Jaguar’s Predation and Human Shield, a Tapir Story
Abstract
1. Introduction
2. Material and Methods
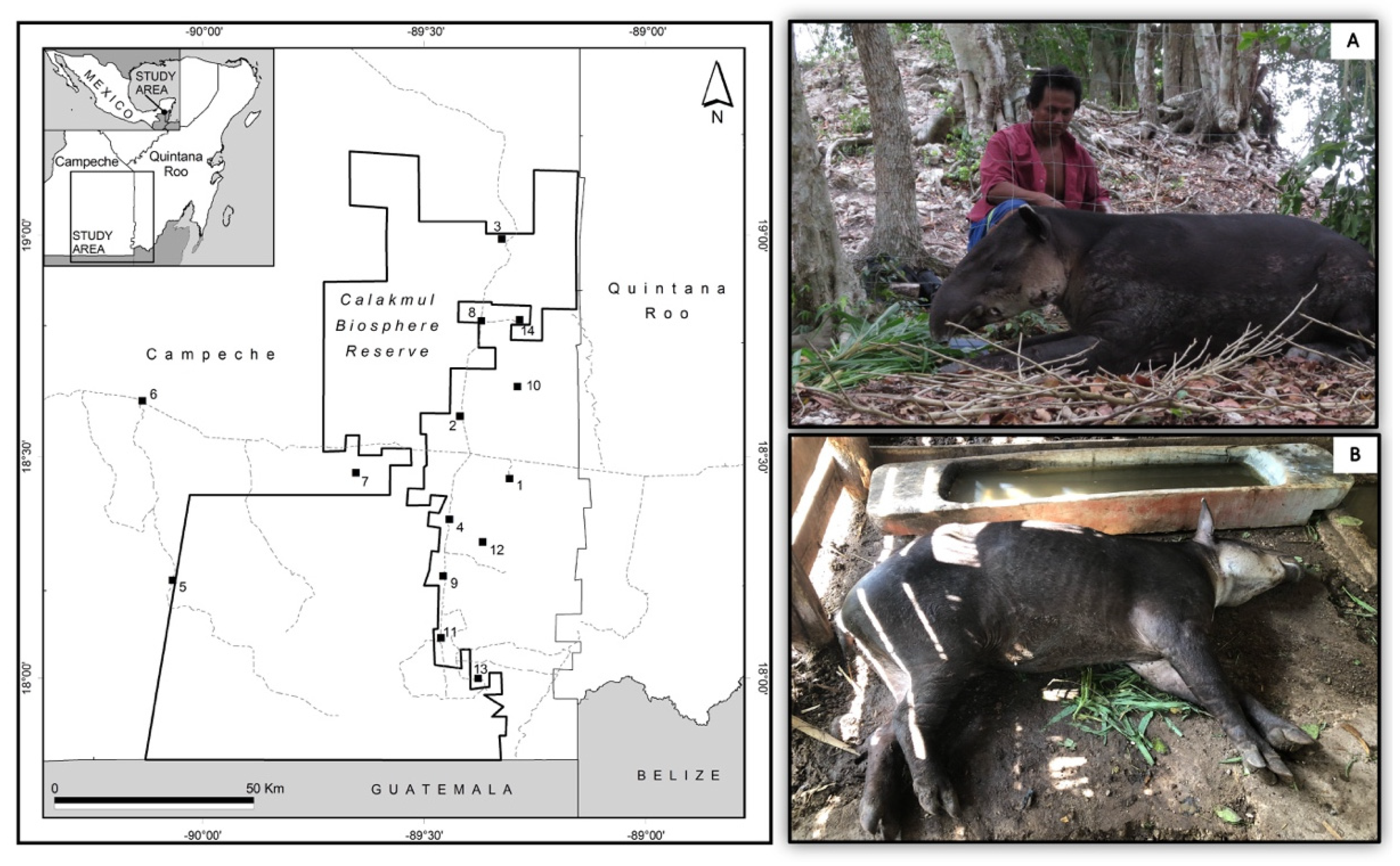
2.1. Study Site
2.2. Tapir Sightings Close to Human Settlements
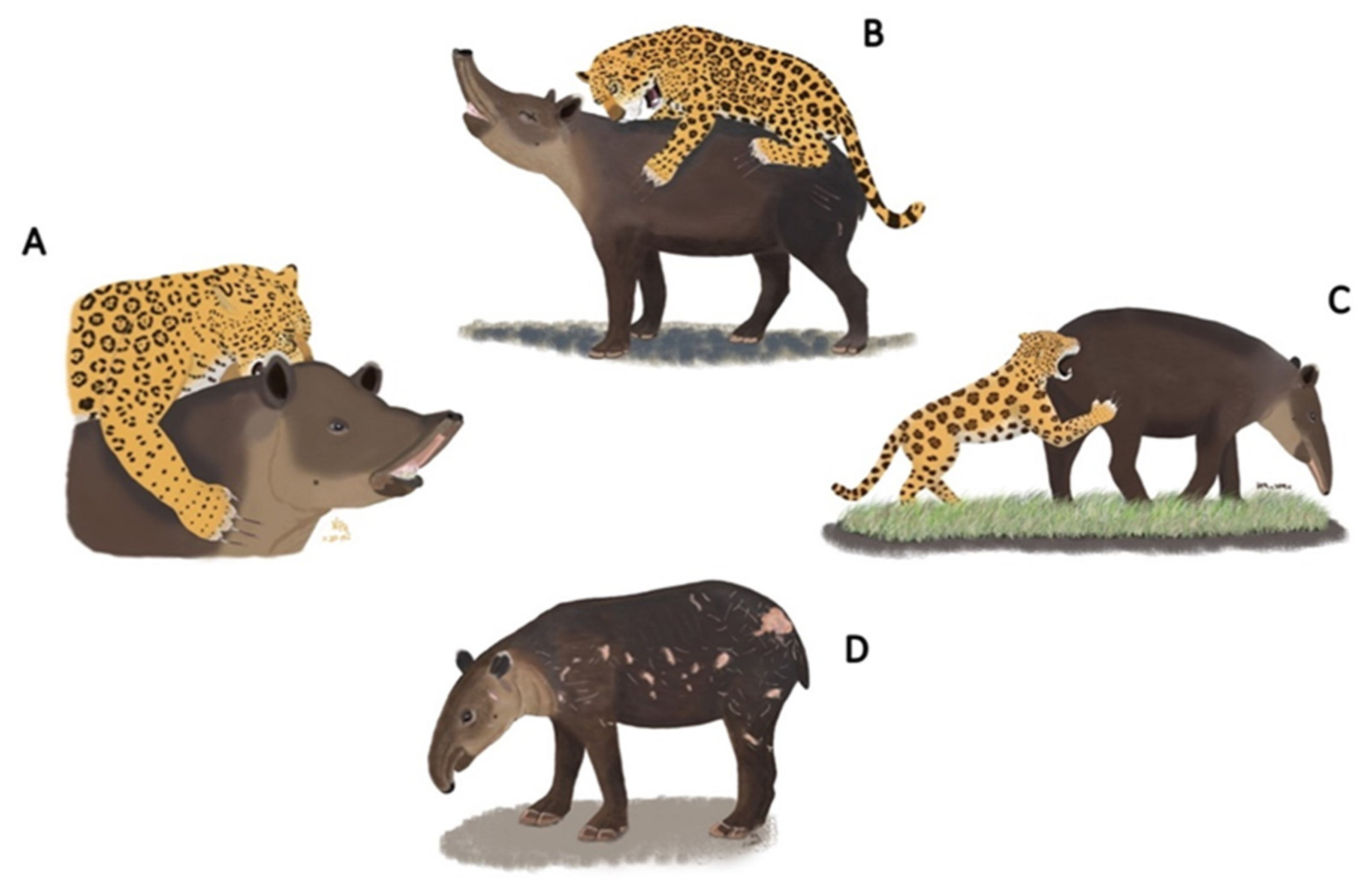
2.3. How Do We Recognize a Jaguar’s Attack on a Tapir?
2.4. Human Shield
2.5. Statistical Analyses
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lima, S.L.; Bednekoff, P.A. Temporal variation in danger drives anti-predator behavior: The predation risk allocation hypothesis. Am. Nat. 1999, 153, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Allendorf, F.W.; Hard, J.J. Human-induced evolution caused by unnatural selection through harvest of wild animals. Proc. Natl. Acad. Sci. USA 2009, 106, 9987–9994. [Google Scholar] [CrossRef] [PubMed]
- Lone, K.; Loe LEMeisingset, E.L.; Stamnes, I.; Mysteryd, A. An adaptive behavioural response to hunting: Surviving male red deer shift habitat at the onset of the hunting season. Anim. Behav. 2015, 102, 127–138. [Google Scholar] [CrossRef]
- Creel, S.; Winnie, J.; Maxwell, B.; Hamlin, K.; Creel, M. Elk Alter Habitat Selection as an Antipredator Response to Wolves. Ecology 2005, 86, 3387–3397. [Google Scholar] [CrossRef]
- Kuijper, D.P.; Verwijmeren, M.; Churski, M.; Zbyryt, A.; Schmidt, K.; Jdrzejewska, B.; Smit, C. What cues do ungulates use to assess predation risk in dense temperate forests? PLoS ONE 2014, 9, e84607. [Google Scholar] [CrossRef]
- Hamilton, W. Geometry for the selfish herd. J. Theor. Biol. 1971, 31, 295–311. [Google Scholar] [CrossRef]
- Schmitt, M.H.; Stears, K.; Wilmers, C.C.; Shrader, A.M. Determining the relative importance of dilution and detection for zebra foraging in mixed-species herds. Anim. Behav. 2014, 96, 151–158. [Google Scholar] [CrossRef]
- Schmitt, M.H.; Stears, K.; Shrader, A.M. Zebra reduce predation risk in mixed-species herds by eavesdropping on cues from giraffe. Behav. Ecol. 2016, 27, 1073–1077. [Google Scholar] [CrossRef]
- Cocroft, R.B.; Hambler, K. Observations on a Commensal Relationship of the Microhylid Frog Chiasmocleis ventrimaculata and the Burrowing Theraphosid Spider Xenesthis immanis in Southeastern Peru. Biotropica 1989, 21, 2–8. [Google Scholar] [CrossRef]
- Karunarathna, D.S.; Amarasinghe, A.A. Mutualism in Ramanella nagaoi Manamendra-Arachchi & Pethiyagoda, 2001 (Amphibia: Microhylidae) and Poecillotheria species (Aracnida: Thereposidae) from Sri Lanka. Taprabonica 2009, 1, 16–18. [Google Scholar] [CrossRef]
- Hénaut, Y.; Machkour-M’Rabet, S. Predation and Other Interactions. In New World Tarantulas: Taxonomy, Biogeography and Evolutionary Biology of Theraphosidae (Zoological Monographs); Pérez-Miles, F., Ed.; Springer: Cham, Switzerland, 2020; Volume 6, pp. 230–270. [Google Scholar]
- Berger, J. Fear, human shields and the redistribution of prey and predators in protected areas. Biol. Lett. 2007, 3, 620–623. [Google Scholar] [CrossRef] [PubMed]
- Muhly, T.B.; Semeniuk, C.; Massolo, A.; Hickman, L.; Musiani, M. Human Activity Helps Prey Win the Predator-Prey Space Race. PLoS ONE 2011, 6, e17050. [Google Scholar] [CrossRef] [PubMed]
- Atickem, A.; Loe, L.E.; Stenseth, N.C. Individual Heterogeneity in Use of Human Shields by Mountain Nyala. Int. J. Behav. Biol. 2014, 120, 715–725. [Google Scholar] [CrossRef]
- Bagchi, S.; Mishra, C. Living with large carnivores: Predation on livestock by the snow leopard (Uncia uncia). J. Zool. 2006, 268, 217–224. [Google Scholar] [CrossRef]
- Lagendijk, D.D.G.; Gusset, M. Human-carnivore coexistence on communal land bordering the greater Kruger area, South Africa. Environ. Manag. 2008, 42, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Linnell, J.D.C.; Swenson, J.E.; Anderson, R. Predators and people: Conservation of large carnivores is possible at high human densities if management policy is favourable. Anim. Conserv. 2001, 4, 345–349. [Google Scholar] [CrossRef]
- Hoogesteijn, R. Manual Sobre Problemas de Depredación Causados por Jaguares y Pumas en Hatos Ganaderos; Wildlife Conservation Society: New York, NY, USA, 2003. [Google Scholar]
- Brown, J.H. Why are there so many species in the tropics? J. Biogeogr. 2014, 41, 8–22. [Google Scholar] [CrossRef]
- Kitching, R.L. Those delusive food webs. Invited Essay review. Trends Ecol. Evol. 2004, 19, 294–295. [Google Scholar] [CrossRef]
- Hebblewhite, M.; Merrill, E.H.; McDonald, T.L. Spatial decomposition of predation risk using resource selection functions: An example in a wolf-elk predator-prey system. Oikos 2005, 111, 101–111. [Google Scholar] [CrossRef]
- Schwartz, C.C.; Cain, S.L.; Podruzny, S.; Cherry, S.; Frattaroli, L. Contrasting Activity Patterns of Sympatric and Allopatric Black and Grizzly Bears. J. Wildl. Manag. 2010, 74, 1628–1638. [Google Scholar] [CrossRef]
- Waser, N.M.; Price, M.V.; Blumstein, D.T.; Arózqueta, S.R.; Escobar, B.D.C.; Pickens, R.; Pistoia, A. Coyotes, deer, and wildflowers: Diverse evidence points to a trophic cascade. Naturwissenschaften 2014, 101, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Sarmento, W.M.; Berger, J. Human visitation limits the utility of protected areas as ecological baselines. Biol. Conserv. 2017, 212, 316–326. [Google Scholar] [CrossRef]
- Basille, M.; Herfindal, I.; Santin-Janin, H.; Linnell, J.D.C.; Odden, J.; Andersen, R.; Høgda, K.A.; Gaillard, J.M. What shapes Eurasian lynx distribution in human dominated landscapes: Selecting prey or avoiding people? Ecography 2009, 32, 683–691. [Google Scholar] [CrossRef]
- Hayward, M.W.; Henschel, P.; O’Brien, J.; Hofmeyr, M.; Balme, G.; Kerley, G.I.H. Prey preferences of the leopard (Panthera pardus). J. Zool. 2006, 270, 298–313. [Google Scholar] [CrossRef]
- Hayward, M.W.; Kamler, J.F.; Montgomery, R.A.; Newlove, A.; Rostro-García, S.; Sales, L.P.; Van Valkenburgh, B. Prey Preferences of the Jaguar Panthera onca Reflect the Post-Pleistocene Demise of Large Prey. Front. Ecol. Evol. 2016, 3, 148. [Google Scholar] [CrossRef]
- Aranda, M.; Sánchez-Cordero, V. Prey spectra of jaguar (Panthera onca) and puma (Puma concolor) in tropical forests of Mexico. Stud. Neotrop. Fauna Environ. 1996, 31, 65–67. [Google Scholar] [CrossRef]
- Núñez, R.; Miller, B.; Lindzey, F. Food habits of jaguars and pumas in Jalisco, Mexico. J. Zool. 2000, 252, 373–379. [Google Scholar] [CrossRef]
- Novack, A.J.; Main, M.B.; Sunquist, M.E.; Labisky, R.F. Foraging ecology of jaguar (Panthera onca) and puma (Puma concolor) in hunted and non-hunted sites within the Maya Biosphere Reserve, Guatemala. J. Zool. 2005, 267, 167–178. [Google Scholar] [CrossRef]
- Sollmann, R.; Betsch, J.; Furtado, M.M.; Hofer, H.; Jácomo, A.T.A.; Palomares, F.; Roques, S.; Tôrres, N.M.; Vynne, C.; Silveira, L. Note on the diet of the jaguar in central Brazil. Eur. J. Wildl. Res. 2013, 59, 445–448. [Google Scholar] [CrossRef]
- Iserson, K.V.; Francis, A.M. Jaguar Attack on a Child: Case Report and Literature Review. West. J. Emerg. Med. 2015, 16, 303–309. [Google Scholar] [CrossRef]
- Perilli, M.L.L.; Lima, F.; Rodrigues, F.H.G.; Cavalcanti, S.M.C. Can Scat Analysis Describe the Feeding Habits of Big Cats? A Case Study with Jaguars (Panthera onca) in Southern Pantanal, Brazil. PLoS ONE 2016, 11, e0151814. [Google Scholar] [CrossRef]
- Pérez-Flores, J.; Arias-Domíngues, H.; Arias-Domínguez, N. First documented predation of a Baird’s tapir by a jaguar in the Calakmul region, Mexico. Neotrop. Biol. Conserv. 2020, 15, 453–461. [Google Scholar] [CrossRef]
- Brock, S.E. The jaguar Panthera onca. J. Brit. Guiana Mus. Zoo 1963, 37, 46–48. [Google Scholar]
- Jedrzejewski, W.; Abarca, M.; Viloria, Á.; Cerda, H.; Lew, D.; Takiff, H.; Abadía, É.; Velozo, P.; Schmidt, K. Jaguar conservation in Venezuela against the backdrop of current knowledge on its biology and evolution. Interciencia Caracas 2011, 36, 954–966. [Google Scholar]
- Weckel, M.; Giuliano, W.; Silver, S. Jaguar (Panthera onca) feeding ecology: Distribution of predator and prey through time and space. J. Zool. 2006, 270, 25–30. [Google Scholar] [CrossRef]
- Naranjo, E.J. Ecology and Conservation of Baird’s Tapir in Mexico. Trop. Conserv. Sci. 2009, 4, 140–158. [Google Scholar] [CrossRef]
- Schaller, G.B.; Vasconcelos, J.M.C. Jaguar predation on capybara. Z. Säugetierk 1978, 43, 296–301. [Google Scholar]
- Crawshaw, P.G.; Quigley, H.B. Hábitos alimentarios del jaguar y el puma en el Pantanal, Brasil, con implicaciones para su manejo y conservación. In El Jaguar en el Nuevo Milenio; Medellín, R.A., Equihua, C., Chetkiewicz, C.L., Crawshaw, P.G., Jr., Rabinowitz, A., Redford, K.H., Robinson, J.G., Sanderson, E.W., Taber, A.B., Eds.; Universidad Nacional Autónoma de México, Wildlife Conservation Society y Fondo de Cultura Económica: Ciudad de México, Mexico, 2002; pp. 223–235. [Google Scholar]
- Pérez-Flores, J. Predation of an adult female Morelet’s crocodile (Crocodylus moreletii) by a jaguar (Panthera onca) in the Calakmul region, Mexico. Herpetol. Notes 2018, 11, 613–616. [Google Scholar]
- García-Gil, G.; Palacio, J.L.; Ortiz, M.A. Reconocimiento geomorfológico e hidrográfico de la Reserva de la Biosfera Calakmul, México. Investig. Geogr. 2002, 48, 7–23. [Google Scholar]
- Reyna-Hurtado, R.; Rojas-Flores, E.; Tanner, G.W. Home range and habitat preferences of white-lipped peccary groups (Tayassu pecari) in a seasonal tropical forest of the Yucatan Peninsula, Mexico. J. Mammal. 2009, 90, 1199–1209. [Google Scholar] [CrossRef]
- Reyna-Hurtado, R.; Sanvicente-López, M.; Pérez-Flores, J.; Carrillo-Reyna, N.; Calmé, S. Insights into the multiannual home range of a Baird’s tapir (Tapirus bairdii) in the Maya Forest. Therya 2016, 7, 271–276. [Google Scholar] [CrossRef]
- Pérez-Flores, J.; Calmé, S.; Reyna-Hurtado, R. Scoring body condition in wild Baird’s tapir (Tapirus bairdii) using camera traps and opportunistic photographic material. Trop. Conserv. Sci. 2016, 9, 1–12. [Google Scholar] [CrossRef]
- Pérez-Flores, J.; Mardero, S.; López-Cen, A.; Contreras-Moreno, F.M. Human-wildlife conflicts and drought in the greater Calakmul Region, Mexico: Implications for tapir conservation. Neotrop. Biol. Conserv. 2021, 16, 539–563. [Google Scholar] [CrossRef]
- O’Farrill, G.; Gauthier-Schampaert, K.; Rayfield, B.; Bodin, Ö.; Calmé, S.; Sengupta, R.; Gonzalez, A. The potential connectivity of waterhole networks and the effectiveness of a protected area under various drought scenarios. PLoS ONE 2014, 9, e95049. [Google Scholar] [CrossRef] [PubMed]
- Ericson, J.; Freudenberger, M.S.; Boege, E. Population dynamics, migration, and the future of the Calakmul Biosphere Reserve. In Biological Diversity: Balancing Interests trough Adaptive Collaborative Management; Buck, L.E., Geisler, C.C., Schelhas, J., Wollenberg, E., Eds.; CRC Press: New York, NY, USA, 2001. [Google Scholar]
- Pérez-Flores, J.; Weissenberger, H.; López-Cen, A.; Calmé, S. Environmental Factors influencing the occurrence of unhealthy tapirs in the southern Yucatan Peninsula. Ecohealth 2020, 17, 359–369. [Google Scholar] [CrossRef]
- Porter-Bolland, L.P.; Drew, A.P.; Vergara-Tenorio, C. Analysis of a natural resources management system in the Calakmul Biosphere Reserve. Landsc. Urban Plan. 2006, 74, 223–241. [Google Scholar] [CrossRef]
- Hayward, M.W.; Kerley, G.I.H. Prey preferences of the lion (Panthera leo). J. Zool. 2005, 267, 309–322. [Google Scholar] [CrossRef]
- Mallon, D.; Harris, R.B.; Wegge, P. Snow leopard prey and diet. In Snow Leopards; McCarthy, T., Mallon, D., Eds.; Elsevier: New York, NY, USA, 2016; pp. 43–55. [Google Scholar]
- Pakpien, S.; Simcharoen, A.; Duangchantrasiri, S.; Chimchome, V.; Pongpattannurak, N.; Smith, J.L.D. Ecological Covariates at Kill Sites Influence Tiger (Panthera tigris) Hunting Success in Huai Kha Khaeng Wildlife Sanctuary, Thailand. Trop. Conserv. Sci. 2017, 10, 1940082917719000. [Google Scholar] [CrossRef]
- Simcharoen, A.; Simcharoen, S.; Duangchantrasiri, S.; Bump, J.; Smith, J.L. Tiger and leopard diets in western Thailand: Evidence for overlap and potential consequences. Food Webs 2018, 15, e00085. [Google Scholar] [CrossRef]
- Escamilla, A.; Sanvicente, M.; Sosa, M.; Galindo-Leal, C. Habitat mosaic, wildlife availability, and hunting in the tropical forest of Calakmul. Conserv. Biol. 2000, 14, 1592–1601. [Google Scholar] [CrossRef]
- Ciuti, S.; Muhly, T.B.; Paton, D.G.; McDevitt, A.D.; Musiani, M.; Boyce, M.S. Human selection of elk behavioural traits in a landscape of fear. Proc. R. Soc. B Biol. Sci. 2012, 279, 4407–4416. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, M.; Dussault, C.; St-Laurent, M.-H. Behavioural strategies towards human disturbances explain individual performance in woodland caribou. Oecologia 2014, 176, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Steyaert, S.M.J.G.; Zedrosser, A.; Elfström, M.; Ordiz, A.; Leclerc, M.; Frank, S.C.; Kindberg, J.; Støen, O.; Brunberg, S.; Swenson, J.E. Ecological implications from spatial patterns in human-caused brown bear mortality. Wildl. Biol. 2016, 22, 144–152. [Google Scholar] [CrossRef]
- Pence, D.B.; Windberg, L.A. Impact of a Sarcoptic Mange Epizootic on a Coyote Population. J. Wildl. Manag. 1994, 58, 624. [Google Scholar] [CrossRef]
- Bradley, C.A.; Altizer, S. Urbanization and the ecology of wildlife diseases. Trends Ecol. Evol. 2007, 22, 95–102. [Google Scholar] [CrossRef]
- Newman, A.A. Do Animals Ask for Help? Nonfiction Minute, iNK Think Tank. 2018. Available online: https://www.nonfictionminute.org/the-nonfiction-minute/do-animals-ask-for-help (accessed on 14 September 2021).
- Haddad-Junior, V.; Assunção, M.C.; de Mello, R.C.; Duarte, M.R. A fatal attack caused by a lowland tapir (Tapirus terrestris) in southeastern Brazil. Wilderness Environ. Med. 2005, 16, 97–100. [Google Scholar] [CrossRef]
- Association of Zoos and Aquariums. Tapir (Tapiridae) Care Manual; AZA Tapir TAG: Silver Spring, MD, USA, 2013; 65p. [Google Scholar]
- Castellanos, A.; Gomez, L. Reintroduced Andean Tapir Attacks A Person In The Antisana Ecological Reserve, Ecuador. Tapir Conserv. 2015, 24, 11–12. [Google Scholar] [CrossRef]
- Caro, T.M. Ungulate anti-predator behaviour: Preliminary and comparative data from African bovids. Behaviour 1994, 128, 189–228. [Google Scholar] [CrossRef]
- Georgiev, A.; Klimczuk, A.C.E.; Traficonte, D.M.; Maestripieri, D. When Violence Pays: A Cost-Benefit Analysis of Aggressive Behavior in Animals and Humans. Evol. Psychol. 2014, 11, 678–699. [Google Scholar] [CrossRef]
- Guerra-Silveira, F.; Abad-Franch, F. Sex Bias in Infectious Disease Epidemiology: Patterns and Processes. PLoS ONE 2013, 8, e62390. [Google Scholar] [CrossRef]
- McDonald, J.L.; Smith, G.; McDonald, R.; Delahay, R.J.; Hodgson, D. Mortality trajectory analysis reveals the drivers of sex-specific epidemiology in natural wildlife–disease interactions. Proc. R. Soc. B Boil. Sci. 2014, 281, 20140526. [Google Scholar] [CrossRef] [PubMed]
- Schuurs, A.; Verheul, H. Effects of gender and sex steroids on the immune response. J. Steroid Biochem. 1990, 35, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Folstad, I.; Karter, A.J. Parasites, Bright Males, and the Immunocompetence Handicap. Am. Nat. 1992, 139, 603–622. [Google Scholar] [CrossRef]
- Zuk, M.; Stoehr, A.M. Immune defense and host life history. Am. Nat. 2002, 160, S9–S22. [Google Scholar] [CrossRef] [PubMed]
- Medici, E.P.; Mangini, P.R.; Fernandes-Santos, R.C. Health assessment of wild lowland tapir (Tapirus terrestris) populations in the atlantic forest and pantanal biomes, Brazil (1996–2012). J. Wildl. Dis. 2014, 50, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Quse, V.; Fernandes-Santos, R.C. Tapir Veterinary Manual, 2nd ed.; IUCN/SSC Tapir Specialist Group (TSG): Missouri, MO, USA, 2014. [Google Scholar]
- Colchero, F.; Conde, D.A.; Manterola, C.; Chávez, C.; Rivera, A.; Ceballos, G. Jaguars on the move: Modeling movement to mitigate fragmentation from road expansion in the Mayan Forest. Anim. Conserv. 2010, 14, 158–166. [Google Scholar] [CrossRef]
- Hebblewhite, M.; Merrill, E. Trade-offs between predation risk and forage differ between migrant strategies in a migratory ungulate. Ecology 2009, 90, 3445–3454. [Google Scholar] [CrossRef]




| Area | Number of Individuals | ||
|---|---|---|---|
| Total | Unhealthy n (%) | Healthy n (%) | |
| HS (0 to 0.5 km) | 10 | 9 (90%) | 1 (10%) |
| HA (>0.5 km to 5 km) | 8 | 5 (62%) | 48 (3%) |
| LHA (>5 km) | 5 | 2 (40%) | 3 (60%) |
| Predator Species (Body Mass) (kg) | Prey | ||
|---|---|---|---|
| Species | Body Mass (kg) | Injured Anatomical Region | |
| P. leo (110–225) | Tragelaphus oryx | 350–1000 | N, SP, R, P, HL |
| Syncerus caffer | 500–1000 | H, N, SH, SP, R, P, HL | |
| Giraffa camelopardalis | 800–1200 | N, P, HL | |
| Diceros bicornis | 800–1400 | SP, P, HL | |
| Hippopotamus amphibious | 1300–1500 | SP, P, HL | |
| Ceratotherium simum | 1700–2300 | P, HL | |
| Loxodonta africana | 3000–6000 | H, SP, P, HL | |
| P. tigris (65–300) | Tapirus indicus | 250–540 | -- |
| Alces alces | 270–771 | -- | |
| Bos javanicus | 590–800 | H, N, SH | |
| Bubalus bubalis | 300–1000 | N | |
| Bos gaurus | 440–1500 | N, SP, P, HL | |
| Rhinoceros unicornis | 1500–2000 | P, HL | |
| Elephas maximus | 2000–6000 | H | |
| P. pardus (25–80) | Tapirus indicus | 250–540 | -- |
| P. onca (40–60) | +Eretmochelys imbricata | 35–127 | H, N, FL |
| +Chelonia mydas | 150–200 | H, N, FL | |
| Tapirus bairdii | 150–300 | H, N, SH, FL, SP, R, P, HL | |
| +Dermochelys coriacea | 250–900 | H, N, FL | |
| P. uncia (22–75) | Capra ibex sibirica | 30–130 | H, FL |
| Ovis ammon | 60–185 | -- | |
| Sus scrofa | 66–272 | -- | |
| Equus hemionus | 200–262 | -- | |
| Bos mutus | 305–1200 | N | |
| Individual Identification | Sex | Body Condition | Anatomical Region Injured | Severity of Lesions |
|---|---|---|---|---|
| 20 de Noviembre 1 | M | U | H, N, P, HL | 3, 1, 2, 2 |
| 20 de Noviembre 2 | M | U | H, N, SH, R, P, HL | 3, 1, 2, 5, 2, 4 |
| 20 de Noviembre 3 | M | U | H, SH, SP, R, P, HL | 2, 2, 5, 3, 5, 5 |
| Alvaro Obregon | M | U | H, SH, SP, R, P, HL | 1, 2, 2, 2, 3, 3 |
| Nuevo Becal | M | U | P, HL | 2, 2 |
| Nueva Vida | F | U | H, N, SH, FL, R, P, HL | 2, 1, 3, 2, 2, 3, 2 |
| Once de Mayo | M | U | H, N, SH, FL, SP, R, P, HL | 4, 2, 5, 5, 5, 5, 5, 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Flores, J.; Hénaut, Y.; Sanvicente, M.; Pablo-Rodríguez, N.; Calmé, S. Jaguar’s Predation and Human Shield, a Tapir Story. Diversity 2022, 14, 1103. https://doi.org/10.3390/d14121103
Pérez-Flores J, Hénaut Y, Sanvicente M, Pablo-Rodríguez N, Calmé S. Jaguar’s Predation and Human Shield, a Tapir Story. Diversity. 2022; 14(12):1103. https://doi.org/10.3390/d14121103
Chicago/Turabian StylePérez-Flores, Jonathan, Yann Hénaut, Mauro Sanvicente, Nereyda Pablo-Rodríguez, and Sophie Calmé. 2022. "Jaguar’s Predation and Human Shield, a Tapir Story" Diversity 14, no. 12: 1103. https://doi.org/10.3390/d14121103
APA StylePérez-Flores, J., Hénaut, Y., Sanvicente, M., Pablo-Rodríguez, N., & Calmé, S. (2022). Jaguar’s Predation and Human Shield, a Tapir Story. Diversity, 14(12), 1103. https://doi.org/10.3390/d14121103








