Dietary Association with Midgut Microbiota Components of Eocanthecona furcellata (Wolff)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Insects
2.2. Preparation of Artificial Diet
2.3. Treatments
2.4. Performance of E. furcellata Fed with Three Diets for Three Successive Generations
2.5. Sequencing of the 16S rRNA and Microbial Characterization
2.6. α- and β-Diversity of Microbial Community
2.7. Statistical Analyses
3. Results
3.1. Performance of E. furcellata Fed with Three Diets for Three Successive Generations
3.2. Sequencing of the 16S rRNA and Microbial Characterization
3.3. α- and β- Diversity of Midgut Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Phylum | Family | Genus | Species (OTU) |
|---|---|---|---|
| OTUs unique to Spodoptera litura larvae and Tenebrio molitor pupae diets (7) | |||
| Bacteroidetes | Prevotellaceae | Prevotellaceae NK3B31 group | uncultured species 1 (OTU60) |
| Prevotellaceae UCG-003 | uncultured species 2 (OTU141) | ||
| Proteobacteria | Sphingomonadaceae | Sphingobium | uncultured species 3 (OTU387) |
| Burkholderiaceae | Burkholderia-Caballeronia-Paraburkholderia | Paraburkholderia tropica (OTU309) | |
| Enterobacteriaceae | Serratia | uncultured species 4 (OTU337) | |
| Spirochaetes | Spirochaetaceae | Treponema 2 | uncultured species 5 (OTU198) |
| unassigned | unassigned | unassigned | unassigned species 1 (OTU27) |
| OTUs unique to the artificial diet (74) | |||
| Acidobacteria | Solibacteraceae Subgroup 3 | Bryobacter | uncultured species 6 (OTU120) |
| uncultured species 7 (OTU121) | |||
| uncultured species 8 (OTU152) | |||
| Candidatus Solibacter | uncultured species 9 (OTU168) | ||
| uncultured species 10 (OTU273) | |||
| uncultured species 11 (OTU491) | |||
| Paludibaculum | uncultured species 12 (OTU161) | ||
| uncultured species 13 (OTU163) | |||
| Holophagaceae | Holophaga | uncultured species 14 (OTU472) | |
| Bacteroidetes | Bacteroidaceae | Bacteroides | uncultured species 15 (OTU26) |
| uncultured species 16 (OTU79) | |||
| uncultured species 17 (OTU135) | |||
| uncultured species 18 (OTU528) | |||
| Barnesiellaceae | unknown genus 1 | uncultured species 19 (OTU43) | |
| Muribaculaceae | unknown genus 2 | uncultured species 20 (OTU28) | |
| uncultured species 21 (OTU54) | |||
| uncultured species 22 (OTU56) | |||
| uncultured species 23 (OTU104) | |||
| uncultured species 24 (OTU173) | |||
| uncultured species 25 (OTU220) | |||
| uncultured species 26 (OTU246) | |||
| uncultured species 27 (OTU249) | |||
| Prevotellaceae | Alloprevotella | uncultured species 28 (OTU29) | |
| Prevotellaceae NK3B31 group | uncultured species 29 (OTU289) | ||
| Prevotellaceae UCG-001 | uncultured species 30 (OTU86) | ||
| Rikenellaceae | Alistipes | uncultured species 31 (OTU74) | |
| Tannerellaceae | Macellibacteroides | uncultured species 32 (OTU45) | |
| uncultured species 33 (OTU94) | |||
| uncultured species 34 (OTU122) | |||
| Ignavibacteria | BSV26 | unknown genus 3 | uncultured species 35 (OTU127) |
| Firmicutes | Carnobacteriaceae | Carnobacterium | uncultured species 36 (OTU13) |
| Streptococcaceae | Lactococcus | Lactococcus garvieae subsp. Garvieae (OTU5) | |
| Clostridiaceae 1 | Clostridium sensu stricto 10 | uncultured species 37 (OTU87) | |
| Eubacteriaceae | Acetobacterium | uncultured species 38 (OTU91) | |
| Eubacterium | bacterium NLAE-zl-P455 (OTU131) | ||
| Lachnospiraceae | Blautia | uncultured species 39 (OTU50) | |
| Epulopiscium | uncultured species 40 (OTU97) | ||
| Lachnospira | uncultured species 41 (OTU178) | ||
| Lachnospiraceae NC2004 group | uncultured species 42 (OTU133) | ||
| Lachnospiraceae NK4A136 group | uncultured species 43 (OTU495) | ||
| uncultured species 44 (OTU541) | |||
| uncultured species 45 (OTU589) | |||
| uncultured species 46 (OTU606) | |||
| unknown genus 4 | uncultured species 47 (OTU137) | ||
| uncultured species 48 (OTU232) | |||
| Ruminococcaceae | Ruminiclostridium 9 | uncultured species 49 (OTU155) | |
| Ruminococcaceae UCG-014 | uncultured species 50 (OTU126) | ||
| Ruminococcus 1 | uncultured species 51 (OTU132) | ||
| Subdoligranulum | uncultured species 52 (OTU449) | ||
| unknown genus 5 | uncultured species 53 (OTU71) | ||
| uncultured species 54 (OTU281) | |||
| uncultured species 55 (OTU488) | |||
| Erysipelotrichaceae | [Anaerorhabdus] furcosa group | uncultured species 56 (OTU33) | |
| Allobaculum | uncultured species 57 (OTU73) | ||
| Fusobacteria | Fusobacteriaceae | Cetobacterium | uncultured species 58 (OTU225) |
| Gemmatimonadetes | Gemmatimonadaceae | Gemmatimonas | bacterium enrichment culture clone auto9_4W (OTU174) |
| unknown genus 5 | uncultured species 59 (OTU58) | ||
| uncultured species 60 (OTU75) | |||
| uncultured species 61 (OTU229) | |||
| Nitrospirae | Unknown family 1 | uncultured species 62 (OTU226) | |
| uncultured species 63 (OTU578) | |||
| Patescibacteria | Unknown family 2 | uncultured species 64 (OTU88) | |
| Proteobacteria | Kiloniellaceae | Pelagibius | Pelagibius litoralis (OTU102) |
| Unknown family 3 | uncultured species 65 (OTU138) | ||
| Desulfovibrionaceae | Bilophila | uncultured species 66 (OTU171) | |
| Unknown family 4 | uncultured species 67 (OTU571) | ||
| Nannocystaceae | Nannocystis | uncultured species 68 (OTU65) | |
| Polyangiaceae | Pajaroellobacter | uncultured species 69 (OTU244) | |
| Burkholderiaceae | Paenalcaligenes | uncultured species 70 (OTU426) | |
| Pandoraea | uncultured species 71 (OTU41) | ||
| Rhodocyclaceae | Zoogloea | uncultured species 72 (OTU37) | |
| Enterobacteriaceae | Morganella | uncultured species 73 (OTU90) | |
| Pseudomonadaceae | Pseudomonas | uncultured species 74 (OTU19) | |
| Rhodanobacteraceae | Rhodanobacter | uncultured species 75 (OTU433) | |
References
- Keerthi, M.C.; Sravika, A.; Mahesha, H.S.; Gupta, A.; Bhargavi, H.A.; Ahmed, S. Performance of the native predatory bug, Eocanthecona furcellata (Wolff) (Hemiptera: Pentatomidae), on the fall armyworm, Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae), and its limitation under field condition. Egypt. J. Biol. Pest Control 2020, 30, 1–4. [Google Scholar] [CrossRef]
- Yasuda, T.; Wakamura, S. Rearing of the Predatory Stink Bug, Eocanthecona furcellata (Wolff) (Heteroptera: Pentatomidae), on Frozen Larvae of Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae). Appl. Entomol. Zool. 1992, 27, 303–305. [Google Scholar] [CrossRef] [Green Version]
- He, X.N.; Xian, J.D.; Chen, R.; Zhang, Z.Y.; Zheng, R. Effects of Four Insect Feed on Development and Reproduction of Cantheconidea furcellata (Hemiptera: Asopinae). J. Environ. Entomol. 2013, 35, 799–803. [Google Scholar]
- Lee, K.S.; Lee, J.H. Rearing of Chrysopa pallens (Rambur) (Neuroptera: Chrysopidae) on Artificial Diet. Èntomol. Res. 2005, 35, 183–188. [Google Scholar] [CrossRef]
- Collier, T.; Van Steenwyk, R. A critical evaluation of augmentative biological control. Biol. Control 2004, 31, 245–256. [Google Scholar] [CrossRef]
- Ye, J.; Dai, J.; Li, J.; Li, Z.; Lu, Y.; Han, S.; Zeng, L. Development and Reproduction of Mallada basalis (Neuroptera: Chrysopidae) on Artificial Diets. Fla. Èntomol. 2015, 98, 1072–1076. [Google Scholar] [CrossRef] [Green Version]
- Gong, J.Y.; Chen, K.W.; Wen, J.; Liu, J.; Zhu, Y.J. Predatory Capacity of Eocanthecona furcellate (Wolff) Reared with Artificial Diet. J. Environ. Entomol. 2019, 41, 471–478. [Google Scholar] [CrossRef]
- Lemos, W.P.; Ramalho, F.S.; Serrão, J.E.; Zanuncio, J.C. Effects of diet on development of Podisus nigrispinus (Dallas) (Het., Pentatomidae), a predator of the cotton leafworm. J. Appl. Èntomol. 2003, 127, 389–395. [Google Scholar] [CrossRef]
- Montoro, M.; De Clercq, P.; Overgaard, J.; Sigsgaard, L. Fitness consequences of artificial diets with different macronutrient composition for the predatory bug Orius majusculus. Èntomol. Exp. Appl. 2020, 168, 492–501. [Google Scholar] [CrossRef]
- Riddick, E.W. Benefits and limitations of factitious prey and artificial diets on life parameters of predatory beetles, bugs, and lacewings: A mini-review. BioControl 2008, 54, 325–339. [Google Scholar] [CrossRef]
- Lee, K.S.; Lee, J.H. Rearing of Orius strigicollis (Heteroptera: Anthocoridae) on Artificial Diet. Èntomol. Res. 2004, 34, 299–303. [Google Scholar] [CrossRef]
- Dillon, R.J.; Dillon, V.M. The Gut Bacteria of Insects: Nonpathogenic Interactions. Annu. Rev. Èntomol. 2004, 49, 71–92. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Hosokawa, T.; Fukatsu, T. Insect-Microbe Mutualism without Vertical Transmission: A Stinkbug Acquires a Beneficial Gut Symbiont from the Environment Every Generation. Appl. Environ. Microbiol. 2007, 73, 4308–4316. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.M. Generating novelty by symbiosis. Nature 1989, 341, 284–285. [Google Scholar] [CrossRef]
- Moran, N.A.; McCutcheon, J.P.; Nakabachi, A. Genomics and Evolution of Heritable Bacterial Symbionts. Annu. Rev. Genet. 2008, 42, 165–190. [Google Scholar] [CrossRef] [Green Version]
- Douglas, A.E. Nutritional Interactions in Insect-Microbial Symbioses: Aphids and Their Symbiotic Bacteria Buchnera. Annu. Rev. Èntomol. 1998, 43, 17–37. [Google Scholar] [CrossRef] [Green Version]
- Moran, N.A. Symbiosis. Curr. Biol. 2006, 16, R866–R871. [Google Scholar] [CrossRef] [Green Version]
- Hosokawa, T.; Kikuchi, Y.; Fukatsu, T. How many symbionts are provided by mothers, acquired by offspring, and needed for successful vertical transmission in an obligate insect-bacterium mutualism? Mol. Ecol. 2007, 16, 5316–5325. [Google Scholar] [CrossRef]
- Kikuchi, Y. Endosymbiotic Bacteria in Insects: Their Diversity and Culturability. Microbes Environ. 2009, 24, 195–204. [Google Scholar] [CrossRef] [Green Version]
- Hirose, E.; Panizzi, A.R.; De Souza, J.T.; Cattelan, A.J.; Aldrich, J.R. Bacteria in the Gut of Southern Green Stink Bug (Heter-optera: Pentatomidae). Ann. Entomol. Soc. Am. 2006, 99, 91–95. [Google Scholar] [CrossRef] [Green Version]
- Prado, S.S.; Hung, K.Y.; Daugherty, M.P.; Almeida, R.P.P. Indirect Effects of Temperature on Stink Bug Fitness, via Maintenance of Gut-Associated Symbionts. Appl. Environ. Microbiol. 2010, 76, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- Engel, P.; Moran, N.A. The gut microbiota of insects–diversity in structure and function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staudacher, H.; Kaltenpoth, M.; Breeuwer, J.A.J.; Menken, S.B.J.; Heckel, D.G.; Groot, A.T. Variability of Bacterial Communities in the Moth Heliothis virescens Indicates Transient Association with the Host. PLoS ONE 2016, 11, e0154514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paniagua Voirol, L.R.; Frago, E.; Kaltenpoth, M.; Hilker, M.; Fatouros, N.E. Bacterial Symbionts in Lepidoptera: Their Diversity, Transmission, and Impact on the Host. Front. Microbiol. 2018, 9, 556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanuncio, J.C.; Ferreira, A.M.R.M.; Tavares, W.S.; Torres, J.B.; Serrão, J.E.; Zanuncio, T.V. Rearing the Predator Brontocoris tabidus (Heteroptera: Pentatomidae) with Tenebrio molitor (Coleoptera: Tenebrionidae) Pupa on Eucalyptus grandis in the Field. Am. J. Plant Sci. 2011, 2, 449–456. [Google Scholar] [CrossRef] [Green Version]
- Wen, J.; Chen, K.W.; Fu, L.; Chen, Y. Exposure of Eocanthecona furcellata (Hemiptera: Pentatomidae) nymphs and adults to high temperatures induces an aestivo-hibernal egg diapause: A strategy for surviving hot summers. Appl. Èntomol. Zoöl. 2017, 52, 457–467. [Google Scholar] [CrossRef]
- Chen, Q.J.; Li, G.H.; Pang, Y.A. Simple Artificial Diet for Mass Rearing of Some Noctuid Species. Chin. Bull. Entomol. 2000, 37, 325–327. [Google Scholar]
- Cohen, A.C.; Smith, L.K. A New Concept in Artificial Diets for Chrysoperla rufilabris: The Efficacy of Solid Diets. Biol. Control 1998, 13, 49–54. [Google Scholar] [CrossRef]
- Fadrosh, D.W.; Ma, B.; Gajer, P.; Sengamalay, N.; Ott, S.; Brotman, R.M.; Ravel, J. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2014, 2, 6. [Google Scholar] [CrossRef] [Green Version]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Heberle, H.; Meirelles, G.V.; Da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A Web-Based Tool for the Analysis of Sets through Venn Diagrams. BMC Bioinform. 2015, 16, 169. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [Green Version]
- Grice, E.A.; Kong, H.H.; Conlan, S.; Deming, C.B.; Davis, J.; Young, A.C.; NISC Comparative Sequencing Program; Bouffard, G.G.; Blakesley, R.W.; Murray, P.R.; et al. Topographical and Temporal Diversity of the Human Skin Microbiome. Science 2009, 324, 1190–1192. [Google Scholar] [CrossRef] [Green Version]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [Green Version]
- Tago, K.; Kikuchi, Y.; Nakaoka, S.; Katsuyama, C.; Hayatsu, M. Insecticide applications to soil contribute to the development of Burkholderia mediating insecticide resistance in stinkbugs. Mol. Ecol. 2015, 24, 3766–3778. [Google Scholar] [CrossRef]
- Luo, J.; Cheng, Y.; Guo, L.; Wang, A.; Lu, M.; Xu, L. Variation of gut microbiota caused by an imbalance diet is detrimental to bugs' survival. Sci. Total. Environ. 2021, 771, 144880. [Google Scholar] [CrossRef]
- Gales, A.; Chatellard, L.; Abadie, M.; Bonnafous, A.; Auer, L.; Carrere, H.; Godon, J.-J.; Hernandez-Raquet, G.; Dumas, C. Screening of Phytophagous and Xylophagous Insects Guts Microbiota Abilities to Degrade Lignocellulose in Bioreactor. Front. Microbiol. 2018, 9, 2222. [Google Scholar] [CrossRef]
- de León, A.V.-P.; Jahnes, B.C.; Duan, J.; Camuy-Vélez, L.A.; Sabree, Z.L. Cultivable, Host-Specific Bacteroidetes Symbionts Exhibit Diverse Polysaccharolytic Strategies. Appl. Environ. Microbiol. 2020, 86, e00091-20. [Google Scholar] [CrossRef]
- Palomo-Briones, R.; Razo-Flores, E.; Bernet, N.; Trably, E. Dark-fermentative biohydrogen pathways and microbial networks in continuous stirred tank reactors: Novel insights on their control. Appl. Energy 2017, 198, 77–87. [Google Scholar] [CrossRef]
- Lagkouvardos, I.; Lesker, T.R.; Hitch, T.C.A.; Gálvez, E.J.C.; Smit, N.; Neuhaus, K.; Wang, J.; Baines, J.F.; Abt, B.; Stecher, B.; et al. Sequence and cultivation study of Muribaculaceae reveals novel species, host preference, and functional potential of this yet undescribed family. Microbiome 2019, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Machtelinckx, T.; Van Leeuwen, T.; Van De Wiele, T.; Boon, N.; De Vos, W.H.; Sanchez, J.-A.; Nannini, M.; Gheysen, G.; De Clercq, P. Microbial community of predatory bugs of the genus Macrolophus (Hemiptera: Miridae). BMC Microbiol. 2012, 12, S9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanna, A. Serratia Marcescens—A Rare Opportunistic Nosocomial Pathogen and Measures to Limit its Spread in Hospitalized Patients. J. Clin. Diagn. Res. 2013, 7, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Steinhaus, E. Serratia marcescens Bizio as an Insect Pathogen. Hilgardia 1959, 28, 351–380. [Google Scholar] [CrossRef] [Green Version]
- Sikorowski, P.P.; Lawrence, A.M.; Inglis, G.D. Effects of Serratia marcescens on Rearing of the Tobacco Budworm (Lepidoptera: Noctuidae). Am. Èntomol. 2001, 47, 51–60. [Google Scholar] [CrossRef]
- Lamelas, A.; Gosalbes, M.J.; Marín, A.M.; Peretó, J.; Moya, A.; Latorre, A. Serratia symbiotica from the Aphid Cinara cedri: A Missing Link from Facultative to Obligate Insect Endosymbiont. PLOS Genet. 2011, 7, e1002357. [Google Scholar] [CrossRef] [Green Version]
- Renoz, F.; Pons, I.; Vanderpoorten, A.; Bataille, G.; Noël, C.; Foray, V.; Pierson, V.; Hance, T. Evidence for Gut-Associated Serratia symbiotica in Wild Aphids and Ants Provides New Perspectives on the Evolution of Bacterial Mutualism in Insects. Microb. Ecol. 2018, 78, 159–169. [Google Scholar] [CrossRef]
- Montllor, C.B.; Maxmen, A.; Purcell, A.H. Facultative bacterial endosymbionts benefit pea aphids Acyrthosiphon pisum under heat stress. Ecol. Èntomol. 2002, 27, 189–195. [Google Scholar] [CrossRef]
- Oliver, K.M.; Russell, J.A.; Moran, N.A.; Hunter, M.S. Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc. Natl. Acad. Sci. USA 2003, 100, 1803–1807. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Yuan, E.; Ling, X.; Zhu-Salzman, K.; Guo, H.; Ge, F.; Sun, Y. An aphid facultative symbiont suppresses plant defence by manipulating aphid gene expression in salivary glands. Plant Cell Environ. 2020, 43, 2311–2322. [Google Scholar] [CrossRef]
- Zhou, X.; Ling, X.; Guo, H.; Zhu-Salzman, K.; Ge, F.; Sun, Y. Serratia symbiotica Enhances Fatty Acid Metabolism of Pea Aphid to Promote Host Development. Int. J. Mol. Sci. 2021, 22, 5951. [Google Scholar] [CrossRef]
- Hu, Y.; Xie, H.; Gao, M.; Huang, P.; Zhou, H.; Ma, Y.; Zhou, M.; Liang, J.; Yang, J.; Lv, Z. Dynamic of Composition and Di-versity of Gut Microbiota in Triatoma rubrofasciata in Different Developmental Stages and Environmental Conditions. Front. Cell. Infect. Microbiol. 2020, 10, 587708. [Google Scholar] [CrossRef]
- Lin, Z.; Pang, S.; Zhou, Z.; Wu, X.; Li, J.; Huang, Y.; Zhang, W.; Lei, Q.; Bhatt, P.; Mishra, S.; et al. Novel pathway of acephate degradation by the microbial consortium ZQ01 and its potential for environmental bioremediation. J. Hazard. Mater. 2021, 426, 127841. [Google Scholar] [CrossRef]
- Breznak, J.A. Phylogenetic Diversity and Physiology of Termite Gut Spirochetes. Integr. Comp. Biol. 2002, 42, 313–318. [Google Scholar] [CrossRef] [Green Version]
- Delalibera, J.I.; Handelsman, J.; Raffa, K.F. Contrasts in Cellulolytic Activities of Gut Microorganisms Between the Wood Borer, Saperda vestita (Coleoptera: Cerambycidae), and the Bark Beetles, Ips pini and Dendroctonus frontalis (Coleoptera: Curculionidae). Environ. Èntomol. 2005, 34, 541–547. [Google Scholar] [CrossRef]
- Calderón-Cortés, N.; Quesada, M.; Watanabe, H.; Cano-Camacho, H.; Oyama, K. Endogenous Plant Cell Wall Digestion: A Key Mechanism in Insect Evolution. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 45–71. [Google Scholar] [CrossRef]
- Erkosar, B.; Yashiro, E.; Zajitschek, F.; Friberg, U.; Maklakov, A.A.; van der Meer, J.R.; Kawecki, T.J. Host diet mediates a negative relationship between abundance and diversity of Drosophila gut microbiota. Ecol. Evol. 2018, 8, 9491–9502. [Google Scholar] [CrossRef]
- Kešnerová, L.; Emery, O.; Troilo, M.; Liberti, J.; Erkosar, B.; Engel, P. Gut microbiota structure differs between honeybees in winter and summer. ISME J. 2019, 14, 801–814. [Google Scholar] [CrossRef] [Green Version]
- McLean, A.H.C.; Parker, B.J.; Hrček, J.; Kavanagh, J.C.; Wellham, P.A.D.; Godfray, H.C.J. Consequences of Symbiont Co-Infections for Insect Host Phenotypes. J. Anim. Ecol. 2018, 87, 478–488. [Google Scholar] [CrossRef]

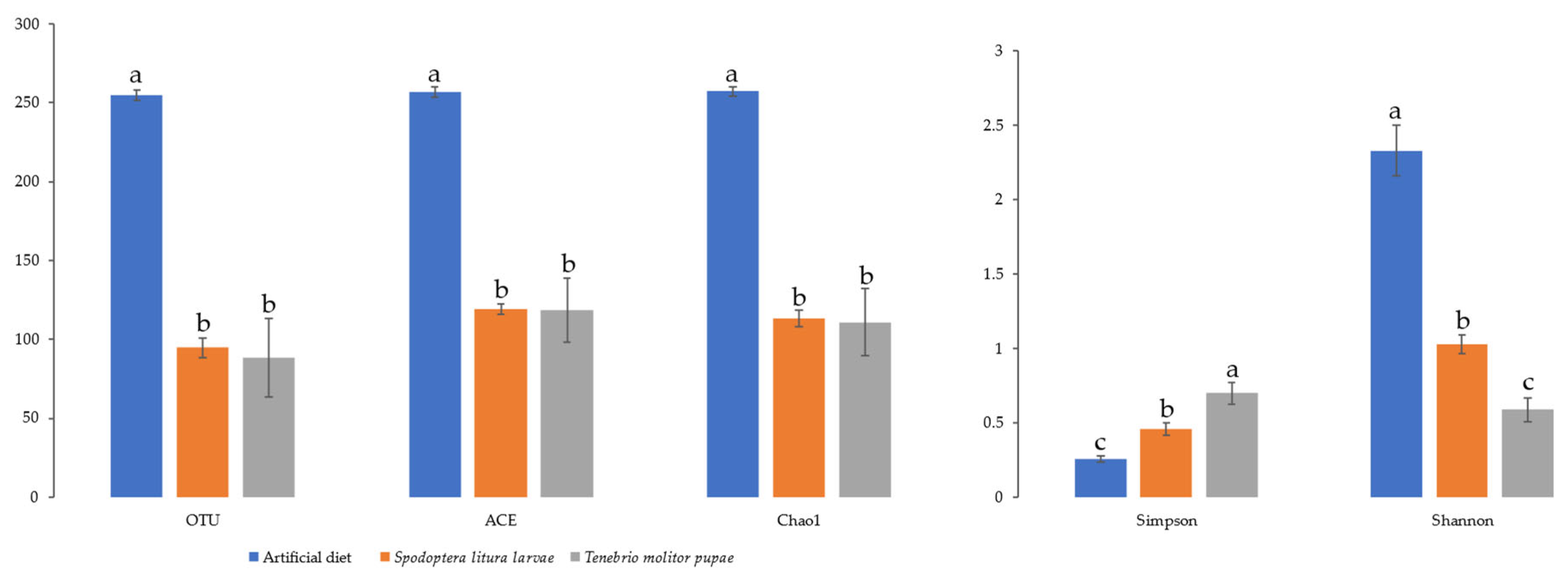
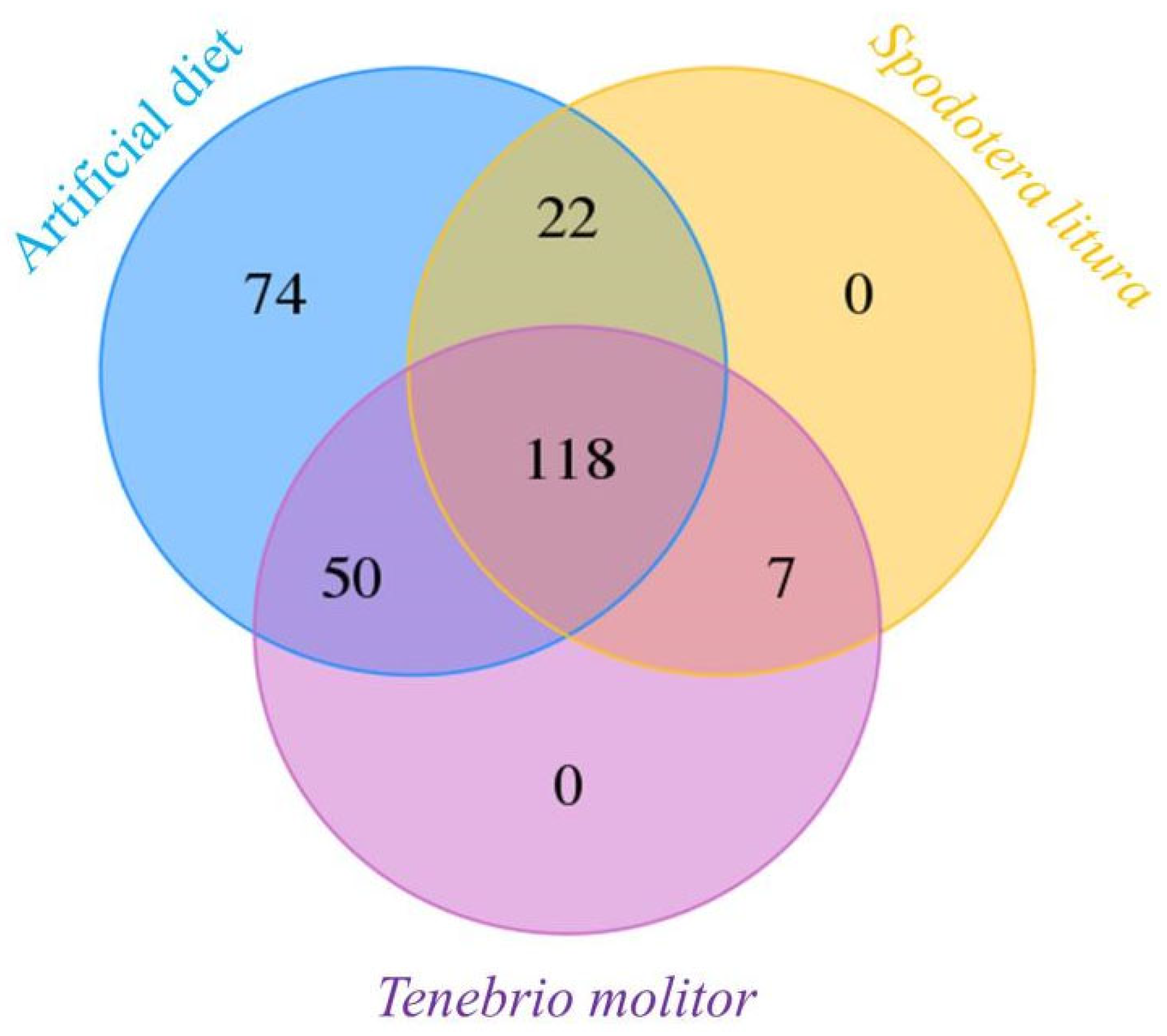

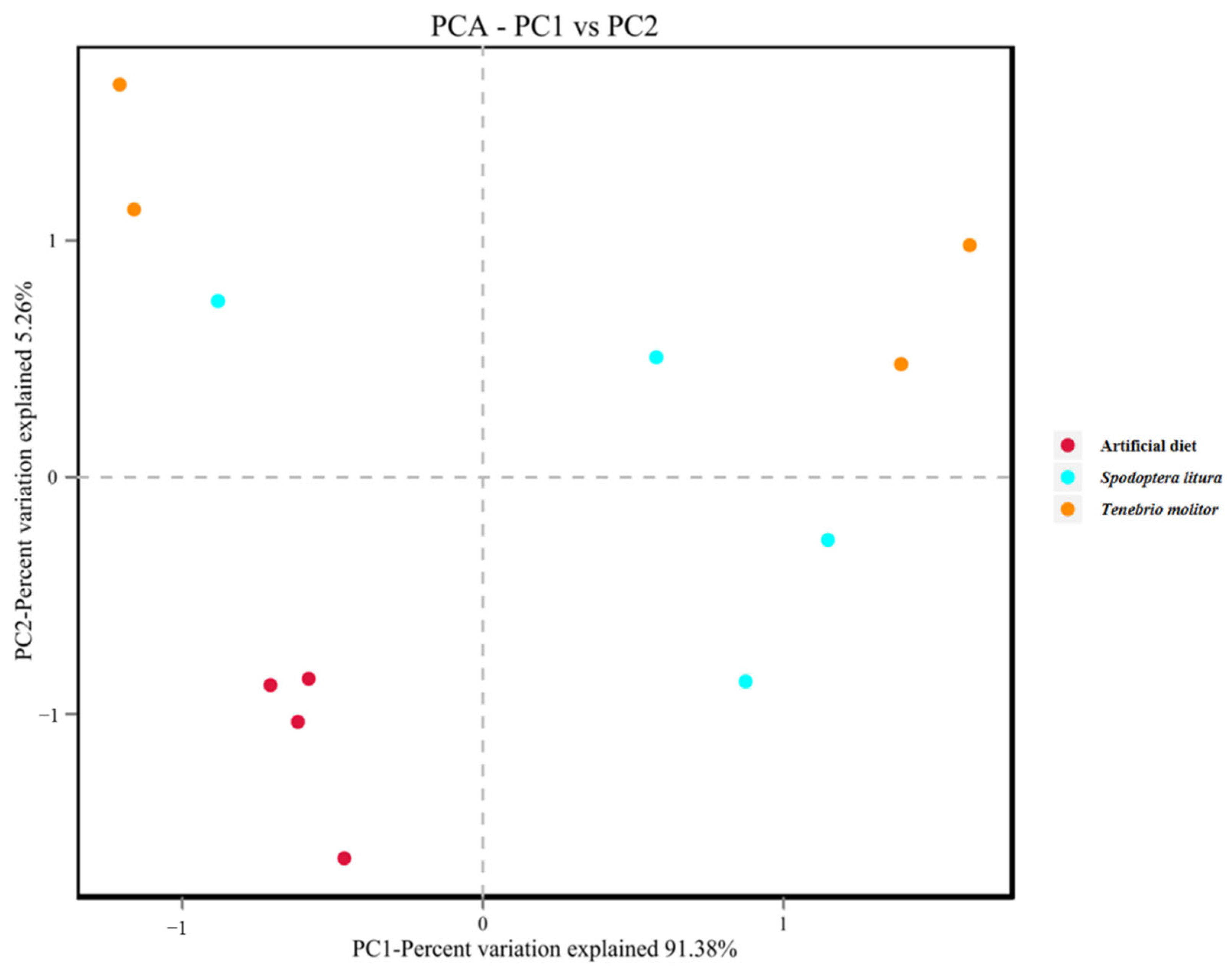
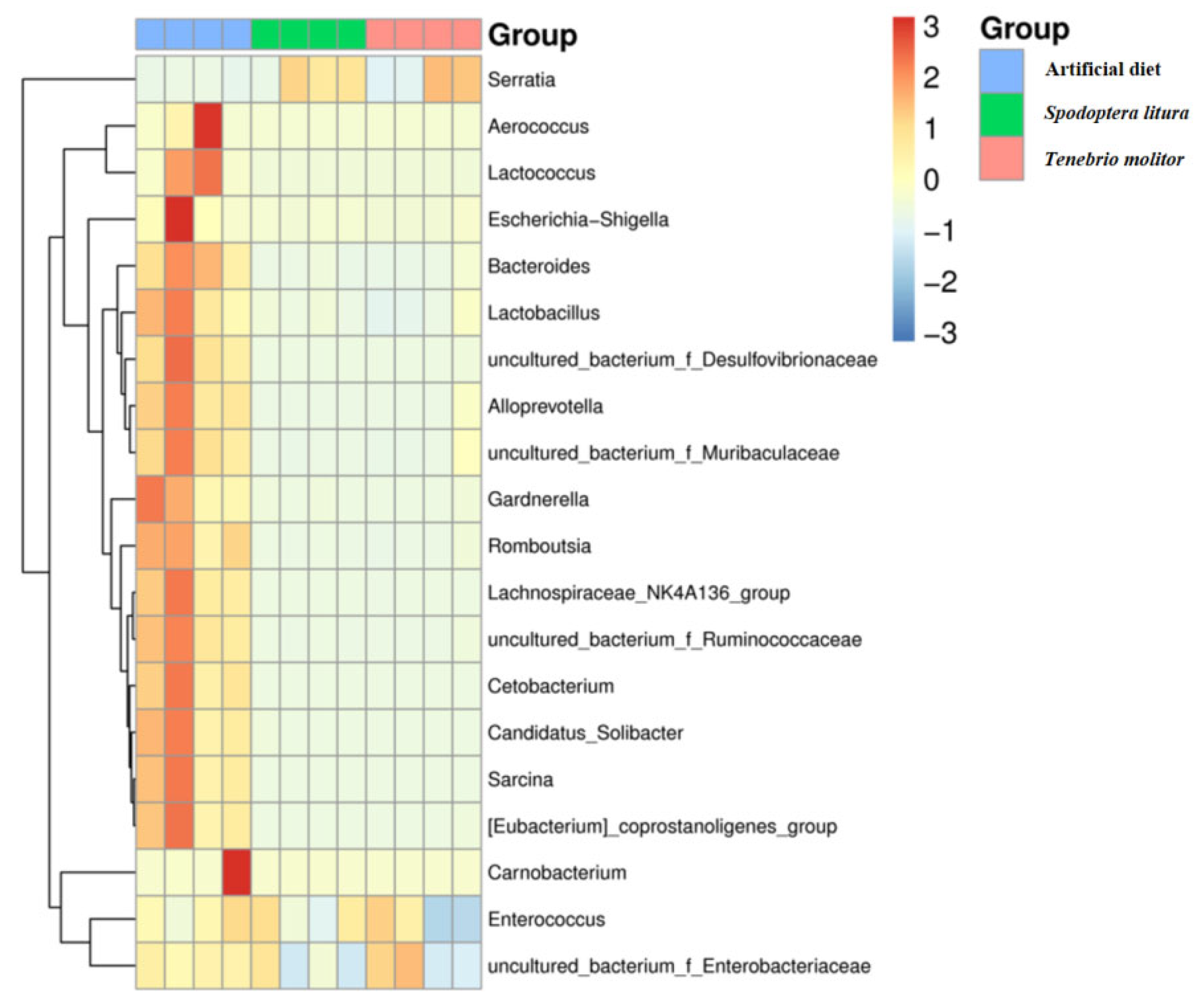

| Diet | 2nd Instar | 3rd Instar | 4th Instar | 5th Instar | Immature * |
|---|---|---|---|---|---|
| Artificial diet | 4.27 ± 0.15 a | 6.32 ± 0.19 a | 7.96 ± 0.49 a | 9.33 ± 0.80 a | 36.33 ± 0.86 a |
| Spodoptera litura larvae | 2.75 ± 0.04 c | 2.58 ± 0.07 c | 3.22 ± 0.12 b | 6.48 ± 0.16 b | 24.96 ± 0.21 b |
| Tenebrio molitor pupae | 3.33 ± 0.50 b | 3.00 ± 0.00 b | 3.64 ± 0.06 b | 5.11 ± 0.27 c | 24.08 ± 0.29 b |
| Diet | Preoviposition Period (d) | Female Oviposition (Eggs Female−1) | Female Longevity (d) | Egg Hatching Rate | ♀:(♀ + ♂) |
|---|---|---|---|---|---|
| Artificial diet | 42.5 ± 8.30 a | 22.00 ± 7.04 b | 61.67 ± 4.44 a | 36.67% b | 0.46 a |
| Spodoptera litura larvae | 16.33 ± 1.44 b | 154.09 ± 25.20 a | 47.74 ± 3.27 b | 100.00% a | 0.37 a |
| Tenebrio molitor pupae | 13.63 ± 2.46 b | 115.57 ± 42.98 a | 38.73 ± 2.39 b | 85.71% a | 0.47 a |
| Diet | Paired-End Reads | Raw Tags | Clean Tags | Effective Tags | Average Length (bp) | Effective (%) * |
|---|---|---|---|---|---|---|
| Artificial diet | 79,792.75 ± 113.75 a | 78,148.50 ± 158.42 a | 75,922.00 ± 183.27 a | 71,001.50 ± 335.83 b | 448.75 ± 0.75 b | 88.98 ± 0.29 b |
| Spodoptera litura larvae | 79,904.25 ± 35.14 a | 76,382.00 ± 302.24 b | 76,161.00 ± 259.88 a | 74,643.00 ± 64.98 a | 453.00 ± 0.00 a | 93.42 ± 0.08 a |
| Tenebrio molitor pupae | 79,981.50 ± 139.71 a | 76,196.50 ± 584.43 b | 75,826.75 ± 465.19 a | 74,980.50 ± 596.86 a | 453.00 ± 0.00 a | 93.75 ± 0.66 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuang, Z.; Wen, J.; Zhu, Y.; He, X.; Chen, K. Dietary Association with Midgut Microbiota Components of Eocanthecona furcellata (Wolff). Diversity 2022, 14, 1130. https://doi.org/10.3390/d14121130
Kuang Z, Wen J, Zhu Y, He X, Chen K. Dietary Association with Midgut Microbiota Components of Eocanthecona furcellata (Wolff). Diversity. 2022; 14(12):1130. https://doi.org/10.3390/d14121130
Chicago/Turabian StyleKuang, Zhaolang, Jian Wen, Yongji Zhu, Xiaofang He, and Kewei Chen. 2022. "Dietary Association with Midgut Microbiota Components of Eocanthecona furcellata (Wolff)" Diversity 14, no. 12: 1130. https://doi.org/10.3390/d14121130
APA StyleKuang, Z., Wen, J., Zhu, Y., He, X., & Chen, K. (2022). Dietary Association with Midgut Microbiota Components of Eocanthecona furcellata (Wolff). Diversity, 14(12), 1130. https://doi.org/10.3390/d14121130








