hardness Implications on Oviposition of the Acorn Weevil Curculio glandium Marsham, 1802 (Coleoptera: Curculionidae). Diversity, 14(11), 922. https://doi.org/10.3390/d14110922






