Abstract
Many forested freshwater wetlands have been altered or destroyed, and wetlands are constructed to offset loss. However, they do not always replace the function of natural wetlands. It is important to understand how features of the habitat differ between types of wetlands and whether constructed wetlands provide an adequate habitat for species adapted to natural wetlands. Our objectives were to measure the characteristics of Four-toed Salamanders’ nesting habitat and determine which factors contribute to the abundance of eggs and nests in natural and constructed upland-embedded wetlands within a ridgetop ecosystem in eastern Kentucky. We located and examined characteristics for 207 nests in twelve wetlands and measured variables at the nest level and at the wetland level. The best predictor of the number of eggs and number of nests was amount of moss at the wetland. These measures of reproductive effort were similar between types of wetlands, but the number of eggs per nest was higher in constructed wetlands and inversely related to amount of moss, highlighting a deficit in nesting habitat. Research of embryonic and larval survival is needed but based on data from other amphibian species in this system, we predict that the survival of Four-toed Salamanders’ larvae is low in constructed wetlands with permanent hydrology. Restoration of constructed wetlands should address the need for moss as nesting substrate and drying of the wetland to reduce the abundance and diversity of predators of larvae.
1. Introduction
Concern about the effects of loss of wetlands on populations of amphibians dates back 100 years [1]. In the United States, forested freshwater wetlands lost more area than any other type of wetland from 1974 to 2009 [2,3]. Consequently, wetlands have been constructed as mitigation or to enhance populations of wildlife. However, constructed wetlands do not replicate natural systems, and floral and faunal assemblages in constructed wetlands often differ from those in natural wetlands and fall short of conservation goals [4].
Construction of wetlands as mitigation tools for conservation of amphibians has had mixed results. This occurs for several reasons. For example, the structure of amphibian assemblages typically differs among sites because of disparities in hydrology, habitat connectivity, floral assemblages, and the ability of species to exploit new wetlands [4,5,6,7]. Some species, particularly generalists, may benefit, whereas rare species or habitat-specialists may have difficulty colonizing. Furthermore, colonization of ponds does not imply reproductive success. Some species using wetlands do not reproduce, their life history traits (e.g., larval duration and size at metamorphosis) may be altered leading to unsuccessful or reduced recruitment, and predation upon embryos and larvae by invertebrates or other amphibian species may be enhanced [8,9,10,11].
Four-toed Salamanders (Hemidactylium scutatum) have specific requirements for nesting and embryonic development [12,13,14]. Females may nest communally, depositing their clutch in the same nest as others [15,16,17]. Females typically select nesting sites on slopes facing north to northeast and with steeper banks; these factors are correlated with increased embryonic survival and may facilitate the entry of larvae into the water [18,19]. Mosses (e.g., Sphagnum and Thiuidium spp.) are important for nesting, presumably because of moisture required at nesting sites and the loose, deep structure they provide [18,19]. Identifying favorable nesting site characteristics, especially in constructed wetlands, is important because sites used for oviposition can affect reproductive fitness in amphibians by influencing both embryonic and larval success [20]. Additionally, because wetlands are constructed to offset the loss of habitat, it is critical to understand how characteristics of the terrestrial and aquatic habitat differ between natural and constructed wetlands and how this impacts the distribution and abundance of species. Therefore, our objectives were to describe characteristics of Four-toed Salamanders’ nests and determine which environmental factors contribute to the abundance of eggs and nests in natural and constructed upland-embedded wetlands within a ridgetop ecosystem in eastern Kentucky. We hypothesized that we would find differences between natural and constructed wetlands in the type of nesting habitat used by Four-toed Salamanders in this system as well as in reproductive effort.
2. Materials and Methods
In the Cumberland Ranger District of the Daniel Boone National Forest (DBNF), Kentucky, more than 700 wetlands have been constructed on ridgetops for the management of wildlife and enhancement of habitat [11,21]. The area encompassing these wetlands consists of the unglaciated portions of the Western Allegheny Plateau Ecoregion and is dominated by mixed mesophytic forest [22]. We identified 18 prospective study sites (nine natural and nine constructed wetlands) based on the presence of Four-toed Salamanders from previous research of wetlands that were systematically selected based on pairing of natural and constructed wetlands on the landscape [6,7]. We excluded sites if no nests were found during this study or if humans recently altered the site. This resulted in six natural and six constructed wetlands, all of which were fishless and located on forested ridgetops and had either ephemeral hydrology (all the natural wetlands) or permanent hydrology (all the constructed wetlands) (Figure 1).

Figure 1.
Natural wetlands (left) showing trees in the interior surrounded by thick clumps of moss. Constructed wetlands (right) showing no trees in the interior, some downed woody debris, and little (if any) mossy cover. The natural wetlands dried during the summer (e.g., bottom left) while the constructed wetlands had a permanent hydroperiod.
We measured the length and width of each wetland to calculate area and measured the perimeter of the wetland to the nearest 0.1 m. We determined the total amount of moss in each wetland by summing the lengths that clumps of moss intersected the water’s edge around the perimeter of the wetland and in the interior of the wetland (e.g., clumps surrounding trees in the wetland’s interior). After full leaf out, beginning 15 May 2011, we determined the closure of the canopy at each study site using a spherical densiometer; we measured this in the center of each wetland and at the perimeter of the wetland in the cardinal and ordinal directions, and averaged the measurements [6,23,24]. Additionally, we scored wetlands using the Kentucky Wetland Rapid Assessment Method (KY-WRAM), which is an assessment of the condition of the wetland that ranges from 0 to 100 from lowest to highest condition [25].
We searched all moss, herbaceous plants, leaf litter, and downed woody debris along the water’s edge to 1 m past the highwater line of each wetland for Four-toed Salamanders’ nests at approximately every two weeks during the nesting period, beginning 1 March 2011 and continuing until no viable embryos remained (12 June 2011). During mid-May to late May 2011, after egg laying had ended, the region experienced heavy and prolonged rainfall and 83% of the nests were inundated, with most eggs being washed into the wetland. Accordingly, thereafter, we limited our focus to nesting ecology because we were unable to determine success of hatching.
When a nest was found, we counted the number of eggs and number of females per nest. To discern between females ovipositing and leaving a nest from those attending the nest, we used the maximum number of females found at a nest after egg laying had ceased at that nest as the number of females attending a nest. To determine the percent of ground cover by type of plant (moss, grass, sedge, and herbaceous plants) and nonliving material (decaying wood, leaflitter, pine needles, soil, gravel, and dead vegetation), we centered a small plot (10 cm2) on the nesting site and made a visual estimate of cover by type. We also identified mosses (to genus). We measured aspect (the direction that the slope faced toward the water from a nest) with a compass to the nearest degree. We measured the slope from the nest to the water line (using a clinometer, accuracy to 1°), with 0° being flat and 90° being vertical. We also measured the distance from the center of the nest to the water to the nearest 0.5 cm. One extreme outlier for distance was removed from the dataset after box plot analysis revealed its value was much greater than all the other ones (248 cm compared to a maximum of 26.5 cm for the remaining distances). We measured the moisture of the soil at each nest as volumetric water content in standard mode using a Fieldscout TDR 100 Soil Moisture Meter (Spectrum Technologies, Inc.); this value is the ratio of water volume to total soil volume. We used measurements of moisture from weeks 6 and 7 (14–28 April 2011) in the analyses because some earlier measurements were missed due to difficulty with the equipment. We measured the pH of the soil (Kelway soil acidity and moisture tester, model HB-2; Kel Instruments Company, Inc., Wyckoff, NJ, USA) to the nearest 0.1 when each nest was initially found. When no soil was present at a nest, we measured pH and moisture of the soil in the nearest soil within 1 m in any direction; if there was no soil within that distance, we did not take a measurement.
The following statistical tests were performed using SPSS (v 24, IBM Corp, Armonk, NY, USA). Because the data were not distributed normally and the sample size was small, we used Mann–Whitney U to determine whether the site-level variables measured in each wetland differed between types of wetlands (natural or constructed): perimeter, area, total moss in the wetland, canopy-closure, and KY-WRAM score. We used Chi-square contingency table analyses to determine whether the following variables were associated with types of wetlands: number of nests with females present, the number of females attending a nest, the amount of moss as nesting substrate (% cover), and the number of wetlands with a uniform distribution of the aspect of the nests. When results were significant, we performed post hoc analysis using Bonferroni correction. We used nested ANOVAs, with individual wetlands nested within types of wetlands, to determine whether the following nesting characteristics varied between types: slope, distance to water, moisture of soil, and pH of soil (each log-transformed). We performed Rayleigh’s Test using Program Oriana (v 3.21, Kovach Computing Services, Anglesey, UK) to determine whether the distribution of the aspect of the nests were uniform [26].
We performed multiple linear regression with a model selection approach using a second-order Akaike’s Information Criterion (AICc) in R (v 3.3.2, R Core Team, Vienna, Austria; ‘AICcmodavg’ package; see Mazerolle [27]) to determine which factors best predicted the number of eggs per wetland, the number of nests per wetland, and the number of eggs per nest. We used Spearman correlations to determine multicollinearity between all pairs of explanatory variables and excluded variables from statistical models when r > 0.70. KY-WRAM was correlated with total moss amount with an r > 0.70, so KY-WRAM was excluded from models. Because our response variables were counts, we determined the appropriate distribution for modeling each variable by comparing log-likelihood ratios of the global model with a Poisson distribution, negative binomial distribution, and a normal distribution with log-transformed data. For both the number of eggs per wetland and the number of nests per wetland, a normal distribution with log-transformed data fit best. For the number of eggs per nest, a negative binomial distribution fit best, and because this response variable was nested within type of wetland, we used a generalized linear mixed model approach in package ‘lme4′ [28], in which the site was included as a random explanatory variable.
We tested all combinations of explanatory variables and an intercept-only model for the number of eggs per wetland and number of nests per wetland, including type of wetland (natural or constructed), canopy-closure, and total amount of moss in the wetland. The model set for the number of eggs per nest included the type of wetland, total amount of moss, canopy-closure, and the following measurements at the nest: number of females present, substrate used for nesting, slope to water, distance to water, moisture of the soil, and pH of the soil as explanatory variables. Our candidate models for eggs per nest included the global model, a model for each single variable, models for all pairs of variables, and an intercept-only model. For all three model sets of response variables, we used model averaging to conduct multi-model inference [29]. Variables whose model-averaged estimate and 85% confidence interval did not overlap zero were interpreted as statistically significant; we used this value because Arnold [30] argued that this procedure promotes compatibility between the information-theoretic approach and statistical inference.
3. Results
3.1. Analyses at the Wetland Level
We located and examined characteristics of 207 nests in 12 wetlands: 133 nests in natural wetlands and 74 nests in constructed wetlands. The number of nests and eggs was similar between types of wetlands. The perimeter, area, and total amount of moss in a wetland was also similar between natural and constructed wetlands. However, canopy-closure and KY-WRAM scores were higher in natural wetlands than in constructed wetlands (Table 1).

Table 1.
Comparison of wetland-level factors, using a Mann–Whitney U, and nest-level factors, using ANOVA, measured for nests of Four-toed Salamanders (Hemidactylium scutatum) in natural and constructed ridgetop wetlands in the Daniel Boone National Forest, Kentucky. Factors are presented as mean ± SE; bold values are statistically significant at an α = 0.05.
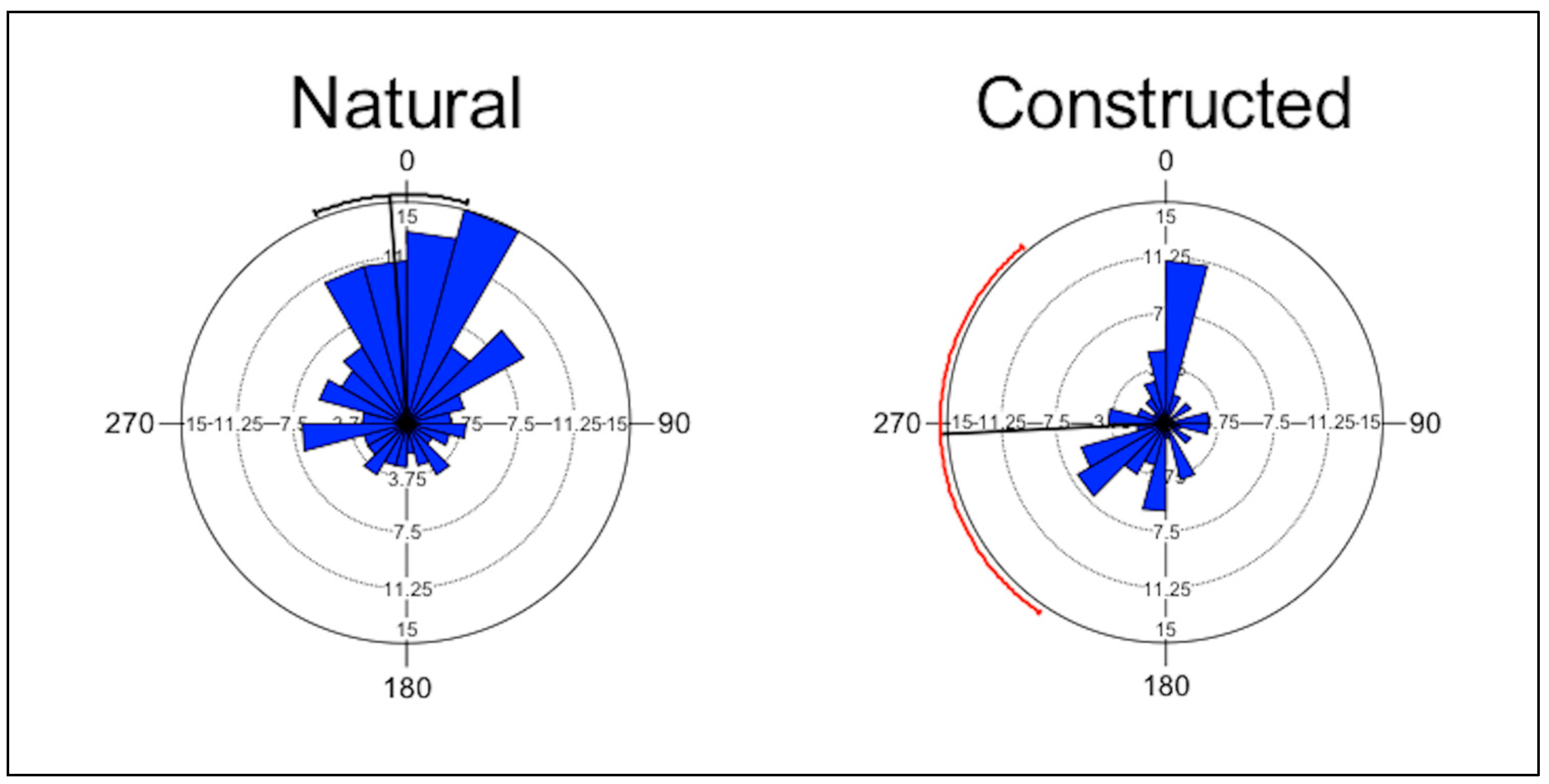
In natural wetlands, many nests faced north, but this pattern was less apparent in constructed wetlands (Figure 2). The results from Rayleigh’s test showed that the aspect of the nests was not uniformly distributed at three natural wetlands and two constructed wetlands (Table 2). There was no difference between types of wetlands in the uniformity of the distribution of the aspect of the nest (Χ2 = 0.3, p = 0.56).
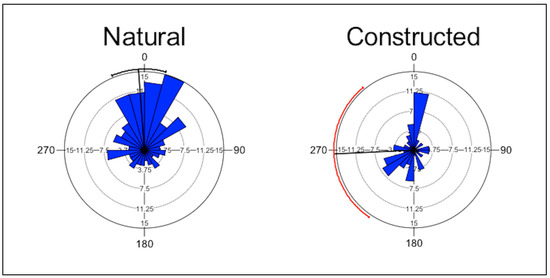
Figure 2.
Rose diagram showing the aspect of the nests of Four-toed Salamanders (Hemidactylium scutatum) in natural and constructed upland-embedded wetlands in the Daniel Boone National Forest, Kentucky. Numbers around the perimeter represent compass direction; bold lines represent mean vector and circular standard deviation.

Table 2.
Comparison of the uniformity of the distribution of the aspect of the nests of Four-toed Salamanders (Hemidactylium scutatum) in natural and constructed ridgetop wetlands of the Daniel Boone National Forest, Kentucky. Bold values are statistically significant at an α = 0.05.
The top three models for analyses of the number of eggs per wetland had a total weight of 0.86, and all included the total amount of moss at the wetland (Table 3). The total amount of moss and the extent of canopy-closure both were positively related to, and significantly contributed to, explaining the number of eggs per wetland (Table 4). The top two models for analyses of the number of nests per wetland had a total weight of 0.97, and both included the total amount of moss at the wetland (Table 3). Only the total amount of moss significantly contributed to the model and had a positive association; therefore, there was no significant difference between constructed and natural wetlands in the number of nests or the number of eggs (Table 4).

Table 3.
Top models with weight > 0 for number of eggs per wetland, number of nests per wetland, and number of eggs per nest of Four-toed Salamanders (Hemidactylium scutatum) in natural and constructed ridgetop wetlands of the Daniel Boone National Forest, Kentucky.

Table 4.
Model-averaged parameters across models for total number of eggs, total number of nests, and eggs per nest of Four-toed Salamanders (Hemidactylium scutatum) in natural and constructed ridgetop wetlands of the Daniel Boone National Forest, Kentucky. Bold values are statistically significant at an α = 0.05.
3.2. Analyses at the Nest Level
There were significantly more eggs per nest in constructed wetlands (Mean ± SE = 61.3 ± 7.0) than in natural wetlands (Mean ± SE = 36.47 ± 3.0) (Table 4; see statistics below). The number of nests with a female present was 39 of 133 nests (29.3% ± 0.04) in natural wetlands and 17 of 74 nests (23.0% ± 0.05) in constructed wetlands, and this did not differ between types of wetlands (Χ2 = 1.0, p = 0.32). The number of females present per nest ranged from 0 to 4 and did not differ between natural (0.71 ± 0.07) and constructed (0.70 ± 0.10) wetlands (Χ2 = 4.8, p = 0.31). Moss was the substrate used most for nesting: 78% of nests were found in areas with 100% moss and 82% were found in areas of >50% moss. There was a significant difference in use of moss as nesting substrate among natural and constructed wetlands (Χ2 = 65.1, p < 0.001). Post hoc analysis with Bonferroni correction indicated that there were more nests found with no mossy substrate in constructed wetlands (n = 30 of 74; 41%) than in natural wetlands (n = 2 of 133; 0.02%) and that there were more nests found in 100% moss substrate in natural wetlands (n = 122 of 133; 92%) than in constructed wetlands (n = 39 of 74; 53%) (Χ2 = 65.1, p < 0.001). In fact, one constructed wetland did not have any moss, with nesting occurring in leaf litter; during heavy rain, however, in this case, many eggs and much of the substrate washed into the wetland (Figure 3). We also observed that some moss (Thuidium spp.) in constructed wetlands was very thin and dried earlier than did thicker mats of moss in natural wetlands (Sphagnum spp. and Thuidium spp.) (Figure 4). Other moss (Leucobryum spp.) was very dense, which made laying eggs within the moss difficult. The slopes, soil moisture, and distance to the water where nests were found were similar between types of wetland, but the pH was lower at nests in natural wetlands (Table 1).

Figure 3.
In constructed wetlands that had no mossy substrate, Four-toed Salamanders (Hemidactylium scutatum) used leaf litter or other substrates for nesting (left). A nest that had been in leafy substrate washed down into the wetland during heavy rainfall, eggs are visible at the edge of the water and soil is exposed where leafy substrate is missing (right).

Figure 4.
Four-toed Salamanders’ (Hemidactylium scutatum) nests in a thick, moist bed of moss in a natural wetland (left) and in a thin, dry bed of moss in a constructed wetland (right).
The top model for analyses of the number of eggs per nest had a total weight of 0.99 and was the global model with all explanatory variables (Table 3). The number of eggs per nest was positively and significantly associated with moisture, presence of females, and canopy-closure and negatively and significantly associated with distance to water and total extent of moss. Natural wetlands had significantly fewer eggs per nest than did constructed wetlands (Table 4).
4. Discussion
We found no difference in the number of Four-toed Salamanders’ nests or eggs at constructed versus natural wetlands; however, due to the low sample size and large variation within types of wetlands, we believe the differences in the mean number of eggs and nests do reflect differences that exist between natural wetlands and many of the constructed wetlands. These measures of reproductive effort were best predicted by the total amount of moss at a wetland and greater closure of the canopy. In natural wetlands, the use of 100% moss as substrate for nesting was greater than in constructed wetlands, which corresponds to other studies that found the presence of high-quality moss to be an indicator of Four-toed Salamander presence on the scale of the landscape [18,31].
Our hypothesis that there would be differences between types of wetlands in Four-toed Salamander’s nesting habitat was supported; primarily, there was a greater use of moss as nesting substrate in natural wetlands. The importance of moss as nesting substrate has been shown previously [18,19,31]. There was greater closure of the canopy in natural wetlands. Previous researchers reported that constructed wetlands with permanent hydroperiods in open-canopy areas did not contain Four-toed Salamanders, but they were found in nearby natural wetlands [32]. Canopy-closure was not a predictor for the number of nests in a wetland, but it was important, along with the total amount of moss, in predicting the number of eggs. This is because the number of eggs per nest differed between types of wetlands; there were more eggs per nest in constructed wetlands because there was less moss used for nesting, possibly because closure of the canopy was lower, and pH was higher in constructed wetlands. This ‘deficit’ in moss is further highlighted by our findings that in constructed wetlands, there was increased use of other substrates for nesting. This underscores how constructed wetlands are lacking in thick beds of moss (particularly Sphagnum spp.).
Furthermore, the number of females present at a nest did not differ between types of wetlands, but the number of eggs per nest did, indicating that more females were ‘egg dumpers’ in communal nests at constructed wetlands than at nests in natural wetlands. We observed few instances of communal nesting at the highest quality natural wetlands with plenty of thick moss. Breitenbach [33] concluded communal nesting would occur less frequently in wetlands with more suitable nesting sites. Communal nesting likely occurred more often in constructed wetlands because of the lack of suitable nesting sites comprised of thick beds of moss.
When wetlands were constructed, the canopy was removed in the process [34]. Canopy and cover by low vegetation are important for the retention of moisture and growth of moss [35,36,37]. Understory vegetative communities differ between wetland-types in this system, with more shade-tolerant species found in the understory of natural wetlands [38]. Most nests faced north in natural wetlands and in wetlands where the aspect of the nest was not uniformly distributed. It is possible that closure of the canopy, low cover, aspect, and pH are more important for the growth of moss than as a cue for nesting by Four-toed Salamanders. We hypothesize that closure of the canopy may occur much more rapidly at constructed wetlands than moss can colonize and populate them, so the extent of moss is limited in these wetlands, at least initially. With favorable nesting sites limited, communal nesting increases and eggs are oviposited at fewer sites, thus significantly increasing the number of eggs per nest, with unknown consequences for embryonic survival at the population level. Perhaps as constructed wetlands age, the canopy closes, and more downed woody debris accumulates, more moss will grow in thicker clumps, although it is unclear what impact the difference in pH might have on the growth of moss. Consequently, north-facing shorelines should become increasingly different from those facing south. Nests on the moist, north-facing shorelines should be at a selective advantage over those on the drier, south-facing shorelines [19].
Natural wetlands had significantly higher KY-WRAM scores than did constructed wetlands, and these scores were positively correlated with the total amount of moss in a wetland. We used abundance of moss in our statistical analyses because it is more directly important for Four-toed Salamanders’ nesting. The amount of moss in the wetland was associated with number of eggs per wetland, number of nests per wetland, and number of eggs per nest, suggesting that KY-WRAM can be an indicator of wetland condition and might be a proxy for indicating suitable nesting habitat for Four-toed Salamanders in ridgetop systems. Rapid assessment methods have been suggested as useful in discerning habitat suitability for other amphibians in this system [6]. However, it is important to note that KY-WRAM scores can be high in wetlands with no moss or Four-toed Salamanders and that these salamanders can inhabit other types of wetlands that may not score high on the KY-WRAM.
Implications for management: Our findings, taken in consideration with the findings from other research conducted in this system, indicate there are differences in the ecological functioning of constructed wetlands when compared to the natural, ephemeral wetlands [6,7,11,38]. For example, we found there was more moss used for nesting, which is very important for a habitat specialist such as the Four-toed Salamander, and greater closure of the canopy in natural wetlands than in constructed wetlands. Closure of the canopy may not only lead to increased growth of moss, but a closed canopy can impact the hydroperiod of the wetland by increasing evapotranspiration [39]. Constructed, permanent wetlands have greater abundance and diversity of predators of larval amphibians that may impact survivorship and population dynamics of amphibians than do natural wetlands or those with variable hydroperiods [6,11,32]. It is possible that Four-toed Salamanders choose to nest in constructed wetlands, but that these wetlands serve as sinks, unable to support populations, as was found for wood frogs (Lithobates sylvaticus) in this system [11]. Unless constructed wetlands provide an optimal habitat for nesting by Four-toed Salamanders and development of their larvae, these wetlands may not mitigate the loss of critical natural habitat.
We believe our results can be applied to similar wetland complexes throughout the Appalachians and other areas of the Eastern United States where similar floral and faunal assemblages are found. However, interpretation of our data, especially for management, needs to consider that constructed wetlands in our study were selected based on previous research that documented the presence of Four-toed Salamanders at each site [6,7]. Otherwise, the amphibian and plant communities of these wetlands were representative of other wetlands in the Cumberland Ranger District based on the research of Fedders [38] who randomly selected study sites across the entire district. It is important to note that most constructed wetlands did not support populations of Four-toed Salamanders: larvae were found in only 7 of 38 constructed wetlands [38]. Therefore, our study potentially overestimates the suitability of constructed wetlands for this species. Restrictive requirements for habitat in addition to the low numbers detected in wetlands across this system mean that suitable habitat for Four-toed Salamanders may be limited. Monitoring of populations, particularly in wetlands where the conditions of the habitat are less than ideal (i.e., permanent hydrology or lack of Sphagnum moss), is recommended.
Long-term monitoring is essential for detecting changes in populations of Four-toed Salamanders at constructed wetlands [4,40]. We urge strong caution be taken in using the presence of nests as an indicator of population status; we were unable to assess embryonic and larval survivorship, which is required to determine this. In the absence of these data, we recommend using nest surveys over long periods of time to monitor populations and determine the impact of constructed wetlands on Four-toed Salamanders’ demography and population viability. Ultimately, data on the survival of offspring are required to determine whether populations are self-sustaining, and whether a nearby natural wetland constitutes a source for recruitment of adults.
Constructed wetlands may be a valuable tool for replacing lost wetlands. However, current construction techniques for upland-embedded wetlands often fail to replicate the natural, favorable conditions for many amphibian species [4,6]. Hopefully, the techniques used when constructing wetlands can address these differences. For example, maintaining trees in the interior of the wetland during construction would address deficits with the closure of the canopy and may alter the hydroperiod to exclude predators. Creating wetlands of irregular shape, with a wandering or meandering edge, would more closely approximate natural wetlands and may provide better nesting for Four-toed Salamanders by increasing the amount of north-facing shoreline. Maintaining downed woody debris and trees in the interior of a wetland also promotes its drying, something that other research has indicated to be important in this system [6,7]. Adding hummocks by uplifting clumps of soil could increase microhabitat complexity and promote the colonization and growth of moss [4,12,33]. Transplanting or ‘head-starting’ moss to assist in colonization may also be beneficial. The United States Forest Service recently began constructing wetlands in DBNF with more irregularity of shorelines, more trees in the interior, and more downed woody debris which, if covered with moss, will provide steep slopes and suitable sites for nesting by Four-toed Salamanders.
Author Contributions
Conceptualization, S.K.K. and S.C.R.; methodology, S.K.K. and S.C.R.; formal analysis, S.K.K. and S.C.R.; data curation, S.C.R.; writing—original draft preparation, S.K.K. and S.C.R.; writing—review and editing, S.K.K. and S.C.R.; supervision, S.C.R.; project administration, S.C.R.; funding acquisition, S.K.K. and S.C.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Sigma Xi Grants in Aid of Research (grant ID#: G20110315157175), an American Society of Ichthyologists and Herpetologists’ Gaige Award, a Northern Kentucky Fly Fisher’s Association Red Barrington Scholarship, a Kentucky Society of Natural History Research Grant, and the Department of Biological Sciences at Eastern Kentucky University.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Animal Care and Use Committee of Eastern Kentucky University (protocol number 04-2012, approved 02/22/2012) and the Kentucky Department of Fish and Wildlife Resources (collecting permit SC1111099).
Data Availability Statement
Data are reported in tables in the article and are held and available upon request from EKU.
Acknowledgments
We thank V. Peters for assistance with statistical analyses, D. Brown, K. Dodd, C. Elliott, H. Heatwole, and anonymous reviewers for comments on an earlier version of the manuscript, and A. Drayer and T. Biebighauser for assistance with the design of the study and locating constructed wetlands. We extend special thanks to the U.S. Forest Service, especially Christy Wampler, for continued support in this research system.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Wright, A.H. Notes on the Muhlenberg’s turtle. Copeia 1918, 1918, 5–7. [Google Scholar] [CrossRef]
- Dahl, T.E. Status and Trends of Wetlands in the Conterminous United States 1986 to 1997; U.S. Department of the Interior, Fish and Wildlife Service: Washington, DC, USA, 2000. [Google Scholar]
- Dahl, T.E. Status and Trends of Wetlands in the Conterminous United States 2004 to 2009; U.S. Department of the Interior, Fish and Wildlife Service: Washington, DC, USA, 2011. [Google Scholar]
- Calhoun, A.J.K.; Arrigoni, J.; Brooks, R.P.; Hunter, M.L.; Richter, S.C. Creating successful vernal pools: A literature review and advice for practitioners. Wetlands 2014, 34, 1027–1038. [Google Scholar] [CrossRef]
- Pechmann, J.H.K.; Estes, R.A.; Scott, D.E.; Gibbons, J.W. Amphibian colonization and use of ponds created for trial mitigation of wetland loss. Wetlands 2001, 21, 93–111. [Google Scholar] [CrossRef]
- Denton, R.D.; Richter, S.C. Amphibian communities in natural and constructed ridge top wetlands with implication for wetland construction. J. Wildl. Manag. 2013, 77, 886–896. [Google Scholar] [CrossRef]
- Drayer, A.N.; Richter, S.C. Physical wetland characteristics influence amphibian community composition in constructed wetlands. Ecol. Eng. 2016, 93, 166–174. [Google Scholar] [CrossRef]
- Semlitsch, R.D.; Scott, D.E.; Pechmann, J.H.K. Time and size at metamorphosis related to adult fitness in Ambystoma talpoideum. Ecology 1988, 69, 184–192. [Google Scholar] [CrossRef]
- Rowe, C.L.; Dunson, W.A. Impacts of hydroperiod on growth and survival of larval amphibians in temporary ponds of central Pennsylvania, USA. Oecologia 1995, 102, 397–403. [Google Scholar] [CrossRef]
- Vasconcelos, D.; Calhoun, A.J.K. Monitoring created seasonal pools for functional success: A six-year case study of amphibian responses, Sears Island, Maine, USA. Wetlands 2006, 26, 992–1003. [Google Scholar] [CrossRef]
- Kross, C.S.; Richter, S.C. Species interactions in constructed wetlands result in population sinks for wood frogs (Lithobates sylvaticus) while benefitting eastern newts (Notophthalmus viridescens). Wetlands 2016, 36, 385–393. [Google Scholar] [CrossRef]
- Petranka, J.W. Salamanders of the United States and Canada; Smithsonian Institution Press: Washington, DC, USA, 1998. [Google Scholar]
- Beazley, K.; Cardinal, N. A systematic approach for selecting focal species for conservation in the forests of Nova Scotia and Maine. Environ. Conserv. 2004, 31, 91–101. [Google Scholar] [CrossRef]
- Harris, R.N. Four-toed salamander, Hemidactylium scutatum. In Amphibian Declines: The Conservation Status of United States Species; Lannoo, M.J., Ed.; University of California Press: Berkeley, CA, USA, 2005; pp. 780–781. [Google Scholar]
- Harris, R.N.; Gill, D.E. Communal nesting, brooding behavior, and embryonic survival of the four-toed salamander Hemidactylium scutatum. Herpetologica 1980, 36, 141–144. [Google Scholar]
- Harris, R.N.; Hames, W.W.; Knight, I.T.; Carreño, C.A.; Vess, T.J. Experimental analysis of joint nesting in the salamander Hemidactylium scutatum (Caudata: Plethodontidae), the effects of population density. Anim. Behav. 1995, 50, 1309–1316. [Google Scholar] [CrossRef]
- Harris, R.N. Body condition and order of arrival affect cooperative nesting behaviour in four-toed salamanders, Hemidactylium scutatum. Anim. Behav. 2008, 75, 229–233. [Google Scholar] [CrossRef]
- Chalmers, R.J.; Loftin, C.S. Wetland and microhabitat use by nesting four-toed salamanders in Maine. J. Herpetol. 2006, 40, 478–485. [Google Scholar] [CrossRef]
- Wahl, G.W.; Harris, R.N.; Nelms, T. Nest site selection and embryonic survival in four-toed salamanders, Hemidactylium scutatum (Caudata, Plethodontidae). Herpetologica 2008, 64, 12–19. [Google Scholar] [CrossRef]
- Resetarits, W.J.; Wilbur, H.M. Choice of oviposition site by Hyla chrysoscelis: Role of predators and competitors. Ecology 1989, 70, 220–228. [Google Scholar] [CrossRef]
- Brown, D.R.; Richter, S.C. Meeting the challenges to preserving Kentucky’s biodiversity. Sustain 2012, 25, 22–33. [Google Scholar]
- Woods, A.J.; Omernik, J.M.; Martin, W.H.; Pond, G.J.; Andrews, W.M.; Call, S.M.; Comstock, J.A.; Taylor, D.D. Ecoregions of Kentucky (Color Poster with Map, Descriptive Text, Summary Tables and Photographs); Map Scale 1:1,000,000; Geological Survey: Reston, VA, USA, 2002. [Google Scholar]
- Skelly, D.K.; Freidenburgh, L.K.; Kiesecker, J.M. Forest canopy and the performance of larval amphibians. Ecology 2002, 83, 983–992. [Google Scholar] [CrossRef]
- Schiesari, L. Pond canopy cover: A resource gradient for anuran larvae. Freshw. Biol. 2006, 51, 412–423. [Google Scholar] [CrossRef]
- Guidugli-Cook, M.; Richter, S.C.; Scott, B.J.; Brown, D.R. Field-based assessment of wetland condition, wetland extent, and the National Wetlands Inventory in Kentucky, USA. Wetl. Ecol. Manag. 2017, 25, 517–532. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Prentice Hall: Hoboken, NJ, USA, 2010. [Google Scholar]
- Mazerolle, M.J. AICcmodavg: Model Selection and Multimodel Inference Based on (Q)AIC(c). R Package Version 2.1-0. 2016. Available online: https://cran.r-project.org/package=AICcmodavg (accessed on 11 October 2022).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Arnold, T.W. Uninformative parameters and model selection using Akaike’s Information Criterion. J. Wildl. Manag. 2010, 74, 1175–1178. [Google Scholar] [CrossRef]
- Blanchard, F.N. The life history of the four-toed salamander. Am. Nat. 1923, 57, 262–268. [Google Scholar] [CrossRef]
- Porej, D.; Hetherington, T.E. Designing wetlands for amphibians: The importance of predatory fish and shallow littoral zones in structuring of amphibian communities. Wetl. Ecol. Manag. 2005, 13, 445–455. [Google Scholar] [CrossRef]
- Breitenbach, G.L. The frequency of communal nesting and solitary brooding in the salamander, Hemidactylium scutatum. J. Herpetol. 1982, 16, 341–346. [Google Scholar] [CrossRef]
- Biebighauser, T.R. Wetland Restoration and Construction, A Technical Guide; Upper Susquehanna Coalition: Owego, NY, USA, 2011. [Google Scholar]
- Fenton, N.J.; Frego, K.A. Bryophyte (moss and liverwort) conservation under remnant canopy in managed forests. Biol. Conserv. 2005, 122, 41–7430. [Google Scholar] [CrossRef]
- Botting, R.S.; Fredeen, A.L. Contrasting terrestrial lichen, liverwort, and moss diversity between old-growth and young second-growth forest on two soil textures in central British Columbia. Botany 2006, 84, 120–132. [Google Scholar] [CrossRef]
- Stewart, K.J.; Mallik, A.U. Bryophyte responses to microclimatic edge effects across riparian buffers. Ecol. Appl. 2006, 16, 1474–1486. [Google Scholar] [CrossRef]
- Fedders, R.B. Amphibian and Plant Communities of Natural and Constructed Upland-Embedded Wetlands in the Daniel Boone National Forest. Master’s Thesis, Eastern Kentucky University, Richmond, KY, USA, 2018. [Google Scholar]
- Lott, R.B.; Hunt, R.J. Estimating evapotranspiration in natural and constructed wetlands. Wetlands 2001, 21, 614–628. [Google Scholar] [CrossRef]
- Corser, J.D.; Dodd, C.K., Jr. Fluctuations in a metapopulation of nesting four-toed salamanders, Hemidactylium scutatum, in the Great Smoky Mountains National Park, USA, 1999–2003. Nat. Areas J. 2004, 24, 135–140. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).