Karst Dolines Support Highly Diversified Soil Collembola Communities—Possible Refugia in a Warming Climate?
Abstract
:1. Introduction
2. Materials and Methods
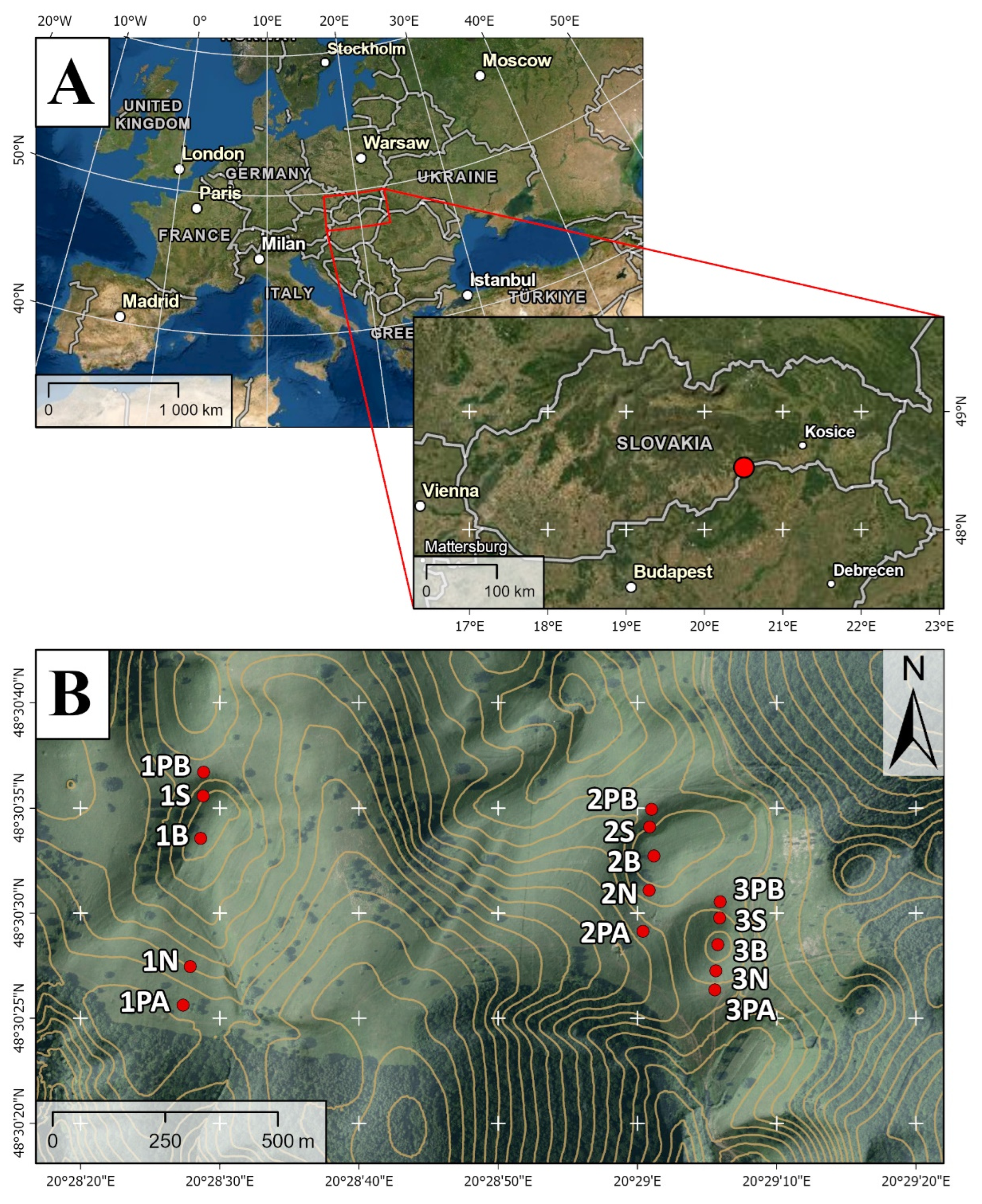
2.1. Description of Study Area
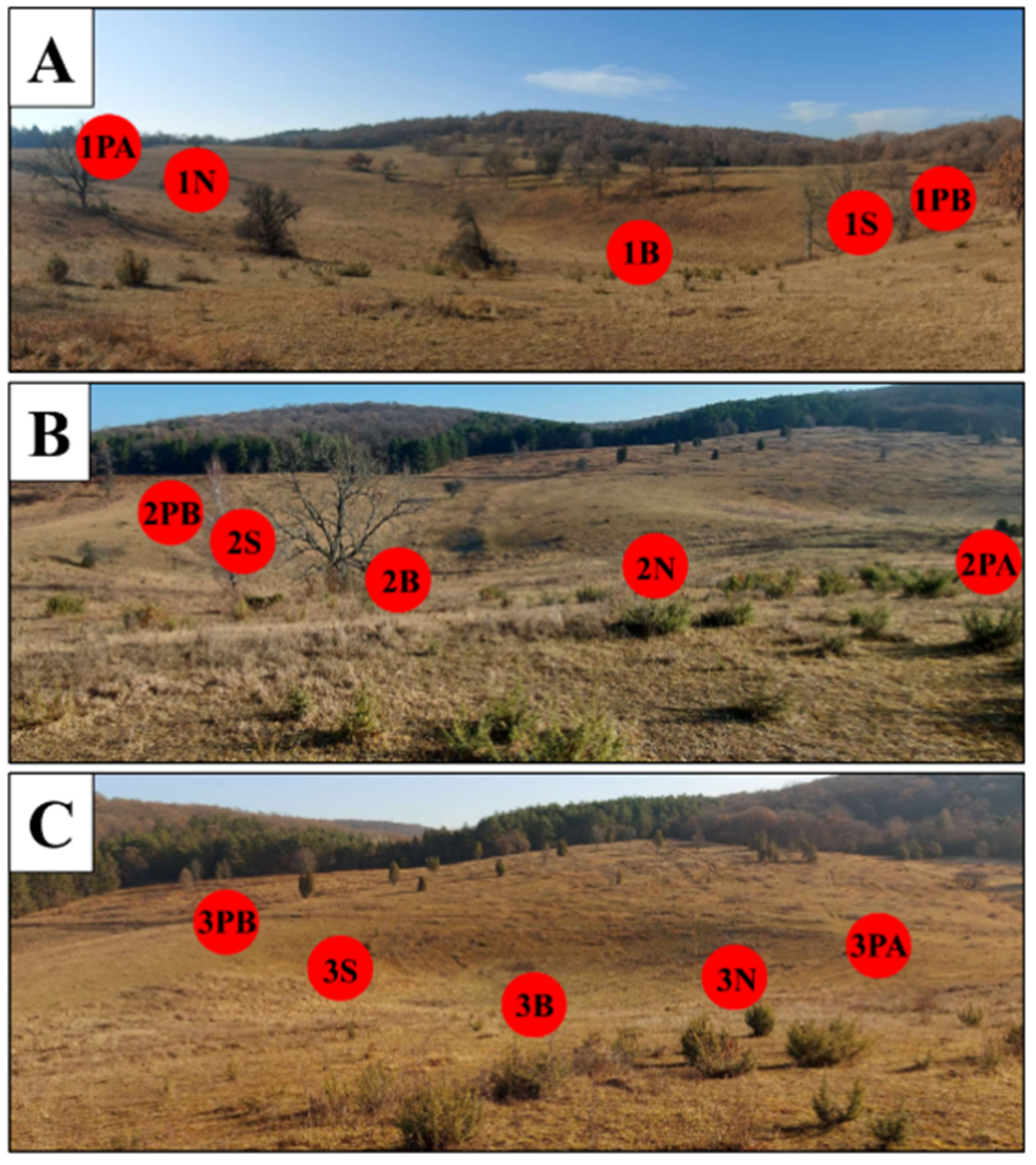
2.2. Study Sites and Sampling Design
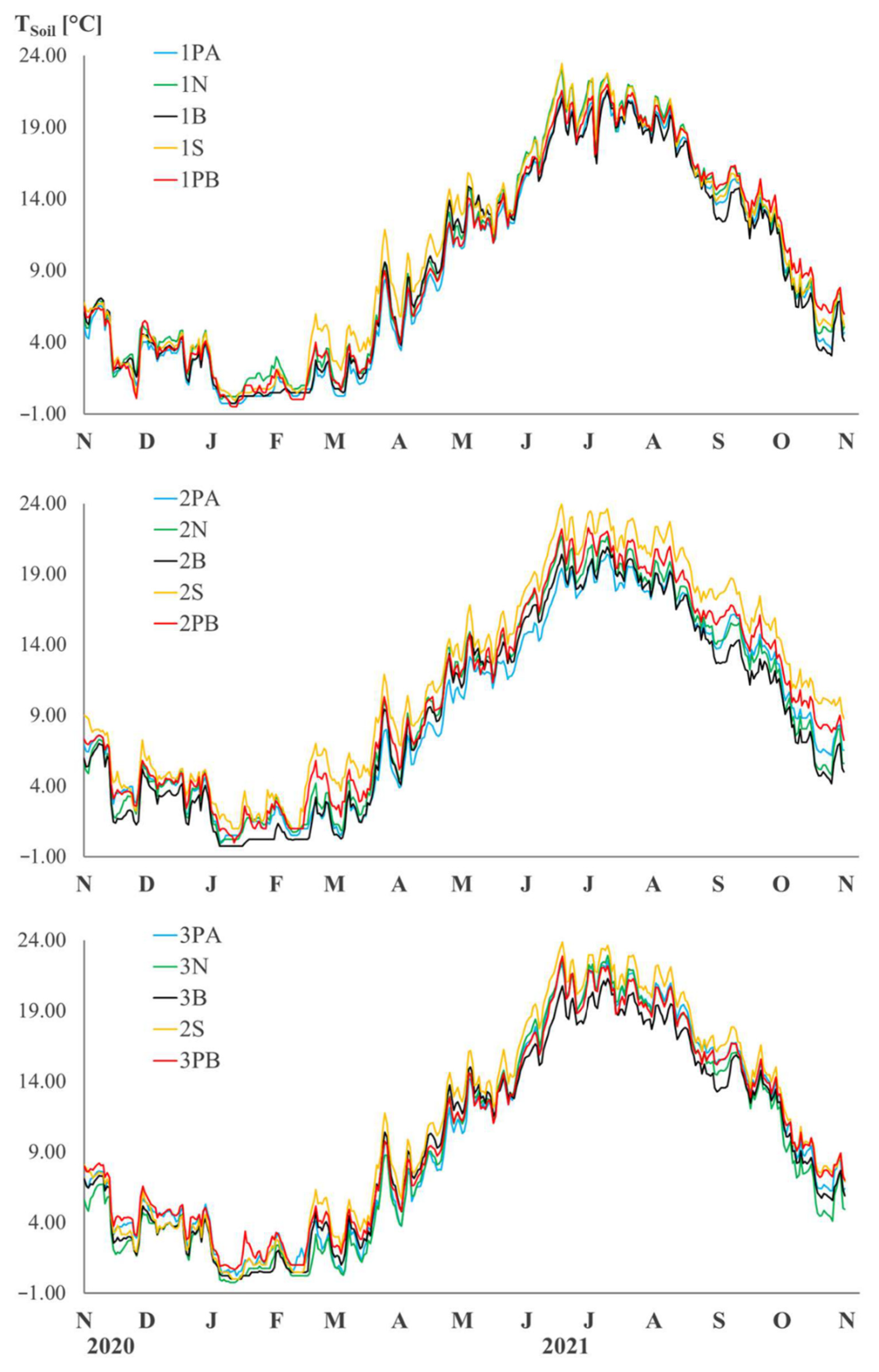
2.3. Soil Topographic, Vegetation, Microclimatic and Chemical Data
2.4. Community Data
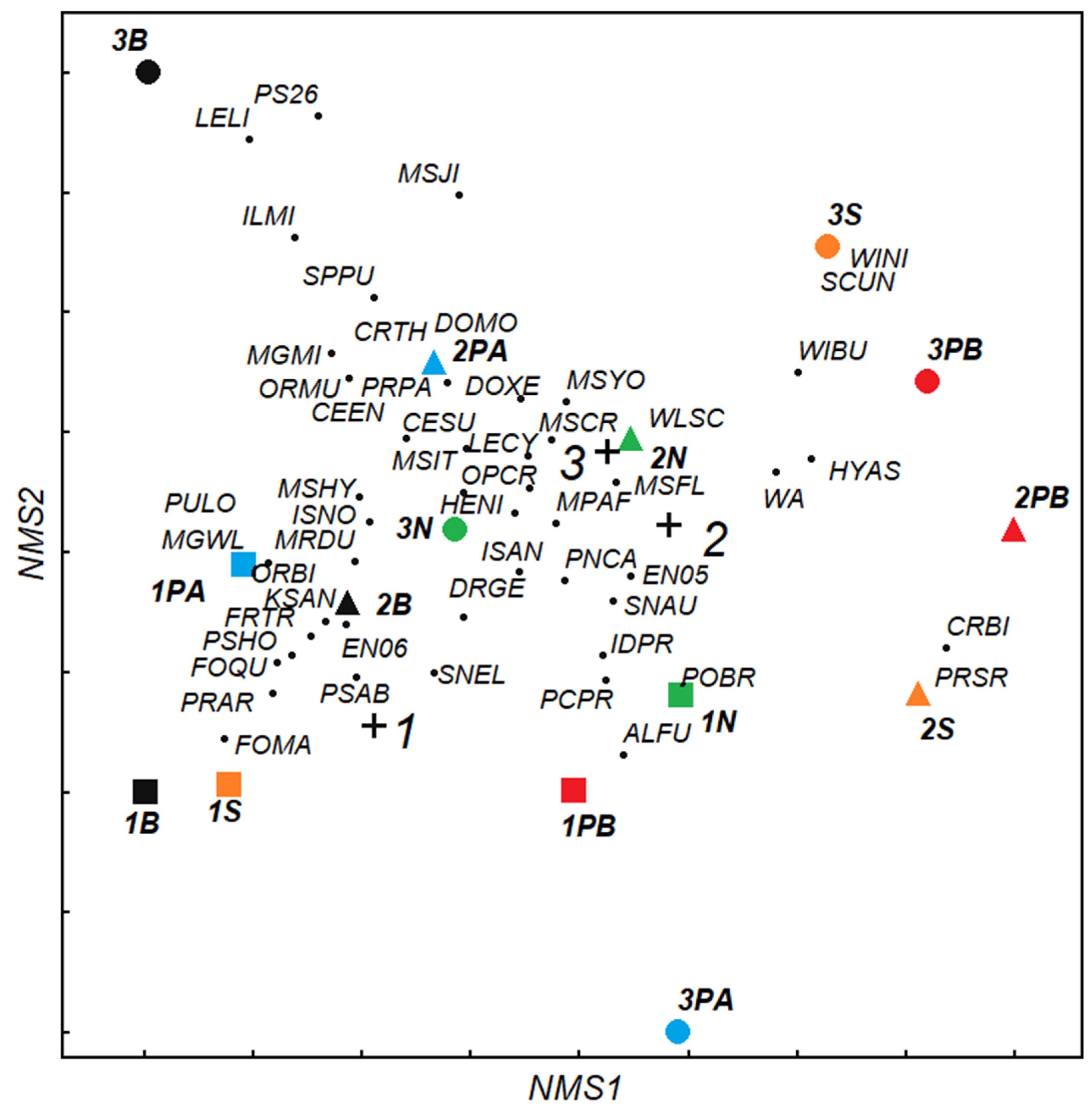
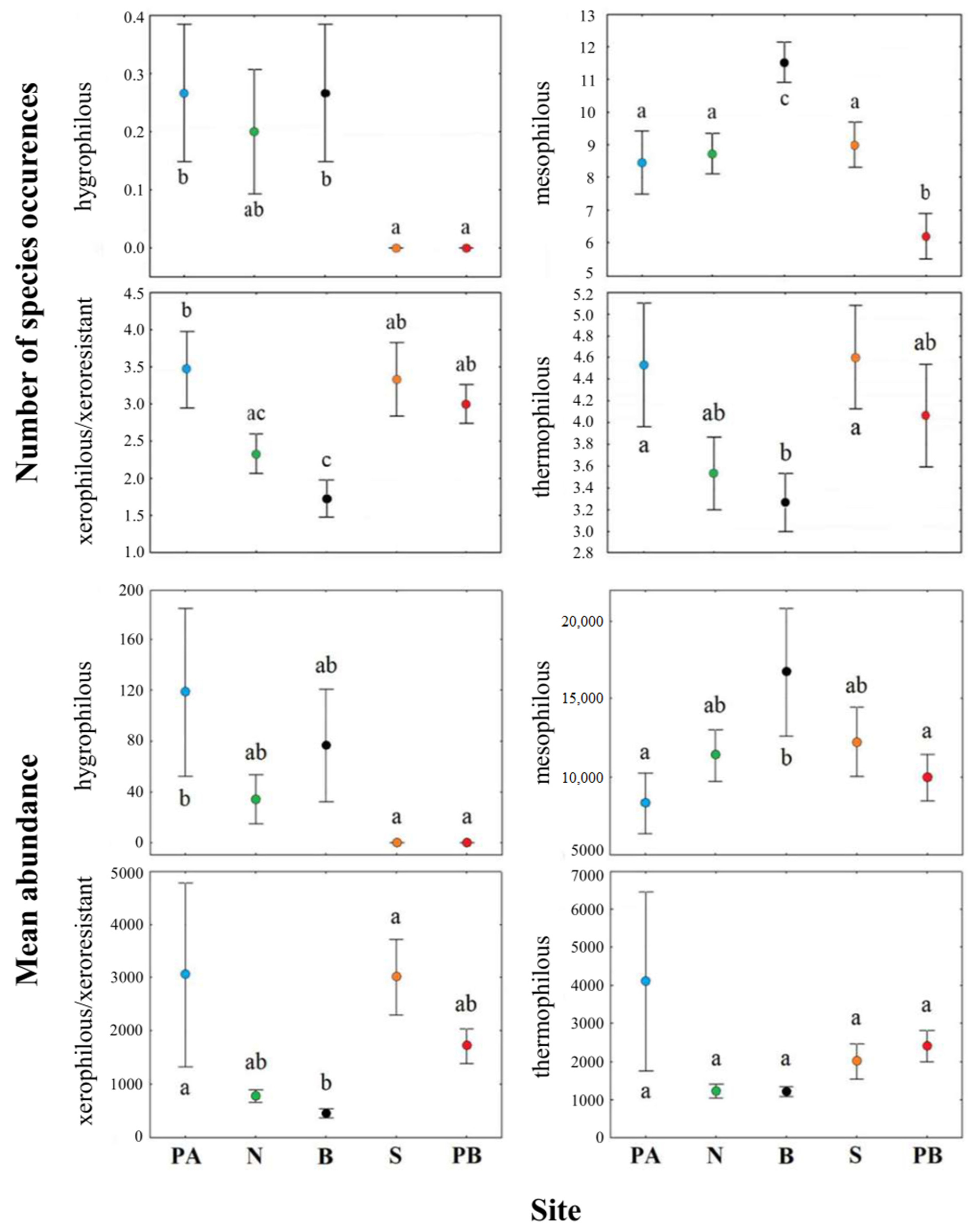
3. Results
4. Discussion
4.1. Characteristics of the Sites at the Dolines
4.2. Effect of Complex Habitat Conditions on Communities
4.3. Distributional Patterns of the Functional Groups
4.4. Endemic and Relict Species vs. Karst Dolines as Microrefugia
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Ecol. Category | Species | 1PA | 1N | 1B | 1S | 1PB | 2PA | 2N | 2B | 2S | 2PB | 3PA | 3N | 3B | 3S | 3PB |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| e 1,2, m 3 | Allacma fusca (Linné, 1758) | 25 | – | 25 | – | 76 | – | 25 | – | 76 | – | 51 | – | – | – | – |
| t 4,5, x 4,6 | Axenyllodes bayeri (Kseneman, 1935) | – | – | – | – | – | – | – | – | – | – | 51 | – | – | – | – |
| e 4,7, m 7 | Ceratophysella engadinensis (Gisin, 1949) | – | – | – | – | – | 331 | – | 153 | – | – | – | – | – | – | – |
| e 7, m 7 | Ceratophysella succinea (Gisin, 1949) | – | – | – | – | 204 | 1605 | – | 510 | – | – | 25 | – | 25 | – | 204 |
| e 8, m 8 | Desoria germanica (Hüther et Winter, 1961) | 382 | 204 | 586 | 25 | 611 | – | 280 | 306 | 25 | – | – | 204 | 25 | 255 | 127 |
| e 9, m 9 | Deutonura conjuncta (Stach, 1926) | 25 | – | – | – | – | – | – | – | – | – | – | – | 25 | – | – |
| t 10,11, x 10,11 | Doutnacia mols Fiellberg, 1998 | – | – | – | – | – | 408 | – | – | – | – | – | – | – | – | – |
| t 4,10, x 4,10 | Doutnacia xerophila Rusek, 1974 | 204 | 25 | 25 | 102 | 25 | 2981 | 51 | 25 | 25 | – | – | 153 | 25 | 25 | 866 |
| t 12, x 12,13 | Entomobrya handschini Stach, 1922 | – | – | – | – | – | – | – | – | – | – | 25 | – | – | – | – |
| t 12, m 13 | Entomobrya quinquelineata Börner, 1901 | – | – | – | – | – | – | 25 | – | 25 | – | – | – | – | – | – |
| un | Entomobrya sp. 1 | – | – | – | – | 51 | 25 | 76 | 76 | 51 | 76 | 178 | 357 | 25 | 76 | 127 |
| un | Entomobrya sp. 2 | 331 | 76 | 51 | 102 | – | 25 | 51 | – | – | – | 25 | 51 | – | – | – |
| e 4,8, m 8 | Folsomia manolachei Bagnall, 1939 | – | – | 892 | 459 | 76 | – | – | – | – | – | – | – | 51 | 76 | – |
| e 4,8, m 13 | Folsomia penicula Bagnall, 1939 | – | – | – | 25 | – | – | – | – | – | – | – | – | – | – | – |
| e 13,14, h 13 | Folsomia quadrioculata (Tullberg, 1871) | 331 | 102 | 229 | – | – | – | – | – | – | – | – | – | – | – | – |
| e 14, m 13 | Friesea mirabilis (Tullberg, 1871) | – | – | – | – | – | – | – | – | – | – | – | 25 | – | – | – |
| e 14,15, m 13 | Friesea truncata Cassagnau, 1958 | – | – | 255 | – | – | – | – | 1147 | – | – | – | – | – | – | – |
| t 8,16, x 13 | Hemisotoma thermophila (Axelson, 1900) | – | – | – | – | – | 1656 | – | – | – | – | – | – | – | – | – |
| t 17, m 13 | Heteromurus nitidus (Templeton, 1835) | 127 | 25 | – | 127 | 25 | 51 | 331 | 331 | 153 | – | – | 76 | 76 | 153 | 25 |
| e 7,18, m 19 | Hypogastrura assimilis (Krasusbauer, 1898) | 459 | 3440 | 76 | – | 1325 | 1197 | 5885 | 1045 | 4433 | 9376 | 1096 | 1707 | 51 | 13554 | 10650 |
| t 4,8, x 8,20 | Isotoma anglicana Lubbock, 1862 | 204 | 204 | 357 | 586 | 535 | 1197 | 255 | 153 | – | 484 | 484 | 357 | 280 | 76 | 459 |
| e 21, m 8,22 | Isotomiella minor (Schäffer, 1896) | 1605 | 51 | 841 | 51 | – | – | 1299 | 1758 | – | – | – | 2064 | 15593 | 2930 | 102 |
| t 4,8, x 4,8 | Isotomodes productus (Axelson, 1906) | – | – | – | – | 306 | 102 | – | – | – | – | 25 | – | – | – | 76 |
| e 10, m 10 | Karlstejnia annae Rusek, 1974 | 153 | – | – | – | 51 | – | – | – | – | – | – | – | – | – | – |
| e 13, m 13 | Lepidocyrtus cyaneus Tullberg, 1871 | 229 | 51 | 866 | 943 | 561 | 943 | 5580 | 1860 | 255 | 433 | – | 917 | 1427 | 2497 | 102 |
| e 20, m 20 | Lepidocyrtus lignorum (Fabricius, 1775) | – | – | – | – | – | 51 | – | 25 | – | – | – | 25 | 611 | 76 | – |
| e 23, m 23 | Megalothorax minimus Willem, 1900 | 1809 | 306 | – | 1045 | 102 | 535 | 1503 | 459 | 153 | 204 | 25 | 306 | 4790 | 382 | 51 |
| e 24, m 24 | Megalothorax willemi Schneider et d’Haese, 2013 | 51 | – | – | 102 | – | 51 | – | – | – | – | – | – | 25 | – | – |
| t 4,10, m 13 | Mesaphorura critica Ellis, 1976 | 153 | 204 | 178 | 102 | 127 | 2369 | 76 | 102 | 102 | 25 | 229 | 25 | 357 | 51 | 1248 |
| e 2,4, m 10 | Mesaphorura florae Simón, Ruiz, Martin et Luciáňez, 1994 | 76 | – | – | 127 | – | – | 51 | – | – | – | – | – | – | 102 | 178 |
| e 10, m 13 | Mesaphorura hylophila Rusek, 1982 | – | – | 25 | – | – | 76 | – | 25 | – | – | – | – | – | – | – |
| e 4, m 14 | Mesaphorura italica (Rusek, 1971) | – | – | – | – | – | 25 | 25 | 25 | – | – | – | 51 | – | – | – |
| e 10, m 10 | Mesaphorura jirii Rusek, 1982 | – | – | – | – | – | – | – | – | – | – | – | – | 76 | – | 51 |
| un | Mesaphorura rudolfi Rusek, 1987 | – | – | – | – | – | – | – | – | – | – | – | – | – | 51 | – |
| e 10, m 10 | Mesaphorura yosii (Rusek, 1967) | – | 25 | – | – | 25 | – | – | – | – | 25 | – | – | 51 | 25 | – |
| t 10,25, x 4,10 | Metaphorura affinis (Börner, 1902) | – | – | 25 | – | 76 | 306 | – | – | 127 | – | – | – | – | – | – |
| e 26, m 23,26 | Micranurida pygmaea Börner, 1901 | – | – | – | – | – | 51 | – | – | – | – | – | – | – | – | – |
| t 27, x 27 | Microgastrura duodecimoculata Stach, 1922 | 739 | – | – | – | – | – | – | – | 102 | – | – | – | – | 51 | – |
| e 2,4, m 13 | Oncopodura crassicornis Shoebotham, 1911 | – | – | – | 25 | 51 | – | – | – | – | – | – | – | 25 | 51 | – |
| e 4, m 28 | Orchesella bifasciata Nicolet, 1842 | – | – | – | – | – | – | – | 76 | – | – | – | – | – | – | – |
| t 29, x 13, 29 | Orchesella multifasciata Stscherbakow, 1898 | – | – | – | 25 | – | – | 25 | 102 | 51 | – | – | 25 | 178 | – | – |
| c 4,30, h 4,30 | Orthonychiurus rectopapillatus (Stach, 1933) | – | – | – | – | – | – | – | – | – | – | 25 | – | – | – | – |
| e 8, m 8 | Parisotoma notabilis (Schäffer, 1896) | 790 | 535 | 739 | 408 | 25 | 1096 | 229 | 815 | 25 | 51 | 306 | 1325 | 1045 | 25 | – |
| t 14, x 13,20 | Pratanurida cassagnaui Rusek, 1973 | 76 | 408 | – | 25 | – | 76 | – | 51 | – | 51 | 204 | 459 | 102 | 102 | 76 |
| e 8, m 8 | Proisotoma brevidens Stach, 1947 | – | 76 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| t 8,31, x 8,31 | Proisotomodes bipunctatus (Axelson, 1903) | – | – | – | – | – | – | – | – | 2573 | 994 | – | – | – | – | – |
| e 4,30, m 4,30 | Protaphorura armata (Tullberg, 1869) | – | 51 | 127 | 2420 | 25 | 25 | 331 | – | – | – | – | – | 229 | – | – |
| e 30, m 30 | Protaphorura pannonica (Haybach, 1960) | 943 | 968 | 229 | 204 | 1045 | 4255 | 1121 | – | 306 | 127 | – | 2217 | 4561 | 510 | 1554 |
| t 4, m 13 | Protaphorura serbica (Lokša et Bogojevič, 1967) | – | – | – | – | – | – | – | – | 153 | – | – | – | – | – | – |
| t 4,5, x4,13 | Pseudachorutes pratensis Rusek, 1973 | 25 | – | – | – | 713 | 76 | 51 | 25 | 76 | – | – | – | – | 51 | 51 |
| e 2, m 2 | Pseudosinella albida (Stach, 1930) | 1783 | 943 | 2854 | 484 | 102 | 127 | 280 | 1197 | 739 | – | 76 | 153 | 25 | 25 | – |
| un | Pseudosinella cf. csafordi Winkler et Mateos, 2018 | – | – | – | – | – | – | – | – | – | – | – | – | 76 | 25 | – |
| e 2, m 13 | Pseudosinella horaki Rusek, 1985 | 892 | – | 102 | 1401 | 25 | – | 178 | 892 | 25 | – | 25 | – | 51 | 25 | – |
| t 32, m 2 | Pumilinura loksai (Dunger, 1973) | 102 | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| e 7, x 25 | Schoettella ununguiculata (Tullberg, 1869) | – | – | – | – | – | – | – | – | – | 102 | – | – | – | 4408 | – |
| e 1, m 20 | Sminthurinus aureus (Lubbock, 1862) | 179 | – | 102 | – | – | 204 | 25 | 127 | 561 | 153 | 51 | 331 | – | – | – |
| t 2, m 1,13 | Sminthurinus elegans (Fitch, 1863) | 25 | 178 | 841 | 331 | 586 | – | 382 | 357 | – | – | 76 | – | – | 204 | 102 |
| t 1, x 1 | Sminthurus maculatus Tömösváry, 1883 | – | – | – | – | 51 | – | – | – | – | – | – | – | – | – | – |
| e 1, m 13 | Sphaeridia pumilis (Krausbauer, 1898) | 102 | – | – | – | – | – | – | 25 | 459 | – | 25 | 51 | 1096 | – | – |
| un | Wankeliella sp. juv. | – | – | – | – | 25 | – | – | – | – | – | – | – | – | 25 | 25 |
| t 14, x 14 | Willemia scandinavica Stach, 1949 | – | 51 | – | – | – | 306 | 127 | – | – | – | 25 | 153 | – | – | 280 |
| t 20, x 6,20 | Willowsia buski (Lubbock, 1869) | – | – | – | – | – | – | – | 25 | 51 | – | – | – | – | 178 | – |
| t 20, x 13,20 | Willowsia nigromaculata (Lubbock, 1873) | – | – | – | – | – | – | – | – | – | – | – | – | – | 382 | – |
References
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.N.; de Siqueira, M.F.; Grainger, A.; Hannah, L.; et al. Extinction risk from climate change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenzweig, C.; Casassa, G.; Karoly, D.J.; Imeson, A.; Liu, C.; Menzel, A.; Rawlins, S.; Root, T.L.; Seguin, B.; Tryjanowski, P. Assessment of observed changes and responses in natural and managed systems. In Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, 1st ed.; Parry, M.L., Canziani, O.F., Palutikof, J.P., Van Der Linden, P.J., Hansen, C.E., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 79–131. [Google Scholar] [CrossRef]
- Blankinship, J.C.; Niklaus, P.A.; Hungate, B.A. A meta-analysis of responses of soil biota to global change. Oecologia 2011, 165, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Keppel, G.; Van Niel, K.P.; Wardell-Johnson, G.W.; Yates, C.J.; Byrne, M.; Mucina, L.; Schut, A.G.T.; Hopper, S.D.; Franklin, S.E. Refugia: Identifying and understanding safe havens for biodiversity under climate change. Glob. Ecol. Biogeogr. 2012, 21, 393–404. [Google Scholar] [CrossRef]
- Bátori, Z.; Gallé, R.; Erdős, L.; Körmöczi, L. Ecological conditions, flora and vegetation of a large doline in the Mecsek Mountains (South Hungary). Acta Bot. Croat. 2011, 70, 147–155. [Google Scholar] [CrossRef]
- Bátori, Z.; Lengyel, A.; Maróti, M.; Körmöczi, L.; Tölgyesi, C.; Bíró, A.; Tóth, M.; Kincses, Z.; Cseh, V.; Erdős, L. Microclimate-vegetation relationships in natural habitat islands: Species preservation and conservation perspectives. Idojaras 2014, 118, 257–281. [Google Scholar]
- Bátori, Z.; Vojtkó, A.; Farkas, T.; Szabó, A.; Havadtői, K.; Vojtkó, A.E.; Tölgyesi, C.; Cseh, V.; Erdős, L.; Maák, I.E.; et al. Large-and small-scale environmental factors drive distributions of cool-adapted plants in karstic microrefugia. Ann. Bot. 2017, 119, 301–309. [Google Scholar] [CrossRef]
- Bátori, Z.; Vojtkó, A.; Maák, I.E.; Lőrinczi, G.; Farkas, T.; Kántor, N.; Tanács, E.; Kiss, P.J.; Juhász, O.; Módra, G.; et al. Karst dolines provide diverse microhabitats for different functional groups in multiple phyla. Sci. Rep. 2019, 9, e7176. [Google Scholar] [CrossRef] [Green Version]
- Bátori, Z.; Erdős, L.; Gajdács, M.; Barta, K.; Tobak, Z.; Tölgyesi, C. Managing climate change microrefugia for vascular plants in forested karst landscapes. For. Ecol. Manag. 2021, 496, 119446. [Google Scholar] [CrossRef]
- Su, Y.; Tang, Q.; Mo, F.; Xue, Y. Karst tiankengs as refugia for indigenous tree flora amidst a degraded landscape in southwestern China. Sci. Rep. 2017, 7, e4249. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Zhang, Z.; Shen, J.; Wang, Z. Bryophyte diversity in karst sinkholes affected by different degrees of human disturbance. Acta Soc. Bot. Pol. 2019, 88, 3620. [Google Scholar] [CrossRef]
- Kiss, P.J.; Tölgyesi, C.; Bóni, I.; Erdős, L.; Vojtkó, A.; Maák, I.E.; Bátori, Z. The effects of intensive logging on the capacity of karst dolines to provide potential microrefugia for cool-adapted plants. Acta Geogr. Slov. 2020, 60, 37–48. [Google Scholar] [CrossRef]
- Öztürk, M.Z.; Savran, A. An oasis in the Central Anatolian steppe: The ecology of a callopse doline. Acta Biologica Turcica 2020, 33, 100–113. [Google Scholar]
- Shui, W.; Chen, Y.; Jian, X.; Jiang, C.; Wang, Q.; Zeng, Y.; Zhu, S.; Guo, P.; Li, H. Original karst tiankeng with underground virgin forest as an inaccessible refugia originated from a degraded surface flora in Yunnan, China. Sci. Rep. 2022, 12, 1–14. [Google Scholar] [CrossRef]
- Shui, W.; Liu, Y.; Jiang, C.; Sun, X.; Jian, X.; Guo, P.; Li, H.; Zhu, S.; Zong, S.; Ma, M. Are degraded karst tiankengs coupled with microclimatic underground forests the refugia of surface flora? Evidence from China’s Yunnan. Front. Ecol. Evol. 2022, 10, 1015468. [Google Scholar] [CrossRef]
- Yáñez-Espinosa, L.; Flores, J.; Fortanelli-Martínez, J.; Quintero-Ruiz, J.R.; De Nova-Vázquez, J.A.; Reyes-Hernández, H. Leaf traits variation of Myriocarpa longipes and Brosimum alicastrum in relation to microclimatic gradient in tropical dolines of Mexico. J. Torrey Bot. Soc. 2022, 149, 262–272. [Google Scholar] [CrossRef]
- Raschmanová, N.; Kováč, Ľ.; Miklisová, D. The effect of mesoclimate on Collembola diversity in the Zádiel Valley, Slovak Karst (Slovakia). Eur. J. Soil Biol. 2008, 44, 463–472. [Google Scholar] [CrossRef]
- Raschmanová, N.; Miklisová, D.; Kováč, Ľ. Soil Collembola communities along a steep microclimatic gradient in the collapse doline of the Silická ľadnica Cave, Slovak Karst (Slovakia). Biologia 2013, 68, 470–478. [Google Scholar] [CrossRef]
- Raschmanová, N.; Miklisová, D.; Kováč, Ľ.; Šustr, V. Community composition and cold tolerance of soil Collembola in a collapse karst doline with strong microclimate inversion. Biologia 2015, 70, 802–811. [Google Scholar] [CrossRef]
- Raschmanová, N.; Miklisová, D.; Kováč, Ľ. A unique small–scale microclimatic gradient in a temperate karst harbours exceptionally high diversity of soil Collembola. Int. J. Speleol. 2018, 47, 247–262. [Google Scholar] [CrossRef]
- Sólymos, P.; Farkas, R.; Kemencei, Z.; Páll-Gergely, B.; Vilisics, F.; Nagy, A.; Kisfali, M.; Hornung, E. Micro-habitat scale survey of land snails in dolines of the Alsó-hegy, Aggtelek National Park, Hungary. Mollusca 2009, 27, 167–171. [Google Scholar]
- Vilisics, F.; Sólymos, P.; Nagy, A.; Farkas, R.; Kemencei, Z.; Hornung, E. Small scale gradient effects on isopods (Crustacea: Oniscidea) in karstic sinkholes. Biologia 2011, 66, 499–505. [Google Scholar] [CrossRef]
- Kemencei, Z.; Farkas, R.; Páll-Gergely, B.; Vilisics, F.; Nagy, A.; Hornung, E.; Sólymos, P. Microhabitat associations of land snails in forested dolinas: Implications for coarse filter conservation. Community Ecol. 2014, 15, 180–186. [Google Scholar] [CrossRef]
- Schlaghamerský, J.; Devetter, M.; Háňel, L.; Tajovský, K.; Starý, J.; Tuf, I.H.; Pižl, V. Soil fauna across Central European sandstone ravines with temperature inversion: From cool and shady to dry and hot places. Appl. Soil Ecol. 2014, 83, 30–38. [Google Scholar] [CrossRef]
- Růžička, V.; Mlejnek, R.; Juřičková, L.; Tajovský, K.; Šmilauer, P.; Zajíček, P. Invertebrates of the Macocha Abyss (Moravian Karst, Czech Republic). Acta Carsologica 2016, 45, 71–84. [Google Scholar] [CrossRef] [Green Version]
- Bátori, Z.; Lőrinczi, G.; Tölgyesi, C.; Módra, G.; Juhász, O.; Aguilon, D.J.; Vojtkó, A.; Valkó, O.; Deák, B.; Erdős, L.; et al. Karstic microrefugia host functionally specific ant assemblages. Front. Ecol. Evol. 2020, 8, 613738. [Google Scholar] [CrossRef]
- Bátori, Z.; Gallé, R.; Gallé-Szpisjak, N.; Császár, P.; Nagy, D.D.; Lőrinczi, G.; Torma, A.; Tölgyesi, C.; Maák, I.E.; Frei, K.; et al. Topographic depressions provide potential microrefugia for ground-dwelling arthropods. Elementa 2022, 10, e00084. [Google Scholar] [CrossRef]
- Marcin, M.; Raschmanová, N.; Miklisová, D.; Kováč, Ľ. Microclimate and habitat heterogeneity as important drivers of soil Collembola in a karst collapse doline in the temperate zone. Invertebr. Biol. 2021, 140, e12315. [Google Scholar] [CrossRef]
- Dobrowski, S.Z. A climatic basis for microrefugia: The influence of terrain on climate. Glob. Chang. Biol. 2011, 17, 1022–1035. [Google Scholar] [CrossRef]
- Ashcroft, M.B.; Gollan, J.R.; Warton, D.I.; Ramp, D. A novel approach to quantify and locate potential microrefugia using topoclimate, climate stability, and isolation from the matrix. Glob. Chang. Biol. 2012, 18, 1866–1879. [Google Scholar] [CrossRef] [Green Version]
- Keppel, G.; Mokany, K.; Wardell-Johnson, G.W.; Phillips, B.L.; Welbergen, J.A.; Reside, A.E. The capacity of refugia for conservation planning under climate change. Front. Ecol. Environ. 2015, 13, 106–112. [Google Scholar] [CrossRef]
- Keppel, G.; Ottaviani, G.; Harrison, S.; Wardell-Johnson, G.W.; Marcantonio, M.; Mucina, L. Towards an eco-evolutionary understanding of endemism hotspots and refugia. Ann. Bot. 2018, 122, 927–934. [Google Scholar] [CrossRef]
- Yao, Z.; Dong, T.; Zheng, G.; Fu, J.; Li, S. High endemism at cave entrances: A case study of spiders of the genus Uthina. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Suggitt, A.J.; Wilson, R.J.; Isaac, N.J.B.; Beale, C.M.; Auffret, A.G.; August, T.; Bennie, J.J.; Crick, H.Q.P.; Duffield, S.; Fox, R.; et al. Extinction risk from climate change is reduced by microclimatic buffering. Nat. Clim. Change 2018, 8, 713–717. [Google Scholar] [CrossRef] [Green Version]
- Sinclair, B.J. Effects of increased temperatures simulating climate change on terrestrial invertebrates on Ross Island, Antarctica. Pedobiologia 2002, 46, 150–160. [Google Scholar] [CrossRef]
- Bokhorst, S.; Huiskes, A.; Convey, P.; Van Bodegom, P.M.; Aerts, R. Climate change effects on soil arthropod communities from the Falkland Islands and the Maritime Antarctic. Soil Biol. Biochem. 2008, 40, 1547–1556. [Google Scholar] [CrossRef]
- Alatalo, J.M.; Jägerbrand, A.K.; Čuchta, P. Collembola at three alpine subarctic sites resistant to twenty years of experimental warming. Sci. Rep. 2015, 5, 18161. [Google Scholar] [CrossRef] [Green Version]
- Greenslade, P.; Slatyer, R. Montane Collembola at risk from climate change in Australia. Eur. J. Soil Biol. 2017, 80, 85–91. [Google Scholar] [CrossRef]
- Holmstrup, M.; Ehlers, B.K.; Slotsbo, S.; Ilieva-Makulec, K.; Sigurdsson, B.D.; Leblans, N.I.; Ellers, I.; Berg, M.P. Functional diversity of Collembola is reduced in soils subjected to short-term, but not long-term, geothermal warming. Funct. Ecol. 2018, 32, 1304–1316. [Google Scholar] [CrossRef]
- Koltz, A.M.; Schmidt, N.M.; Høye, T.T. Differential arthropod responses to warming are altering the structure of Arctic communities. R. Soc. Open Sci. 2018, 5, 171503. [Google Scholar] [CrossRef] [Green Version]
- Mammola, S.; Piano, E.; Cardoso, P.; Vernon, P.; Domínguez-Villar, D.; Culver, D.C.; Pipan, T.; Isaia, M. Climate change going deep: The effects of global climatic alterations on cave ecosystems. Anthr. Rev. 2019, 6, 98–116. [Google Scholar] [CrossRef]
- Sauro, U. Closed depressions in karst areas. In Encyclopedia of caves, 3rd ed.; White, W.B., . Culver, D.C., Pipan, T., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 285–300. [Google Scholar] [CrossRef]
- Hopkin, S.P. Biology of Springtails: (Insecta: Collembola), 1st ed.; Oxford University press: Oxford, UK, 1997; pp. 1–330. [Google Scholar]
- Rusek, J. Biodiversity of Collembola and their functional role in the ecosystem. Biodivers. Conserv. 1998, 7, 1207–1219. [Google Scholar] [CrossRef]
- Potapov, A.; Bellini, B.; Chown, S.; Deharveng, L.; Janssens, F.; Kováč, Ľ.; Kuznetsova, N.; Ponge, J.F.; Potapov, M.; Querner, P.; et al. Towards a global synthesis of Collembola knowledge—challenges and potential solutions. Soil Org. 2020, 92, 161–188. [Google Scholar] [CrossRef]
- Kærsgaard, C.W.; Holmstrup, M.; Malte, H.; Bayley, M. The importance of cuticular permeability, osmolyte production and body size for the desiccation resistance of nine species of Collembola. J. Insect Physiol. 2004, 50, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Raschmanová, N.; Šustr, V.; Kováč, Ľ.; Parimuchová, A.; Devetter, M. Testing the climatic variability hypothesis in edaphic and subterranean Collembola (Hexapoda). J. Therm. Biol. 2018, 78, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, N.; Engtsson, J.B.; Persson, N.T. Effects of experimental irrigation and drought on the composition and diversity of soil fauna in a coniferous stand. J. Appl. Ecol. 2002, 39, 924–936. [Google Scholar] [CrossRef]
- Kardol, P.; Reynolds, W.N.; Norby, R.J.; Classen, A.T. (2011). Climate change effects on soil microarthropod abundance and community structure. Appl. Soil Ecol. 2011, 47, 37–44. [Google Scholar] [CrossRef]
- Jakál, J. Kras Silickej Planiny (The Karst of the Silická Planina Plateau), 1st ed.; Osveta: Martin, Czechoslovakia, 1975; pp. 1–152. (In Slovak) [Google Scholar]
- Rozložník, M.; Szőllős, F.; Uhrin, M.; Karasová, E. Slovenský kras—Slovak Karst Biosphere Reserve. In Biosphere Reserves on the Crossroads of Central Europe, Czech Republic—Slovak Republic. Czech National Committee for UNESCO’s MaB Programme, 1st ed.; Jenik, J., Price, F., Eds.; Empora: Prague, Czech Republic, 1994; pp. 113–128. [Google Scholar]
- Bárány-Kevei, I. Microclimate of karstic dolines. Acta Climatologica 1999, 32–33, 19–27. [Google Scholar]
- Crossley, D.A.; Blair, J.M. A high efficiency, low-technology Tullgren type extractor for soil microarthropods. Agric. Ecosyst. Environ. 1991, 34, 187–192. [Google Scholar] [CrossRef]
- Bretfeld, G. Symphypleona. In Synopses on Palearctic Collembola, 1st ed.; Dunger, W., Ed.; Senckenberg Museum of Natural History Görlitz: Görlitz, Germany, 1999; Volume 2, pp. 1–318. [Google Scholar]
- Fjellberg, A. The Collembola of Fennoscandia and Denmark. Part I: Poduromorpha. In Fauna Entomologica Scandinavica, 1st ed.; Kristensen, N.P., Michelsen, V., Eds.; Brill: Leiden, The Netherlands, 1998; Volume 35, pp. 1–184. [Google Scholar]
- Gisin, H. Collembolenfauna Europas, 1st ed.; Museum D‘ Histoire Naturelle: Geneva, Germany, 1960; pp. 1–297. [Google Scholar]
- Potapov, M. Isotomidae. In Synopses on Palearctic Collembola, 1st ed.; Dunger, W., Ed.; State Museum of the Natural History Museum of Görlitz: Görlitz, Germany, 2001; Volume 3, pp. 1–603. [Google Scholar]
- Thibaud, J.M.; Schulz, H.J.; da Gama Assalino, M.M. Hypogastruridae. In Synopses on Palearctic Collembola, 1st ed.; Dunger, W., Ed.; State Museum of the Natural History Museum of Görlitz: Görlitz, Germany, 2004; Volume 4, pp. 1–287. [Google Scholar]
- Zimdars, B.; Dunger, W. Tullberginae Bagnall, 1935. In Synopses on Palearctic Collembola, 1st ed.; Dunger, W., Ed.; Abhandlungen und Berichte des Naturkundemuseums Görlitz: Görlitz, Germany, 1994; Volume 1, pp. 1–71. [Google Scholar]
- Jordana, R.; Arbea, J.; Simón, C.; Luciáñez, M. Fauna Iberica. Collembola Poduromorpha, 1st ed.; Museo Nacional de Ciencias Naturales: Madrid, Spain, 1997; pp. 1–807. [Google Scholar]
- Jordana, R. Capbryinae & Entomobryini. In Synopses on Palaearctic Collembola, 1st ed.; Dunger, W., Burkhardt, U., Eds.; Senckenberg Museum of Natural History Görlitz: Görlitz, Germany, 2012; Volume 7, pp. 1–390. [Google Scholar]
- Stach, J. The Apterygotan Fauna of Poland in Relation to the World-Fauna of this Group of Insects: Tribe: Orchesellini, 1st ed.; Polska Akademia Nauk: Kraków, Poland, 1960; pp. 1–151. [Google Scholar]
- Stach, J. The Apterygotan Fauna of Poland in Relation to the World-Fauna of this Group of Insects. Tribe: Entomobryini, 1st ed.; Polska Akademia Nauk: Kraków, Poland, 1963; pp. 1–126. [Google Scholar]
- Quinn, P.; Beven, K.; Chevallier, P.; Planchon, O. The prediction of hillslope flow paths for distributed hydrological modelling using digital terrain models. Hydrol. Process. 1991, 5, 59–79. [Google Scholar] [CrossRef]
- Hegedüšová-Vantarová, K.; Škodová, I. Rastlinné Spoločenstvá Slovenska. 5. Travinno-Bylinná Vegetácia (Plant Communities of Slovakia 5. Grassland Vegetation), 1st ed.; Veda: Bratislava, Slovakia, 2014; pp. 1–580. [Google Scholar]
- ISO 10390:2005; Soil Quality—Determination of pH. International Standards Organization: Geneva, Switzerland, 2005.
- ISO 10694:1995; Soil Quality—Determination of Organic and Total Carbon after Dry Combustion (Elementary Analysis). International Standards Organization: Geneva, Switzerland, 1995.
- Kobza, J.; Hrivňáková, K.; Makovníková, J.; Barančíková, G.; Bezák, P.; Bezáková, Z.; Dodok, R.; Grečo, V.; Chlpík, J.; Lištjak, M.; et al. Jednotné Pracovné Postupy Rozborov Pôd, 1st ed.; Výskumný ústav pôdoznalectva a ochrany pôdy: Bratislava, Slovakia, 2011; pp. 1–136. [Google Scholar]
- StatSoft, Inc. STATISTICA (Data Analysis Software System) Version 12. 2013. Available online: www.statsoft.com/ (accessed on 7 March 2022).
- Tischler, W. Synökologie der Landtiere, 1st ed.; Fischer: Stuttgart, Germany, 1955; pp. 1–414. [Google Scholar]
- McCune, B.; Grace, J.B.; Urban, D.L. Analysis of Ecological Communities; MjM Software Design: Gleneden Beach, OR, USA, 2002; Volume 28, pp. 1–300. [Google Scholar]
- McCune, B.; Mefford, M.J. PC-ORD. Multivariate Analysis of Ecological Data; MjM Software Design: Gleneden Beach, OR, USA, 2016; pp. 1–34. [Google Scholar]
- Fridley, J.D. Downscaling climate over complex terrain: High finescale (<1000 m) spatial variation of near-ground temperatures in a montane forested landscape (Great Smoky Mountains). JAMC 2009, 48, 1033–1049. [Google Scholar] [CrossRef] [Green Version]
- Bárány-Kevei, I.; Zboray, Z.; Kiss, M. Data for the geoecology of solution karst dolines, with particular attention to climatic, soil and biogenic environment. Acta Climatol. Chorol. 2021, 4, 27–71. [Google Scholar] [CrossRef]
- Ǻström, M.; Dynesius, M.; Hylander, K.; Nilsson, C. Slope aspect modifies community responses to clear-cutting in boreal forests. Ecology 2007, 88, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Hishi, T.; Urakawa, R.; Saitoh, S.; Maeda, Y.; Hyodo, F. Topography is more important than forest type as a determinant for functional trait composition of Collembola community. Pedobiologia 2022, 90, e150776. [Google Scholar] [CrossRef]
- Quintero-Ruiz, J.R.; Yáñez-Espinosa, L.; Flores, J.; Fortanelli, J.; De-Nova, A.; Reyes-Hernandez, H.; Rodas-Ortíz, J.P. Analysis of the soil and microclimate relationship in two dolines of Carso Huasteco, Mexico. JNRD 2019, 9, 25–33. [Google Scholar] [CrossRef]
- Hortal, J.; Triantis, K.A.; Meiri, S.; Thébault, E.; Sfenthourakis, S. Island species richness increases with habitat diversity. Am. Nat. 2009, 174, 205–217. [Google Scholar] [CrossRef] [Green Version]
- Stein, A.; Gerstner, K.; Kreft, H. Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecol. Lett. 2014, 17, 866–880. [Google Scholar] [CrossRef]
- Báldi, A. Habitat heterogeneity overrides the species–area relationship. J. Biogeogr. 2008, 35, 675–681. [Google Scholar] [CrossRef]
- Seibold, S.; Bässler, C.; Brandl, R.; Büche, B.; Szallies, A.; Thorn, S.; Ulyshen, M.D.; Müller, J. Microclimate and habitat heterogeneity as the major drivers of beetle diversity in dead wood. J. Appl. Ecol. 2016, 53, 934–943. [Google Scholar] [CrossRef] [Green Version]
- Jureková, N.; Raschmanová, N.; Miklisová, D.; Kováč, Ľ. Mesofauna at the soil-scree interface in a deep karst environment. Diversity 2021, 13, 242. [Google Scholar] [CrossRef]
- Wallwork, J.A. Ecology of Soil Animals; McGraw-Hill: London, UK, 1970; pp. 1–283. [Google Scholar]
- Coleman, D.C.; Crossley, D.C. Fundamentals of Soil Ecology, 1st ed.; Academic Press: Cambridge, MA, USA, 1995; pp. 1–205. [Google Scholar] [CrossRef]
- Maclean, I.M.; Hopkins, J.J.; Bennie, J.; Lawson, C.R.; Wilson, R.J. Microclimates buffer the responses of plant communities to climate change. Glob. Ecol. Biogeogr. 2015, 24, 1340–1350. [Google Scholar] [CrossRef]
- Chen, Y.P.; Jiang, C.; Jian, X.M.; Shui, W.; Hu, Y.; Ma, T.; Xiang, Z.Y. Spatial distribution characteristics of grassland plant communities in a moderately degraded tiankeng in Zhanyi, Yunnan. Acta Ecol. Sin. 2018, 38, 8008–8021. [Google Scholar] [CrossRef]
- Kováč, Ľ. Ice caves. In Cave Ecology, 1st ed.; Moldovan, O.T., Kováč, Ľ., Halse, S., Eds.; Springer: Cham, Switzerland, 2018; Volume 235, pp. 331–349. [Google Scholar] [CrossRef]
- Perşoiu, A.; Lauritzen, S.-E. Ice Caves, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–752. [Google Scholar]
- Bátori, Z.; Vojtkó, A.; Keppel, G.; Tölgyesi, C.; Čarni, A.; Zorn, M.; Farkas, T.; Erdős, L.; Kiss, P.J.; Módra, G.; et al. Anthropogenic disturbances alter the conservation value of karst dolines. Biodivers. Conserv. 2019, 29, 503–525. [Google Scholar] [CrossRef] [Green Version]
- Papáč, V.; Raschmanová, N.; Kováč, Ľ. New species of the genus Megalothorax Willem, 1900 (Collembola: Neelipleona) from a superficial subterranean habitat at Dobšinská Ice Cave, Slovakia. Zootaxa 2019, 4648, 165–177. [Google Scholar] [CrossRef]
- Ford, D.C.; Williams, P. Karst Hydrogeology and Geomorphology, 1st ed.; John Wiley & Sons: Chichester, UK, 2007; pp. 1–562. [Google Scholar]
- Mosblech, N.A.S.; Bush, M.B.; van Woesik, R. On metapopulations and microrefugia: Palaeoecological insights. J. Biogeogr. 2011, 38, 419–429. [Google Scholar] [CrossRef]
- Fanciulli, P.P.; Carapelli, A.; Belloni, M.; Dallai, R.; Frati, F. Allozyme variation in the springtails Allacma fusca and A. gallica (Collembola, Sminthuridae). Pedobiologia 2009, 52, 309–324. [Google Scholar] [CrossRef]
- Smolis, A. Review of the Polish Deutonura CASSAGNAU, 1979 (Collembola: Neanuridae: Neanurinae) with redescription of D. conjuncta (STACH, 1926). Acta zool. cracov. Ser. B Invertebrata 2008, 51, 43–82. [Google Scholar] [CrossRef] [Green Version]
- Dunger, W.; Schlitt, B. Synopses on Palaearctic Collembola: Tullbergiidae. Soil Org. 2011, 83, 1–168. [Google Scholar]
- Traser, G. Xerothermic Collembola from the Bükk–Mts NE–Hungary. Folia Hist.–Nat. Musei Matra. 2002, 26, 159–161. [Google Scholar]
- Rusek, J. Collembola. In Terrestrial Invertebrates of the Pálava Biosphere Reserve of UNESCO, I. Folia Facultatis Scientarium Naturalium Universitatis Masarykianae Brunensis, Biologia, 1st ed.; Rozkošný, R., Vaňhara, J., Eds.; Masarykova univerzita: Praha, Czech Republic, 1995; Volume 92, pp. 103–109. [Google Scholar]
- Auclerc, A.; Ponge, J.F.; Barot, S.; Dubs, F. Experimental assessment of habitat preference and dispersal ability of soil springtails. Soil Biol. Biochem. 2009, 41, 1596–1604. [Google Scholar] [CrossRef] [Green Version]
- Petersen, H. Collembolan communities in shrublands along climatic gradients in Europe and the effect of experimental warming and drought on population density, biomass and diversity. Soil Org. 2011, 83, 463–488. [Google Scholar]
- Gruia, M. Sur la faune de collemboles de l’ecosysteme exokarstique et karstique de Movile (Dobrogea du Sud, Mangalia, Romania). Mem. Biospeol. 1998, 25, 45–52. [Google Scholar]
- Kuznetsova, N.A. Long-term dynamics of Collembola in two contrasting ecosystems. Pedobiologia 2006, 50, 157–164. [Google Scholar] [CrossRef]
- Skarżyński, D. The springtails (Collembola) of epilittoral of selected rivers and streams of Lower Silesia. Wiad. Entomol. 1999, 17, 133–143, (In Polish with English summary). [Google Scholar]
- Fjellberg, A. The Collembola of Fennoscandia and Denmark, Part II: Entomobryomorpha and Symphypleona, 1st ed.; Brill: Leiden, The Netherlands, 2007; pp. 1–265. [Google Scholar]
- Sterzyńska, M. Communities of Collembola in natural and transformed soils of the linden-oak-hornbeam sites of the Mazovian Lowland. Fragm. Faunist. 1990, 34, 165–262. [Google Scholar] [CrossRef] [Green Version]
- Makkonen, M.; Berg, M.P.; van Hal, J.R.; Callaghan, T.V.; Press, M.C.; Aerts, R. Traits explain the responses of a sub-arctic Collembola community to climate manipulation. Soil Biol. Biochem. 2011, 43, 377–384. [Google Scholar] [CrossRef]
- Sławska, M.; Sławski, M. Springtails (Collembola, Hexapoda) in Bogs of Poland, 1st ed.; Warsaw University of Life Sciences: Warsaw, Poland, 2009; pp. 1–83. [Google Scholar]
- Schneider, C.; D’Haese, C.A. Morphological and molecular insights on Megalothorax: The largest Neelipleona genus revisited (Collembola). Invertebr. Syst. 2013, 27, 317–364. [Google Scholar] [CrossRef]
- Kuznetsova, N.A. Collembolan guild structure as an indicator of tree plantation conditions in urban areas. Memorab. Zool. 1994, 49, 197–205. [Google Scholar]
- Sławski, M.; Sławska, M. The forest edge as a border between forest and meadow. Vegetation and Collembola communities. Pedobiolgia 2000, 44, 442–450. [Google Scholar] [CrossRef]
- Szeptycki, A. Fauna of the springtails (Collembola) of the Ojców National Park in Poland. Acta Zool. Cracov. 1967, 12, 219–280. [Google Scholar]
- Kuznetsova, N.A.; Kresťyaninova, A.I. Dynamics of Springtail Communities (Collembola) in Hydrological Series of Pine Forests in Southern Taiga. Entomol. Rev. 1998, 78, 969–981. [Google Scholar]
- Ponge, J.F. Biocenoses of Collembola in atlantic temperate grass-woodland ecosystems. Pedobiologia 1993, 37, 223–244. [Google Scholar]
- Pomorski, R.J. Onychiurinae of Poland (Collembola); Polish Taxonomical Society: Wroclaw, Poland, 1998; Volume 9, pp. 1–201. [Google Scholar]
- Traser, G.; Szűcs, P.; Winkler, D. Collembola diversity of moss habitats in the Sopron Region, NW–Hungary. Acta Silv. Lignaria Hung. 2006, 2, 69–80. [Google Scholar]
- Traser, G. Springtails of the Aggtelek National Park (Hexapoda: Collembola). In The fauna of the Aggtelek National Park; Mahunka, S., Ed.; Magyar Nemzeti Muzeum: Budapest, Hungary, 1999; pp. 49–59. [Google Scholar]
| Doline/Site | Coordinates | Distance [m] | Altitude [m a.s.l.] | Slope [°] | Exposition | Topographic Index | Solar Radiation [kWh.m−2.year −1] | Insolation [h.year−1] | Tmean [°C] | Tmin [°C] | Tmax [°C] | C [%] | N [%] | Soil pHH2O |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1PA | 48°30′24.71″ N, 20°28′21.87″ E | 0 | 468 | 3 | N | 7.08 | 952.93 | 2906.26 | 8.96 ± 7.00 | −0.25 | 23.25 | 4.98 | 0.46 | 6.09 |
| 1N | 48°30′26.55″ N, 20°28′22.39″ E | 56.5 | 460 | 12 | NE | 5.30 | 917.05 | 3616.94 | 9.75 ± 7.15 | 0 | 26.25 | 7.25 | 0.64 | 7.33 |
| 1B | 48°30′32.65″ N, 20°28′23.12″ E | 246 | 433 | 1 | N | 7.66 | 986.48 | 3225.26 | 9.11 ± 6.87 | −0.25 | 23.25 | 4.83 | 0.45 | 5.90 |
| 1S | 48°30′34.67″ N, 20°28′23.29″ E | 309 | 436 | 18 | SE | 4.52 | 1068.95 | 3098.40 | 10.08 ± 7.02 | −0.25 | 26.25 | 4.87 | 0.42 | 5.90 |
| 1PB | 48°30′35.79″ N, 20°28′23.33″ E | 339 | 442 | 2 | E | 3.60 | 1032.59 | 3635.64 | 9.57 ± 7.05 | −0.5 | 24.50 | 7.74 | 0.75 | 6.90 |
| 2PA | 48°30′28.23″ N, 20°28′54.88″ E | 0 | 432 | 7 | NE | 5.11 | 958.75 | 3664.84 | 9.27 ± 6.31 | 0 | 21.75 | 7.49 | 0.64 | 7.21 |
| 2N | 48°30′30.18″ N, 20°28′55.35″ E | 59.5 | 422 | 11 | NE | 6.59 | 911.19 | 3361.04 | 9.73 ± 6.78 | −0.5 | 24.50 | 8.16 | 0.72 | 6.91 |
| 2B | 48°30′31.81″ N, 20°28′55.69″ E | 104.5 | 416 | 1 | NE | 11.08 | 978.46 | 3102.45 | 9.00 ± 6.74 | −0.5 | 22.50 | 6.15 | 0.61 | 5.90 |
| 2S | 48°30′33.20″ N, 20°28′55.36″ E | 148.5 | 421 | 18 | S | 3.91 | 1143.93 | 3538.48 | 11.63 ± 7.09 | 1 | 26.75 | 9.34 | 0.91 | 7.24 |
| 2PB | 48°30′34.03″ N, 20°28′55.50″ E | 174.5 | 424 | 4 | SE | 4.20 | 1058.86 | 3757.31 | 10.42 ± 6.83 | 0 | 26.25 | 8.81 | 0.87 | 6.80 |
| 3PA | 48°30′25.44″ N, 20°29′00.06″ E | 0 | 426 | 3 | N | 5.04 | 1000.38 | 3670.67 | 10.05 ± 7.00 | 0 | 26.00 | 10.50 | 1.07 | 7.40 |
| 3N | 48°30′26.35″ N, 20°29′00.12″ E | 30 | 422 | 9 | N | 5.73 | 932.86 | 3596.80 | 9.56 ± 7.31 | −0.25 | 25.75 | 7.78 | 0.67 | 7.41 |
| 3B | 48°30′27.59″ N, 20°29′00.26″ E | 66.7 | 419 | 0 | N | 13.32 | 1032.42 | 3578.97 | 9.57 ± 6.61 | 0 | 22.50 | 8.00 | 0.72 | 6.10 |
| 3S | 48°30′28.87″ N, 20°29′00.41″ E | 103.7 | 423 | 10 | S | 5.90 | 1113.83 | 3654.93 | 10.90 ± 7.36 | 0 | 26.75 | 7.68 | 0.66 | 6.81 |
| 3PB | 48°30′29.63″ N, 20°29′00.45″ E | 129.7 | 427 | 5 | SW | 4.84 | 1070.80 | 3736.66 | 10.32 ± 6.65 | 0.5 | 26.00 | 10.60 | 0.93 | 7.04 |
| Doline/Site | A | S | H | J |
|---|---|---|---|---|
| 1PA | 11,822 ± 6878 | 27 | 2.70 | 0.82 |
| 1N | 7924 ± 1440 | 20 | 2.03 | 0.68 |
| 1B | 9427 ± 7980 | 21 | 2.37 | 0.78 |
| 1S | 9121 ± 4035 | 22 | 2.38 | 0.77 |
| 1PB | 6828 ± 1662 | 26 | 2.56 | 0.79 |
| 2PA | 20,153 ± 14,810 | 28 | 2.54 | 0.76 |
| 2N | 18,268 ± 4224 | 25 | 2.00 | 0.62 |
| 2B | 11,694 ± 2131 | 27 | 2.63 | 0.80 |
| 2S | 10,548 ± 2541 | 23 | 1.93 | 0.61 |
| 2PB | 12,102 ± 2078 | 13 | 0.97 | 0.38 |
| 3PA | 3032 ± 1453 | 20 | 2.19 | 0.73 |
| 3N | 11,032 ± 5876 | 22 | 2.36 | 0.76 |
| 3B | 30,905 ± 19,300 | 27 | 1.68 | 0.51 |
| 3S | 26,395 ± 9695 | 29 | 1.67 | 0.50 |
| 3PB | 16,357 ± 7546 | 20 | 1.42 | 0.48 |
| Hygrophilous | Mesophilous | Xerophilous/Xeroresistant | Thermophilous | |
|---|---|---|---|---|
| F; p | F; p | F; p | F; p | |
| Number of species | 3.26; 0.017 | 7.15; <0.001 | 4.01; 0.005 | 1.79; 0.141 |
| Mean abundance | 2.38; 0.060 | 1.81; 0.136 | 2.07; 0.094 | 1.20; 0.319 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcin, M.; Raschmanová, N.; Miklisová, D.; Šupinský, J.; Kaňuk, J.; Kováč, Ľ. Karst Dolines Support Highly Diversified Soil Collembola Communities—Possible Refugia in a Warming Climate? Diversity 2022, 14, 1037. https://doi.org/10.3390/d14121037
Marcin M, Raschmanová N, Miklisová D, Šupinský J, Kaňuk J, Kováč Ľ. Karst Dolines Support Highly Diversified Soil Collembola Communities—Possible Refugia in a Warming Climate? Diversity. 2022; 14(12):1037. https://doi.org/10.3390/d14121037
Chicago/Turabian StyleMarcin, Michal, Natália Raschmanová, Dana Miklisová, Jozef Šupinský, Ján Kaňuk, and Ľubomír Kováč. 2022. "Karst Dolines Support Highly Diversified Soil Collembola Communities—Possible Refugia in a Warming Climate?" Diversity 14, no. 12: 1037. https://doi.org/10.3390/d14121037
APA StyleMarcin, M., Raschmanová, N., Miklisová, D., Šupinský, J., Kaňuk, J., & Kováč, Ľ. (2022). Karst Dolines Support Highly Diversified Soil Collembola Communities—Possible Refugia in a Warming Climate? Diversity, 14(12), 1037. https://doi.org/10.3390/d14121037









