Diversity and Resilience of Seed-Removing Ant Species in Longleaf Sandhill to Frequent Fire
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design
Prescribed Fire
2.3. Specimen Sampling
2.3.1. Ant Identification and Characterization
2.3.2. Seed Trials
2.3.3. Tuna–Honey Bait Trials
2.3.4. Leaf Litter Sampling
2.3.5. Corroborative Samples
2.4. Statistical Analyses
3. Results
3.1. Seed-Removing Species
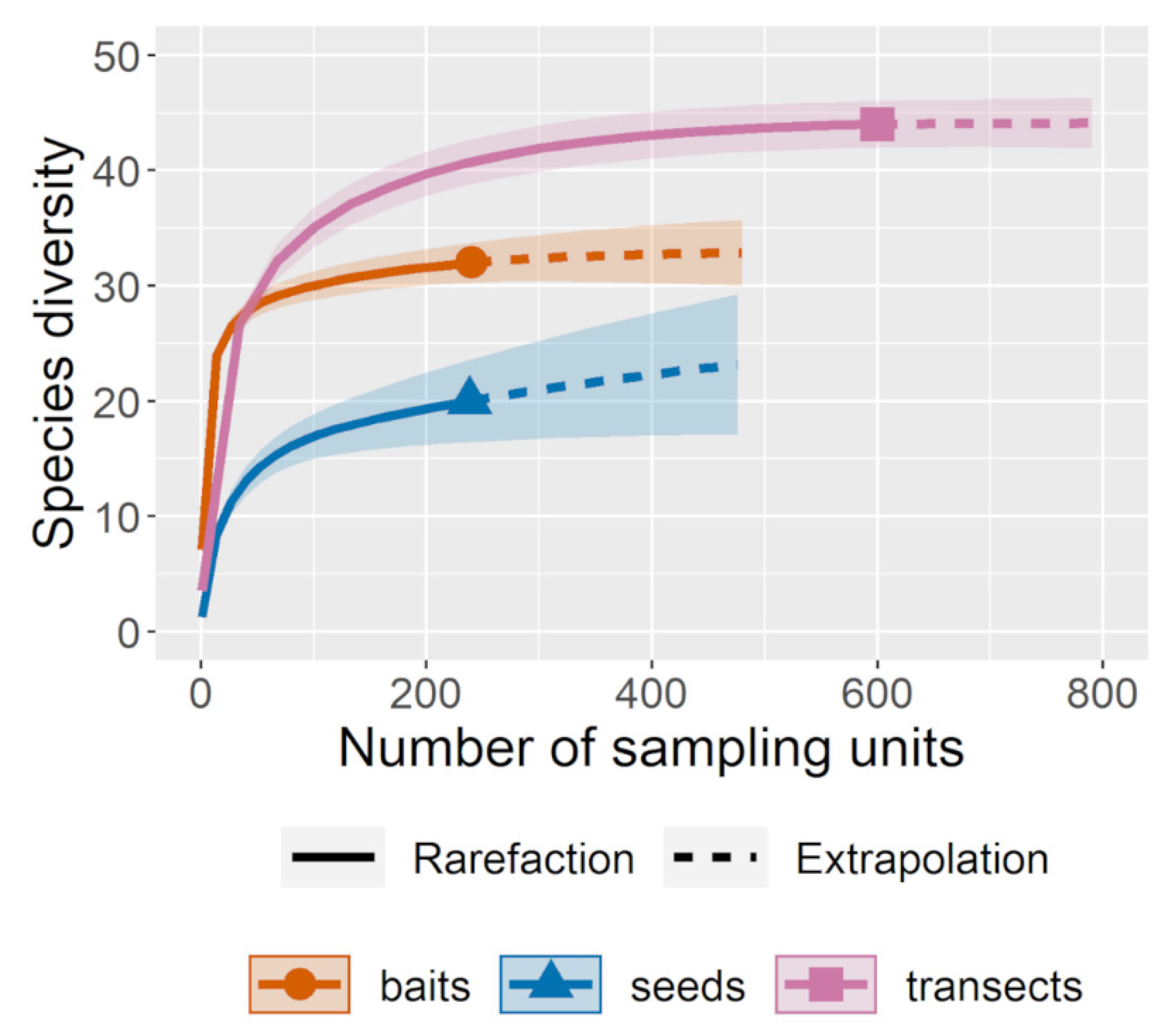
3.2. Community Resiliency to Fire
3.3. Seed Removal Activity
3.3.1. Ant Richness
3.3.2. Proportion of Seeds Removed
4. Discussion
4.1. Seed-Removing Species
4.2. Community Resilience to Fire
4.3. Seed Removal Activity
4.4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ryan, K.C.; Knapp, E.E.; Varner, J.M. Prescribed fire in North American forests and woodlands: History, current practice, and challenges. Front. Ecol. Environ. 2013, 11, e15–e24. [Google Scholar] [CrossRef]
- Freeman, J.; Kobziar, L.; Rose, E.W.; Cropper, W. A critique of the historical-fire-regime concept in conservation. Conserv. Biol. 2017, 31, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.R.; BassiriRad, H. Diminishing effects of ant mounds on soil heterogenity across a chronodequence of prairie restoration sites. Pedobiologia 2005, 49, 359–366. [Google Scholar] [CrossRef]
- De Almeida, T.; Blight, O.; Mesléard, F.; Bulot, A.; Provost, E.; Dutoit, T. Harvester ants as ecological engineers for Mediterranean grassland restoration: Impacts on soil and vegetation. Biol. Conserv. 2020, 245, 108547. [Google Scholar] [CrossRef]
- Del Toro, I.; Ribbons, R.R.; Pelini, S.L. The little things that run the world revisited: A review of ant-mediated ecosystem services and disservices (Hymenoptera: Formicidae). Myrmecol. News 2012, 17, 133–146. [Google Scholar]
- Handel, S.N.; Beattie, A.J. Seed dispersal by ants. Sci. Am. 1990, 263, 76–83B. [Google Scholar] [CrossRef]
- Gove, A.D.; Majer, J.D.; Dunn, R.R. A keystone ant species promotes seed dispersal in a “diffuse” mutualism. Oecologia 2007, 153, 687–697. [Google Scholar] [CrossRef]
- Canner, J.E.; Dunn, R.R.; Giladi, I.; Gross, K. Redispersal of seeds by a keystone ant augments the spread of common wildflowers. Acta Oecol. 2012, 40, 31–39. [Google Scholar] [CrossRef]
- Ben-Zvi, G.; Seifan, M.; Giladi, I. Ant guild identity determines seed fate at the post-removal seed dispersal stages of a desert perennial. Insects 2021, 12, 147. [Google Scholar] [CrossRef]
- Christianini, A.V.; Mayhe-Nunes, A.J.; Oliveira, P.S. Exploitation of fallen diaspores by ants: Are there ant-plant partner choices. Biotropica 2012, 44, 360–367. [Google Scholar] [CrossRef]
- Agaldo, J.A.; Christianini, A.V.; Chapman, H.M. Interactions between ants and non-myrmecochorous diaspores in a West African montane landscape. J. Trop. Ecol. 2021, 37, 1–9. [Google Scholar] [CrossRef]
- Leal, I.R.; Leal, L.C.; Andersen, A.N. The benefits of myrmecochory: A matter of stature. Biotropica 2015, 47, 281–285. [Google Scholar] [CrossRef]
- Lengyel, S.; Gove, A.D.; Latimer, A.M.; Majer, J.D.; Dunn, R.R. Convergent evolution of seed dispersal by ants, and phylogeny and biogeography in flowering plants: A global survey. Perspect. Plant Ecol. Evol. Syst. 2010, 12, 43–55. [Google Scholar] [CrossRef]
- Christian, C.E. Consequences of a biological invasion reveal the importance of mutualism for plant communities. Nature 2001, 413, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Penn, H.J.; Crist, T.O. From dispersal to predation: A global synthesis of ant-seed interactions. Ecol. Evol. 2018, 8, 9122–9138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levey, D.J.; Byrne, M.M. Complex ant-plant interactions: Rain-forest ants as secondary dispersers and post-dispersal seed predators. Ecology 1993, 74, 1802–1812. [Google Scholar] [CrossRef]
- Retana, J.; Picó, F.X.; Rodrigo, A. Dual role of harvesting ants as seed predators and dispersers of a non-myrmechorous Mediterranean perennial herb. Oikos 2004, 105, 377–385. [Google Scholar] [CrossRef]
- Griffiths, H.M.; Ashton, L.A.; Walker, A.E.; Hasan, F.; Evans, T.A.; Eggleton, P.; Parr, C.L. Ants are the major agents of resource removal from tropical rainforests. J. Anim. Ecol. 2018, 87, 293–300. [Google Scholar] [CrossRef] [Green Version]
- Folgarait, P. Ant biodiversity to ecosystem functioning: A review. Biodivers. Conserv. 1998, 7, 1221–1244. [Google Scholar] [CrossRef]
- Christian, C.E.; Stanton, M.L. Cryptic consequences of a dispersal mutualism: Seed burial, elaiosome removal, and seed-bank dynamics. Ecology 2004, 85, 1101–1110. [Google Scholar] [CrossRef]
- Parr, C.L.; Eggleton, P.; Davies, A.B.; Evans, T.A.; Holdsworth, S. Suppression of savanna ants alters invertebrate composition and influences key ecosystem processes. Ecology 2016, 97, 1611–1617. [Google Scholar] [CrossRef] [PubMed]
- Andersen, A.N. Responses of ant communities to disturbance: Five principles for understanding the disturbance dynamics of a globally dominant faunal group. J. Anim. Ecol. 2019, 88, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, H.L.; Maravalhas, J.B.; Cornelissen, T. Effects of fire disturbance on ant abundance and diversity: A global meta-analysis. Biodivers. Conserv. 2017, 26, 177–188. [Google Scholar] [CrossRef]
- Swengel, A.B. A literature review of insect responses to fire, compared to other conservation managements of open habitat. Biodivers. Conserv. 2001, 10, 1141–1169. [Google Scholar] [CrossRef]
- Parr, C.L.; Andersen, A.N.; Chastagnol, C.; Duffaud, C. Savanna fires increase rates and distances of seed dispersal by ants. Oecologia 2007, 151, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, K.P.; Mackay, D.A.; Whalen, M.A. Interactions between ants and seeds of two myrmecochorous plant species in recently burnt and long-unburnt forest sites. Austral Ecol. 2011, 36, 767–778. [Google Scholar] [CrossRef]
- Beaumont, K.P.; Mackay, D.A.; Whalen, M.A. Multiphase myrmecochory: The roles of different ant species and effects of fire. Oecologia 2013, 172, 791–803. [Google Scholar] [CrossRef]
- Harmon, M.E.; Franklin, J.F.; Swanson, F.J.; Sollins, P.; Gregory, S.V.; Lattin, J.D.; Anderson, N.H.; Cline, S.P.; Aumen, N.G.; Sedell, J.R.; et al. Ecology of coarse woody debris in temperate ecosystems. Adv. Ecol. Res. 1986, 15, 133–302. [Google Scholar] [CrossRef]
- Hartley, M.J. Rationale and methods for conserving biodiversity in plantation forests. For. Ecol. Manag. 2002, 155, 81–95. [Google Scholar] [CrossRef]
- Seibold, S.; Bässler, C.; Brandl, R.; Gossner, M.M.; Thorn, S.; Ulyshen, M.D.; Müller, J. Experimental studies of dead-wood biodiversity—A review identifying global gaps in knowledge. Biol. Conserv. 2015, 191, 139–149. [Google Scholar] [CrossRef]
- Stoklund, J.; Siitonen, J.; Jonsson, B.G. Biodiversity in Dead Wood; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Ulyshen, M.D.; Lucky, A.; Work, T.T. Effects of prescribed fire and social insects on saproxylic beetles in a subtropical forest. Sci. Rep. 2020, 10, 9630. [Google Scholar] [CrossRef] [PubMed]
- Brown Jr, W.L. Diversity of ants. In Ants: Standard Methods for Measuring and Monitoring Biodiversity; Agosti, D., Majer, J.D., Alonso, L.E., Schultz, T.R., Eds.; Smithsonian Institution Press: Washington, DC, USA, 2000; pp. 45–79. [Google Scholar]
- Warren, R.J.; Bradford, M.A. Ant colonization and coarse woody debris decomposition in temperate forests. Insectes Soc. 2012, 59, 215–221. [Google Scholar] [CrossRef]
- Deyrup, M. Ants of Florida: Identification and Natural History; CRC Press: Boca Raton, FL, USA; Taylor and Francis Group: Boca Raton, FL, USA, 2017. [Google Scholar]
- Andrew, N.; Rodgerson, L.; York, A. Frequent fuel-reduction burning: The role of logs and associated leaf litter in the conservation of ant biodiversity. Austral Ecol. 2000, 25, 99–107. [Google Scholar] [CrossRef]
- Hanula, J.L.; Ulyshen, M.D.; Wade, D.D. Impacts of prescribed fire frequency on coarse woody debris volume, decomposition and termite activity in the longleaf pine flatwoods of Florida. Forests 2012, 3, 317–331. [Google Scholar] [CrossRef]
- Noss, R.F. Fire Ecology of Florida and the Southeastern Coastal Plain; University Press of Florida: Tallahassee, FL, USA, 2018. [Google Scholar]
- Oswalt, C.M.; Cooper, J.A.; Brockway, D.G.; Brooks, H.W.; Walker, J.L.; Connor, K.F.; Oswalt, S.N.; Conner, R.C. History and Current Condition of Longleaf Pine in the Southern United States; USDA Forest Service, Southeastern Forest Experimental Station: Asheville, NC, USA, 2012.
- Kirkman, L.K.; Goebel, P.C.; Palik, B.J.; West, L.T. Predicting plant species diversity in a longleaf pine landscape. Écoscience 2004, 11, 80–93. [Google Scholar] [CrossRef]
- Van Lear, D.H.; Carroll, W.D.; Kapeluck, P.R.; Johnson, R. History and restoration of the longleaf pine-grassland ecosystem: Implications for species at risk. For. Ecol. Manag. 2005, 211, 150–165. [Google Scholar] [CrossRef]
- Alba, C.; Skalova, H.; McGregor, K.F.; D’Antonio, C.; Pysek, P. Native and exotic plant species respond differently to wildfire and prescribed fire as revealed by meta-analysis. J. Veg. Sci. 2015, 26, 102–113. [Google Scholar] [CrossRef]
- Howze, J.M.; Smith, L.L. The influence of prescribed fire on site selection in snakes in the longleaf pine ecosystem. For. Ecol. Manag. 2021, 481, 118703. [Google Scholar] [CrossRef]
- Darracq, A.K.; Boone, W.W.; McCleery, R.A. Burn regime matters: A review of the effects of prescribed fire on vertebrates in the longleaf pine ecosystem. For. Ecol. Manag. 2016, 378, 214–221. [Google Scholar] [CrossRef]
- Hanula, J.L.; Wade, D.D. Influence of long-term dormant-season burning and fire exclusion on ground-dwelling arthropod populations in longleaf pine flatwoods ecosystems. For. Ecol. Manag. 2003, 175, 163–184. [Google Scholar] [CrossRef]
- Izhaki, I.; Levey, D.J.; Silva, W.R. Effects of prescribed fire on an ant community in Florida pine savanna. Ecol. Entomol. 2003, 28, 439–448. [Google Scholar] [CrossRef]
- Lubertazzi, D.; Tschinkel, W.R. Ant community change across a ground vegetation gradient in north Florida’s longleaf pine flatwoods. J. Insect Sci. 2003, 3, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stuble, K.L.; Kirkman, L.K.; Carroll, C.R. Patterns of abundance of fire ants and native ants in a native ecosystem. Ecol. Entomol. 2009, 34, 520–526. [Google Scholar] [CrossRef]
- Colby, D.; Prowell, D. Ants (Hymenoptera: Formicidae) in wet longleaf pine savannas in Louisiana. Fla. Entomol. 2016, 89, 266–269. [Google Scholar] [CrossRef]
- Atchison, R.A.; Hulcr, J.; Lucky, A. Managed fire frequency significantly influences the litter arthropod community in longleaf pine flatwoods. Environ. Entomol. 2018, 47, 575–585. [Google Scholar] [CrossRef]
- Stamp, N.E.; Lucas, J.R. Spatial patterns and dispersal distances of explosively dispersing plants in Florida sandhill vegetation. J. Ecol. 1990, 78, 589–600. [Google Scholar] [CrossRef]
- Stuble, K.L.; Kirkman, L.K.; Carroll, C.R. Are red imported fire ants facilitators of native seed dispersal? Biol. Invasions 2010, 12, 1661–1669. [Google Scholar] [CrossRef]
- Cumberland, M.S.; Kirkman, L.K. The effects of the red imported fire ant on seed fate in the longleaf pine ecosystem. Plant Ecol. 2013, 214, 717–724. [Google Scholar] [CrossRef]
- Cumberland, M.S.; Kirkman, L.K. The effects of disturbance on the red imported fire ant (Solenopsis invicta) and the native ant community. For. Ecol. Manag. 2012, 279, 27–33. [Google Scholar] [CrossRef]
- Kipyatkov, V.E. Annual cycles of development in ants: Diversity, evolution, regulation. Proc. Colloq. Soc. Insects 1993, 2, 25–48. [Google Scholar]
- Kwapwich, C.; Tschinkel, W. Demography, demand, death, and the seasonal allocation of labor in the Florida harvester ant (Pogonomyrmex badius). Behav. Ecol. Sociobiol. 2013, 67, 2011–2027. [Google Scholar] [CrossRef]
- Ohyama, L. Asynchrony in seasonal patterns of taxonomic and functional diversity in an aboveground ant (Hymenoptera: Formicidae) community (Florida, USA). Environ. Entomol. 2022, 51, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Tschinkel, W.R. Seasonal life history and nest architecture of a winter-active ant, Prenolepis imparis. Insectes Soc. 1987, 34, 146–164. [Google Scholar] [CrossRef]
- Florida Natural Areas Inventory. Guide to the Natural Communities of Florida: 2010 Edition; Florida Natural Areas Inventory: Tallahassee, FL, USA, 2010.
- Rappe, A.; University of Florida, Gainesville, FL, USA. Personal communication. 2017. [Google Scholar]
- Del Toro, I.; Silva, R.R.; Ellison, A.M. Predicted impacts of climatic change on ant functional diversity and distributions in eastern North American forests. Divers. Distrib. 2015, 21, 781–791. [Google Scholar] [CrossRef] [Green Version]
- Frost, C.C. Four centuries of changing landscape patterns in the longleaf pine ecosystem. In Proceedings of the Tall Timbers Fire Ecology Conference; Hermann, S.M., Ed.; Tall Timbers Research Station: Tallahassee, FL, USA, 1993; pp. 17–33. [Google Scholar]
- Rappe, A.; University of Florida, Gainesville, FL, USA. Personal communication. 2020. [Google Scholar]
- Rappe, A.; University of Florida, Gainesville, FL, USA. Personal communication. 2022. [Google Scholar]
- Thaxton, J.M.; Platt, W.J. Small-scale fuel variation alters fire intensity and shrub abundance in a pine savanna. Ecology 2006, 87, 1331–1337. [Google Scholar] [CrossRef]
- Crandall, R.M.; Platt, W.J. Habitat and fire heterogeneity explain the co-occurrence of congeneric resprouter and reseeder Hypericum spp. along a Florida pine savanna ecocline. Plant Ecol. 2012, 213, 1643–1654. [Google Scholar] [CrossRef]
- Loudermilk, E.L.; Achtemeier, G.L.; O’Brien, J.J.; Hiers, J.K.; Hornsby, B.S. High-resolution observations of combustion in heterogeneous surface fuels. Int. J. Wildland Fire 2014, 23, 1016–1026. [Google Scholar] [CrossRef] [Green Version]
- Robertson, K.M.; Platt, W.J.; Faires, C.E. Patchy fires promote regeneration of longleaf pine (Pinus palustris Mill.) in pine savannas. Forests 2019, 10, 367. [Google Scholar] [CrossRef] [Green Version]
- MacGown, J.A. Ants (Formicidae) of the Southeastern United States. Identification Keys. Available online: https://www.mississippientomologicalmuseum.org.msstate.edu/Researchtaxapages/Formicidaepages/Identification.Keys.htm (accessed on 2 October 2018).
- Hilley, E.; Thiet, R. Vulnerable broom crowberry (Corema conradii) benefits from ant seed dispersal in coastal US heathlands. Plant Ecol. 2015, 216, 1091–1101. [Google Scholar] [CrossRef]
- Beattie, A.J.; Culver, D.C. The guild of myrmecochores in the herbaceous flora of West Virginia forests. Ecology 1981, 62, 107–115. [Google Scholar] [CrossRef]
- Ness, J.H. Forest edges and fire ants alter the seed shadow of an ant-dispersed plant. Oecologia 2004, 192, 119–132. [Google Scholar] [CrossRef]
- Bale, M.T.; Zettler, J.A.; Robinson, B.A.; Spira, T.P.; Allen, C.R. Yellow jackets may be an underestimated component of an ant-seed mutualism. Southeast. Nat. 2003, 2, 609–614. [Google Scholar] [CrossRef]
- Giladi, I. The Role of Habitat-Specific Demography, Habitat-Specific Dispersal, Habitat-Specific Dispersal, and the Evolution of Dispersal Distances in Determining Current and Future Distributions of the Ant-Dispersed Forest Herb, Hexastylis arifolia. Dissertation Thesis, University of Georgia, Athens, GA, USA, 2004. [Google Scholar]
- Tschinkel, W.R.; Domínguez, D.J. An illustrated guide to seeds found in nests of the Florida harvester ant, Pogonomyrmex badius. PLoS ONE 2017, 12, e0171419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, C.R. Energy use and allocation in the Florida harvester ant, Pogonomyrmex badius: Are stored seeds a buffer? Behav. Ecol. Sociobiol. 2007, 61, 1479–1487. [Google Scholar] [CrossRef]
- Harmon, G.D.; Stamp, N.E. Effects of postdispersal seed predation on spatial inequality and size variability in an annual plant, Erodium cicutarium (Geraniaceae). Am. J. Bot. 1992, 79, 300–305. [Google Scholar] [CrossRef]
- Horvitz, C.C. Analysis of how ant behaviors affect germination in a tropical myrmeco chore Calathea microcephala (P. & E.) Koernicke (Marantaceae): Microsite selection and aril removal by neotropical ants, Odontomachus, Pachycondyla, and Solenopsis (Formicidae). Oecologia 1981, 51, 47–52. [Google Scholar]
- Horvitz, C.C.; Schemske, D.W. Seed dispersal of a neotropical myrmecochore: Variation in removal rates and dispersal distance. Biotropica 1986, 18, 319–323. [Google Scholar] [CrossRef]
- Carroll, R.C.; Risch, S.J. The dynamics of seed harvesting in early successional communities by a tropical ant, Solenopsis geminata. Oecologia 1984, 61, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Kaspari, M. Body size and microclimate use in neotropical granivorous ants. Oecologia 1993, 96, 500–507. [Google Scholar] [CrossRef]
- Kaspari, M. Worker size and seed size selection by harvester ants in a neotropical forest. Oecologia 1996, 105, 397–404. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Migo, T.; Westerman, P.R.; Johnson, D.E. Post-dispersal predation of weed seeds in rice fields. Weed Res. 2010, 50, 553–560. [Google Scholar] [CrossRef]
- Motzke, I.; Tscharntke, T.; Sodhi, N.S.; Klein, A.-M.; Wanger, T.C. Ant seed predation, pesticide applications and farmers’ income from tropical multi-cropping gardens. Agric. For. Entomol. 2013, 15, 245–254. [Google Scholar] [CrossRef]
- Hernandez-Cumplido, J.; Forter, B.; Moreira, X.; Heil, M.; Benrey, B. Induced floral and extrafloral nectar production affect ant-pollinator interactions and plant fitness. Biotropica 2016, 48, 342–348. [Google Scholar] [CrossRef]
- Cuautle, M.; Rico-Gray, V.; Diaz-Castelazo, C. Effects of ant behaviour and presence of extrafloral nectaries on seed dispersal of the neotropical myrmecochore Turnera ulmifolia L. (Turneraceae). Biol. J. Linn. Soc. 2005, 86, 67–77. [Google Scholar] [CrossRef]
- Kimmel, C.B. The importance of Fire Management for Conserving Flower-Visiting Insect Diversity in a Longleaf Pine Sandhill Forest. Dissertation Thesis, University of Florida, Gainesville, FL, USA, 2017. [Google Scholar]
- Zuckerberg, B.; Cohen, J.M.; Nunes, L.A.; Bernath-Plaisted, J.; Clare, J.D.J.; Gilbert, N.A.; Kozidis, S.S.; Nelson, S.B.M.; Shipley, A.A.; Thompson, K.L.; et al. A review of overlapping landscapes: Pseudoreplication or a red herring in landscape ecology? Curr. Landscape Ecol. Rep. 2020, 5, 140–148. [Google Scholar] [CrossRef]
- Bestelmeyer, B.T.; Agosti, D.; Alonso, L.E.; Brandão, C.R.F.; Brown Jr, W.L.; Delabie, J.H.C.; Silvestre, R. Field techniques for the study of ground-dwelling ants. In Ants: Standard Methods for Measuring and Monitoring Biodiversity; Agosti, D., Majer, J.D., Alonso, L.E., Schultz, T.R., Eds.; Smithsonian Institution Press: Washington, DC, USA, 2000; pp. 122–144. [Google Scholar]
- Carval, D.; Cotte, V.; Resmond, R.; Perrin, B.; Tixier, P. Dominance in a ground-dwelling ant community of banana agroecosystem. Ecol. Evol. 2016, 6, 8617–8631. [Google Scholar] [CrossRef]
- Fisher, B.L. Ant diversity patterns along an elevational gradient in the Reserve Naturelle Integrate d’Andringitra, Madagascar. Fieldiana Zool. 1996, 85, 93–108. [Google Scholar]
- LeVan, K. TOS protocol and procedure: Ground beetle sampling. In NEON TOS Protocol NEON.DOC.0; NEON: Boulder, CO, USA, 2019; pp. 1–136. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Dray, S.; Bauman, D.; Blanchet, G.; Borcard, D.; Clappe, S.; Guenard, G.; Jombart, T.; Larocque, G.; Legendre, P.; Madi, N.; et al. adespatial: Multivariate Multiscale Spatial Analysis, R Package Version 0.3-8. 2020. Available online: https://CRAN.R-project.org/package=adespatial (accessed on 26 October 2020).
- Legendre, P.; De Caceres, M. Beta diversity as the variance of community data: Dissimilarity coefficients and partitioning. Ecol. Lett. 2013, 16, 951–963. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Michael, F.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package, R Package Version 2.5-6. 2019. Available online: https://CRAN.R-project.org/package=vegan (accessed on 21 April 2020).
- Hervé, M. RVAideMemoire: Testing and Plotting Procedures for Biostatistics, R Package Version 0.9-74. 2020. Available online: https://CRAN.R-project.org/package=RVAideMemoire (accessed on 21 April 2020).
- Barton, K. MuMIn: Multi-Model Inference, R Package Version 1.46.0. 2022. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 16 March 2022).
- Lenth, R.V. Emmeans: Estimated Marginal Means, aka Least-Squares Means, R Package Version 1.7.3. 2022. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 16 March 2022).
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R, 2nd ed.; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar]
- Pozzobom, U.M.; Heino, J.; Brito, M.T.S.; Landeiro, V.L. Untangling the determinants of macrophyte beta diversity in tropical floodplain lakes: Insights from ecological uniqueness and species contributions. Aquatic Sci. 2020, 82, 56. [Google Scholar] [CrossRef]
- Schmidt, C.A.; Shattuck, S.O. The higher classification of the ant subfamily Ponerinae (Hymenoptera: Formicidae), with a review of ponerine ecology and behavior. Zootaxa 2014, 3817, 1–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ness, J.H.; Morin, D.F.; Giladi, I. Uncommon specialization in a mutualism between a temperate herbaceous plant guild and an ant: Are Aphaenogaster ants keystone mutualists? Oikos 2009, 118, 1793–1804. [Google Scholar] [CrossRef]
- Meadley-Dunphy, S.A.; Prior, K.M.; Frederickson, M.E. Invasive ants disperse seeds farther than native ants, affecting the spatial pattern of seedling recruitment and survival. Oecologia 2020, 192, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Thom, M.D.; Daniels, J.C.; Kobziar, L.N.; Colburn, J.R. Can butterflies evade fire? Pupa location and heat tolerance in fire prone habitats of Florida. PLoS ONE 2015, 10, e0126755. [Google Scholar] [CrossRef]
- Hill, K.C.; Bakker, J.D.; Dunwiddie, P.W. Prescribed fire in grassland butterfly habitat: Targeting weather and fuel conditions to reduce soil temperatures and burn severity. Fire Ecol. 2017, 13, 24–41. [Google Scholar] [CrossRef] [Green Version]
- Trager, J.C. Revision of Conomyrma (Hymenoptera: Formicidae) from the southeastern United States, especially Florida, with keys to the species. Fla. Entomol. 1988, 71, 11–29. [Google Scholar] [CrossRef]
- Tschinkel, W.R. Florida harvester ant nest architecture, nest relocation and soil carbon dioxide gradients. PLoS ONE 2013, 8, e59911. [Google Scholar] [CrossRef]
- Atchison, R.A.; Lucky, A. Ant Species Collected in Ordway-Swisher Biological Station Sandhill Habitat (2017–2018). 2021. Available online: https://knb.ecoinformatics.org/view/doi:10.5063/F1891493.




| Sampling Period | |||
|---|---|---|---|
| Sampling Method | Pre-Burn 2017 | Post-Burn 2017 | 1-Year Post-Burn 2018 |
| Seed trials | 13 June to 9 July | 2 Aug to 6 Nov | 22 May to 28 June |
| Tuna–honey trials | 4 May to 5 July | 31 July to 15 Oct | 5 June to 25 July |
| Leaf litter sampling | 12 June to 9 July | 1 Aug to 26 Sept | 31 May to 30 July |
| Species Name | Our Study | Stamp and Lucus [51] | Stuble et al. [52] | Cumberland and Kirkman [54] | Studies in Non-Longleaf Habitat |
|---|---|---|---|---|---|
| Aphaenogaster floridana M.R. Smith | ✓ | ||||
| Aphaenogaster treatae Forel | ✓ | ✓ | Disperser: Hilley and Thiet [70] | ||
| Brachymyrmex depilis Emery | ✓ | ✓ | |||
| Crematogaster lineolate (Say) | ✓ | ||||
| Cyphomyrmex rimosus (Spinola) | ✓ | ||||
| Dorymyrmex bossutus (Trager) | ✓ | ||||
| Dorymyrmex bureni (Trager) | ✓ | ✓ | ✓ | ||
| Forelius pruinosus (Roger) | ✓ | ✓ | ✓ | ||
| Forelius sp A | ✓ | ||||
| Formica pallidefulva Latreille | ✓ | Disperser: Beattie and Culver, Bale et al., Giladi, Ness [71,72,73,74] | |||
| Nylanderia arenivaga (Wheeler) | ✓ | ✓ | |||
| Nylanderia parvula (Mayr) | ✓ | Disperser: Beattie and Culver [71] | |||
| Nylanderia wojciki (Trager) | ✓ | ||||
| Odontomachus brunneus (Patton) | ✓ | ||||
| Pheidole bilimeki Mayr | top | ✓ | |||
| Pheidole dentata Mayr | ✓ | ✓ | ✓ | ✓ | Disperser: Giladi [74] |
| Pheidole metallescens Emery | ✓ | top | |||
| Pheidole morrisi Forel | ✓ | ✓ | |||
| Pheidole navigans Wheeler | ✓ | ||||
| Pogonomyrmex badius (Latreille) | ✓ | ✓ | Predator: Harmon and Stamp, Smith, Tschinkel and Domínguez [75,76,77] | ||
| Solenopsis carolinensis Forel | ✓ | ||||
| Solenopsis geminata (Fabricius) | ✓ | Predator: Horvitz, Carroll, and Risch, Horvitz and Schemske, Kaspari, Cuautle et al., Chauhan et al., Motzke et al., Hernandez-Cumplido et al. [78,79,80,81,82,83,84,85,86] | |||
| Solenopsis invicta Buren | top | top | |||
| Solenopsis nickersoni Thompson | ✓ | ||||
| Solenopsis truncorum Forel | ✓ | ||||
| Tapinoma sessile (Say) | ✓ | ||||
| Trachymyrmex septentrionalis (McCook) | ✓ |
| Seed Removing Species | Whole Ant Community | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DF | Sum Sqs | Mean Sqs | F-Model | R2 | p-Value | Sum Sqs | Mean Sqs | F- Model | R2 | p-Value | |
| Burn treatment | 1 | 0.8 | 0.8 | 4.6 | 0.07 | 0.001 * | 0.71 | 0.71 | 6.3 | 0.09 | 0.001 * |
| Sampling period | 2 | 0.45 | 0.22 | 1.3 | 0.04 | 0.225 | 0.51 | 0.26 | 2.3 | 0.07 | 0.002 * |
| Burn x | |||||||||||
| Sampling period | 2 | 0.31 | 0.15 | 0.89 | 0.03 | 0.585 | 0.29 | 0.14 | 1.3 | 0.04 | 0.179 |
| Residuals | 53 | 9.3 | 0.17 | 0.86 | 6.0 | 0.11 | 0.8 | ||||
| Total | 58 | 10.8 | 1 | 7.5 | 1 | ||||||
| DF | Deviance | Residual DF | Residual Deviance | |
|---|---|---|---|---|
| Null model | 47 | 547.83 | ||
| Sampling period * | 2 | 252.88 | 45 | 294.94 |
| Coarse woody debris (CWD) | 1 | 22.93 | 44 | 272.01 |
| Sampling period x CWD | 2 | 10.0 | 42 | 262.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atchison, R.A.; Lucky, A. Diversity and Resilience of Seed-Removing Ant Species in Longleaf Sandhill to Frequent Fire. Diversity 2022, 14, 1012. https://doi.org/10.3390/d14121012
Atchison RA, Lucky A. Diversity and Resilience of Seed-Removing Ant Species in Longleaf Sandhill to Frequent Fire. Diversity. 2022; 14(12):1012. https://doi.org/10.3390/d14121012
Chicago/Turabian StyleAtchison, Rachel A., and Andrea Lucky. 2022. "Diversity and Resilience of Seed-Removing Ant Species in Longleaf Sandhill to Frequent Fire" Diversity 14, no. 12: 1012. https://doi.org/10.3390/d14121012
APA StyleAtchison, R. A., & Lucky, A. (2022). Diversity and Resilience of Seed-Removing Ant Species in Longleaf Sandhill to Frequent Fire. Diversity, 14(12), 1012. https://doi.org/10.3390/d14121012






