A New Large †Pachycormiform (Teleosteomorpha: †Pachycormiformes) from the Lower Jurassic of Germany, with Affinities to the Suspension-Feeding Clade, and Comments on the Gastrointestinal Anatomy of Pachycormid Fishes
Abstract
:1. Introduction
1.1. Pachycormiform Evolution
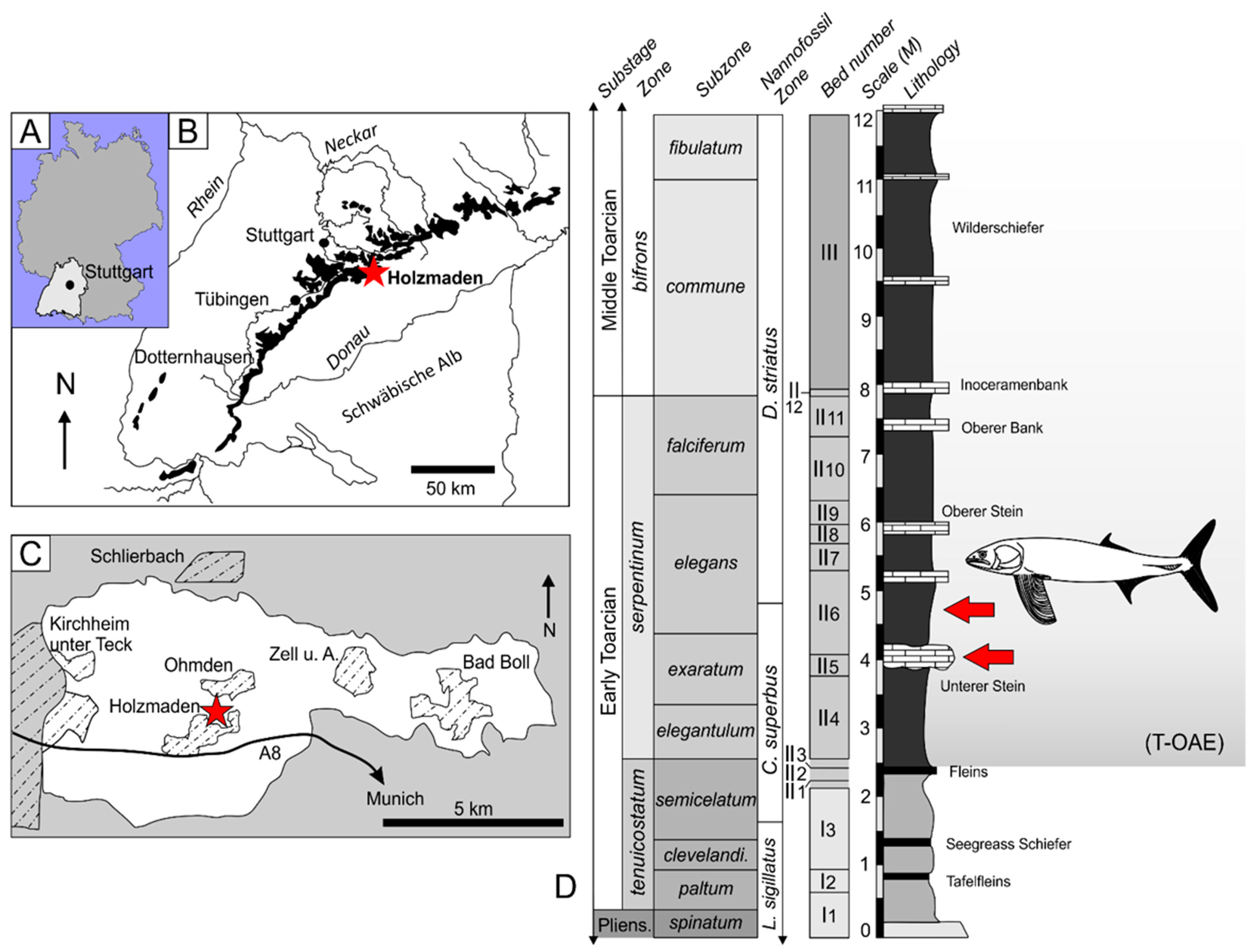
1.2. Geological Context
2. Materials and Methods
2.1. Examined Material
2.2. Anatomical Terminologies
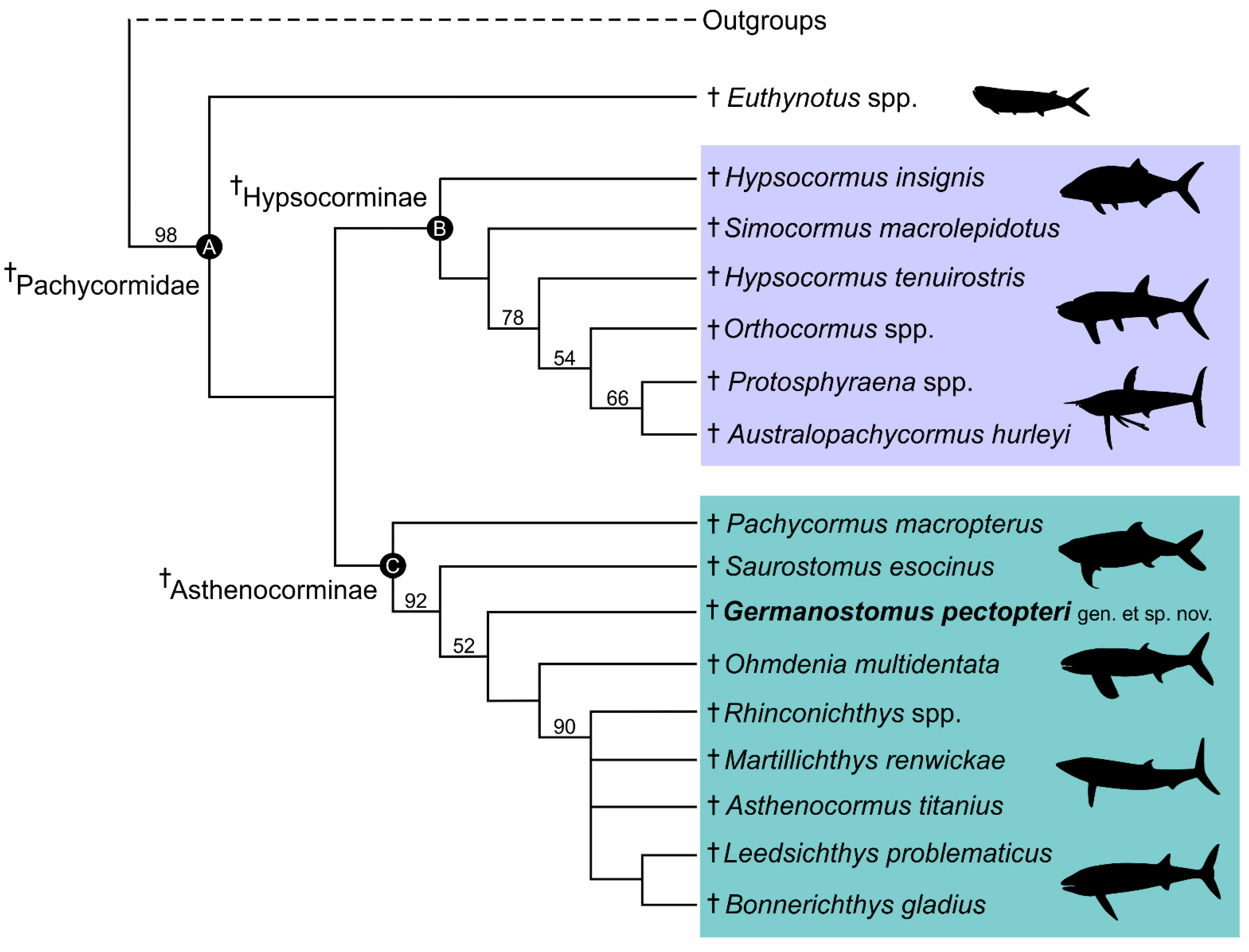
2.3. Phylogenetic Analysis
2.4. CT Scanning and Visualization
- -
- BRLSI.1383 and BRLSI.1384: 225 kV, 284 uA; 63.9 W; 2.83 s exposure; 3 mm Cu filter; 3141 projections with 4 frames averaged per projection; voxel size 46.43 μm;
- -
- BRLSI.1297: 225 kV, 519 uA, 116.8 W; 1.42 s exposure, 2.5 mm Cu filter; 3141 projections with 4 frames averaged per projection; voxel size 90 μm.
3. Results
3.1. Systematic Paleontology
- GERMANOSTOMUS gen. nov.
- ZooBank LSID. urn:lsid:zoobank.org:act:B2B56C1D-D753-4731-9C86-26DBD492487C
- Type species. Germanostomus pectopteri gen. et sp. nov.; see below.
- Diagnosis. As for the type and only species, G. pectopteri (below).
- Etymology. Genus named for the Southwestern Germanic basin where the taxon originates; with the suffix stomus (Greek) for ‘mouth’ deriving from the closely related genus Saurostomus.
- Germanostomus pectopteri gen. et sp. nov.
- ZooBank LSID: urn:lsid:zoobank.org:act:B2B56C1D-D753-4731-9C86-26DBD492487C
- Type locality. ‘Holzmaden’, Baden-Württemberg, SW Germany.
- Type horizon. Posidonienschiefer Formation, Lias Epsilon II 6, exaratum–elegans Subzones, serpentinum Zone, Early Toarcian, Lower Jurassic.
- Stratigraphic range. exaratum–elegans Subzones, middle of serpentinum Zone, presently restricted to the locality of ‘Holzmaden’, Baden-Württemberg, Germany.
- Etymology. pectopteri = pecto (pectoral) with pteros (wing), referencing the highly distinctive wing-shaped hydrofoil morphology of the large pectoral fin.
3.2. Description
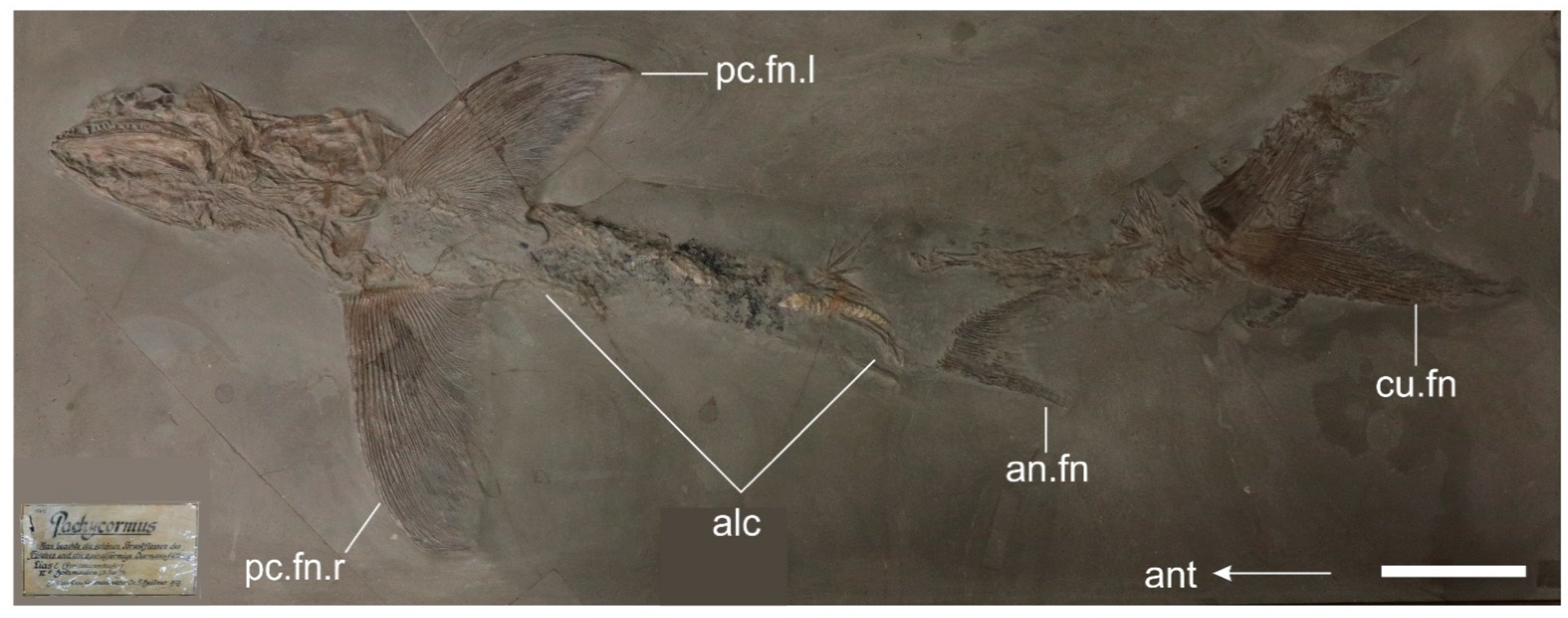
3.2.1. General Features
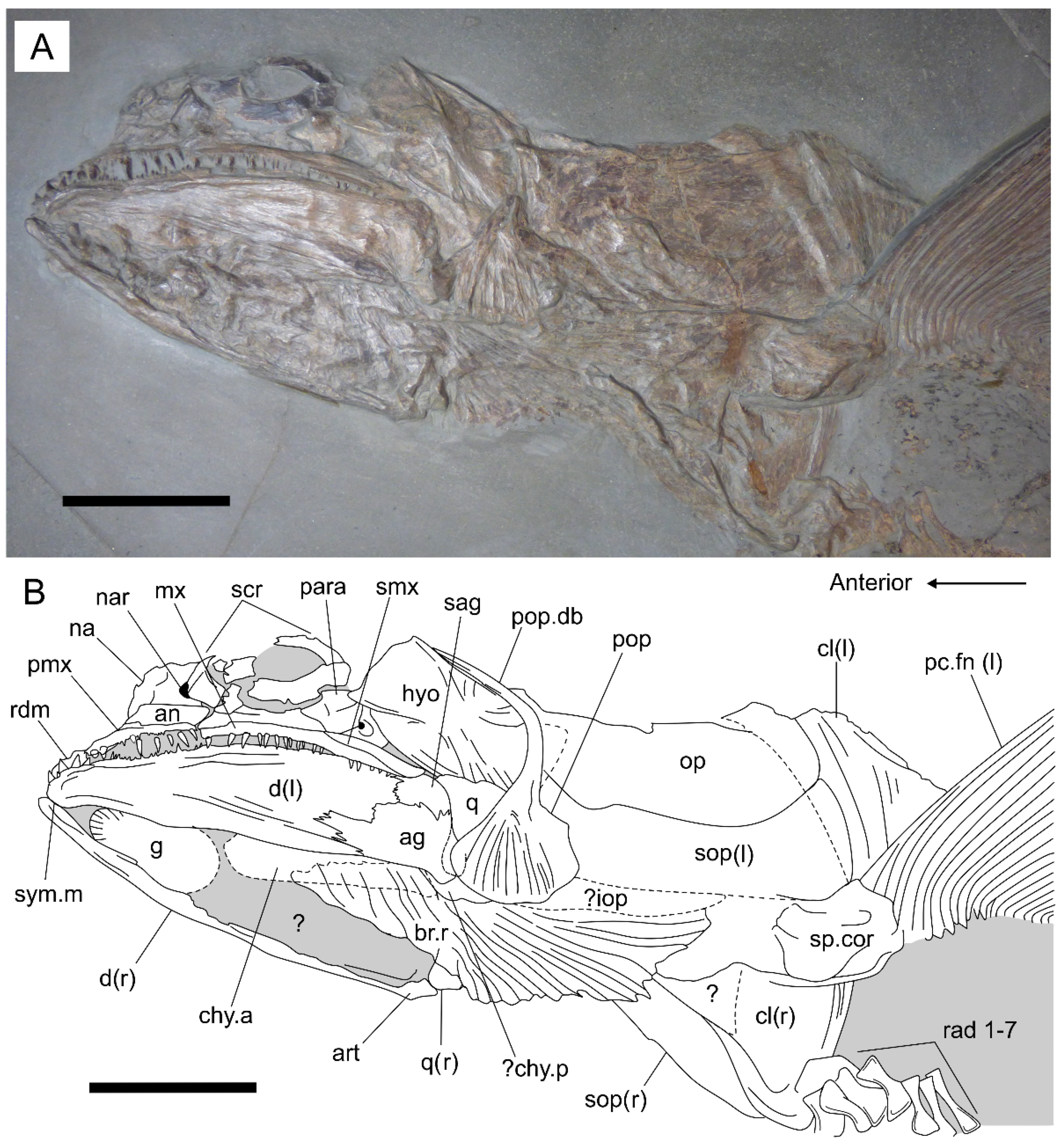
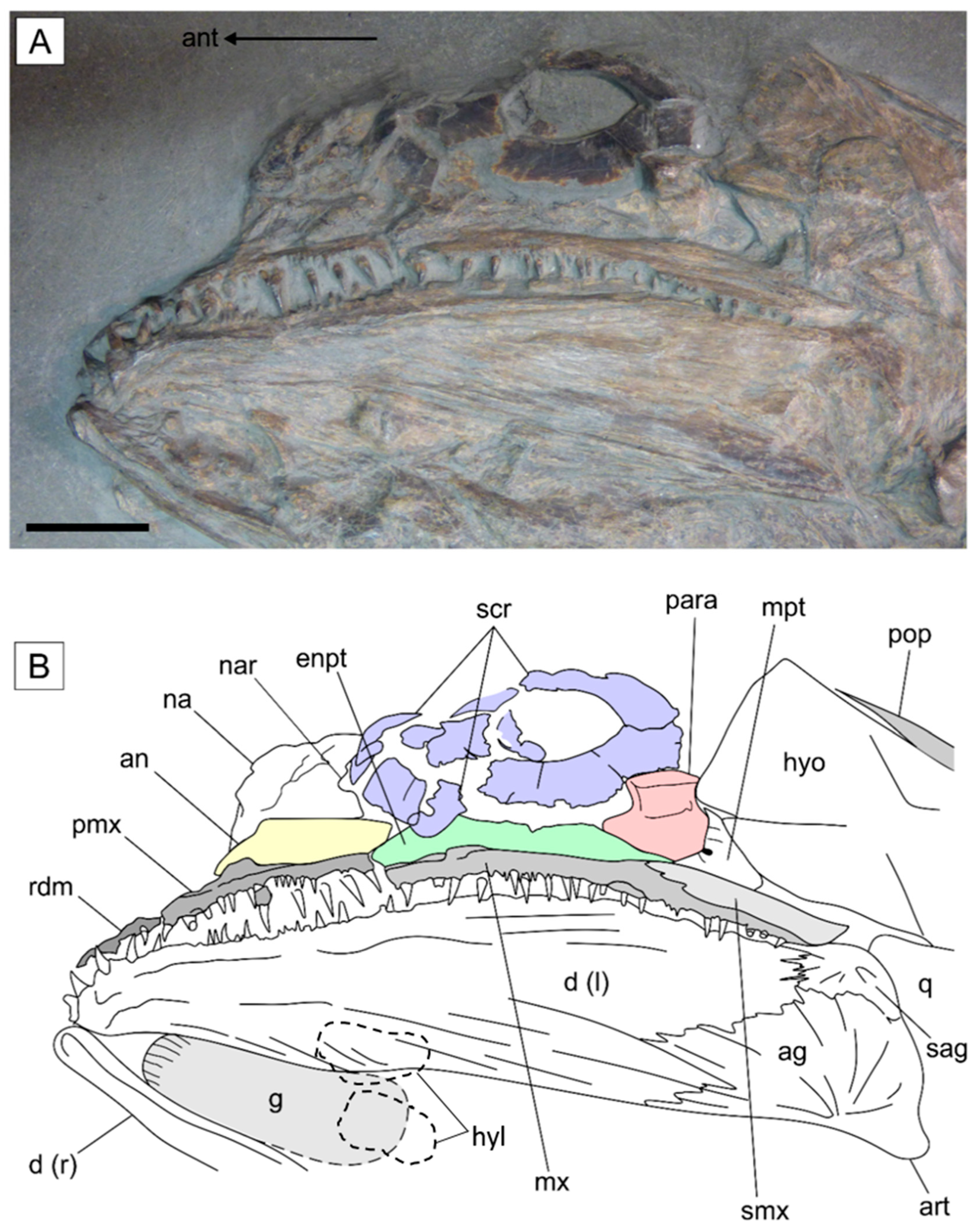
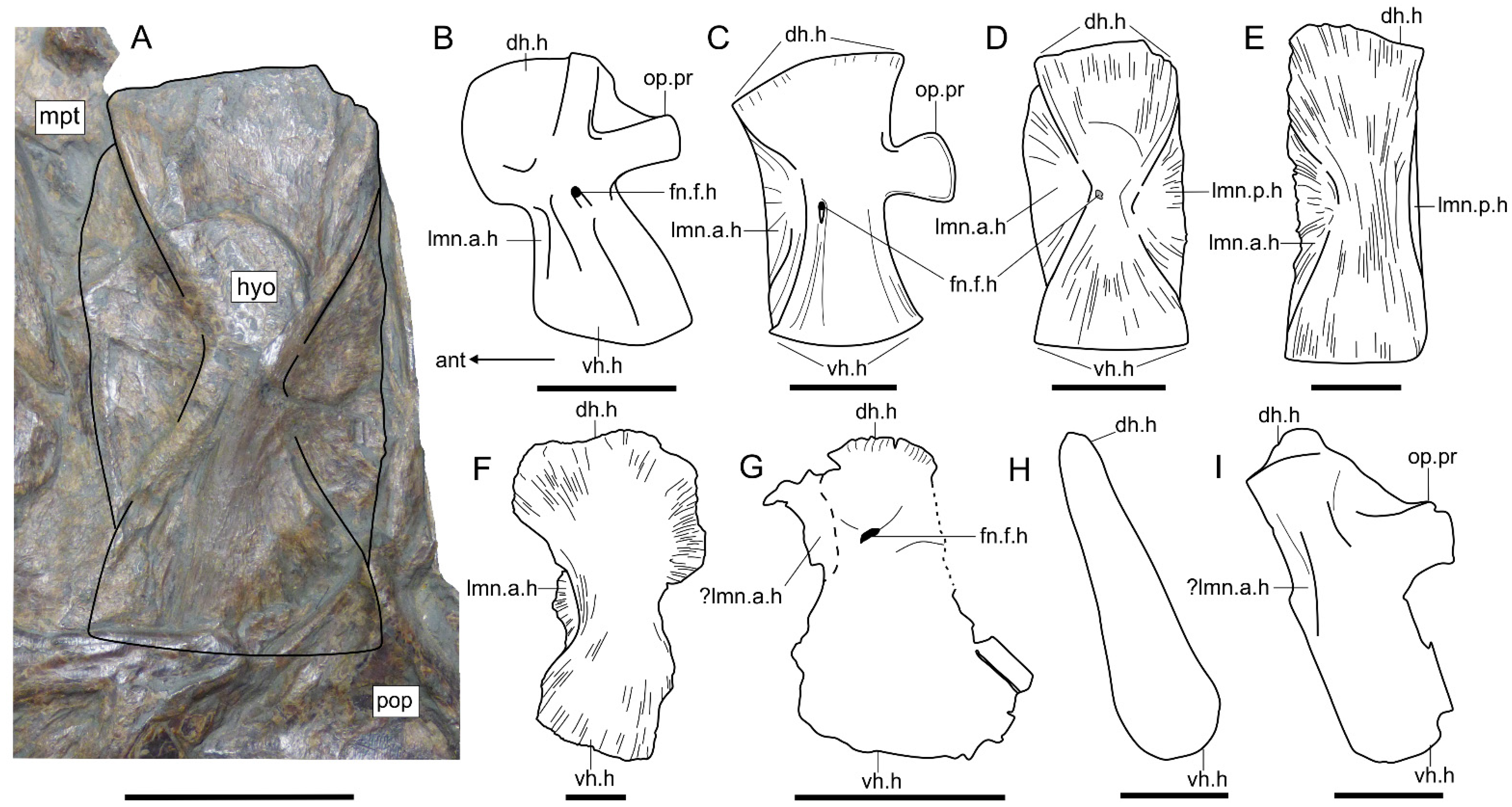
3.2.2. Cranium
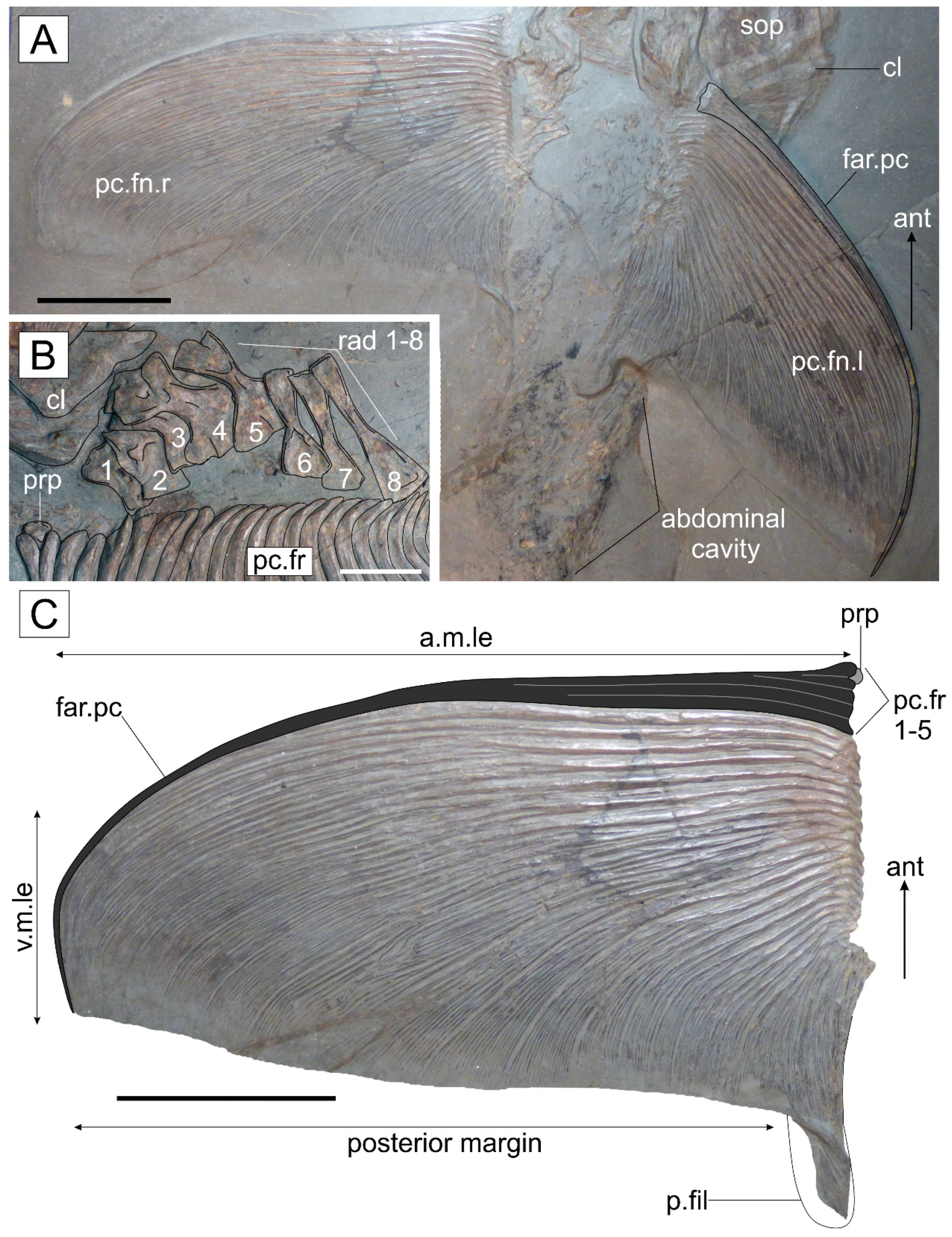
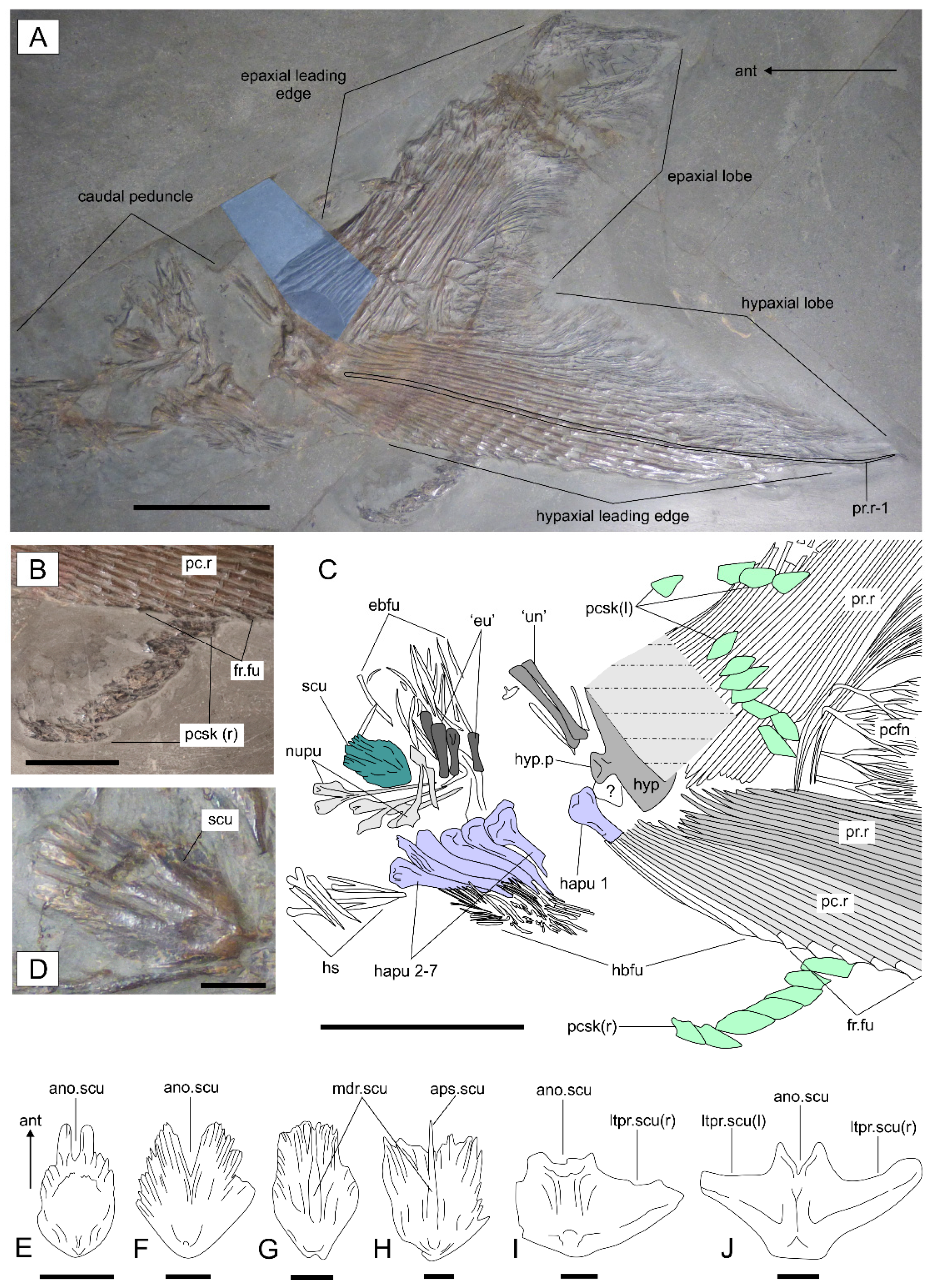
3.2.3. Postcranium
4. Discussion
4.1. Phylogenetic Interrelations of Germanostomus gen. nov., within †Pachycormiformes
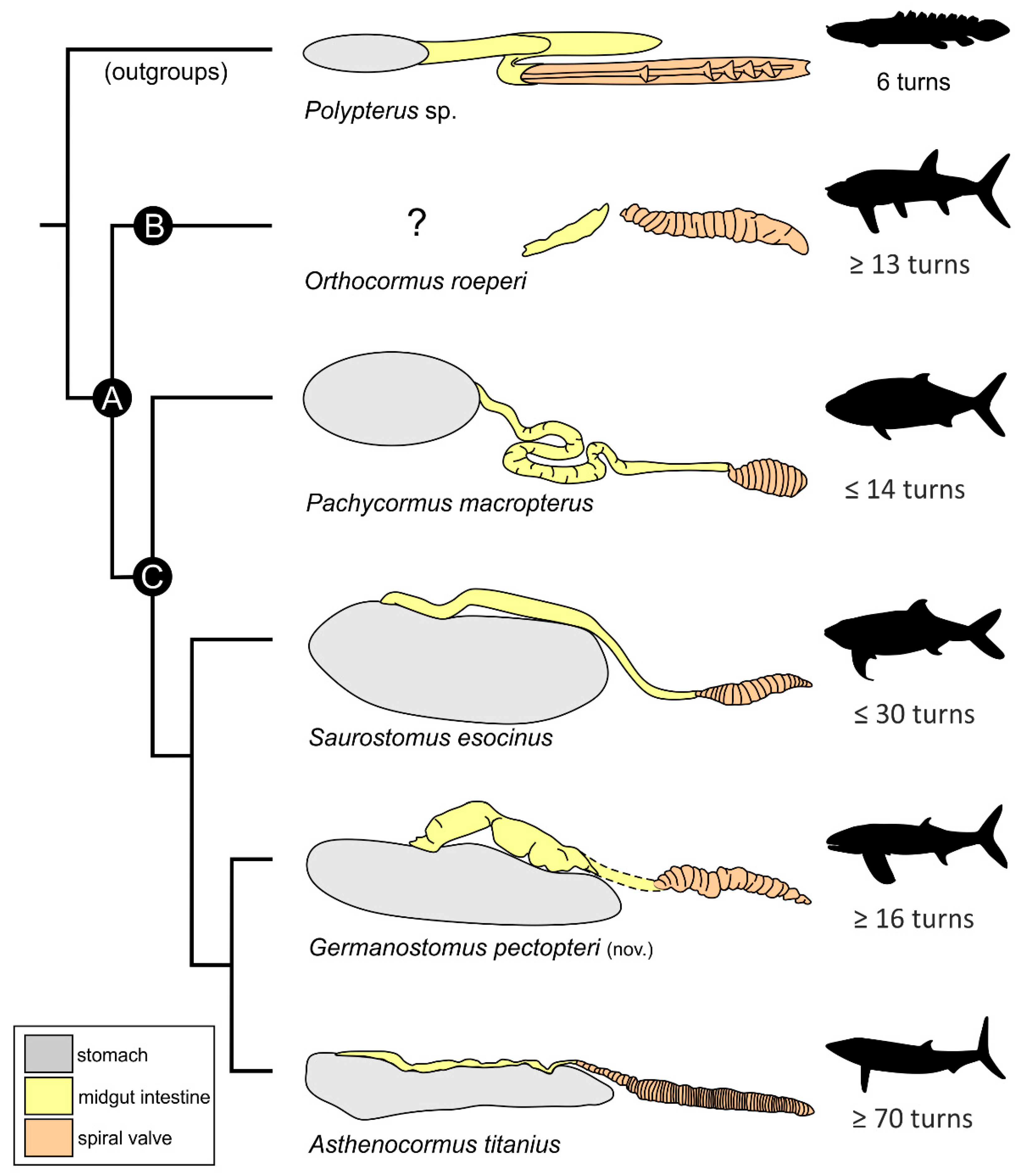
4.2. Affinities with the Suspension-Feeding Clade
- Hyomandibula that is tall and superficially rectangular with a posterior lamina. The hyomandibula of Saurostomus is strongly waisted and does not possess a posterior lamina; in Ohmdenia the bone is perfectly rectangular with a well-developed posterior lamina.
- The opercular process of the hyomandibula is entirely absent in Germanostomus, Ohmdenia, and most successive taxa with the possible exception of Bonnerichthys and Martillichthys (see Section 4.3.4); this process is well developed in Saurostomus.
- Scales highly reduced and possibly absent over much of the body in Germanostomus.
- Increased elongation of the upper and lower jaw relative to the posterior skull (see Section 4.3.2). This character is more extreme in Ohmdenia, but in Germanostomus there is already significant elongation of the upper and lower jaw in comparison to Saurostomus esocinus, including disproportionate elongation of the premaxilla (relative to maxilla) and the dentary (relative to the angular and surangular).
4.3. Comparison of Germanostomus to Other Pachycormid Fishes
4.3.1. Dentition
4.3.2. Elongation of the Premaxilla
4.3.3. Pectoral Fin Morphology
4.3.4. Morphological Disparity of the Hyomandibulae
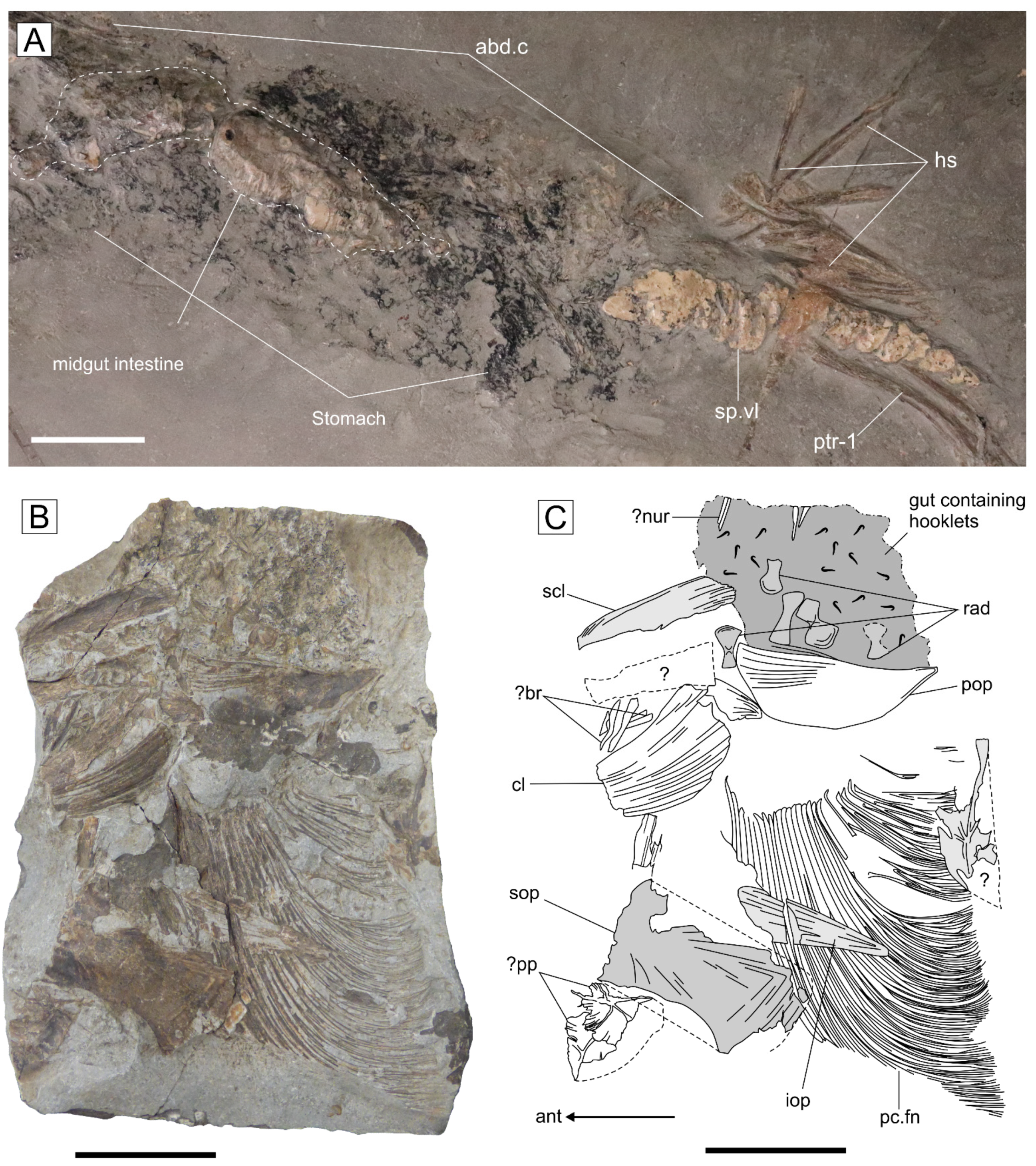
4.4. Diet and Gastrointestinal Anatomy of Germanostomus Pectopteri gen. et sp. nov.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lambers, P.H. On the Ichthyofauna of the Solnhofen Lithographic Limestone (Upper Jurassic, Germany). Ph.D. Thesis, Rijkunuversiteit Groningen, Groningen, The Netherlands, 1992, unpublished. [Google Scholar]
- Tanimoto, M.; Kaede, T. Protosphyraena pectoral fin from the Upper Cretaceous of Nakagawa, Hokkaido, Japan. Cretac. Vertebr. Hokkaido Konseki 2006, 29, 62–67. [Google Scholar]
- Kear, B.P. First record of a pachycormid fish (Actinopterygii: Pachycormiformes) from the Lower Cretaceous of Australia. J. Vertebr. Paleontol. 2007, 27, 1033–1038. [Google Scholar] [CrossRef]
- Liston, J.J. The occurrence of the Middle Jurassic pachycormid fish Leedsichthys. Oryctos 2010, 9, 1–36. [Google Scholar]
- Friedman, M.; Shimada, K.; Martin, L.D.; Everhart, M.J.; Liston, J.; Maltese, A.; Triebold, M. 100-million-year dynasty of giant planktivorous bony fishes in the Mesozoic seas. Science 2010, 327, 990–993. [Google Scholar] [CrossRef] [Green Version]
- Gouiric-Cavalli, S.; Arratia, G. A new †Pachycormiformes (Actinopterygii) from the Upper Jurassic of Gondwana sheds light on the evolutionary history of the group. J. Syst. Palaeontol. 2022, 19, 1517–1550. [Google Scholar] [CrossRef]
- Quenstedt, F.A. Der Jura; Laupp: Tübingen, Germany, 1858; 824p. [Google Scholar]
- Winkler, T.C. Description d’une nouvelle espèce de Pachycormus. Archive du Musée Teyler 1878, 5, 1–9. [Google Scholar]
- Sauvage, H.E. Note sur quelques poissons du Lias Supérieur de l’Yonne. Bull Soc sc hist et nat de l’Yonne 1891, 45, 31–38. [Google Scholar]
- Hauff, B. Untersuchung der Fossilfundstätten von Holzmaden im Posidonienschiefer des oberen Lias Württembergs. Palaeontographica 1921, 64, 1–42. [Google Scholar]
- Hauff, B.; Hauff, R.B. Das Holzmadenbuch; REPRO-DRUCK GmbH: Fellbach, Germany, 1981; p. 138. [Google Scholar]
- Liston, J.J. An overview of the pachycormiform Leedsichthys. In Mesozoic Fishes 3—Systematics, Paleoenvironments and Biodiversity; Arratia, G., A. Tintori, A., Eds.; Verlag Dr. Friedrich Pfeil: München, Germany, 2004; pp. 379–390. [Google Scholar]
- Liston, J.J. A review of the characters of the edentulous pachycormiforms Leedsichthys, Asthenocormus, and Martillichthys nov. gen. In Mesozoic Fishes 4—Homology and Phylogeny; Arratia, G., Schultze, H.P., Wilson, M.V.H., Eds.; Verlag Dr. Friedrich Pfeil: München, Germany, 2008; pp. 181–191. [Google Scholar]
- Arratia, G.; Schultze, H.P. Outstanding features of a new Late Jurassic pachycormiforms fish from the Kimmeridgian of Brunn, Germany and comments on current understanding of pachycormiforms. In Mesozoic Fishes 5—Global Diversity and Evolution; Verlang Dr. Friedrich Pfeil: München, Germany, 2013; pp. 87–120. [Google Scholar]
- Maxwell, E.E.; Lambers, P.H.; López-Arbarello, A.; Schweigert, G. Re-evaluation of pachycormid fishes from the Late Jurassic of Southwestern Germany. Acta Palaeontol. Pol. 2020, 65, 429–453. [Google Scholar] [CrossRef]
- Friedman, M. Parallel evolutionary trajectories underlie the origins of giant suspension-feeding whales and bony fishes. Proc. R. Soc. Lond. B 2012, 279, 944–951. [Google Scholar] [CrossRef]
- Schumacher, B.A.; Shimada, K.; Liston, J.; Maltese, A. Highly specialised suspension-feeding bony fish Rhinconichthys (Actinopterygii: Pachycormiformes) from the mid-Cretaceous of the United States, England, and Japan. Cretac. Res. 2016, 61, 71–85. [Google Scholar] [CrossRef] [Green Version]
- Liston, J.J. A Fish Fit for Ozymandius? The Ecology, Growth and Osteology of Leedsichthys (Pachycormidae, Actinopterygii). Ph.D. Thesis, University of Glasgow, Glasgow, UK, 2006, unpublished. [Google Scholar]
- Gouiric-Cavalli, S.; Cione, A. Notodectes is the first endemic pachycormiform genus (Osteichthys, Actinopterygii, Pachycormiformes) in the Southern Hemisphere. J. Vertebr. Paleontol. 2015, 35, e933738. [Google Scholar] [CrossRef]
- Dobson, C.E.; Giles, S.; Johanson, Z.; Liston, J.; Friedman, M. Cranial osteology of the Middle Jurassic (Callovian) Martillichthys renwickae (Neopterygii, Pachycormiformes) with comments of the evolution of ecology of edentulous pachycormids. Pap. Palaeontol. 2021, 7, 111–136. [Google Scholar] [CrossRef]
- Johanson, Z.; Liston, J.; Davesne, D.; Challands, T.; Smith, M.M. Mechanisms of dermal bone repair after predatory attack in the giant stem-teleost Leedsichthys problematicus Woodward, 1889a (Pachycormiformes). J. Anat. 2022, 241, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Wretman, L.; Blom, H.; Kear, B.P. Resolution of the Early Jurassic actinopterygian fish Pachycormus and dispersal hypothesis for Pachycormiformes. J. Vertebr. Paleontol. 2016, 36, e12060221-8. [Google Scholar] [CrossRef]
- Cawley, J.J.; Kriwet, J.; Klug, S.; Benton, M.J. The stem group teleost Pachycormus (Pachycormiformes: Pachycormidae) from the Upper Lias (Lower Jurassic) of Strawberry Bank, UK. PalZ 2018, 93, 285–302. [Google Scholar] [CrossRef] [Green Version]
- Cooper, S.L.A.; Maxwell, E.E. Revision of the pachycormid fish Saurostomus esocinus AGASSIZ from the Early Jurassic (Toarcian) of Europe with new insight into the origins of suspension-feeding in Pachycormidae. Pap. Palaeontol. 2022, 8, e1467. [Google Scholar] [CrossRef]
- De Blainville, H.D. Poissons fossiles. In Nouveau dictionnaire d’Histoire Naturelle; Deterville: Paris, France, 1818; Volume 27, pp. 310–395. [Google Scholar]
- Lehman, J.P. Étude d’un Pachycormus du Lias de Normandie; Almqvist & Wiksells Bocktryckeri AB: Uppsala, Sweden, 1947; Volume 2, pp. 1–44. [Google Scholar]
- Mainwaring, A.J. Anatomical and Systematic Revision of Pachycormidae, a Family of Mesozoic Fossil Fishes. Ph.D. Thesis, Westfield College, London, UK, 1978, unpublished. [Google Scholar]
- Dobson, C.E. Morphology, Systematics and Palaeoecology of Pachycormid Fishes. Ph.D. Thesis, Department of Earth Sciences, St Hugh’s College, University of Oxford, Oxford, UK, 2019, unpublished. [Google Scholar]
- Agassiz, L. Recherches sur les Poissons fossiles (Volume 2); Imprimerie de Petitpierre: Neuchatel, Switzerland, 1843; p. 366. [Google Scholar]
- Wagner, A. Zur charakteristik der Gattungen Sauropsis und Pachycormus nebst ihren Verwandten. Gelehrte Anzeigen (München) 1860, 26, 209–227. [Google Scholar]
- Hauff, B., Jr. Ohmdenia multidentata nov. gen. et nov sp. Ein neuer großer Fischfund aus den Posidonienschiefern des Lias von Ohmden /Holzmaden in Württemberg. N. Jb. Geol. Paläontol. Abh. 1953, 97, 39–50. [Google Scholar]
- Delsate, D. Haasichthys michelsi, nov. gen., nov. sp., un nouveau Pachycormiforme (Osteichthyes, Actinopterygii) du Toarcian inférieur (Jurassique) luxembourgeois. Trav. Sci. Mus. Nat. Hist. Nat. Lux. 1999, 32, 87–140. [Google Scholar]
- Wenz, S. Complements a l’étude des poissons actinopterygiens du Jurassique Français. In Cahiers de Paléontologie; Éditions du Centre national de la recherche scientifique: Paris, France, 1968; 276p. [Google Scholar]
- Maxwell, E.E.; Cooper, S.L.A.; Mujal, E.; Miedema, F.; Serafini, G.; Schweigert, G. Evaluating the existence of vertebrate deadfall communities in the Early Jurassic Posidonienschiefer Formation. Geosciences 2022, 12, 158. [Google Scholar] [CrossRef]
- Maxwell, E.E.; Vincent, P. Effects of the early Toarcian Oceanic Anoxic Event on ichthyosaur body size and faunal composition in the Southwest German Basin. Paleobiology 2015, 42, 117–126. [Google Scholar] [CrossRef]
- Delsate, D. L’ichthyofaune du Toarcien luxembourgeois. Cadre général et catalogue statistique. Trav. Sci. Mus. Nat. Hist. Nat. Lux. 1998, 3, 1–101. [Google Scholar]
- Röhl, H.-J.; Schmid-Röhl, A.; Oschmann, W.; Frimmel, A.; Schwark, L. Erratum to “The Posidonia Shale (Lower Toarcian) of SW-Germany: An oxygen-depleted ecosystem controlled by sea level and palaeoclimate. Palaeogeogr. Palaeoclim. Palaeoecol. 2001, 169, 273–299. [Google Scholar] [CrossRef]
- Röhl, H.-J.; Schmid-Röhl, A. Lower Toarcian (Upper Liassic) black shales I Central European epicontinental basin: A sequence stratigraphic case study from the SW-German Posidonia Shale. SEPM Spec. Publ. 2005, 82, 165–189. [Google Scholar]
- Bour, I.; Mattioli, E.; Pittet, B. Nannofacies analysis as a tool to reconstruct paleoenvironmental changes during the Early Toarcian anoxic event. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007, 249, 58–79. [Google Scholar] [CrossRef]
- Trabucho-Alexandre, J.; Dirkx, R.; Veld, H.; Klaver, G.; De Boer, P.L. Toarcian black shales in the Dutch Central Graben: Record of energetic, variable depositional conditions during an oceanic anoxic event. J. Sediment Res. 2012, 82, 104–120. [Google Scholar] [CrossRef]
- Brignon, A. Le saumon pétrifé de Beaune: Histoire de la découverte de l’holotype de Pachycormus macropterus (Blainville, 1818). Geodiversitas 2017, 39, 691–703. [Google Scholar] [CrossRef]
- Mönnig, E.; Fraz, M.; Schweigert, G. Der Jura in der Stratigraphischen Tabelle von Deutschland (STD 2016). Z. Dt. Ges. Geowiss. 2018, 169, 225–246. [Google Scholar]
- Urliches, M.; Wild, R.; Ziegler, B. Der Posidonien-Schiefer und seine Fossilien. Stuttgarter Beitr Naturk Ser C 1994, 36, 1–95. [Google Scholar]
- Jäger, M. The Museum of Fossils in the Werkforum: Guidebook of the Exhibition of Jurassic Fossils; Holcim: Dotternhausen, Germany, 2005; 149p. [Google Scholar]
- Quenstedt, F.A. Das Flözgebirge Würtembergs mit besonderer Rücksicht auf den Jura, 1st ed.; Laupp: Tübingen, Germany, 1843; 558p. [Google Scholar]
- Riegraf, W.; Werner, G.; Lörcher, F. Der Posidonienschiefer. Biostratigraphie, Fauna und Fazies des südwestdeutschen Untertoarciums (Lias ε); Ferdinand Enke: Stuttgart, Germany, 1984; 195p. [Google Scholar]
- Kauffman, E.G. Ecological reappraisal of the German Posidonienschiefer (Toarcian) and the stagnant basin model. In Communities of the Past; Gray, J., Boucot, A.J., Berry, W.B.N., Eds.; Hutchinson Ross: Stroudsburg, PA, USA, 1981; pp. 311–381. [Google Scholar]
- Hess, H. Lower Jurassic Posidonia Shale of Southern Germany. Foss. Crinoids 1999, 3, 183–196. [Google Scholar]
- Hooker, J.N.; Ruhl, M.; Dickson, A.J.; Hansen, L.N.; Idiz, E.; Hesselbo, S.P.; Cartwright, J. Shale anisotropy and natural hydraulic fracture propagation: An example from the Jurassic (Toarcian) Posidonienschiefer, Germany. J. Geophys. Res. Solid Earth 2020, 125, e2019JB018442. [Google Scholar] [CrossRef] [Green Version]
- Seilacher, A. Die Holmadener Posidonienschiefer Entstshung der Fossillagerstätte und eines Erdölmuttergesteins. Klassische Fundstellen der Paläontologie 1990, 2, 107–131. [Google Scholar]
- Martill, D.M. Soupy substrates: A medium for the exceptional preservation of ichthyosaurs of the Posidonia Shale (Lower Jurassic) of Germany. Kaupia-Darmstädter Beiträge zur Naturgeschichte 1993, 2, 77–97. [Google Scholar]
- Berckhemer, F. Geschäftliches Angelegenheiten des Vereins, Sammlungsberichte und Nachrufe. Section C: Geologische Abteilung. Jahresh Ver vaterl Naturkd Württemb 1927, 83, 22–24. [Google Scholar]
- Kovar-Eder, J.; Schmid, U. (Eds.) Natur, Museum, Geschichte: 225 Jahre Staatliches Museum für Naturkunde Stuttgart; Staatliches Museum für Naturkunde Stuttgart: Stuttgart, Germany, 2016; 184, ISBN 978-3-00-052764-7. [Google Scholar]
- Smith, A.S.; Vincent, P. A new pliosaur (Reptilia: Sauropterygia) from the Lower Jurassic of Holzmaden, Germany. Palaeontology 2010, 53, 1049–1063. [Google Scholar] [CrossRef]
- White, E.I. Additions to the Upper Liassic Fish-fauna of Holzmaden. Ann. Mag. Nat. Hist. Ser. 9 1925, 15, 601–610. [Google Scholar] [CrossRef]
- Woodward, A.S. III.—Preliminary notes on some new and little-known British Jurassic fishes. Geol. Mag. 1889, 6, 448–455. [Google Scholar] [CrossRef] [Green Version]
- Wagner, A. Monographie der fossilen Fische aus den lithographischen Schiefern Bayerns. Zweite Abtheilung. Abh. Math. Phys. Kl. Königlich Bayer. Akad. Wiss. 1863, 9, 611–748. [Google Scholar]
- Leidy, J. Remarks on Saurocephalus and its allies. Trans Am Philos Soc, n.s. 1857, 11, 91–95. [Google Scholar] [CrossRef]
- Woodward, A.S. The fossil fishes of the English Chalk. Part V. Monogr. Palaeontogr. Soc. 1909, 63, 153–184. [Google Scholar] [CrossRef]
- Woodward, A.S. Catalogue of the Fossil Fishes in the British Museum (Natural History); British Museum (Natural History): London, UK, 1895; Volume 3, 544p. [Google Scholar]
- Schultze, H.-P.; Arsenault, M. The panderichthyid fish Elpistostege: A close ancestor of the tetrapods? Palaeontology 1985, 28, 293–309. [Google Scholar]
- Schultze, H.-P. Nomenclature and homologization of cranial bones in actinopterygians. In Mesozoic Fishes 4—Homology and Phylogeny; Arratia, G., Schultze, H.-P., Wilson, M.V.H., Eds.; Verlag Dr. Friedrich Pfeil: München, Germany, 2008; pp. 23–48. [Google Scholar]
- Arratia, G.; Lambers, P. The caudal skeleton of Pachycormiformes: Parallel evolution? In Mesozoic Fishes—Systematics and Paleoecology; Verlag Dr. Friedrich Pfeil: München, Germany, 1996; pp. 191–218. [Google Scholar]
- Arratia, G. Actinopterygian postcranial skeleton with special reference to the diversity of fin ray elements, and the problem of identifying homologies. In Mesozoic Fishes 4—Homology and Phylogeny; Arratia, G., Schultze, H.P., Wilson, M.V.H., Eds.; Verlag Dr. Friedrich Pfeil: München, Germany, 2008; pp. 49–101. [Google Scholar]
- Liston, J.J.; Maltese, A.E.; Lambers, P.H.; Delsate, D.; Harcout-Smith, W.E.H.; Van Heteren, A.H. Scythes, sickles and other blades: Defining the diversity of pectoral fin morphotypes in Pachycormiformes. PeerJ 2019, 7, e7675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schultze, H.-P.; Arratia, G. Reevaluation of the caudal skeleton of actinopterygian fishes: I. Lepisosteus and Amia. J. Morphol. 1986, 190, 215–241. [Google Scholar] [CrossRef]
- Schultze, H.-P.; Arratia, G. The composition of the caudal skeleton of teleosts (Actinopterygii: Osteichthyes). Zool. J. Linnaean Soc. 1989, 97, 189–231. [Google Scholar] [CrossRef]
- Grande, L.; Bemis, W.E. A comprehensive phylogenetic study of amiid fishes (Amiidae) based on comprehensive skeletal anatomy. An empirical search for interconnected patterns of natural history. Soc. Vert. Paleo. Mem. 1998, 18, 1–696. [Google Scholar] [CrossRef]
- Arratia, G. Identifying patterns of diversity of the actinopterygian fulcra. Acta Zool. 2009, 90, 220–235. [Google Scholar] [CrossRef]
- Schultze, H.-P.; Arratia, G. The caudal skeleton of basal teleosts, its conventions, and some of its major evolutionary novelties in a temporal dimension. In Mesozoic Fishes 5—Global Diversity and Evolution; Arratia, G., Schultze, H.P., Eds.; Verlag Dr. Friedrich Pfeil: München, Germany, 2013; pp. 187–246. [Google Scholar]
- Schultze, H.-P. The scales of Mesozoic actinopterygians. In Mesozoic Fishes—Systematics and Paleocology; Arratia, G., Viohl, G., Eds.; Verlag Dr. F. Pfeil: Munich, Germany, 1996; pp. 83–93. [Google Scholar]
- Goloboff, P.A.; Catalano, S.A. TNT version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics 2016, 32, 221–238. [Google Scholar] [CrossRef]
- Cope, E.D. Zittel’s Manual of Palaeontology. Am. Nat. 1887, 21, 1014–1019. [Google Scholar]
- Arratia, G. The sister-group of Teleostei: Consenus and disagreements. J. Vertebr. Paleontol. 2001, 21, 767–773. [Google Scholar] [CrossRef]
- Berg, L.S. A classification of fish-like vertebrate. Bulletin de l’Académie des Sciences de l’URSS 1937, 4, 1277–1280. [Google Scholar]
- Vetter, B. Die Fische aus dem lithographischen Schiefer im Dresdner Museum. Mitt k. miner-geol prähist Mus Dresden. 1881, 4, 1–118. [Google Scholar]
- Schwenkel, H. Die Steine reden. Monatsschrift Württemberg 1930, 1930, 24–27. [Google Scholar]
- Urlichs, M.; Wild, R.; Ziegler, B. Fossilien aus Holzmaden. Stuttgarter Beitr Naturk Ser C 1979, 11, 1–34. [Google Scholar]
- Jäger, M. Saurier und Seelilien, Versteinerungen aus dem Jurameer Posidonienschiefer. Seekreis Verlag Konstanz. 1985, 65p. [Google Scholar]
- Patterson, C. Interrelationships of holosteans. In Interrelationships of Fishes; Greenwood, P.H., MilesS, R.S., Patterson, C., Eds.; Academic Press: Cambridge, MA, USA, 1973; Volume 53, pp. 233–305. [Google Scholar]
- Patterson, C. Cartilage bones, dermal bones and membrane bones, or the exoskeleton versus the endoskeleton. In Problems in Vertebrate Evolution; Andrews, S.M., Ed.; Linnaean Soc Symposium Ser: London, UK, 1977; Volume 4, pp. 77–123. [Google Scholar]
- Olson, P.E. The skull and pectoral girdle of the parasemionotid fish Watsonulus eugnathoides from the Early Triassic Sakamena Group of Madagascar, with comments on the relationships of the holostean fishes. J. Vertebr. Paleontol. 1984, 4, 481–499. [Google Scholar] [CrossRef] [Green Version]
- Grande, L. An Empirical Synthetic Pattern Study of Gars (Lepisosteiformes) and Closely Related Species, Based Mostly on Skeletal Anatomy. The Resurrection of the Holostei. Cop. Spec. Publ. 2010, 6, 1–871. [Google Scholar]
- Woodward, A.S. I.—On a new specimen of the Liassic pachycormid fish Saurostomus esocinus, Agassiz. Geol. Mag. 1916, 3, 49–51. [Google Scholar] [CrossRef] [Green Version]
- Jessen, H.L. Schultergürtel und Pectoralflosse bei Actinopterygiern. [Shoulder girdle and pectoral fin in actinopterygians]. Foss. Strat. 1971, 1, 1–101. [Google Scholar]
- Smith, L.S. Digestion in teleost fishes. In Aquaculture Development and Coordination Programme. Fish Feed Technology; Smith, L.S., Ed.; Food and Agriculture Organization of the United Nations: Rome, Italy, 1980; pp. 1–19. [Google Scholar]
- Hassampour, M.; Joss, J. Anatomy and histology of the spiral valve intestine in juvenile Australian lungfish, Neoceratodus forsteri. Open Zool. J. 2009, 2, 62–85. [Google Scholar] [CrossRef]
- Argyriou, T.; Clauss, M.; Maxwell, E.E.; Furrer, H.; Sanchez-Villagra, M.R. Exceptional preservation reveals gastrointestinal anatomy and evolution in early actinoptrygian fishes. Sci. Rep. 2016, 6, 18758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burton, D.; Burton, M. Essential Fish Biology: Diversity, Structure and Function; Oxford University Press: Oxford, UK, 2018; 417p. [Google Scholar]
- Ferrón, H.G.; Holgado, B.; Liston, J.J.; Martínez-Pérez, C.; Botella, H. Assessing metabolic constraints on the maximum body size of actinopterygians: Locomotion energetics of Leedsichthys problematicus (Actinopterygii, Pachycormiformes). Palaeontology 2018, 61, 775–783. [Google Scholar] [CrossRef]
- Liston, J.J.; Noè, L. The tail of the Jurassic fish Leedsichthys problematicus (Osteichthyes: Actinoperygii) collected by Alfred Nicholas Leeds—An example of the importance of historical records in palaeontology. Arch. Nat. Hist. 2004, 31, 236–252. [Google Scholar] [CrossRef]
- Kriwet, J. Lancetfish teeth (Neoteleostei, Alepisauroidei) from the Early Cretaceous of Alcaine, NE Spain. Lethaia 2003, 36, 323–331. [Google Scholar] [CrossRef]
- Kriwet, J. A comprehensive study of the skull and dentition of pycnodont fishes. Zitteliana A 2005, 76, 117–123. [Google Scholar]
- Berkovitz, B.; Shellis, P. The Teeth of Non-Mammalian Vertebrates; Elsevier Academic Press: Amsterdam, The Netherlands, 2016; 343p. [Google Scholar]
- Mihalitsis, M.; Bellwood, D. Functional implications of dentition-based morphotypes in piscivorous fishes. R. Soc. Open Sci. 2019, 6, 190040. [Google Scholar] [CrossRef] [Green Version]
- Mihalitsis, M.; Bellwood, D. Morphological and functional diversity of piscivorous fishes on coral reefs. Coral Reefs 2019, 38, 945–954. [Google Scholar] [CrossRef]
- Needham, A.E. On relative growth in the jaws of certain fishes. Proc. Zool. Soc. Lond. 1935, 105, 773–784. [Google Scholar] [CrossRef]
- Stewart, J.D. The stratigraphic distribution of Late Cretaceous Protosphyraena in Kansas and Alabama. 80–94. In Paleontology and Biostratigraphy of Western Kansas: Articles in Honour of Myrl V. Walker. Fort Hays Studies, 3; Nelson, M.E., Ed.; Fort Hays State University: Hays, KS, USA, 1988; 127p. [Google Scholar]
- Friedman, M.; Shimada, K.; Everhart, M.J.; Irwin, K.J.; Grandstaff, B.S.; Stewart, J.D. Geographic and stratigraphic distribution of the Late Cretaceous suspension-feeding bony fish Bonnerichthys gladius (Teleostei, Pachycormiformes). J. Vertebr. Paleontol. 2013, 33, 35–47. [Google Scholar] [CrossRef]
- Martill, D.M. Leedsichthys problematicus, a giant filter-feeding teleost from the Jurassic of England and France. Neues Jahrbuch für Geologie und Paläontologie-Monatshefte 1988, 11, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, B.; Rosen, D.E. Major adaptive levels in the evolution of the actinopterygian feeding mechanism. Am. Zool. 1961, 1, 187–204. [Google Scholar] [CrossRef] [Green Version]
- Wilga, C.A. Evolutionary divergence in the feeding mechanism of fishes. Acta Palaeontol. Pol. 2008, 58, 113–120. [Google Scholar]
- Böttcher, R. Über die Nahrung eines Leptopterygius (Ichthyosauria, Reptilia) aus dem süddeutschen Posidonienschiefer (Unterer Jura) mit Bemerkungen über den Magen der Ichthyosaurier. Stuttgarter Beitr Naturk Ser. B 1989, 155, 1–19. [Google Scholar]
- Dick, D.; Schweigert, G.; Maxwell, E.E. Trophic niche ontogeny and palaeoecology of early Toarcian Stenopterygius (Reptilia: Ichthyosauria). Palaeontology 2016, 59, 423–431. [Google Scholar] [CrossRef]
- Klug, C.; Schweigert, G.; Hoffman, R.; Weis, R.; De Bates, K. Fossilized leftover falls as sources of palaeoecological data: A ‚‘pabulite’ comprising a crustacean, a belemnite and a vertebrate from the Early Jurassic Posidonia Shale. Swiss. J. Palaeontol. 2021, 140, 10. [Google Scholar] [CrossRef]
- Přikryl, T.; Košták, M.; Mazuch, M.; Milkuláš, R. Evidence for fish predation on a coleoid cephalopod from the Lower Jurassic Posidonia Shale of Germany. N. Jb. Geol. Paläontol. Abh. 2012, 263, 25–33. [Google Scholar] [CrossRef]
- Aldinger, H. Zur Ökologie und Stratinomie der Fische des Posidonienschiefers (Lias Epsilon). Sencken. Leth. 1965, 46, 1–12. [Google Scholar]
- Alesci, A.; Capillo, G.; Fumia, A.; Messina, E.; Albano, M.; Aragona, M.; Locascio, P.; Spano, N.; Pargolizzi, S.; Lauriano, E.R. Confocal characterization of intestinal dendritic cells from myxines to teleosts. Biology 2022, 11, 1045. [Google Scholar] [CrossRef]
- Neumayer, L. Vergleichend anatomische Untersuchungen über den Darmkanal fossiler Fische. Abh d. b. Ak Wiss Math-Phys. Kl 1919, 29, 1–28. [Google Scholar]
- Harder, W. Anatomy of Fishes; Schweizerbart’sche Verlagsbuchhandlung: Stuttgart, Germany, 1975; pp. 1–612. [Google Scholar]
- Miyashita, T. A Paleozoic hagfish Myxinikela siroko—Revised anatomy and implications for evolution of the living jawless vertebrate lineages. Can. J. Zool. 2020, 98, 850–865. [Google Scholar] [CrossRef]
- Gilmore, B. Scroll coprolites from the Silurian of Ireland and the feeding of early vertebrates. Palaeontology 1992, 35, 319–333. [Google Scholar]
- Aldridge, R.J.; Gabbott, S.E.; Sivester, L.J.; Theron, J.N. Bromalites from the Soom Shale Lagerstätte (Upper Ordovician) of South Africa: Palaeoecological and palaeobiological implications. Palaeontology 2006, 49, 857–871. [Google Scholar] [CrossRef]
- Meng, Q.; Zhu, Y. A study of the spiral valves of Chinese cartilaginous fishes. Acta Zool. Sinica 1985, 31, 277–284. [Google Scholar]
- Mcallister, J.A. Phylogenetic distribution and morphological reassessment of the intestines of fossil and modern fishes. Zool. Jahrb. Abt. Anat. Ontog. Tiere 1987, 115, 281–294. [Google Scholar]
- Holmgren, S.; Nilsson, S. Sharks, skates and rays. In The Biology of Elasmobranch Fishes; Hamlett, W.C., Ed.; The John Hopkins University Press: Baltimore, MD, USA, 1999; pp. 144–173. [Google Scholar]
- Trinajstic, K.; Long, J.A.; Sanchez, S.; Biosvert, C.A.; Snitting, D.; Tafforeau, P.; Dupret, V.; Clement, A.M.; Currie, P.D.; Roelofs, B.; et al. Exceptional preservation of organs in Devonian placoderms from the Gogo Lagerstätte. Science 2022, 377, 1311–1314. [Google Scholar] [CrossRef]
- Weisel, G.F. Anatomy and histology of the digestive system of the paddlefish (Polyodon spathula). J. Morphol. 1973, 140, 243–255. [Google Scholar] [CrossRef]
- Weisel, G.F. Histology of the feeding and digestive organs of the shovelnose sturgeon, Scaphirhynchus platorhynchus. Copeia 1979, 518, 518–525. [Google Scholar] [CrossRef]
- Thoney, D.A.; Hargis, W.J., Jr. Juvenile anisakine parasites from the coelacanth Latimeria chalumnae. Environ. Biol. Fishes 1991, 32, 281–283. [Google Scholar] [CrossRef]
- Forey, P. History of the Coelacanth Fishes; Springer Nature: Berlin/Heidelberg, Germany, 1997; 434p, ISBN 978-0-412-78480-4. [Google Scholar]
- Zatoń, M.; Broda, K.; Qvarnström, M.; Niedźwiedzki, G.; Ahlberg, P.E. The first direct evidence of a Late Devonian coelacanth fish feeding on conodont animals. Sci. Nat. 2017, 104, 26. [Google Scholar] [CrossRef] [Green Version]
- Capasso, L. Pycnodonts: An overview and new insights in the Pycnodontomorpha Nursall, 2010. Occas. Pap. Univ. Mus. Chieti Monogr. Publ. 2021, 1, 1–223. [Google Scholar]
- Gengenbaur, C. Vergleichende Anatomie der Wirbelthiere mit Berücksichtigung der Wirbellosen; Verlag Wilhelm Engelmann: Leipzig, Saxony, Germany, 1901; Volume 2, p. 696. [Google Scholar]
- Macullum, A.B. Alimentary canal and pancreas of Acipenser, Amia, and Lepidosteus. J. Anat. Physiol. 1886, 202, 604–636. [Google Scholar]
- Hilton, W.A. On the intestine of Amia calva. Am. Nat. 1900, 34, 717–735. [Google Scholar] [CrossRef]
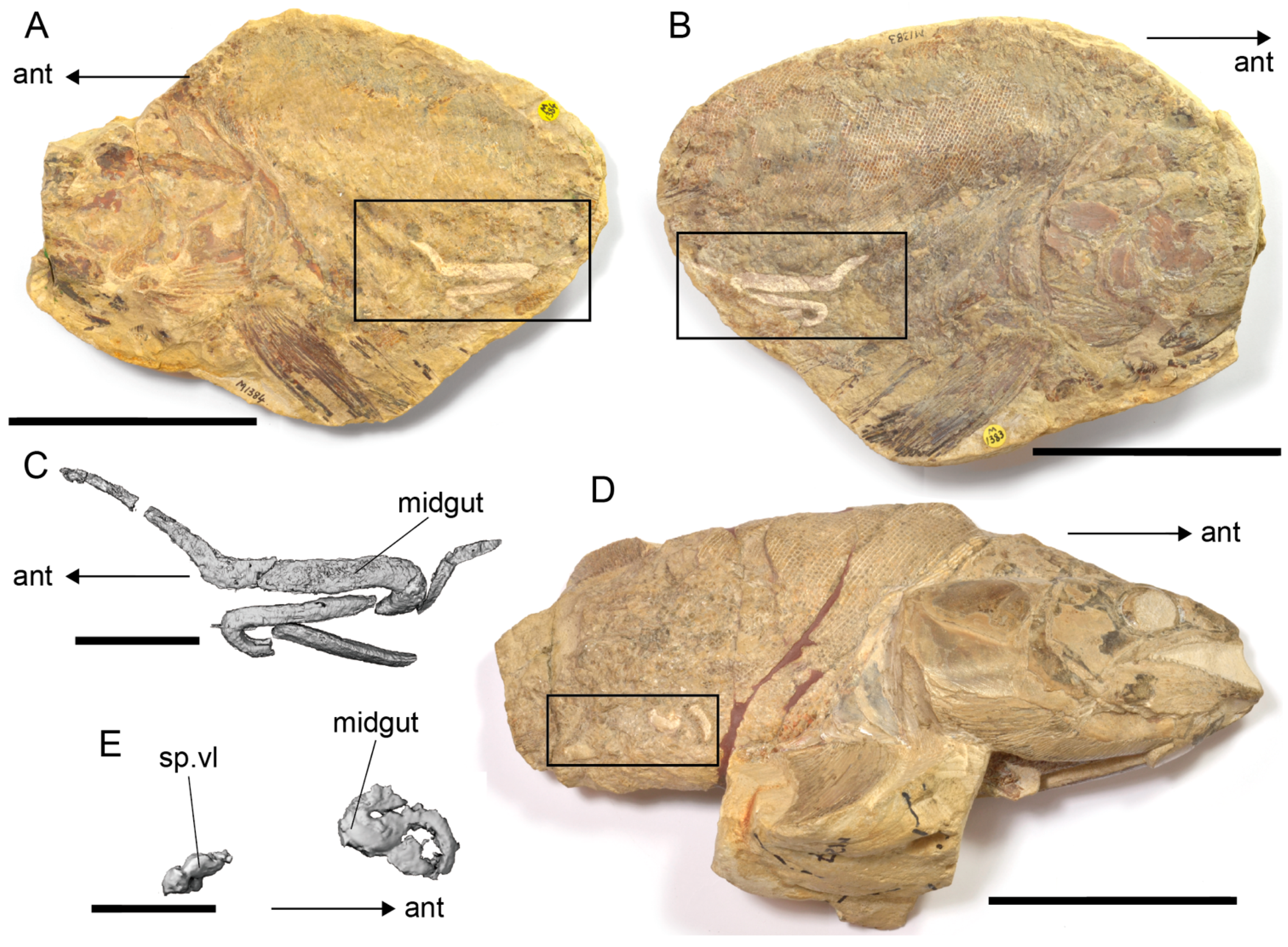
| Specimen no. | Taxon | TL | SL | BL | CFH | HL | ML | MH | POL | OPL | PCFW | PCFL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SMNS 15815 | Germanostomus pectopteri gen. et sp. nov. (Holotype) | 1060 | 850 | 605 | 90 | 245 | 125 | 25 | 55 | 120 | 130 | 190 |
| SMNS 56344 | Germanostomus pectopteri gen. et sp. nov. | - | - | - | - | - | - | - | - | - | 85 | 140 |
| GPIT-PV-31531 | Ohmdenia multidentata (Holotype) | - | - | - | - | - | 598 | 95 | - | 165 | 100 | 335 |
| SMNS 51144 | Saurostomus esocinus (neotype) | 750 | 630 | 480 | 212 | 182 | 90 | 20 | 40 | 47 | 100 | 150 |
| SMNS 12576 | Saurostomus esocinus | 1260 | 1100 | 830 | 350 | 280 | 140 | 30 | 58 | 120 | 177 | - |
| SMNS 18189 | Pachycormus macropterus | 1005 | 816 | 575 | 263 | 250 | 142 | 31 | 65 | 110 | 104 | 222 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cooper, S.L.A.; Giles, S.; Young, H.; Maxwell, E.E. A New Large †Pachycormiform (Teleosteomorpha: †Pachycormiformes) from the Lower Jurassic of Germany, with Affinities to the Suspension-Feeding Clade, and Comments on the Gastrointestinal Anatomy of Pachycormid Fishes. Diversity 2022, 14, 1026. https://doi.org/10.3390/d14121026
Cooper SLA, Giles S, Young H, Maxwell EE. A New Large †Pachycormiform (Teleosteomorpha: †Pachycormiformes) from the Lower Jurassic of Germany, with Affinities to the Suspension-Feeding Clade, and Comments on the Gastrointestinal Anatomy of Pachycormid Fishes. Diversity. 2022; 14(12):1026. https://doi.org/10.3390/d14121026
Chicago/Turabian StyleCooper, Samuel L. A., Sam Giles, Holly Young, and Erin E. Maxwell. 2022. "A New Large †Pachycormiform (Teleosteomorpha: †Pachycormiformes) from the Lower Jurassic of Germany, with Affinities to the Suspension-Feeding Clade, and Comments on the Gastrointestinal Anatomy of Pachycormid Fishes" Diversity 14, no. 12: 1026. https://doi.org/10.3390/d14121026
APA StyleCooper, S. L. A., Giles, S., Young, H., & Maxwell, E. E. (2022). A New Large †Pachycormiform (Teleosteomorpha: †Pachycormiformes) from the Lower Jurassic of Germany, with Affinities to the Suspension-Feeding Clade, and Comments on the Gastrointestinal Anatomy of Pachycormid Fishes. Diversity, 14(12), 1026. https://doi.org/10.3390/d14121026






