Diversity of Coral Reef Fishes in the Western Indian Ocean: Implications for Conservation
Abstract
:1. Introduction
2. Materials and Methods
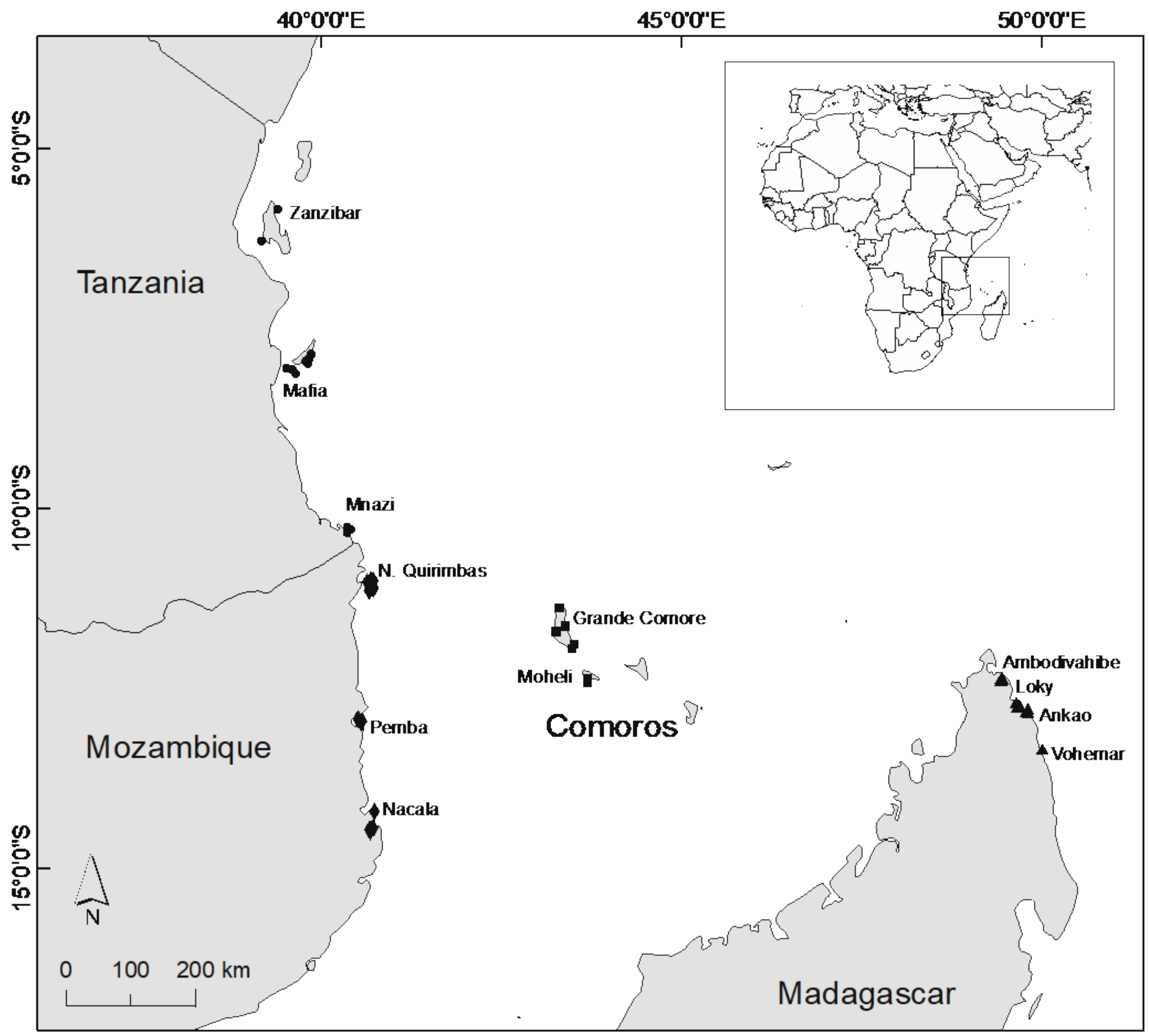
2.1. Study Sites
2.2. Survey Method
2.3. Data Analyses
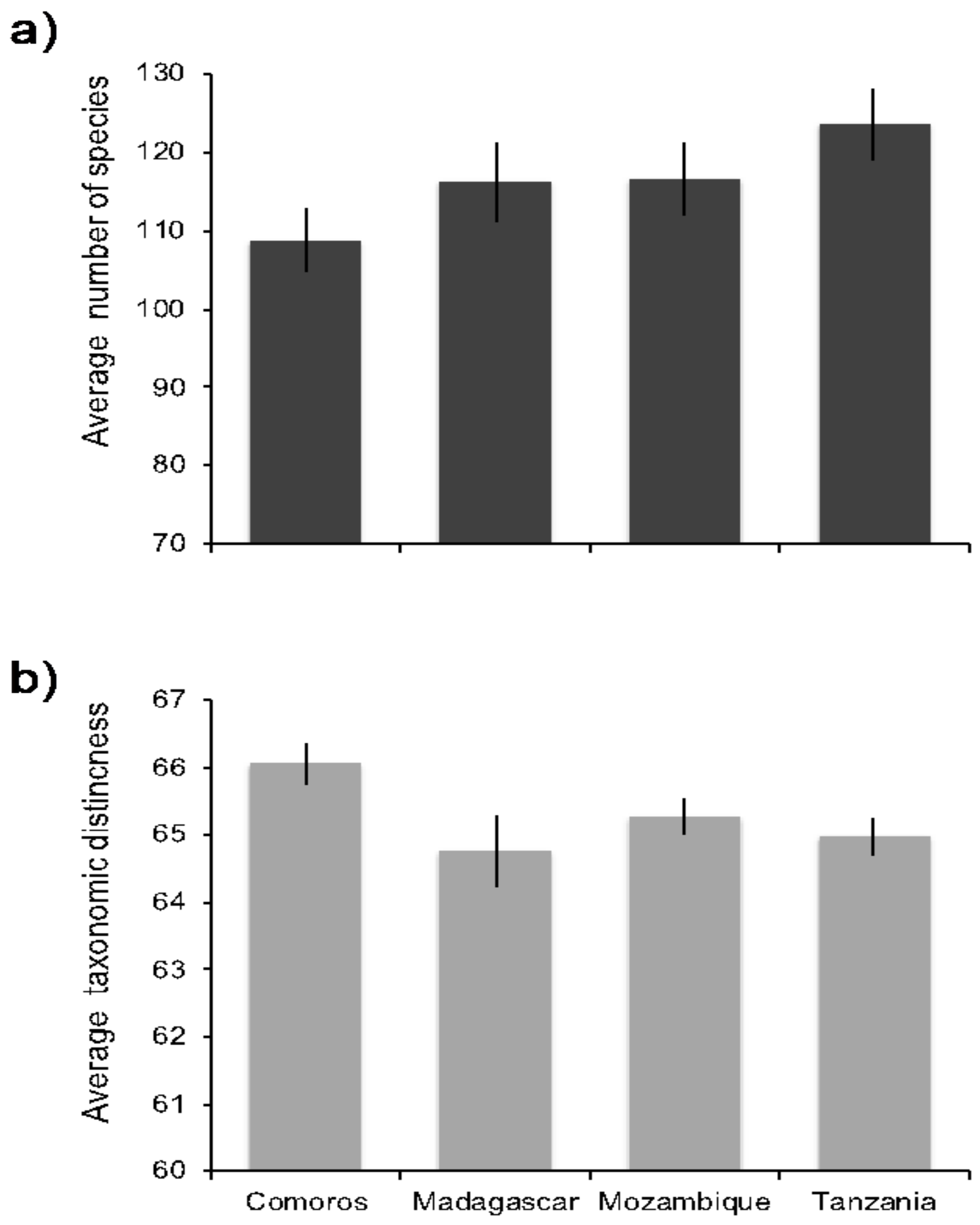
3. Results

- •
- the five most significant species in explaining differences between Comoros and Madagascar were very common in Madagascar but not sighted in Comoros: Plectropomus punctatus, Plectorhinchus gaterinus, Hipposcarus harid, Epibulis insidiator and Chaetodon vagabundus. All were moderately common in mainland countries except C. vagabundus, which was rare;
- •
- other species explained differences between Comoros and Madagascar but did not appear in the top 20 species in other paired geographic zone comparisons, such as Pomacentrus baenschi and P. caeruleopunctatus, or were species common in Madagascar (in 80% of sites) but either rare or absent in mainland;
- •
- high ranking species in Comoros were not sighted in Madagascar, e.g., Anampses twistii and Pygoplites diacanthus. Abudefduf vaigiensis, though less significant, was also common in Comoros and not sighted in Madagascar. Other species common in Comoros but rare/absent in Madagascar included Coris formosa, Mulloidichthys flavolineatus, and Parupeneus trifasciatus;
- •
- other species that contributed to both Madagascar—Comoros and Madagascar—mainland comparisons were common or relatively common across sites in Madagascar and mainland but were rare or absent in Comoros, such as Neoglyphidodon melas, Scarus ghobban, Cheilinus fasciatus and Cheilinus oxycephalus, and Chaetodon melannotus. With a similar distribution pattern though less significant was Lutjanus fulviflamma which was rare in Comoros. Scarus ghobban, (ranked 10th overall), was a key species distinguishing Comoros, where it was rare, from both Madagascar (8th rank) and mainland (20th rank, Table S3);
- •
- species that were top ranking species contributing to the Comoros—mainland differences were not highly ranked in the other SIMPER results such as Aprion virescens, Ostracion meleagris and Heniochus acuminatus. The first two were more common in Comoros compared with mainland or Madagascar, whereas H. acuminatus was not sighted in Comoros. Chaetodon interruptus and Anampses lineatus were also common in Comoros and less common in mainland though were less significant in explaining differences;
- •
- Amphiprion allardi and Cephalopholis argus were the only species significant in both island—mainland comparisons: A. allardi was only observed in mainland sites and C. argus was much more common in mainland sites.
4. Discussion
4.1. Biogeographic Patterns in Species Diversity
4.2. Anthropogenic Influences
4.3. Species Level Differences
4.4. Methods for Species Richness Surveys
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mora, C. Large-scale patterns and processes in reef fish richness. In Ecology of Fishes on Coral Reefs; Mora, C., Ed.; Cambridge University Press: Cambridge, UK, 2015; pp. 88–96. [Google Scholar]
- Hubert, N.; Dettai, A.; Pruvost, P.; Cruaud, C.; Kulbicki, M.; Myers, R.F.; Borsa, P. Geography and life history traits account for the accumulation of cryptic diversity among Indo-West Pacific coral reef fishes. Mar. Ecol. Prog. Ser. 2017, 583, 179–193. [Google Scholar] [CrossRef] [Green Version]
- Bellwood, D.R.; Wainright, P.C. The history and biogeography of fishes on coral reefs. In Coral Reef Fishes; Sale, P., Ed.; Academic Press: Cambridge, MA, USA, 2002; pp. 5–32. [Google Scholar]
- Stow, D. Vanished Ocean: How Tethys Reshaped the World; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Parravicini, V.; Kulbicki, M.; Bellwood, D.R.; Friedlander, A.M.; Arias-Gonzalez, J.E.; Chabanet, P.; Floeter, S.; Myers, R.; Vigliola, L.; D’Agata, S.; et al. Global patterns and predictors of tropical reef fish species richness. Ecography 2013, 36, 1254–1262. [Google Scholar] [CrossRef]
- Heron, S.F.; Maynard, J.A.; Van Hooidonk, R.; Eakin, C.M. Warming Trends and Bleaching Stress of the World’s Coral Reefs 1985–2012. Sci. Rep. 2016, 6, 38402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, J.P.W.; Wilson, S.; Jennings, S.; Graham, N.A. Thermal stress induces persistently altered coral reef fish assemblages. Glob. Chang. Biol. 2019, 25, 2739–2750. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.P.; Kerry, J.T.; Baird, A.H.; Connolly, S.R.; Dietzel, A.; Eakin, C.M.; Heron, S.; Hoey, A.S.; Hoogenboom, M.O.; Liu, G.; et al. Global warming transforms coral reef assemblages. Nature 2018, 556, 492–496. [Google Scholar] [CrossRef]
- Graham, N.A.J.; Chabanet, P.; Evans, R.D.; Jennings, S.; Letourneur, Y.; Macneil, M.A.; McClanahan, T.R.; Öhman, M.C.; Polunin, N.V.C.; Wilson, S.K. Extinction vulnerability of coral reef fishes. Ecol. Lett. 2011, 14, 341–348. [Google Scholar] [CrossRef] [Green Version]
- Graham, N.A.J.; McClanahan, T.R.; MacNeil, M.A.; Wilson, S.K.; Cinner, J.E.; Huchery, C.; Holmes, T.H. Human Disruption of Coral Reef Trophic Structure. Curr. Biol. 2017, 27, 231–236. [Google Scholar] [CrossRef] [Green Version]
- Dana, J.D. On an isothermal oceanic chart illustrating the geographical distribution of marine animals. Am. J. Sci. 1853, 16, 153–167, 314–327. [Google Scholar]
- Forbes, E. The Natural History of the European Seas; Goodwin-Austin, R., Ed.; Facsimile Publisher: London, UK, 1859. [Google Scholar]
- Briggs, J.C. Marine Zoogeography; McGraw-Hill: New York, NY, USA, 1974. [Google Scholar]
- Ekman, S. Zoogeography of the Sea; Sidgwick and Jackson: London, UK, 1953. [Google Scholar]
- Briggs, J.C.; Bowen, B.W. A realignment of marine biogeographic provinces with particular reference to fish distributions. J. Biogeogr. 2012, 39, 12–30. [Google Scholar] [CrossRef]
- Spalding, M.D.; Fox, H.E.; Allen, G.R.; Davidson, N.; Ferdaña, Z.A.; Finlayson, M.; Halpern, B.S.; Jorge, M.A.; Lombana, A.; Lourie, S.A.; et al. Marine ecoregions of the world: A bioregionalization of coastal and shelf areas. Bioscience 2007, 57, 573–583. [Google Scholar] [CrossRef] [Green Version]
- IUCN. Managing Marine Protected Areas: A Toolkit for the Western Indian Ocean; IUCN Eastern Africa Regional Programme: Nairobi, Kenya, 2004. [Google Scholar]
- McClanahan, T.R.; Sheppard, C.R.C.; Obura, D.O. (Eds.) Coral Reefs of the Indian Ocean; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Barber, P.H.; Meyer, C.P. Pluralism explains diversity in the Coral Triangle. In Ecology of Fishes on Coral Reefs; Mora, C., Ed.; Cambridge University Press: Cambridge, UK, 2015; pp. 258–263. [Google Scholar]
- Carpenter, K.E.; Springer, V.G. The center of the center of marine shore fish biodiversity: The Philippine Islands. Environ. Biol. Fishes. 2005, 72, 467–480. [Google Scholar] [CrossRef]
- Obura, D.O. An Indian Ocean centre of origin revisited: Palaeogene and Neogene influences defining a biogeographic realm. J. Biogeogr. 2015, 43, 229–242. [Google Scholar] [CrossRef]
- Obura, D.O. The Diversity and Biogeography of Western Indian Ocean Reef-Building Corals. PLoS ONE 2012, 7, e45013. [Google Scholar] [CrossRef] [PubMed]
- Obura, D.O.; Church, J.E.; Gabrié, C. Assessing Marine World Heritage from an Ecosystem Perspective: The Western Indian Ocean; UNESCO World Heritage Centre: Paris, France, 2012. [Google Scholar]
- Veron, J.; Stafford-Smith, M.; DeVantier, L.; Turak, E. Overview of distribution patterns of zooxanthellate Scleractinia. Front. Mar. Sci. 2015, 2, 81. [Google Scholar] [CrossRef] [Green Version]
- Burgess, N.D.; DAHales, J.D.A.; Underwood, E.; Dinerstein, E. (Eds.) Terrestrial Ecoregions of Africa and Madagascar: A Conservation Assessment; Island Press: Washington, DC, USA, 2004. [Google Scholar]
- Mora, C.; Chittaro, P.M.; Sale, P.F.; Kritzer, J.P.; Ludsin, S.A. Patterns and processes in reef fish diversity. Nature 2003, 421, 933–936. [Google Scholar] [CrossRef]
- Allen, G.R. Reef fishes of Northwestern Madagascar. In A Rapid Marine Biodiversity Assessment of the Coral Reefs of Northwest Madagascar; McKenna, S., Allen, G.R., Eds.; RAP Bullet; Conservation International: Washington, DC, USA, 2005; pp. 39–48. [Google Scholar]
- Samoilys, M.; Randriamanantsoa, B. Reef fishes of northeast Madagascar. In A Rapid Marine Biodiversity Assessment of the Coral Reefs of Northeast Madagascar; Obura, D., Di Carlo, G., Rabearisoa, A., Oliver, T., Eds.; Conservation International: Washington, DC, USA, 2011; pp. 29–39. [Google Scholar]
- Cowburn, B.; Samoilys, M.A.; Obura, D. The current status of coral reefs and their vulnerability to climate change and multiple human stresses in the Comoros Archipelago, Western Indian Ocean. Mar. Pollut. Bull. 2018, 133, 956–969. [Google Scholar] [CrossRef]
- Fricke, R.; Durville, P.; Bernardi, G.; Borsa, P.; Mou-Tham, G.; Chabanet, P. Checklist of the shore fishes of Europa Island, Mozambique Channel, southwestern Indian Ocean, including 302 new records. Stuttgarter Beiträge zur Naturkd A Neue Ser. 2013, 6, 247–276. [Google Scholar]
- Chabanet, P.; Durville, P. Reef Fish Inventory of Juan De Nova’s Natural Park (Western Indian Ocean). West Indian Ocean J. Mar. Sci. 2005, 4, 145–162. [Google Scholar] [CrossRef]
- Fricke, R. Fishes of the Mascarene Islands (Mauritius, Réunion, Rodriguez). An Annotated Checklist, with Descriptions of New Species; Theses Zoo; Koeltz Scientific Books: Königstein, Germany, 1999. [Google Scholar]
- Lieske, E.; Myers, R.F. Coral Reef Fishes, 2nd ed.; Princeton University Press: Princeton, NJ, USA, 2001. [Google Scholar]
- Smith, M.; Heemstra, P.C. Smith’s Sea Fishes; Springer-Verlag: Berlin, Germany, 1996. [Google Scholar]
- Nelson, J.S. Fishes of the World, 4th ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Kulbicki, M.; Vigliola, L.; Wantiez, L.; Floeter, S.; Hubert, N. The biogeography of Chaetodontidae, a model for reef fishes? In The Biology and Ecology of Butterfly-Fishes; Pratchett, M., Berumen, M., Kapoor, B., Eds.; CRC Press: Boca Raton, FL, USA, 2013; pp. 70–106. [Google Scholar]
- Samoilys, M.; Roche, R.; Koldewey, H.; Turner, J. Patterns in reef fish assemblages: Insights from the Chagos Archipelago. PLoS ONE 2018, 13, e0191448. [Google Scholar] [CrossRef]
- Samoilys, M.A.; Osuka, K.; Maina, G.W.; Obura, D.O. Artisanal fisheries on Kenya’s coral reefs: Decadal trends reveal management needs. Fish Res. 2017, 186, 177–191. [Google Scholar] [CrossRef]
- Bellwood, D.R. The Eocene fishes of Monte Bolca: The earliest coral reef fish assemblage. Coral Reefs. 1996, 15, 11–19. [Google Scholar] [CrossRef]
- Choat, J.H.; Bellwood, D.R. Reef fishes: Their history and evolution. In The Ecology of Fishes on Coral Reefs; Sale, P.F., Ed.; Academic Press, Inc.: San Diego, CA, USA, 1991; pp. 39–61. [Google Scholar]
- Munday, P.L.; Jones, G.P.; Pratchett, M.S.; Williams, A.J. Climate change and the future for coral reef fishes. Fish Fish 2008, 9, 261–285. [Google Scholar] [CrossRef]
- Fricke, R.; Eschmeyer, W.N.; van der Laan, R. Eschmeyer’s Catalog of Fishes: Genera, Species, References; Fricke, R., Eschmeyer, W.N., van der Laan, R., Eds.; California Academy of Sciences: San Francisco, CA, USA, 2021. [Google Scholar]
- Clarke, K.R.; Warwick, R.M. A further biodiversity index applicable to species lists: Variation in taxonomic distinctness. Mar. Ecol. Prog. Ser. 2001, 216, 265–278. [Google Scholar] [CrossRef]
- Warwick, R.M.; Clarke, K.R. Taxonomic Distinctness and Environmental Assessment. J. Appl. Ecol. 1998, 35, 532–543. [Google Scholar] [CrossRef]
- Zauca, J.; Gee, K. Maritime Spatial Planning: Past, Present, Future; Zauca, J., Gee, K., Eds.; Springer: Heidelberg, Germany, 2018. [Google Scholar]
- Keith, D.; Rodríguez, J.; Rodríguez-Clark, K.; Nicholson, E.; Aapala, K.; Alonso, A.; Asmussen, M.; Bachman, S.; Basset, A.; Barrow, E.G.; et al. Scientific Foundations for an IUCN Red List of Ecosystems. PLoS ONE 2013, 8, e62111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Agata, S.; Mouillot, D.; Kulbicki, M.; Andréfouët, S.; Bellwood, D.R.; Cinner, J.E.; Cowman, P.; Kronen, M.; Pinca, S.; Vigliola, L. Human-mediated loss of phylogenetic and functional diversity in coral reef fishes. Curr. Biol. 2014, 24, 555–560. [Google Scholar] [CrossRef] [Green Version]
- Barneche, D.R.; Rezende, E.L.; Parravicini, V.; Maire, E.; Edgar, G.J.; Stuart-Smith, R.D.; Arias-González, J.E.; Ferreira, C.E.L.; Friedlander, A.M.; Green, A.L.; et al. Body size, reef area and temperature predict global reef-fish species richness across spatial scales. Glob. Ecol. Biogeogr. 2019, 28, 315–327. [Google Scholar] [CrossRef]
- Bellwood, D.R.; Hughes, T.P.; Connolly, S.R.; Tanner, J. Environmental and geometric constraints on Indo-Pacific coral reef biodiversity. Ecol. Lett. 2005, 8, 643–651. [Google Scholar] [CrossRef]
- Audru, J.C.; Guennoc, P.; Thinon, I.; Abellard, O. Bathymay: La structure sous-marine de Mayotte révélée par l’imagerie multifaisceaux. Comptes. Rendus. Geosci. 2006, 338, 1240–1249. [Google Scholar] [CrossRef] [Green Version]
- Samoilys, M.A.; Halford, A.; Osuka, K. Disentangling drivers of the abundance of coral reef fishes in the Western Indian Ocean. Ecol. Evol. 2019, 9, 4149–4167. [Google Scholar] [CrossRef]
- Crochelet, E.; Roberts, J.; Lagabrielle, E.; Obura, D.; Petit, M.; Chabanet, P. A model-based assessment of reef larvae dispersal in the Western Indian Ocean reveals regional connectivity patterns—Potential implications for conservation policies. Reg. Stud. Mar. Sci. 2016, 7, 159–167. [Google Scholar] [CrossRef] [Green Version]
- Muths, D.; Tessier, E.; Bourjea, J. Genetic structure of the reef grouper Epinephelus merra in the West Indian Ocean appears congruent with biogeographic and oceanographic boundaries. Mar. Ecol. 2015, 36, 447–461. [Google Scholar] [CrossRef]
- Muths, D.; Gouws, G.; Mwale, M.; Tessier, E.; Bourjea, J. Genetic connectivity of the reef fish Lutjanus kasmira at the scale of the western Indian Ocean. Can. J. Fish. Aquat. Sci. 2012, 69, 842–853. [Google Scholar] [CrossRef]
- Jacquet, J.; Fox, H.; Motta, H.; Ngusaru, A.; Zeller, D. Few data but many fish: Marine small-scale fisheries catches for Mozambique and Tanzania. Afr. J. Mar. Sci. 2017, 32, 2338. [Google Scholar] [CrossRef]
- Rehren, J.; Samoilys, M.; Reuter, H.; Jiddawi, N. Integrating resource perception, ecological surveys, and fisheries statistics: A review of the fisheries in Zanzibar. Rev. Fish Sci. Aquac. 2020, 30, 1–18. [Google Scholar] [CrossRef]
- Ateweberhan, M.; McClanahan, T.R.; Graham, N.A.J.; Sheppard, C.R.C. Episodic heterogeneous decline and recovery of coral cover in the Indian Ocean. Coral Reefs. 2011, 30, 739–752. [Google Scholar] [CrossRef]
- DeMartini, E.E.; Friedlander, A.M.; Sandin, S.A.; Sala, E. Differences in fish-assemblage structure between fished and unfished atolls in the northern Line Islands, central Pacific. Mar. Ecol. Prog. Ser. 2008, 365, 199–215. [Google Scholar] [CrossRef]
- DeMartini, E.E.; Smith, J.E. Effects of fishing on the fishes and habitats of coral reefs. In Ecology of Fishes on Coral Reefs; Mora, C., Ed.; Cambridge University Press: Cambridge, UK, 2015; pp. 135–144. [Google Scholar]
- Samoilys, M.A.; Osuka, K.; Mussa, J.; Rosendo, S.; Riddell, M.; Diade, M.; Mbugua, J.; Kawaka, J.; Hill, N.A.O.; Koldewey, H. An integrated assessment of coastal fisheries in Mozambique for conservation planning. Ocean Coast Manag. 2019, 182, 104924. [Google Scholar] [CrossRef]
- Heemstra, P.C.; Randall, J.E. Groupers of the World (Family Serranidae, Subfamily Epinephelinae). An Annotated and Illsutrated Catalogue of the Grouper; Fisheries; FAO: Rome, Italy, 1993. [Google Scholar]
- Wickel, J.; Jamon, A.; Pinault, M. Composition et Structure des Peuplements Ichtyologiques Marins de l ’ île de Mayotte (Sud-Ouest de L ’océan Indien); Société Française d’Ichtyologie: Paris, France, 2014; Volume 38, pp. 179–203. [Google Scholar]
- Strona, G.; Lafferty, K.D.; Fattorini, S.; Beck, P.S.A.; Guilhaumon, F.; Arrigoni, R.; Montano, S.; Seveso, D.; Galli, P.; Planes, S.; et al. Global tropical reef fish richness could decline by around half if corals are lost. Proc. R Soc. B Biol. Sci. 2021, 288, 20210274. [Google Scholar] [CrossRef]
- Fautin, D.G.; Allen, G.R. Field Guide to Anemonefishes and their Host Sea Anemones; West Australian Museum: Perth, Australia, 1992. [Google Scholar]
- Fricke, R.; Mulochau, T.; Durville, P.; Chabanet, P.; Tessier, E.; Letourner, Y. Annotated checklist of the fish species (Pisces) of La Réunion, including a Red List of threatened and declining species. Stuttgarter Beiträge zur Naturkd A Neue Ser. 2009, 2, 1–168. [Google Scholar]
- Durville, P.; Chabanet, P.; Quod, J.P. Visual Census of the Reef Fishes in the Natural Reserve of the Glorieuses Islands (Western Indian Ocean). West. Indian Ocean J. Mar. Sci. 2003, 2, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Chabanet, P.; Tessier, E.; Mulochau, T.; Durville, P.; Rene, F. Peuplement ichtyologique des Bancs des Geyser et Zelee (Ocean Indien Occidental). Cymbium 2002, 26, 11–26. [Google Scholar]
- Bourjon, P.; Crochelet, E.; Fricke, R. First record of the large caerulean damselfish, Pomacentrus caeruleopunctatus (Actinopterygii: Perciformes: Pomacentridae), from reunion island, south-west indian ocean. Acta Ichthyol. Piscat. 2019, 49, 59–63. [Google Scholar] [CrossRef] [Green Version]
- Schott, F.A.; Xie, S.P.; McCreary, J.P. Indian ocean circulation and climate variability. Rev. Geophys. 2009, 47, 1–46. [Google Scholar] [CrossRef]
- Lutjeharms, J.R.E.; Bornman, T.G. The importance of the greater agulhas currentis increasingly being recognized. S. Afr. J. Sci. 2010, 106, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Kulbicki, M.; Parravicini, V.; Bellwood, D.R.; Arias-Gonzàlez, E.; Chabanet, P.; Floeter, S.R.; Friedlander, A.; McPherson, J.; Myers, R.E.; Vigliola, L.; et al. Global biogeography of reef fishes: A hierarchical quantitative delineation of regions. PLoS ONE 2013, 8, e81847. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species. Version 2021-2 [Internet]. 2021. Available online: https://www.iucnredlist.org (accessed on 19 November 2021).
- Stuart-Smith, R.D.; Bates, A.E.; Lefcheck, J.S.; Duffy, J.E.; Baker, S.C.; Thomson, R.J.; Stuart-Smith, J.F.; Hill, N.A.; Kininmonth, S.J.; Airoldi, L.; et al. Integrating abundance and functional traits reveals new global hotspots of fish diversity. Nature. Nature 2013, 501, 539–542. [Google Scholar] [CrossRef]
- Gudka, M.; Obura, D.; Mbugua, J.; Ahamada, S.; Kloiber, U.; Holter, T. Participatory reporting of the 2016 bleaching event in the Western Indian Ocean. Coral Reefs 2020, 39, 1–11. [Google Scholar] [CrossRef]
- Allen, G.R.; Werner, T.B. Coral reef fish assessment in the coral triangle of southeastern Asia. Environ. Biol. Fishes 2002, 65, 209–214. [Google Scholar] [CrossRef]
- Cole, A.C.; Pratchett, M.S.; Jones, J.P. Diversity and functional importance of coral-feeding fishes on tropical coral reefs. Fish Fish 2008, 9, 286–307. [Google Scholar] [CrossRef]
- Samoilys, M.A.; Carlos, G. Determining methods of underwater visual census for estimating the abundance of coral reef fishes. Environ. Biol. Fishes 2000, 57, 289–304. [Google Scholar] [CrossRef]
- Pratchett, M.S.; Munday, P.L.; Wilson, S.K.; Graham, N.A.J.; Cinner, J.E.; Bellwood, D.R.; Jones, G.P.; Polunin, N.V.; Mcclanahan, T.R. Effects of climate-induced coral bleaching on coral-reef fishes ecological and economic consequences. Oceanogr. Mar. Biol. 2008, 46, 251–296. [Google Scholar] [CrossRef]
- Obura, D.O.; Gudka, M.; Abdou Rabi, F.; Bacha Gian, S.; Bigot, L.; Bijoux, J.; Freed, S.; Maharavo, J.; Mwaura, J.; Porter, S.N.; et al. Coral Reef Status Report for the Western Indian Ocean; Global Coral Reef Monitoring Network (GCRMN); International Coral Reef Initiative (ICRI); Indian Ocean Commission: Port Louis, Mauritius, 2017. [Google Scholar]
- Pecl, G.T.; Araújo, M.B.; Bell, J.D.; Blanchard, J.; Bonebrake, T.C.; Chen, I.C.; Clark, T.D.; Colwell, R.K.; Danielsen, F.; Evengård, B.; et al. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 2017, 355, eaai9214. [Google Scholar] [CrossRef] [PubMed]
- Obura, D.O.; Gudka, M.; Samoilys, M.; Osuka, K.; Mbugua, J.; Keith, D.A.; Porter, S.; Roche, R.; van Hooidonk, R.; Ahamada, S.; et al. Vulnerability to collapse of coral reef ecosystems in the Western Indian Ocean. Nat. Sustain. 2021. [Google Scholar] [CrossRef]



| Order | Suborder | Families | CFDI A | Abundance C and B | 10 Coral Reef B | Most Speciose B and W |
|---|---|---|---|---|---|---|
| Perciformes (a) | Labroidei | Labridae (wrasse) | X | X | X | 1 |
| Percoidei | Epinephelidae (groupers) | 3 | ||||
| Labroidei | Pomacentridae (damsel fishes) | X | X | X | 2 | |
| Perciformes (b) | Percoidei | Chaetodontidae (butterfly fishes) | X | X | X | 6 |
| Labroidei | Scarinae (parrot fishes) 1 | X | X | 8 | ||
| Acanthuroidei | Acanthuridae (surgeon fishes) | X | X | X | 7 | |
| Percoidei | Lutjanidae (snappers) | 10 | ||||
| Percoidei | Pomacanthidae (angel fishes) | X | 11 | |||
| Perciformes (c) | Percoidei | Lethrinidae (emperors) | 13 | |||
| Percoidei | Haemulidae (grunts) | 23 | ||||
| Percoidei | Mullidae (goat fishes) | X | 19 | |||
| Acanthuroidei | Siganidae (rabbit fishes) | 21 | ||||
| Percoidei | Nemipteridae (bream) | 24 | ||||
| Percoidei | Carangidae (trevally) | X | N/A | |||
| Perciformes (d) | Percoidei | Caesionidae (fusiliers) | 28 | |||
| Tetraodontiformes | Tetraodontiformes | Balistidae (trigger fishes) | 16 | |||
| Tetraodontiformes | Tetraodontiformes | Monacanthidae (file fishes) | 14 | |||
| Tetraodontiformes | Tetraodontiformes | Ostraciidae (box fishes) | 25 | |||
| Tetraodontiformes | Tetraodontiformes | Tetraodontidae (puffer fishes) | 18 |
| (a) Between Countries Global R = 0.405, p = 0.001 | ||
|---|---|---|
| Pairwise Tests—Groups | R Statistic | p |
| Madagascar, Comoros | 0.864 | 0.001 |
| Madagascar, Tanzania | 0.592 | 0.001 |
| Madagascar, Mozambique | 0.483 | 0.001 |
| Comoros, Tanzania | 0.397 | 0.001 |
| Comoros, Mozambique | 0.354 | 0.007 |
| Tanzania, Mozambique | 0.103 | 0.048 |
| (b) Between Areas within Mainland Countries Global R = 0.21; p = 0.011 | ||
| Pairwise Tests—Groups | R Statistic | p |
| Chumbe, Mafia | 0.617 | 0.056 |
| Chumbe, Nacala | 0.321 | 0.200 |
| Chumbe, Pemba | 0.857 | 0.067 |
| Chumbe, Vamizi | 0.3 | 0.156 |
| Chumbe, Mnazi | 0.583 | 0.100 |
| Mafia, Nacala | 0.418 | 0.012 |
| Mafia, Pemba | 0.489 | 0.009 |
| Mafia, Vamizi | 0.131 | 0.061 |
| Mafia, Mnazi | 0.173 | 0.225 |
| Nacala, Pemba | −0.01 | 0.571 |
| Nacala, Vamizi | 0.21 | 0.113 |
| Nacala, Mnazi | 0.148 | 0.229 |
| Pemba, Vamizi | 0.104 | 0.240 |
| Pemba, Mnazi | 0.5 | 0.029 |
| Vamizi, Mnazi | −0.113 | 0.636 |
| AVERAGE ABUNDANCE | Diss/SD | Cum.Av. Diss.Contrib % | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Family/Sub-Fam | Range | COMOROS | MAINLAND | MADAGASC | Comoros-Mainland | Madagacar-Mainland | Madagascar-Comoros | Comoros-Mainland | Madagacar-Mainland | Madagascar-Comoros | Rank AvDiss/SD |
| Plectropomus_punctatus | Epinephelidae | WIO | 0 | 0.32 | 1 | 0.68 | 1.43 | 11.97 | 76.92 | 6.78 | 0.85 | 1 |
| Plectorhinchus_gaterinus | Haemulidae | WIO | 0 | 0.57 | 0.9 | 1.14 | 0.88 | 2.89 | 13.62 | 59.79 | 1.63 | 5 |
| Hipposcarus_harid | Labridae | RS-IO 1 | 0 | 0.54 | 0.9 | 1.06 | 0.93 | 2.88 | 27.36 | 49.69 | 2.4 | 6 |
| Epibulus_insidiator | Labridae | RS-IWP | 0 | 0.79 | 0.9 | 1.87 | 0.61 | 2.88 | 0.73 | 82.37 | 3.16 | 3 |
| Chaetodon_vagabundus | Chaetodontidae | IWP | 0 | 0.07 | 0.9 | 0.28 | 2.24 | 2.88 | 98.19 | 2.23 | 3.93 | 2 |
| Anampses_twistii | Labridae | RS-IWP | 0.86 | 0.86 | 0 | 0.56 | 2.37 | 2.38 | 84.35 | 1.49 | 4.66 | 4 |
| Neoglyphidodon_melas | Pomacentridae | RS-IWP | 0.14 | 0.75 | 1 | 1.43 | 0.57 | 2.37 | 2.76 | 85.29 | 5.4 | 8 |
| Scarus_ghobban | Scarinae | RS-IWP | 0.14 | 0.61 | 1 | 1.15 | 0.8 | 2.37 | 12.01 | 66.81 | 6.12 | 9 |
| Cheilinus_fasciatus | Labridae | RS-IWP | 0 | 0.61 | 0.8 | 1.23 | 0.87 | 1.95 | 7.58 | 62.1 | 6.8 | 11 |
| Acanthurus_dussumieri | Acanthuridae | IWP | 0.14 | 0.21 | 0.9 | 0.64 | 1.6 | 1.87 | 80.63 | 5.57 | 7.48 | 10 |
| Chaetodon_melannotus | Chaetodontidae | RS-IWP | 0.14 | 0.75 | 0.9 | 1.43 | 0.65 | 1.87 | 3.4 | 80.32 | 8.15 | 13 |
| Pomacanthus_chrysurus | Pomacanthidae | WIO | 0.14 | 0.39 | 0.9 | 0.85 | 1.17 | 1.87 | 63.12 | 12.81 | 8.82 | 15 |
| Pomacentrus_baenschi | Pomacentridae | EA | 0 | 0.36 | 0.8 | 0.74 | 1.17 | 1.96 | 74.43 | 14.88 | 9.49 | 16 |
| Mulloidichthys_flavolineatus | Mullidae | IWP | 0.86 | 0.36 | 0.1 | 1.21 | 0.78 | 1.88 | 8.7 | 68.86 | 10.16 | 17 |
| Parupeneus_trifasciatus | Mullidae | IWP | 0.86 | 0.46 | 0.1 | 1.04 | 0.93 | 1.87 | 24.89 | 54.24 | 10.83 | 18 |
| Lutjanus_fulviflamma | Lutjanidae | RS-IWP | 0.14 | 0.64 | 0.8 | 1.22 | 0.83 | 1.55 | 9.82 | 63.6 | 11.45 | 23 |
| Pygoplites_diacanthus | Pomacanthidae | RS-IWP | 0.71 | 0.86 | 0 | 0.72 | 2.36 | 1.55 | 75.08 | 0.75 | 12.06 | 7 |
| Chlorurus_atrilunula | Scarinae | WIO | 0.14 | 0.43 | 0.8 | 0.89 | 1.07 | 1.55 | 57.87 | 20.73 | 12.67 | 25 |
| Thalassoma_genivittatum | Labridae | (WIO-Mas) | 0.14 | 0.07 | 0.8 | 0.49 | 1.72 | 1.55 | 89.85 | 4.92 | 13.29 | 21 |
| Chromis_ternatensis | Pomacentridae | IWP | 1 | 0.82 | 0.3 | 0.46 | 1.28 | 1.5 | 89.51 | 9.6 | 13.9 | 41 |
| Cheilinus_oxycephalus | Labridae | IWP | 0.14 | 0.75 | 0.8 | 1.43 | 0.73 | 1.55 | 4.02 | 72.46 | 14.5 | 22 |
| Abudefduf_vaigiensis | Pomacentridae | RS-IWP | 0.71 | 0.46 | 0 | 1.02 | 0.92 | 1.55 | 27.85 | 59.01 | 15.11 | 26 |
| Pomacentrus_trichrourus | Pomacentridae | RS-WIO | 0.14 | 0.54 | 0.8 | 1.04 | 0.95 | 1.56 | 26.37 | 50.95 | 15.72 | 24 |
| Pomacentrus_caeruleopunctatus | Pomacentridae | WIO-Mas | 0.14 | 0.04 | 0.8 | 0.45 | 1.83 | 1.55 | 92.02 | 4.27 | 16.32 | 20 |
| Lutjanus_ehrenbergi | Lutjanidae | RS-IWP | 0 | 0.11 | 0.7 | 0.34 | 1.36 | 1.5 | 96.48 | 7.35 | 16.9 | 47 |
| Pervagor_janthinosoma | Labridae | IWP | 0.71 | 0.21 | 0.1 | 1.27 | 0.6 | 1.41 | 7.01 | 82.85 | 17.47 | 32 |
| Coris_formosa | Labridae | RS-WIO 2 | 0.71 | 0.11 | 0.1 | 1.4 | 0.47 | 1.4 | 4.64 | 90.14 | 18.04 | 38 |
| Variola_louti | Epinephelidae | RS-IWP | 0.71 | 0.54 | 0.1 | 0.96 | 1.04 | 1.4 | 42.1 | 30.47 | 18.61 | 27 |
| Amblyglyphidodon_indicus | Pomacentridae | RS-IO | 0.29 | 0.71 | 0.9 | 1.19 | 0.69 | 1.41 | 11.47 | 77.59 | 19.17 | 31 |
| Cheilinus_trilobatus | Labridae | IWP | 0.29 | 0.75 | 0.9 | 1.23 | 0.65 | 1.4 | 9.26 | 79.26 | 19.74 | 36 |
| Additional Significant Species (top 20) in Madagascar-Mainland Comparisons | ||||||||||||
| Cephalopholis_argus | Epinephelidae | RS-IWP | 0.29 | 0.86 | 0.1 | 1.35 | 1.87 | 0.69 | 5.25 | 3.6 | 81.16 | 14 |
| Chaetodon_falcula | Chaeotodontidae | IO | 0.43 | 0.86 | 0.1 | 1.09 | 1.87 | 0.88 | 16.25 | 2.91 | 67.67 | 19 |
| Amphiprion_allardi | Pomacentridae | WIO | 0 | 0.71 | 0 | 1.55 | 1.55 | Undefined | 2.12 | 6.19 | 100 | 114 |
| Acanthurus_xanthopterus | Acanthuridae | IWP | 0.14 | 0.11 | 0.7 | 0.53 | 1.36 | 1.32 | 87.45 | 7.92 | 21.93 | 44 |
| Ctenochaetus_binotatus | Acanthuridae | IWP | 0.71 | 0.36 | 1 | 1.12 | 1.32 | 0.63 | 15.2 | 8.48 | 85.38 | 64 |
| Pomacanthus_semicirculatus | Pomacanthidae | IWP | 0.57 | 0.36 | 1 | 1.03 | 1.32 | 0.86 | 25.88 | 9.05 | 74.18 | 46 |
| Ctenochaetus_truncatus | Acanthuridae | IO | 0.86 | 0.79 | 0.3 | 0.64 | 1.24 | 1.32 | 79.28 | 10.15 | 20.29 | 48 |
| Zebrasoma_velifer | Acanthuridae | IWP | 0.71 | 0.36 | 0.9 | 1.12 | 1.24 | 0.69 | 14.68 | 10.69 | 80.58 | 69 |
| Pomacentrus_caeruleus | Pomacentridae | IO 3 | 0.71 | 0.39 | 1 | 1.08 | 1.22 | 0.63 | 17.28 | 11.23 | 88.06 | >180 |
| Pterocaesio_tile | Caesionidae | IWP | 0.86 | 0.79 | 0.3 | 0.64 | 1.24 | 1.32 | 79 | 11.77 | 20.84 | 49 |
| Calotomus_carolinus | Scarinae | IP | 0.57 | 0.68 | 0.2 | 0.94 | 1.23 | 1.07 | 44.84 | 12.29 | 41.67 | 42 |
| Additional Significant Species (Top 20) in Comoros–Mainland (Note C. argus and A. allardi, above, also Rank Here) | ||||||||||||
| Heniochus_acuminatus | Chaeotodontidae | IWP | 0 | 0.79 | 0.6 | 1.88 | 0.88 | 1.2 | 1.45 | 60.56 | 27.75 | 12 |
| Aprion_virescens | Lutjanidae | IWP | 0.71 | 0.21 | 0.5 | 1.27 | 0.99 | 0.99 | 6.43 | 46.28 | 47.41 | 40 |
| Ostracion_meleagris | Ostraciidae | IP | 0.71 | 0.21 | 0.6 | 1.27 | 1.11 | 0.9 | 5.84 | 19.3 | 60.75 | 37 |
| Chromis_lepidolepis | Pomacentridae | IWP | 0.29 | 0.75 | 0.5 | 1.23 | 0.99 | 0.99 | 8.14 | 32.72 | 55.92 | 45 |
| Chaetodon_interruptus | Chaeotodontidae | IO | 0.71 | 0.29 | 0.7 | 1.19 | 1.17 | 0.83 | 10.37 | 14.36 | 72.76 | 50 |
| Anampses_lineatus | Labridae | RS-IO | 0.71 | 0.32 | 0.3 | 1.15 | 0.86 | 1.17 | 10.92 | 63.97 | 29.77 | 51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samoilys, M.; Alvarez-Filip, L.; Myers, R.; Chabanet, P. Diversity of Coral Reef Fishes in the Western Indian Ocean: Implications for Conservation. Diversity 2022, 14, 102. https://doi.org/10.3390/d14020102
Samoilys M, Alvarez-Filip L, Myers R, Chabanet P. Diversity of Coral Reef Fishes in the Western Indian Ocean: Implications for Conservation. Diversity. 2022; 14(2):102. https://doi.org/10.3390/d14020102
Chicago/Turabian StyleSamoilys, Melita, Lorenzo Alvarez-Filip, Robert Myers, and Pascale Chabanet. 2022. "Diversity of Coral Reef Fishes in the Western Indian Ocean: Implications for Conservation" Diversity 14, no. 2: 102. https://doi.org/10.3390/d14020102
APA StyleSamoilys, M., Alvarez-Filip, L., Myers, R., & Chabanet, P. (2022). Diversity of Coral Reef Fishes in the Western Indian Ocean: Implications for Conservation. Diversity, 14(2), 102. https://doi.org/10.3390/d14020102






