Euchlorocystis marina sp. nov. (Oocystaceae, Trebouxiophyceae), a New Species of Green Algae from a Seawater Shrimp Culture Pond
Abstract
:1. Introduction
2. Results
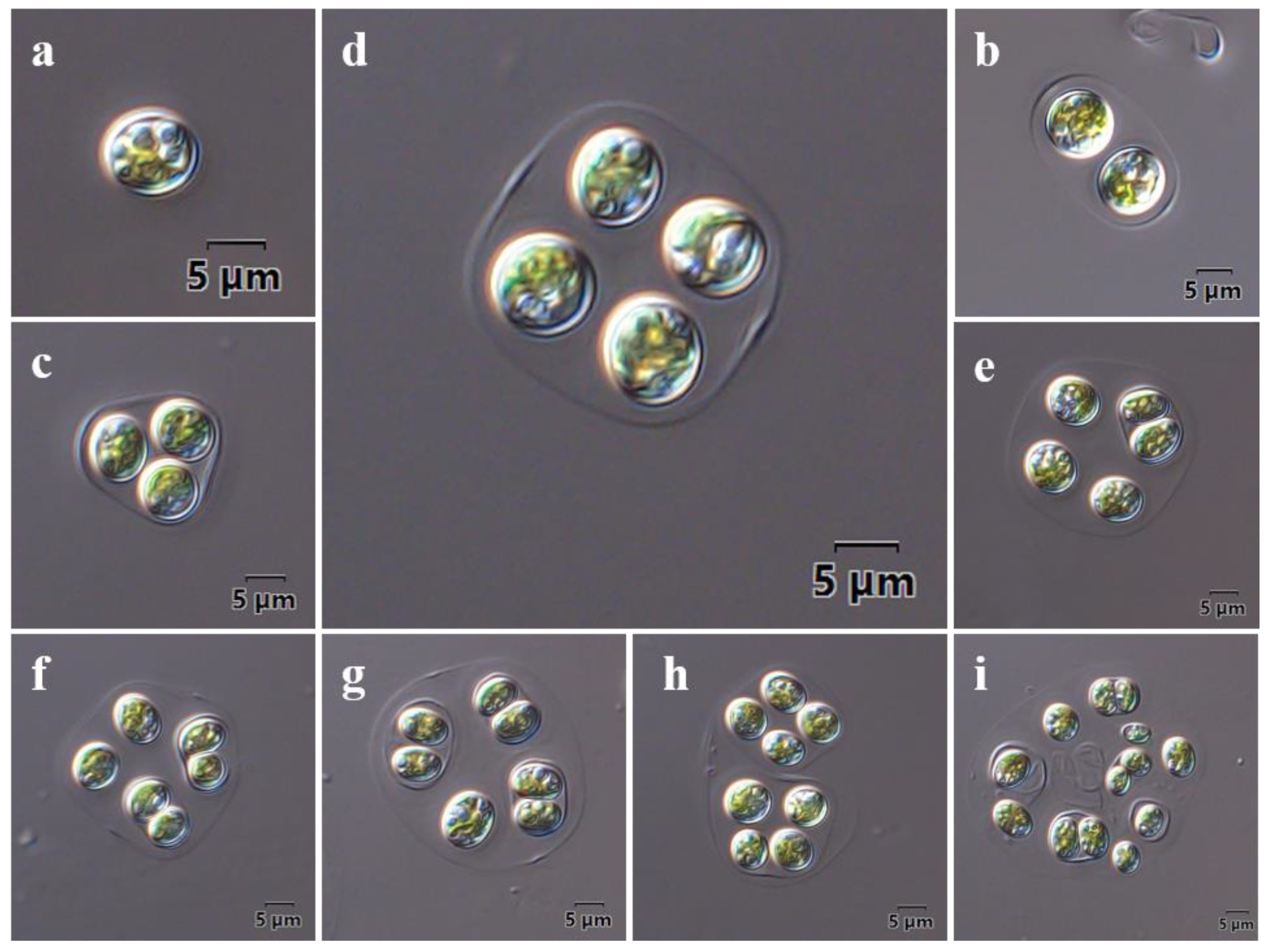
2.1. Morphological Observations
2.2. Molecular Phylogeny
3. Discussion
4. Materials and Methods
4.1. Strains and Culture Conditions
4.2. Morphological Observation
4.3. Molecular Analyses
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, M.; Huang, X.H.; Zhang, R.; Gu, B.H. Uptake of urea nitrogen by Oocystis borgei in prawn (Litopenaeus vannamei) aquaculture ponds. Bull. Environ. Contam. Toxicol. 2018, 101, 586–591. [Google Scholar] [CrossRef]
- Ajala, S.O.; Alexander, M.L. Assessment of Chlorella vulgaris, Scenedesmus obliquus, and Oocystis minuta for removal of sulfate, nitrate, and phosphate in wastewater. Int. J. Energy Environ. Eng. 2020, 11, 311–326. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, T.; Utsunomiya, K.; Kobayashi, K.; Owada, M.; Karube, I. Carbon dioxide fixation by a unicellular green alga Oocystis sp. J. Biotechnol. 1992, 25, 261–267. [Google Scholar] [CrossRef]
- Huang, X.H.; Li, C.L.; Liu, C.W.; Zeng, D.S. Study on the ecological factors of Oocystis borgei. J. Zhanjiang Ocean. Univ. 2002, 22, 8–12. (In Chinese) [Google Scholar]
- Wang, X.Q.; Zhang, Y.L.; Li, C.L.; Huang, X.H.; Li, F.; Wang, X.Y.; Li, G. Allelopathic effect of Oocystis borgei culture on microcystis aeruginosa. Environ. Technol. 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chuka-ogwude, D.; Ogbonna, J.; Borowitzka, M.A.; Moheimani, N.R. Screening, acclimation and ammonia tolerance of microalgae grown in food waste digestate. J. Appl. Phycol. 2020, 32, 3775–3785. [Google Scholar] [CrossRef]
- Vidyashankar, S.; Venugopal, K.S.; Swarnalatha, G.V.; Kavitha, M.D.; Chauhan, V.S.; Ravi, R.; Bansal, A.K.; Singh, R.; Pande, A.; Ravishankar, G.A.; et al. Characterization of fatty acids and hydrocarbons of Chlorophycean microalgae towards their use as biofuel source. Biomass Bioenergy 2015, 77, 75–91. [Google Scholar] [CrossRef]
- Komárek, J.; Fott, B. Chlorophyceae (Grünalgen) Ordnung: Chlorococcales. In Das Phytoplankton des Süßwassers: Systematik und Biologie; Huber-Pestalozzi, G., Ed.; Schweizerbart’sche Verlagsbuchhandlung: Stuttgart, Germany, 1983; Volume 7, pp. 1–1044. [Google Scholar]
- Silva, T.G.; Štenclová, L.; Archanjo, N.C.P.; Bagatini, I.L. Revised phylogenetic position of Nephrocytium nägeli (Sphaeropleales, Chlorophyceae), with the description of Nephrocytiaceae fam. nov. and Nephrocytium vieirae sp. nov. Taxon 2021, 70, 917–930. [Google Scholar] [CrossRef]
- Hepperle, D.; Hegewald, E.; Krienitz, L. Phylogenetic position of the Oocystaceae (Chlorophyta). J. Phycol. 2000, 36, 590–595. [Google Scholar] [CrossRef]
- Pažoutová, M.; Škaloud, P.; Nemjová, K. Phylogenetic position of Ooplanctella planoconvexa, gen. et comb. nova and Echinocoleum elegans (Oocystaceae, Trebouxiophyceae, Chlorophyta). Fottea 2010, 10, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Krienitz, L.; Bock, C. Elongatocystis ecballocystiformis gen. et comb. nov., and some reflections on systematics of Oocystaceae (Trebouxiophyceae, Chlorophyta). Fottea 2011, 11, 271–278. [Google Scholar] [CrossRef] [Green Version]
- Xia, S.; Zhu, H.; Cheng, Y.Y.; Liu, G.X.; Hu, Z.Y. Phylogenetic position of Ecballocystis and Ecballocystopsis (Chlorophyta). Fottea 2013, 13, 65–75. [Google Scholar] [CrossRef] [Green Version]
- Štenclová, L.; Fučíková, K.; Kaštovský, J.; Pažoutová, M. Molecular and morphological delimitation and generic classification of the family Oocystaceae (Trebouxiophyceae, Chlorophyta). J. Phycol. 2017, 53, 1263–1282. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhu, H.; Liu, B.; Liu, G.; Hu, Z. Classification of Planctonema-like algae, including a new genus Planctonemopsis gen. nov., a new species Planctonema gelatinosum sp. nov. and a reinstated genus Psephonema (Trebouxiophyceae, Chlorophyta). J. Phycol. 2017, 53, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhu, H.; Song, H.; Liu, B.; Wang, Q.; Liu, G.; Hu, Z. Quadricoccopsis gen. nov., a new genus of Quadricoccus-like algae in Oocystaceae from China (Trebouxiophyceae, Chlorophyta). Fottea 2018, 18, 189–199. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, H.; Song, H.; Wang, Q.; Xiong, X.; Wu, C.; Liu, G.; Hu, Z. Euchlorocystis gen. nov. and Densicystis gen. nov., two new genera of Oocystaceae algae from high-altitude semi-saline habitat (Trebouxiophyceae, Chlorophyta). J. Eukaryot. Microbiol. 2018, 65, 200–210. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Q.; Zhu, H.; Liu, B.; Rindi, F.; Liu, G.; Xie, S.; Hu, Z. Reticulocystis yunnanense gen. et sp. nov., a new member of freshwater Oocystaceae algae (Trebouxiophyceae, Chlorophyta). Eur. J. Phycol. 2020, 55, 507–516. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. Available online: https://www.algaebase.org (accessed on 25 October 2021).
- Campos, H.; Soto, D.; Parra, O.; Steffen, W.; Aguero, G. Limnological studies of Amarga lagoon, Chile: A saline lake in Patagonian South America. Int. J. Salt Lake Res. 1995, 4, 301–314. [Google Scholar] [CrossRef]
- Wen, Z.; Zhi-Hui, H. Biological and ecological features of inland saline waters in North Hebei, China. Int. J. Salt Lake Res. 1999, 8, 267–285. [Google Scholar] [CrossRef]
- Ramírez-Olvera, M.A.; Alcocer, J.; Merino-Ibarra, M.; Lugo, A. Nutrient limitation in a tropical saline lake: A microcosm experiment. Hydrobiologia 2009, 626, 5–13. [Google Scholar] [CrossRef]
- Chen, X.; Yang, J.; Chen, Y.; Hou, H. Seasonal dynamics of phytoplankton and its relationship with environmental factors of a Chinese Lake. Pol. J. Environ. Stud. 2016, 25, 1427–1433. [Google Scholar] [CrossRef]
- Huang, X.; Li, X.; Wang, Y.; Zhou, M. Effects of environmental factors on the uptake rates of dissolved nitrogen by a salt-water green alga (Oocystis borgei Snow). Bull. Environ. Contam. Toxicol. 2012, 89, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Hindák, F. Studies of the Chlorococcal algae (Chlorophyceae) IV. In Biologické Pracé; Ruzicka, M., Ed.; Veda: Bratislava, Slovakia, 1988; Volume 34, pp. 1–263. [Google Scholar]
- Stoyneva, M.P.; Cocquyt, C.; Gärtner, G.; Vyverman, W. Oocystis lacustris CHOD. (Chlorophyta, Trebouxiophyceae) in Lake Tanganyika (Africa). Linz. Biol. Beitr. 2007, 39, 571–632. [Google Scholar]


| Positions Compared: 1633. | Accession Number | Eremo. viridis | Ecball. dichotomus | Oocystis. marina | Euchloro. subsalina | Euchloro. marina | Oocystella. oogama | Oonephris. obesa |
|---|---|---|---|---|---|---|---|---|
| Eremosphaera viridis | AF387154 | — | 90.0% | 94.6% | 94.5% | 92.6% | 93.9% | 87.6% |
| Ecballocystopsis dichotomus | JX018184 | 163 | — | 90.4% | 90.3% | 88.1% | 90.6% | 82.7% |
| Oocystis marina | MF100784 | 88 | 157 | — | 96.7% | 94.7% | 95.8% | 86.6% |
| Euchlorocystis subsalina | MF100785 | 90 | 158 | 54 | — | 97.5% | 95.8% | 86.8% |
| Euchlorocystis marina | OM413748 | 121 | 194 | 87 | 41 | — | 94.1% | 84.6% |
| Oocystella oogama | KM020080 | 100 | 154 | 69 | 69 | 96 | — | 86.5% |
| Oonephris obesa | KY006558 | 202 | 283 | 219 | 216 | 251 | 220 | — |
| Morphology Character | Euchlorocystis subsalina | Euchlorocystis marina |
|---|---|---|
| Cell shape | Oval to elongated elliptical with round ends round and no thickenings | Round, oval, or slightly reniform without thickening polars |
| Cell size | 11.3–16.6 μm long and 6.3–10.3 μm wide | 6.9–12.3 μm long and 4.3–10.7 μm wide |
| Cell arrangement | Solitary, 2–16 cell colonies | Solitary, 2–16 cell colonies |
| Mucilage envelopment | Lemma- to square-shape | Usually irregular round shape, with or without gelled and thickened poles. |
| Cell wall | Thick, layered. | Thick, layered. |
| Chloroplasts number | Single | Single |
| Chloroplast shape | Wide trough shape, parietal | Horseshoe-shaped |
| Pyrenoids number per chloroplast | 2–6 | 1–4, within mature cells |
| Cell reproduction | Propagation by 2–4 autospores | Asexual reproduction by 2–4 autospores; There exist the asynchronous division in cell colonies |
| Reference | [17] | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Dong, M.; Zhang, N.; Zhang, Y.; Li, Q.; Qian, Z.; Lian, Q.; Luo, J.; Huang, X.; Li, C. Euchlorocystis marina sp. nov. (Oocystaceae, Trebouxiophyceae), a New Species of Green Algae from a Seawater Shrimp Culture Pond. Diversity 2022, 14, 119. https://doi.org/10.3390/d14020119
Li F, Dong M, Zhang N, Zhang Y, Li Q, Qian Z, Lian Q, Luo J, Huang X, Li C. Euchlorocystis marina sp. nov. (Oocystaceae, Trebouxiophyceae), a New Species of Green Algae from a Seawater Shrimp Culture Pond. Diversity. 2022; 14(2):119. https://doi.org/10.3390/d14020119
Chicago/Turabian StyleLi, Feng, Mingbiao Dong, Ning Zhang, Yulei Zhang, Qianru Li, Zuyuan Qian, Qingsheng Lian, Jiansen Luo, Xianghu Huang, and Changling Li. 2022. "Euchlorocystis marina sp. nov. (Oocystaceae, Trebouxiophyceae), a New Species of Green Algae from a Seawater Shrimp Culture Pond" Diversity 14, no. 2: 119. https://doi.org/10.3390/d14020119
APA StyleLi, F., Dong, M., Zhang, N., Zhang, Y., Li, Q., Qian, Z., Lian, Q., Luo, J., Huang, X., & Li, C. (2022). Euchlorocystis marina sp. nov. (Oocystaceae, Trebouxiophyceae), a New Species of Green Algae from a Seawater Shrimp Culture Pond. Diversity, 14(2), 119. https://doi.org/10.3390/d14020119







